Abstract
Background
An unusual 12 lead electrocardiographic pattern may be present in patients with cavotricuspid isthmus-dependent flutter.
Objective
Using baseline patient characteristics and echocardiography we sought to study predictors of unusual ECG characteristics in patients with cavotricuspid isthmus-dependent atrial flutter.
Methods
This was a dual center, retrospective cohort study of 147 patients undergoing electrophysiology study and ablation for cavotricuspid isthmus dependent atrial flutter.
Results
Among this cohort, 23 patients (16%) had unusual 12-lead ECG characteristics. Using multivariate logistic regression, we found two clinical predictors for having an unusual ECG pattern. A clockwise pattern at time of EPS was the strongest predictor of an unusual ECG pattern (odds ratio [OR] 15.3 (95% confidence interval [CI] 4.0–59.4, p<0.005). In addition, patients with decreased systolic function had a 3.5 greater odds (95% CI 1.1–11.5, p=0.037) of having an unusual ECG pattern.
Conclusions
Our data demonstrate that among patients suffering from cavotricuspid isthmus dependent atrial flutter who are referred for ablation, 16% will have unusual ECG patterns. Patients with clockwise atrial activation and LV dysfunction have greater odds of manifesting unusual patterns by surface electrocardiogram.
Keywords: Cavotricuspid isthmus-dependent flutter, Predictors, Unusual 12 lead ECG pattern, Ejection fraction
Introduction
Typical cavotricuspid isthmus-dependent atrial flutter is a common arrhythmia that is routinely cured by catheter ablation, with procedural success rates exceeding 95%1–4. Non-cavotricuspid isthmus dependent (atypical) atrial flutter circuits, conversely, are more challenging to ablate and the ablation procedure itself carries a higher risk of complication. Differentiating typical from unusual atrial flutter by surface ECG or other cinical data is therefore quite valuable.
Electrocardiographically, flutter refers classically to a pattern of regular tachycardia with rate > 240 beats per minute, lacking an isoelectric baseline between deflections5 (Figure 1). However, not all patients demonstrate typical appearing flutter waves on ECG and for those patients, the diagnosis of CTI dependent flutter may not be apparent until the patient is brought for electrophysiology study (EPS). Variable patterns exist for both counterclockwise CTI dependent (typical) and clockwise CTI dependent (reverse typical) flutter circuits and have been extensively described previously6–9. The terms typical and reverse typical are as defined by the NASPE working group 20015. These patterns appear frequently and have been associated with heart disease and left atrial abnormalities6–8. The purpose of our study was to show how often patients with documented cavotricuspid isthmus dependent atrial flutter have unusual ECG patterns.
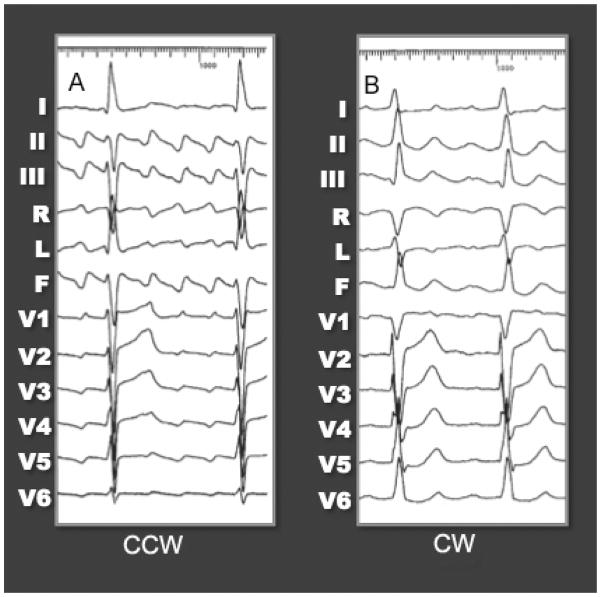
Figure 1.
ECG pattern of CCW (typical) atrial flutter (A) and CW (reverse typical) atrial flutter (B). It is important to remember, that for typical AFL, the flutter waves should have a consistent beat to beat morphology, with minimal variation and cycle length, and should be negative in the inferior leads, positive in V1, and progressively more negative across the precordial leads. However, atrial flutter can go in the CW direction as well, giving a different, appearance to the ECG (B).
We sought to establish the prevalence of these unusual flutter wave patterns among patients referred to tertiary electrophysiology centers for curative ablation procedures and to better characterize this cohort for purposes of aiding in the diagnosis of cavotricuspid isthmus-dependent atrial flutter.
Materials and Methods
Patients
We studied a sample of 147 patients with cavotricuspid isthmus-dependent (CTI) atrial flutter referred to the University of California, San Francisco and San Diego campuses for electrophysiology study (EPS) and ablation from 2001 to 2006. Patients underwent a full EPS prior to ablation. CTI dependent flutter was confirmed by entrainment mapping and successful isthmus ablation. The medical records prior to the ablation procedure were reviewed for baseline characteristics, medical history, and echocardiographic data.
Electrophysiology Study
Informed witnessed consent was obtained from all patients. Standard EPS and ablation of atrial flutter was completed in all patients. All catheters were placed in the standard fashion using right femoral vein and right internal jugular vein access upon the discretion of the attending electrophysiologist. A duodecapolar catheter was placed around the tricuspid annulus, decapolar catheter into the coronary sinus, and quadripolar catheter in the His position.
Atrial flutter was determined to be the arrhythmia diagnosis based on 1) a compatible atrial activation sequence; 2) entrainment with manifest fusion resulting from pacing atrial tissue extrinsic to the flutter circuit, 3) entrainment with concealed fusion resulting from atrial pacing from the cavotricuspid isthmus with resultant orthodromic activation of the reentrant circuit as during the spontaneous atrial flutter, with virtually no or no antidromic activation.
After determination of a target arrhythmia, a deflectable-tip mapping/ablation catheter was advanced to the tricuspid annulus. RF applications were first applied at the 6 o’clock left anterior oblique (LAO) location at the cavotricuspid isthmus. Each application had the following characteristics: the catheter was initially placed on the ventricular aspect of the TA where energy delivery at 50 W targeting 60°C was begun. This was followed by a series of discrete applications along a line between the TA and IVC orifice. Where necessary additional septal or inferior lines were drawn.
At the completion of the ablation line, all patients underwent pacing maneuvers to document bidirectional block in the cavotricuspid isthmus.
ECG Analysis
A 12 lead electrocardiogram was obtained during the electrophysiology study while the patient was in atrial flutter. ECGs were recorded at standard paper speed of 25mm/second and a gain of 1 mV/cm. The sensitivity was increased at times to better define the flutter wave morphologies. When obvious flutter wave morphology was not apparent, atrio-ventricular block maneuvers (e.g. carotid sinus massage or adenosine) were used for clarification.
The electrocardiogram was then reviewed by two independent reviewers and coded on the presence of flutter wave morphology in all 12 leads. Coding of the flutter waves was based on standard criteria (positive +, negative −, isoelectric, or biphasic)10. If the two independent reviewers did not agree than a third expert was consulted. Reviewers were blinded to the clinical scenario and outcomes.
Counterclockwise (CCW or typical) atrial flutter was defined as usual in the presence of negative F waves in the inferior leads (−, biphasic dominant −) and positive flutter waves in precordial lead V1 (+, biphasic dominant +)10. Clockwise (CW or reverse typical) atrial flutter was defined as usual in the presence of positive flutter waves in the inferior leads (+, biphasic dominant +) and negative flutter waves in V1 (−, biphasic dominant −)10.
Absence of typical CCW or CW flutter wave patterns (as described above) were coded as “Unusual.”
Statistics
Continuous variables are expressed as means with standard deviations and were compared using Student’s t-test. Categorical variables are expressed as percentages and were compared using the chi-squared test.
Multivariable logistic regression was used to determine predictors that were independently associated with an unusual ECG pattern. The predictor variables exhibiting differences between groups with a p value of <0.10 in the univariate analysis qualified for entering the multivariate model. A two-tailed p value of <0.05 was considered statistically significant.
Statistical Analysis was perfomed using STATA software, version 10.0 (StatCorp, 4905 Lakeway Drive, College Station, Texas 77845).
Results
The study population consisted of a total of a 147 patients with EPS-defined diagnosis of cavotricuspid isthmus-dependent atrial flutter. 90.5% of these patients had a counterclockwise pattern of atrial activation, while 9.5% had a clockwise pattern. Twenty-three patients (16%) had unusual 12-lead ECG characteristics.
The mean age was 60 ± 13 years of age with a male predominance (85.7%). The mean left ventricular ejection fraction was 56.0± 14%. There was a high prevalence of prior manifest atrial fibrillation (23.8%), structural heart disease (36.7%), left atrial enlargement (24%), and previous cardiothoracic surgery (49.7%). All patient characteristics are shown in Table 1.
Table 1.
Comparison of baseline Characteristics, stratified by ECG pattern
| Characteristic (%) | All (n=147) | Typical (n=124) | Unusual (n=23) | p value* |
|---|---|---|---|---|
| Age (years) | 60 ± 13 | 59±13 | 61±13 | 0.50 |
| Male | 85.7 | 86.3 | 82.6 | 0.64 |
| LVEF | 56.0 ± 14 | 57±13 | 50±16 | 0.03 |
| Atrial Fibrillation | 23.8 | 23.4 | 26.1 | 0.78 |
| Structural Heart Disease | 36.7 | 34.7 | 47.8 | 0.23 |
| Congestive Heart Failure | 15.6 | 12.1 | 34.8 | 0.006 |
| Hypertension | 25.1 | 26.6 | 17.4 | 0.35 |
| Diabetes | 3.4 | 4.0 | 0 | 0.33 |
| Hyperlipidemia | 4.8 | 4.8 | 4.4 | 0.92 |
| Left Atrial Enlargement (ECHO) | 24.1 | 23.0 | 30.4 | 0.44 |
| Left Ventricular Hypertrophy (ECHO) | 10.2 | 11.3 | 4.4 | 0.31 |
| Valve Disease | 2.0 | 2.4 | 0 | 0.45 |
| COPD | 2.7 | 3.2 | 0 | 0.38 |
| Previous PVI | 2.7 | 3.2 | 0 | 0.38 |
| ACEI or ARB | 20.4 | 20.2 | 21.7 | 0.86 |
| Previous CT Surgery | 49.7 | 51.6 | 39.3 | 0.27 |
| CW Pattern on EPS | 9.5 | 4.8 | 34.8 | <0.005 |
| CL (msec) | 260.4 ±35 | 254 ±31 | 286 ±38 | <0.005 |
p value represents comparison of typical versus unusual ECG pattern
Comparative analysis of baseline characteristics based on usual versus unusual ECG criteria demonstrate multiple significant characteristics. Patients with unusual ECG characteristics had a lower ejection fraction (50 ±16 versus 57 ±13, p=0.03), were more likely to have congestive heart failure (34.8% versus 12.1 %, p=0.006), more frequently manifest a clockwise pattern on EPS (34.8% versus 4.8%, p<0.005), and had longer atrial cycle lengths (mean CL 286 ±38 versus 254 ±31 msec, p=<0.005).
Unusual ECG Form
For the entire group of unusual ECG patterns (n=23), the most common unusual pattern was diffuse low amplitude flattening of the flutter wave in I, aVL, V5/6 in 13/23 (57%) of the cases (Figure 2). Flattening was also seen in the inferior leads but at a much lower rate, 10/23 (43%). The morphology in V1 was variable with 10/23 (43%) revealing positive flutter waves (Figure 3), (5/23) 22% flat, and (4/23) 17% fractionated (poorly defined low amplitude signals). Many of the unusual patterns seen mimic atrial fibrillation.
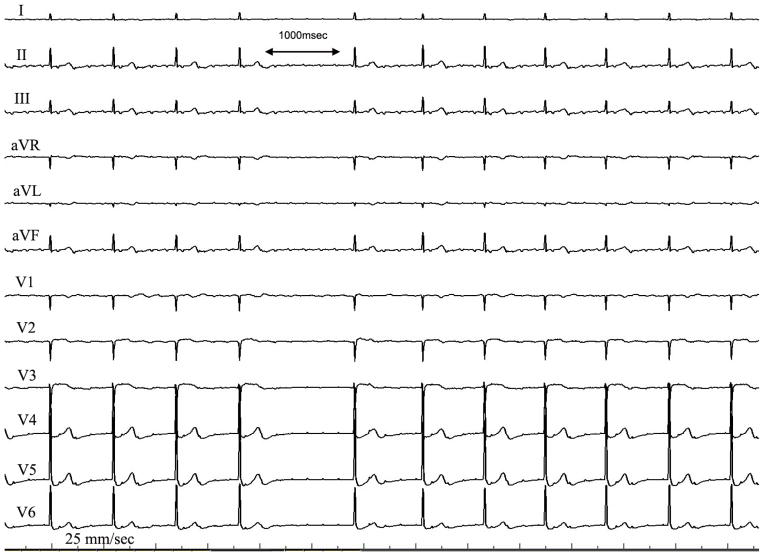
Figure 2.
12 lead ECG of the unusual pattern seen in CTI flutter. Note the diffuse low amplitude flutter waves seen in most leads, but especially prominent in leads I, V5/6. The ECG is recorded at 25 mm/sec with a 1 mV/cm voltage. Note that the low amplitude tracing may mimic that of atrial fibrillation.
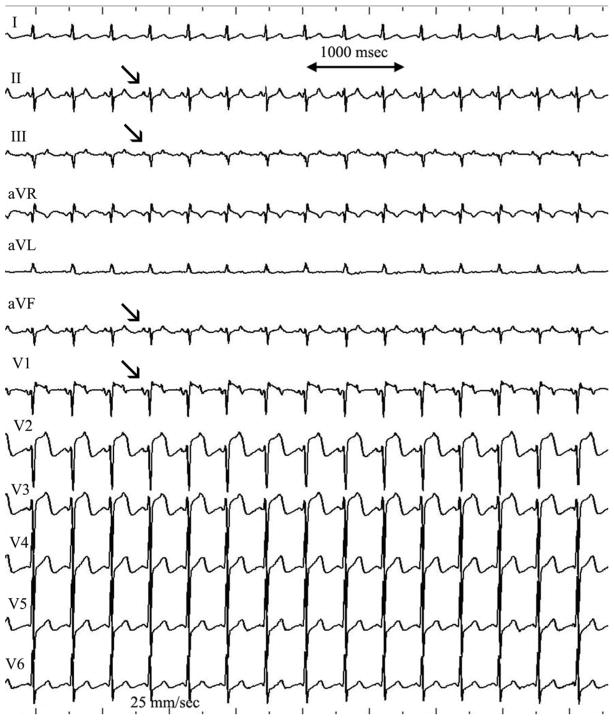
Figure 3.
12 lead electrocardiogram of an unusual pattern seen in clockwise CTI dependent atrial flutter. Note the positive flutter waves seen in both V1 and the inferior leads (arrows). The flutter wave in V1 is biphasic with an initial positive deflection followed by a negative deflection. The ECG is recorded at 25 mm/sec with a 1 mV/cm voltage.
In the subgroup of patients with a clockwise activation pattern (n=8), 5/8 (63%) revealed flattening of the flutter wave in I, aVL, V5/6 and 3/8 (38%) in the inferior leads. There was no clear dominant pattern in V1, with the highest percentage being fractionated 3/8 (38%). Other patterns included positive flutter waves in both V1 and the inferior leads 1/8 (12.5%, see Figure 3) and positive flutter waves in V1 along with prolonged duration of biphasic flutter waves with curtailed diastolic interval in the inferior leads (3/8, 38%, figure 4).
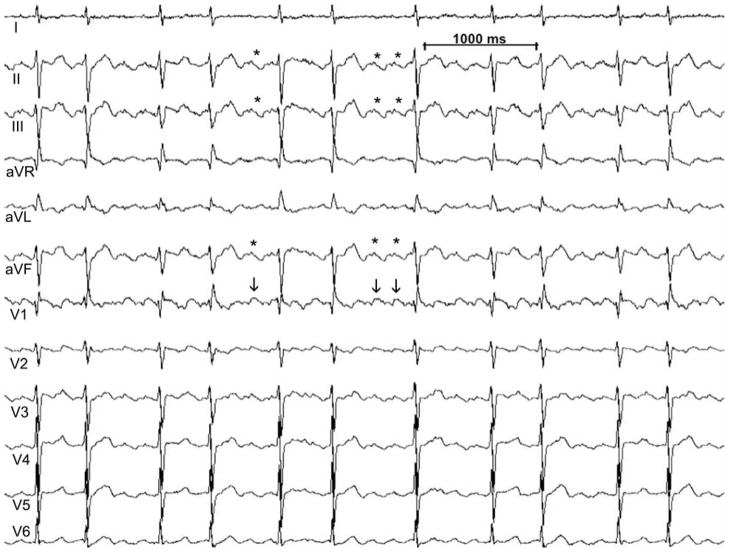
Figure 4.
12 lead electrocardiogram of a pattern seen in clockwise CTI dependent atrial flutter, 3/8 patients. Note the positive flutter waves seen in V1 (vertical arrows) and the prolonged duration of biphasic flutter waves with curtailed diastolic interval in the inferior leads (asterisks). This ECG pattern may be confused with atrial fibrillation. The ECG is recorded at 25 mm/sec with a 1 mV/cm voltage.
In patients with reduced systolic function (n=11), the flattening in the lateral leads was even more prominent with 8/11 (73%) of patients having flattening of the flutter waves in V5/6. V1 was more variable with the most common finding of a fractionated potential 3/11 (27%).
Univariate Analyses
Univariate analayses of baseline and echocardiography provide three statistically significant predictors of unusual ECG flutter wave patterns. These include reduced ejection fraction (EF<55%) (RR of 2.2, p=0.033), congestive heart failure (RR of 2.88, p=0.006), and clockwise pattern on EPS (RR 5.1 and p<0.005). Severely reduced ejection fraction (EF<35%) had a relative risk of 1.85, but was not statistically significant (p=0.22). All relative risk ratios are shown in Table 2.
Table 2.
Univariate Analysis, Relative Risk ratio for Unusual ECG pattern
| Characteristic (%) | RR | 95%CI | p value |
|---|---|---|---|
| Age | 1.01 | 0.978–1.05 | 0.50 |
| Age >50 years | 0.54 | 0.18–1.18 | 0.13 |
| Male | 0.79 | 0.29–2.10 | 0.64 |
| Reduced Ejection fraction (EF<55) | 2.21 | 1.06–4.63 | 0.033 |
| Severely reduced EF<35 | 1.85 | 0.73–4.73 | 0.22 |
| Atrial Fibrillation | 1.13 | 0.48–2.64 | 0.78 |
| Structural Heart Disease | 1.57 | 0.75–3.33 | 0.23 |
| Congestive Heart Failure | 2.88 | 1.38–5.99 | 0.006 |
| Hypertension | 0.63 | 0.23–1.72 | 0.35 |
| Diabetes | N/A | ||
| Hyperlipidemia | 0.91 | 0.14–5.81 | 0.92 |
| Left Atrial Enlargement (ECHO) | 1.38 | 0.62–3.07 | 0.44 |
| Left Ventricular Hypertrophy (ECHO) | 0.40 | 0.58–4.87 | 0.31 |
| Valve Disease | N/A | ||
| COPD | N/A | ||
| Previous PVI | N/A | ||
| ACEI or ARB | 1.1 | 0.44–2.68 | 0.86 |
| Previous CT Surgery | 0.65 | 0.30–1.41 | 0.27 |
| CW Pattern on EPS | 5.1 | 2.62–9.78 | <0.005 |
| CL >250(msec) | 2.44 | 0.77–7.74 | 0.10 |
Multivariate Analysis
Of the three statistically significant variables demonstrated by univariate analysis, only two variables remained statistically significant in multivariate analysis (Table 3). These were reduced ejection fraction (OR 3.5, p=0.037) and CW pattern on EPS (OR 15.3, p<0.005). Although a clinical diagnosis of congestive heart failure was significant in univariate analysis, it failed to reach statistical significance by multivariate analysis (p=0.25).
Table 3.
Multivariate Analysis of variables that were significant by univarate assessment for an unusual surface ECG pattern.
| Characteristic (%)* | OR | 95%CI | p value |
|---|---|---|---|
| Reduced Systolic function (EF<55) | 3.5 | 1.08–11.35 | 0.037 |
| Congestive Heart Failure | 2.04 | 0.60–6.86 | 0.251 |
| CW Pattern on EPS | 15.3 | 3.96–59.4 | <0.005 |
Only variables exhibiting differences between groups with a p value of <0.10 in the univariate analysis qualified for entering the multivariate model
Discussion
Unusual 12 lead ECG patterns in patients with cavotricuspid isthmus-dependent atrial flutter is common; such patterns were seen in about 16% of patients in our cohort. Identifying patients who are likely to have typical CTI AFL despite what may be considered an unusual pattern by surface electrocardiogram, will have significant bearing on the decision of whether or not to consider EPS and ablation. Our study shows that there are clinical parameters which help to significantly modify the pre-test probability that a patient with an unusual ECG pattern may have typical CTI AFL.
Our results suggest that there are two clinical predictors in patients with typical atrial flutter (as diagnosed at EPS) for having an unusual ECG pattern. First, patients with any decrease in systolic function have an odds ratio of 3.5 (p=0.037) of having an unusual ECG pattern. This is clinically important as a diagnosis of congestive heart failure comprised approximately 16% of our population. Although previous studies have showed an unusual ECG pattern correlation with structural heart disease and abnormal left atrial enlargement6, to our knowledge this is the first time that ejection fraction was found to be associated with an unusual flutter pattern on ECG.
Secondly, clockwise pattern at time of EPS in our study was the strongest predictor of an unusual ECG pattern and had an odds ratio of 15.3 (p<0.005).
Waldo showed that the ECG pattern of flutter is due to left atrial activation.11 Unusual patterns may appear when the left atrium is diseased and associated with altered sequence of atrial activation. It is plausible that patients with decreased LV systolic function and other structural heart disease have concomitant atrial fibrosis and atrial dilation. Our study showed a trend towards an increased atrial cycle length (>250 msec) predicting an unusual ECG pattern (p=0.1). Although this was not statistically significant it is hypothesis generating.
Prior studies
Saoudi et al12, described the ECG characteristics among a cohort of 8 patients with clockwise CTI dependent flutter, 20 patients with counterclockwise CTI dependent flutter, and 10 patients who had both counterclockwise and clockwise flutter. The most consistent ECG finding in clockwise CTI dependent flutter in their study was a shorter plateau phase, a widening of the negative component of the flutter wave inferiorly, and a negative f wave in V1. The classic “sawtooth pattern” was seen in 14 of the 18 with a clockwise pattern, and this pattern was defined as “a mostly negative deflection in the inferior leads followed by a low-amplitude positive notch preceding a slightly descending plateau.” Thus the inferior leads were found to be similar to that described for patients with counterclockwise CTI dependent flutter. Among the four clockwise patients who did not have the classic “sawtooth patterns”, 3 showed positive f waves inferiorly and the remaining patient the flutter wave was sinusoidal12. In contrast, our study included a total of 14 patients with clockwise CTI dependent flutter and 6 of the 14 had positive flutter waves in the inferior leads as described by others13. In the remainder, the most common pattern was flattening of the flutter waves. This flattening was most prominent in leads I, aVL, V5/6 (5/8, 63%) but was also recorded in the inferior leads (3/8, 38%).
Other findings were prolonged duration of the flutter wave with a curtailed plateau similar to the that described by Saoudi et al12 (figure 4), and a single patients (1/8, 12.5%) with positive flutter waves in both V1 and the inferior leads. Of interest, none of our patients with clockwise CTI dependent flutter had totally negative deflections in the inferior leads.
Our study, like Milliez et all6, showed that among our cohort, there was a high prevalence of structural heart disease (37%) and left atrial enlargement (24%). However, we did not find these characteristics to independently predict the presence of an unusual flutter wave pattern on surface ECG. Our study adds to the growing body of literature that highly prevalent concomitant cardiovascular conditions may alter the standard electrocardiographic phenotype of cavo-tricuspid isthmus-dependant atrial flutter and that these conditions will need to be weighed when considering the potential therapeutic options available to patients with unusual appearing atrial flutter.
Previous work by Chugh et al14 suggests that CTI dependent flutter that occurs after pulmonary vein isolation often has an unusual ECG pattern due to altered left atrial activation. In our cohort, only 4 patients (3%) had previously undergone this procedure and none of them had unusual ECG characteristics. With so few cases, our study lacks the power to comment on this relationship and was not considered in our univariate or multivariate analyses.
Limitations
Our study is limited by the retrospective nature of the data collection. We started with the gold standard diagnosis of EPS and ablation defined CTI flutter and then evaluated the ECG characteristics. Although this is a limitation, we thought this was important first step in defining our population as those exclusively with CTI flutter. The next step would be to prospectively evaluate these findings in all patients referred for flutter EPS and ablation. This population would include non-CTI flutter patients.
All patients were referred to specialty tertiary care centers specifically for consideration of atrial flutter ablation and therefore there may be selection bias in favor of patients with a typical atrial flutter pattern. Generalizing these results to all patients with flutter seen in clinical practice may be biased.
Conclusions
Our data demonstrate that among patients suffering from typical atrial flutter who are referred for EPS and ablation, a small, but substantial, number of patients will have unusual ECG patterns readily curable with current ablation procedures. Patients with CW atrial activation and LV dysfunction have greater odds of manifesting unusual patterns by surface electrocardiogram. The most common unusual ECG pattern was flattening and/or fractionation of the flutter waves which may mimic atrial fibrillation.
Acknowledgments
Funding Sources: None
Abbreviations
- CTI
Cavotricuspid isthmus-dependent
- ECG
Electrocardiogram
- EF
Ejection fraction
- EPS
Electrophysiology Study
- CW
Clockwise
- CCW
Counterclockwise
Footnotes
Author Contributions
Kurt S. Hoffmayer, MD: main author, concept/design, data analysis/Interpretation, drafting article.
Yanfei Yang, MD: concept/design
Stephen Joseph, MD, PhD: concept/design
McCabe James M MD: Data analysis/Interpretation
Prashant Bhave, MD: Data analysis/Interpretation
Hsu Jonathan, MD: Data analysis/Interpretation
Ramford K. Ng, MD: Data collection
Byron K Lee, MD, MAS: Critical Revision of article
Nitish Badhwar, MBBS: Critical Revision of article
Randall J Lee, MD, PhD: Critical Revision of article
Zian H Tseng, MD, MAS: Critical Revision of article
Jeffrey E Olgin, MD: Critical Revision of article
Sanjiv M. Narayan, MD: Critical Revision of article
Gregory M Marcus, MD, MAS: Data Analysis/Interpretation
Melvin M. Scheinman, MD: Concept/design, critical revisions of manuscript, data analysis/interpretation
References
- 1.Calkins H, Leon AR, Deam AG, Kalbfleisch SJ, Langberg JJ, Morady F. Catheter ablation of atrial flutter using radiofrequency energy. Am J Cardiol. 1994 Feb 15;73(5):353–356. doi: 10.1016/0002-9149(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 2.Lee KW, Yang Y, Scheinman MM University of Califoirnia-San Francisco SF, CA, USA. Atrial flutter: a review of its history, mechanisms, clinical features, and current therapy. Curr Probl Cardiol. 2005 Mar 1;30(3):121–167. doi: 10.1016/j.cpcardiol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Scheinman MMYY. Atrial Flutter: Historical Notes-PArt 1. PACE. 2004 Feb 28;:1–3. doi: 10.1111/j.1540-8159.2004.00446.x. [DOI] [PubMed] [Google Scholar]
- 4.Scheinman MM, Yang Y, Cheng J. Atrial flutter: Part II Nomenclature. Pacing Clin Electrophysiol. 2004 Apr 1;27(4):504–506. doi: 10.1111/j.1540-8159.2004.00472.x. [DOI] [PubMed] [Google Scholar]
- 5.Saoudi N, Cosio F, Waldo A, et al. Classification of atrial flutter and regular atrial tachycardia according to electrophysiologic mechanism and anatomic bases: a statement from a joint expert group from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J Cardiovasc Electrophysiol. 2001 Jul;12(7):852–866. doi: 10.1046/j.1540-8167.2001.00852.x. [DOI] [PubMed] [Google Scholar]
- 6.Milliez P, Richardson AW, Obioha-Ngwu O, Zimetbaum PJ, Papageorgiou P, Josephson ME. Variable electrocardiographic characteristics of isthmus-dependent atrial flutter. J Am Coll Cardiol. 2002 Sep 18;40(6):1125–1132. doi: 10.1016/s0735-1097(02)02070-3. [DOI] [PubMed] [Google Scholar]
- 7.Scheinman MM, Cheng J, Yang Y. Mechanisms and clinical implications of atypical atrial flutter. Journal of Cardiovascular Electrophysiology. 1999 Aug 1;10(8):1153–1157. doi: 10.1111/j.1540-8167.1999.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Cheng J, Bochoeyer A, et al. Atypical right atrial flutter patterns. Circulation. 2001 Jun 26;103(25):3092–3098. doi: 10.1161/01.cir.103.25.3092. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmayer KS, Goldschlager N. Pseudoatrial flutter. J Electrocardiol. 2008 May-Jun;41(3):201. doi: 10.1016/j.jelectrocard.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Kistler PM, Roberts-Thomson KC, Haqqani HM, et al. P-wave morphology in focal atrial tachycardia: development of an algorithm to predict the anatomic site of origin. J Am Coll Cardiol. 2006 Sep 5;48(5):1010–1017. doi: 10.1016/j.jacc.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 11.Waldo AL. Some observations concerning atrial flutter in man. Pacing Clin Electrophysiol. 1983 Sep;6(5 Pt 2):1181–1189. doi: 10.1111/j.1540-8159.1983.tb04456.x. [DOI] [PubMed] [Google Scholar]
- 12.Saoudi N, Nair M, Abdelazziz A, et al. Electrocardiographic patterns and results of radiofrequency catheter ablation of clockwise type I atrial flutter. J Cardiovasc Electrophysiol. 1996 Oct;7(10):931–942. doi: 10.1111/j.1540-8167.1996.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalman JM, Olgin JE, Saxon LA, Lee RJ, Scheinman MM, Lesh MD. Electrocardiographic and electrophysiologic characterization of atypical atrial flutter in man: use of activation and entrainment mapping and implications for catheter ablation. J Cardiovasc Electrophysiol. 1997 Feb;8(2):121–144. doi: 10.1111/j.1540-8167.1997.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 14.Chugh A, Latchamsetty R, Oral H, et al. Characteristics of cavotricuspid isthmus-dependent atrial flutter after left atrial ablation of atrial fibrillation. Circulation. 2006 Feb 7;113(5):609–615. doi: 10.1161/CIRCULATIONAHA.105.580936. [DOI] [PubMed] [Google Scholar]