Summary
Background
It has been reported by multiple laboratories that the quantitation of factor (F)VIII by activity-based assays is influenced by the method, procedure and the quality of reagents used in the assays.
Objective
To evaluate the influence of von Willebrand factor (vWF) on FVIII activity in vitro.
Methods
The APTT and synthetic coagulation proteome assays were used. Citrated FVIII/vWF-depleted substrate plasma (SP; both antigens <0.5%) was used in all APTT assays.
Results
The concentration of FVIII antigen in pooled plasma from healthy donors (NP) was 1.5 nM. The SP reconstituted with 1.5 nM recombinant (r)FVIII clotted in 23.8±0.2 s (S.D.). The addition of 10 μg/ml vWF to the SP increased the clotting time to 28.7±0.1 s, i.e. the activity of rFVIII (FVIIc) decreased to 50%. This inhibitory effect of vWF decreased with decreasing rFVIII concentration in SP and became negligible at rFVIII ≤10% (150 pM). The FVIIIc of 1.5 nM FVIII in two products formulated with vWF (Immunate and Hemofil M) was not affected by the addition of exogenous vWF to the SP, whereas the FVIIIc of the B-domainless rFVIII product ReFacto was decreased 64% by the addition of vWF. In the synthetic coagulation proteome triggered with 5 pM tissue factor, vWF had no effect on thrombin generation.
Conclusions
vWF has an inhibitory effect on the measurement of FVIII clotting activity. This effect depends upon the structure and formulation of the FVIII product.
Keywords: factor VIII, von Willebrand factor, clotting activity, factor VIII products, tissue factor, synthetic coagulation proteome
Introduction
Factor (F) VIII is a nonenzymatic procofactor, which upon activation is converted to its active form FVIIIa. FVIIIa binds FIXa and forms a complex enzyme on the membrane surface, intrinsic FXase, which converts zymogen FX to serine protease FXa(1). FVIII circulates in vivo as a heterodimer consisting of a heterogenous NH2-terminal heavy chain and a COOH-terminal light chain associated in a divalent metal ion-dependent manner(2). FVIII forms a complex through the light chain with von Willebrand factor (vWF), which protects FVIII from proteolysis and concentrates FVIII at the site of active hemostasis(3;4). Upon activation, the light chain of FVIII dissociates from vWF, and the generated FVIIIa is capable to bind FIXa and transiently express cofactor activity(5).
It has been shown in previous publications that vWF inhibits FVIII activation by FXa(6–9). No inhibition of FVIII activation and activity by vWF was observed when thrombin was used as an activator(6;10). All of these activation experiments were performed using purified proteins, supraphysiologic concentrations of FVIII and a preformed activator, i.e. FXa and thrombin at the concentrations selected somewhat randomly. However, it is not clear what effect on FVIII activation/activity vWF has at physiologic conditions when both potential FVIII activators are formed at the same time and both proteins (FVIII and vWF) are present at physiologically relevant concentrations.
In this study, we evaluate the influence of vWF on FVIII activity in the APTT clotting assay using FVIII/vWF-depleted substrate plasma and in the synthetic coagulation proteome triggered with TF(11;12).
Materials and methods
Materials
All FVIII products were provided by Baxter Health Care Corp. (Duarte, CA). Albumin-free recombinant FVIII (rFVIII) produced in CHO cells was used as the calibrator (standard) in all assays. The concentration of this product (0.62 mg/ml) was established by the absorbance at 280 nm using an extinction coefficient E0.1% value of 1.3(13). Three lots of purified human vWF and polyclonal goat anti-vWF antibody were provided as gifts by Dr. R. Jenny from Haematologic Technologies, Inc (Essex Junction, VT). FVIII/vWF-immunodepleted plasma (substrate plasma) was purchased from Precision Biologic (Dartmouth, Canada). Congenital FVIII-deficient plasma (lot #GK 884-17c1) was purchased from George King Bio Medical, Inc; Overland Park, KS). The APTT reagent was purchased from Trinity Biotech PLC Bray (Wicklow, Ireland). 10-donor normal plasma, biotin-labeled monoclonal anti-FVIII heavy-chain antibody 24 (α-FVIII-24) and monoclonal anti-vWF antibody (α-vWF-99) were produced in house. Monoclonal anti-FVIII light-chain antibody 68 (α-FVIII-68) was produced by Dr. D. Fass. HRP-streptavidin was purchased from Sigma (St. Louis, MO), chromogenic substrate from KPL (Gaitherburg, MD) and HRP-goat anti-mouse Ig was purchased from Amersham Life Sciences (Arlington Heights, IL). Human coagulation factors VII, X, IX, and prothrombin were isolated from fresh frozen plasma using the methods of Bajaj et al.(14) and FXI using the monoclonal anti-FXI antibody α-FXI-2. All preparations were purged of trace contaminants and traces of active enzymes as described(15). Human FV and AT-III were isolated from freshly frozen citrated plasma(16;17). Citrated plasma for protein purification was purchased from Haematologic Technologies, Inc. rTF1-242 (expressed in E. coli) was provided as a gift from Drs. Shu Len Liu and Roger Lundblad (Baxter Healthcare Corp). Human rFVIIa was provided as a gift by Dr. Ula Hedner (Novo Nordisk, Denmark). Full-length recombinant tissue factor pathway inhibitor (TFPI) was a gift from Dr. S. Hardy (Chiron Corp, Emeryville, CA). 1,2-Dioleolyl-sn-Glycero-3-Phospho-L-Serine (PS) and 1,2-Dioleoyl-sn-Glycero-3-Phosphocholine (PC) were purchased from Avanti Polar Lipids, Inc (Alabaster, AL).
Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) and β-mercaptoethanol were purchased from Sigma (St Louis, MO). Phospholipid vesicles (PCPS) composed of 25% PS and 75% PC were prepared as described(18). TF was relipidated into PCPS vesicles (molar ratio 1:5000) as described(19). Spectrozyme TH was purchased from American Diagnostica, Inc (Greenwich, CT).
ELISA
FVIII
The assay utilized a double-sandwich format using α-FVIII-68 as a capture antibody and biotin-labeled α-FVIII-24 as a probe (20). α-FVIII-68 was immobilized in the wells of microtiter plate (50 μl/well at 5 μg/ml concentration). To eliminate the influence of vWF on FVIII antigen assay, test samples were reduced with 10 mM β-mercaptoethanol for 2 hr as described previously (21). At this concentration, β-mercaptoethanol had no effect on the quantitation of vWF-free FVIII. Sequential dilutions of reduced FVIII products were added to α-FVIII-68-coated wells. FVIII binding was probed with biotin-labeled α-FVIII-24 (50 μl/well at 5 μg/ml concentration) and detected using HRP-streptavidin and a chromogenic substrate. The concentration of FVIII protein in the products was estimated from the calibration curve built with the albumin-free rFVIII standard. The detection limit of this assay is 30 pM.
vWF
For the double-sandwich assay, polyclonal goat anti-vWF antibody was immobilized in the wells of a microtiter plate (50 μl/well at 5 μg/ml). Sequential dilutions (1.25–80 ng/ml) of purified vWF in PBS were (50 μl) were added to coated wells. 50 μl of α-vWF-99 (10 μg/ml) were added and incubated for an hour. Binding was detected with HRP-goat anti-mouse IgG and a chromogenic substrate. The detection limit of this assay is 2.5 ng/ml (0.025% of mean physiologic vWF concentration).
APTT
FVIII/vWF-immunodepleted substrate plasma was reconstituted with purified vWF and FVIII at the desired final concentrations (total volume of sample was 100 μl and additions to plasma did not exceed 5 μl). When desired, vWF was incubated at room temperature for 5 min with either polyclonal goat anti-vWF antibody or α-vWF-99 (0.1 mg/ml). Alternatively, substrate plasma was mixed with 10-donor normal plasma (contains 1.5 nM FVIII antigen) at various proportions. The reconstituted plasma was incubated for 5 min at 37° with 100 μl of the APTT reagent. Clotting was initiated by the addition of 100 μl 25 mM CaCl2 and clotting times were determined using the ST8 instrument (Diagnostica Stago, Parsippany, NJ). To build a calibration curve, FVIII/vWF-depleted substrate plasma was reconstituted with various concentrations (21–1500 pM) of albumin-free full-length rFVIII.
Synthetic Coagulation Proteome
The procedure used was a modification of Lawson et al.(11) and van ‘t Veer et al.(15). Relipidated TF at 5 pM final concentration was added to the mixture of vWF (omitted when desired), factors V, VII, VIIa, VIII, IX, X, and XI, prothrombin, TFPI and AT-III (all at mean physiologic concentrations) in HBS, 2 mM CaCl2 containing 2 μM PCPS. Thrombin generation over time was measured in a chromogenic assay using Spectrozyme TH and a Molecular Devices (Sunnyvale, CA) THERMOmax microplate reader.
Results
Standard deviations of the clotting times in the APTT assays did not exceed 1 s.
Titration of normal plasma and rFVIII
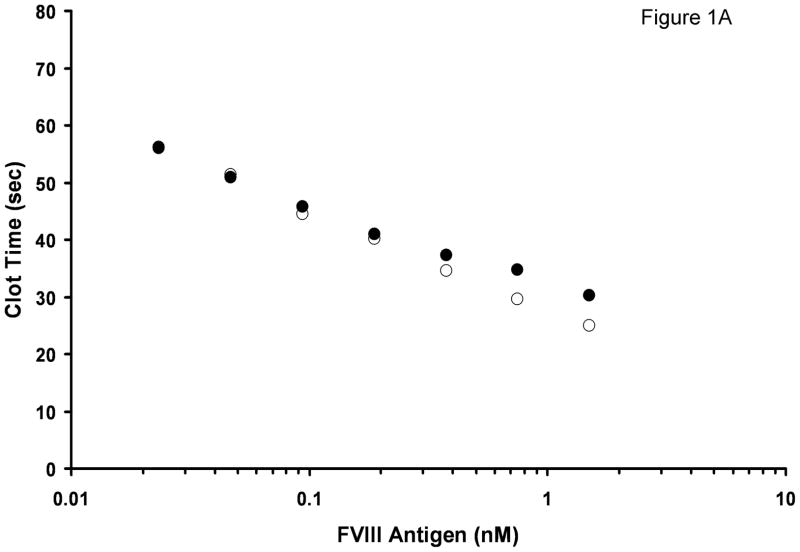
The concentration of FVIII antigen in normal 10-donor plasma was 1.5 nM, and the concentration of vWF was 10 μg/ml (both determined by ELISA). No vWF was detected in FVIII/vWF-immunodepleted substrate plasma. FVIII activity in this plasma was <1% (indicated by manufacturer). In the APTT assay, 10-donor plasma clotted in 30.2 s (Fig. 1A). Mixing this plasma 1:1 v/v with substrate plasma increased the clotting time to 34.4 s. Further dilutions of 10-donor plasma in substrate plasma led to steady increases in the clotting time. At 100% substrate plasma, the clotting time was 79 s. The addition of 1.5 nM albumin-free rFVIII (which was used as a calibrator for the determination of FVIII antigen) to substrate plasma led to a clotting time of 25.0 s, i.e. the clotting time was shorter by 5.2 s than that observed in 10-donor plasma. With decreasing rFVIII concentrations added to the doubly-deficient substrate plasma, the difference in clotting time between substrate plasma reconstituted with rFVIII and substrate plasma mixed with 10-donor plasma also decreased. At 0.19 nM FVIII this difference decreased to 1 s, and at 50 pM FVIII the clotting time in both titrations was identical (51 s).
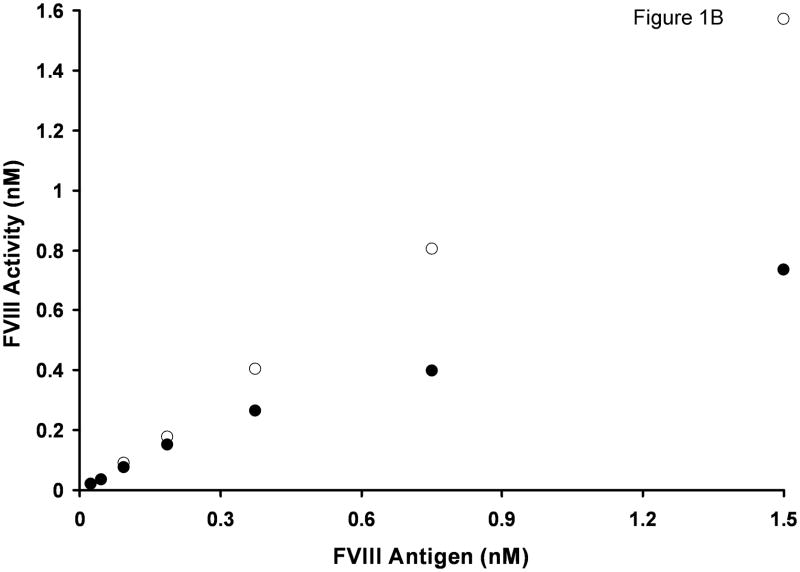
Fig. 1.
Titration of rFVIII (○) and normal pooled plasma (●) into FVIII/vWF-immunodepleted plasma in an APTT assay. A. Clotting times B. Activity. Standard deviation bars for clotting times are smaller than the size of the symbols.
Based on clotting times, the FVIII activity in FVIII/vWF-depleted substrate plasma and in 10-donor normal plasma was evaluated using a calibration curve built by sequential dilutions of albumin-free rFVIII in doubly-deficient substrate plasma. At identical (1.5 nM) FVIII antigen concentrations, the measured apparent FVIII functional concentration in substrate plasma was 1.6 nM (Fig. 1B) and in 10-donor plasma, 0.74 nM, i.e. the difference was 2.2-fold. With decreasing FVIII concentrations, this difference also decreased steadily. For example, at 0.375 nM FVIII antigen, the observed activity of rFVIII in substrate plasma was 0.40 nM and the observed activity of FVIII in normal plasma was 0.27 nM, i.e. an activity ratio of 1.5. At 0.19 nM FVIII antigen, this ratio decreased to 1.2.
The influence of vWF on rFVIII activity
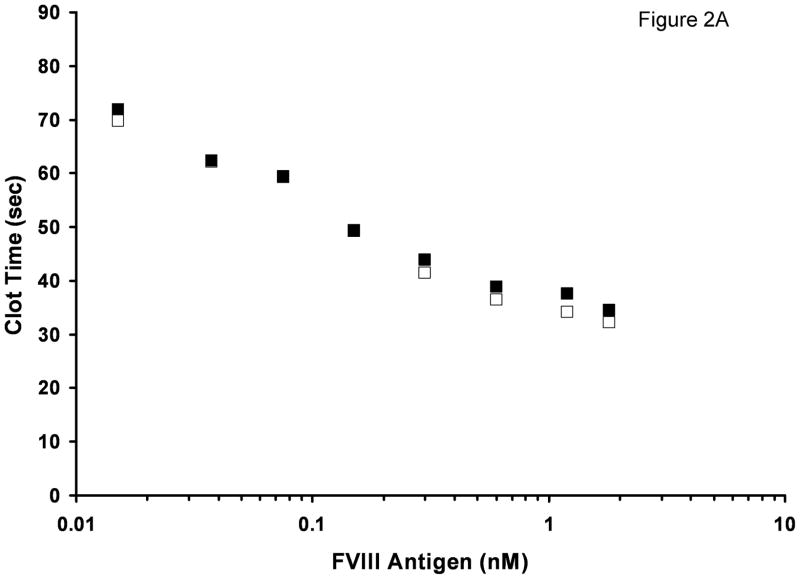
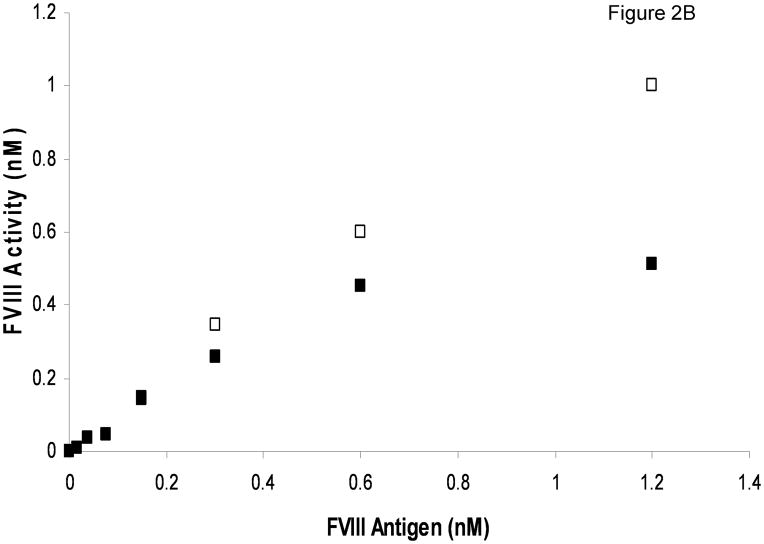
The most significant difference between FVIII/vWF-deficient substrate plasma reconstituted with rFVIII and normal plasma is the absence of vWF in the former. To test if vWF is the cause of prolonged clotting times in normal plasma, we evaluated the influence of purified vWF on FVIII activity. Albumin-free rFVIII was titrated into FVIII/vWF-deficient plasma with or without 10 μg/ml (mean physiologic concentration) vWF added. At near-physiologic rFVIII concentrations, the clotting times were prolonged in the presence of vWF (Fig. 2A). With decreasing rFVIII concentrations, the inhibitory effect of vWF decreased, and at 0.15 nM rFVIII and below the clotting times were almost identical in the presence and absence of vWF. When clotting times were converted to FVIII functional concentration, a pronounced effect of vWF on rFVIII activity was observed at (near)-physiologic rFVIII concentrations (Fig. 2B). For example, in the absence of vWF the activity of 1.2 nM rFVIII antigen was 2-fold higher than in the presence of vWF.
Fig. 2.
Titration of rFVIII into FVIII/vWF-immunodepleted plasma in the APTT assay with (■) and without (□) addition of 10 μg/ml purified vWF. A. Clotting times B. Activity. Standard deviation bars for clotting times are smaller than the size of the symbols.
Overall, the decrease in activity caused by added vWF at various rFVIII concentrations was similar to that observed in plasma mixing experiments (Fig. 1) suggesting that vWF present in 10-donor normal plasma acts as an inhibitor of FVIII clotting activity in plasma measured by the APTT.
The inhibitory effect of vWF can be significantly reduced by polyclonal and monoclonal anti-vWF antibodies used in our study. Thus, FVIII/vWF-depleted plasma reconstituted with 1.5 nM rFVIII clotted in 25.1 s. The addition of 10 μg/ml vWF increased the clotting time to 30.6 s. An addition of polyclonal or monoclonal (α-vWF-99) antibody at 0.1 mg/ml concentration together with 10 μg/ml vWF decreased the clotting times to 26.7 s and 26.6 s, respectively. These antibodies at 0.1 mg/ml had no effect on the APTT clotting time of 10-donor normal plasma.
The influence of vWF on FVIII activity for various FVIII products
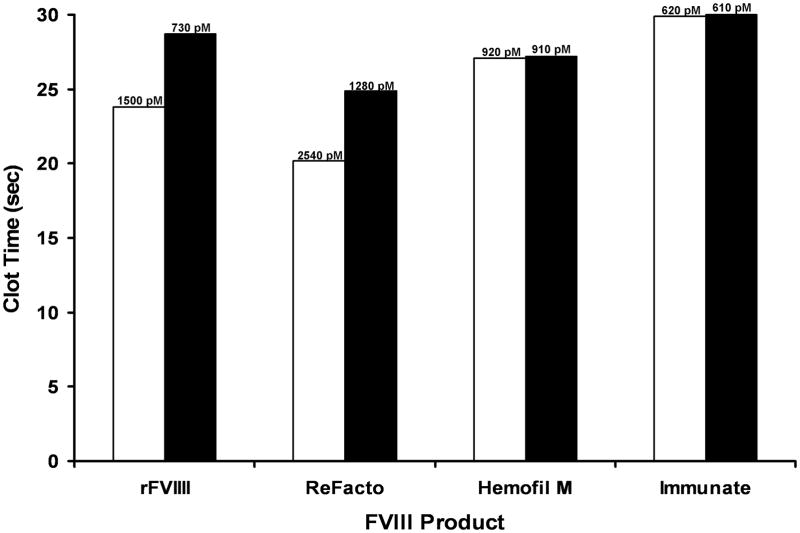
Based upon the effect of vWF on plasma FVIII and rFVIII, we hypothesized that the activity of FVIII in products already containing vWF in their formulations should be less sensitive to the exogenous vWF. Albumin-free rFVIII, ReFacto (does not contain vWF), Hemofil M and Immunate (both contain natural plasma FVIII and 2.5-fold and 62-fold molar excess of vWF, respectively; established by ELISA) were tested for their response to exogenous vWF. For all FVIII products, the final concentration of FVIII antigen in FVIII/vWF-depleted plasma was 1.5 nM and the concentration of purified vWF (when added) was 10 μg/ml. Similar to the data presented in Fig. 2, the clotting activity of rFVIII in the absence of vWF was ~2-fold higher than in the presence of vWF (Fig. 3). The same effect was observed for another vWF-free FVIII product, ReFacto. For the two products already containing vWF (Hemofil M and Immunate), 10 μg/ml of exogenous vWF had no additional effect.
Fig. 3.
The APTT clotting times of FVIII/vWF-immunodepleted plasma reconstituted with various FVIII products containing 1.5 nM FVIII antigen in the absence (open bars) and presence (filled bars) of 10 μg/ml purified vWF. Numbers on the top of bars represent FVIII activity.
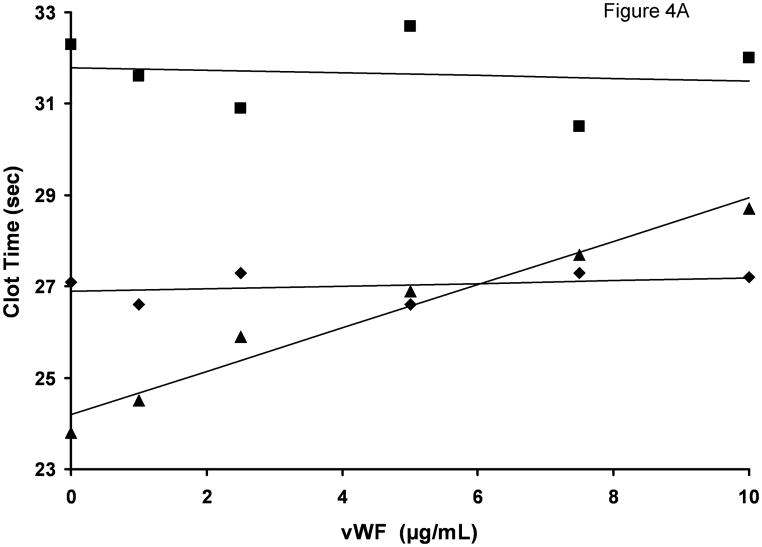
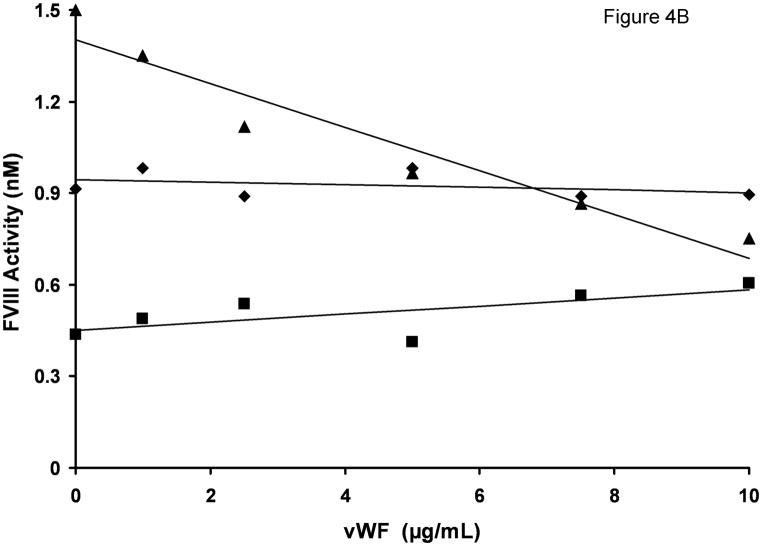
In the experiments presented so far we tested the influence of vWF at a single, physiologic concentration of this protein. To establish if the inhibitory effect of vWF is concentration-dependent, we titrated vWF in the clotting assay using a single (1.5 nM) concentration of FVIII antigen present in various FVIII products. For albumin-free rFVIII, the clotting time increased steadily with increasing vWF concentration (from 23.8 s in the absence of vWF to 28.7 s in the presence of 10 μg/ml vWF (Fig. 4A). These values correspond to a decrease in FVIII functional concentration from 1.5 nM to 0.75 nM (Fig. 4B), a result similar to that observed in rFVIII and plasma titration experiments. The clotting activities of Hemofil M and Immunate were almost not affected by the addition of vWF at any concentration tested. Similarly, the addition of 10 μg/ml exogenous vWF to congenital FVIII-deficient plasma (contained 10.3 μg/ml endogenous vWF) reconstituted with 1.5 nM albumin-free rFVIII did not produce any inhibitory effect (clotting times with and without vWF were 25.4 and 25.7 s, respectively).
Fig. 4.
vWF titration in FVIII/vWF-immunodepleted plasma reconstituted with rFVIII (▲), Immunate (■) and Hemofil M (◆). A. Clotting times; B. Activity. The concentration of FVIII antigen in all assays was 1.5 nM. Standard deviation bars for clotting times are smaller than the size of the symbols.
Thrombin generation in the synthetic coagulation proteome
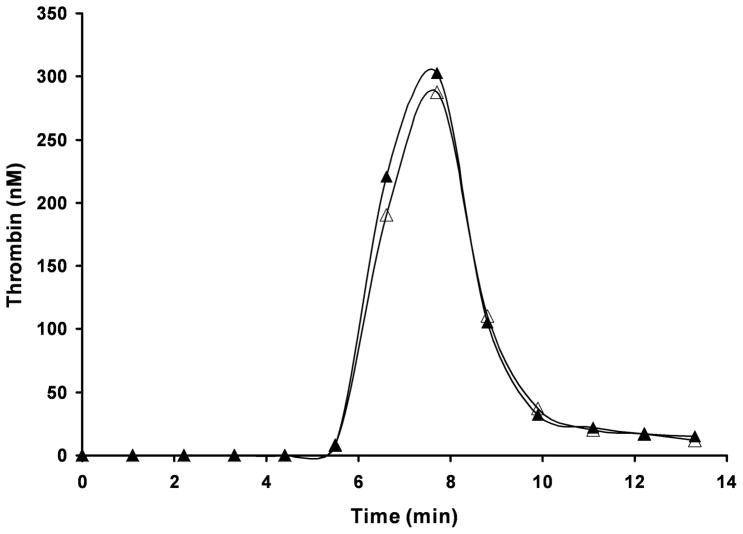
After an inhibitory effect of vWF on FVIII activity in the contact pathway-initiated clotting assay (APTT) was established, the influence of this cofactor on thrombin generation in the synthetic coagulation proteome was tested. vWF had no effect on thrombin generation in a reaction initiated with 5 pM TF (Fig. 5).
Fig. 5.
Thrombin generation in the synthetic coagulation proteome initiated with 5 pM relipidated TF in the absence (△) and in the presence (▲) of 10 μg/ml vWF.
Discussion
The data of this study indicate that vWF at mean physiologic concentration acts as an inhibitor of FVIII in the APTT plasma clotting assay, decreasing apparent FVIII activity to as low as 50% of that observed in the absence of vWF. This observation is valid for both natural FVIII present in pooled plasma from healthy volunteers and for rFVIII reconstituted into FVIII-deficient plasma. This inhibitory effect of vWF explains the discrepancy between the accepted mean physiologic concentration of FVIII (0.7 nM) and the concentration of FVIII antigen in 10-donor plasma observed in the current study (1.5 nM). The former has been established by activity measurements and is influenced by the presence of vWF in plasma, whereas the latter is based upon an objective measurement of FVIII mass and is independent of vWF, which is displaced by the reduction of the FVIII/vWF complex. The inhibitory effect of vWF decreases with decreasing concentrations of FVIII and vWF, presumably due to the dissociation of the FVIII/vWF complex at low concentrations of the components(22;23). This inhibitory effect of vWF is mostly eliminated by anti-vWF antibodies.
No inhibition of FVIII APTT clotting activity was observed when exogenous vWF was added to FVIII/vWF-deficient plasma together with FVIII products containing vWF in their preparations, i.e. Immunate and Hemofil M. Although Immunate contains a molar excess of vWF over FVIII (62-fold) similar to that observed in vivo(24) and Hemofil M has 2.5 moles of vWF for 1 mole of FVIII, both vWF containing FVIII products are not sensitive to additional vWF. It has been suggested by Leyte et al.(22) that only 1–2% of total vWF contains high affinity binding sites for FVIII with the dissociation constant of 0.21 nM. It is reasonable to assume that during an immunoaffinity chromatography of FVIII present in Hemofil M, the high affinity fraction of vWF co-purifies with FVIII. At 1.5 nM FVIII and, correspondingly, 3.7 nM vWF used in our experiments, both components of the FVIII/vWF complex are present at saturating concentrations. As a consequence, an addition of the exogenous vWF does not change the complex equilibrium and has no effect on the clotting activity of FVIII.
The mechanism of vWF inhibitory activity towards FVIII in the clotting assay is not known. There are two possible explanations for this effect: either vWF inhibits FVIII activation or the dissociation of the light chain of FVIII from vWF, which is essential for the expression of FVIIIa cofactor activity(5), is a relatively slow process. The data of our previous publication indicate that in TF-initiated thrombin generation thrombin is the only enzyme responsible for FV and FVIII activation(25). However, the activator(s) of FVIII in the contact pathway-initiated APTT assay has not been established. Similarly, there are no published data related to the influence of vWF on FVIII activation/activity in the APTT assay. There are several publications suggesting that vWF has no effect on FVIII activation by thrombin(6;10). The shortcomings of those studies are that the FVIII activation was done in purified protein mixtures either at supraphysiologic concentrations of FVIII or using preformed thrombin or both. The outcome could be different when FVIII activation occurs in a physiologic medium (plasma) by an activator (thrombin) formed in situ. Alternatively, there could be another FVIII activator than thrombin in the APTT assay. Although FXa is a less efficient activator of FVIII than thrombin(26), it is also possible that initial amounts of FVIIIa in the APTT are generated in plasma by FXa prior to the complete FVIII activation by thrombin. These limited amounts of FVIIIa could be substantial enough to have an effect on the generation of the low concentrations of thrombin required for clot formation(27). The early occurrence of FVIIIa in the reactions leading to thrombin generation in complex systems(11;12) does not contradict this assumption. Additionally, it is in a good agreement with observations that vWF inhibits FVIII activation by FXa(6;7) and that vWF has no effect on thrombin generation in the TF-initiated synthetic coagulation proteome (see Fig. 5).
In summary, the data of this study indicate that vWF has a pronounced inhibitory effect on FVIII procoagulant activity in the APTT assay, a routine assay used for the evaluation of FVIII activity. Although the mechanism of this activity is not clear, the presence and the concentration of vWF in FVIII products and plasma samples should not be ignored when determining the regimen of FVIII-replacement therapy is based on clotting activity measurements of FVIII.
Acknowledgments
This study was supported by P01 HL46703 grant from the National Institutes of Health and a grant from Baxter Healthcare-Bioscience. We thank Dr. R. Jenny for providing us with vWF and polyclonal anti-vWF antibody, Dr. U Hedner for providing rFVIIa and Dr. S. Hardy for providing rTFPI. We also thank J. Amblo and M. Gissel for their technical assistance. This work was in part presented at the XXIst Congress of the International Society on Thrombosis and Haemostasis , July 6–12, 2007, Geneva, Switzerland (abstract number P2547).
Footnotes
Disclosure of Conflict of Interests
This study was in part supported by Baxter Healthcare-Bioscience and K.G.M. is a consultant for Baxter Healthcare-Bioscience.
Reference List
- 1.Mann KG, Brummel-Ziedins K, Orfeo T, Butenas S. Models of blood coagulation. Blood Cells Mol Dis. 2006 doi: 10.1016/j.bcmd.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 2.Vehar GA, Keyt B, Eaton D, Rodriguez H, O'Brien DP, Rotblat F, et al. Structure of human factor VIII. Nature. 1984;312(5992):337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- 3.Lollar P, Hill-Eubanks DC, Parker CG. Association of the factor VIII light chain with von Willebrand factor. J Biol Chem. 1988;263(21):10451–10455. [PubMed] [Google Scholar]
- 4.Hamer RJ, Koedam JA, Beeser-Visser NH, Bertina RM, van Mourik JA, Sixma JJ. Factor VIII binds to von Willebrand factor via its Mr-80,000 light chain. Eur J Biochem. 1987;166(1):37–43. doi: 10.1111/j.1432-1033.1987.tb13480.x. [DOI] [PubMed] [Google Scholar]
- 5.Hill-Eubanks DC, Parker CG, Lollar P. Differential proteolytic activation of factor VIII-von Willebrand factor complex by thrombin. Proc Natl Acad Sci U S A. 1989;86(17):6508–6512. doi: 10.1073/pnas.86.17.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koedam JA, Hamer RJ, Beeser-Visser NH, Bouma BN, Sixma JJ. The effect of von Willebrand factor on activation of factor VIII by factor Xa. Eur J Biochem. 1990;189(2):229–234. doi: 10.1111/j.1432-1033.1990.tb15481.x. [DOI] [PubMed] [Google Scholar]
- 7.Koppelman SJ, Koedam JA, Van Wijnen M, Stern DM, Nawroth PP, Sixma JJ, et al. von Willebrand factor as a regulator of intrinsic factor X activation. J Lab Clin Med. 1994;123(4):585–593. [PubMed] [Google Scholar]
- 8.Nogami K, Shima M, Nishiya K, Sakurai Y, Tanaka I, Giddings JC, et al. Human factor VIII inhibitor alloantibodies with a C2 epitope inhibit factor Xa-catalyzed factor VIII activation: a new anti-factor VIII inhibitory mechanism. Thromb Haemost. 2002;87(3):459–465. [PubMed] [Google Scholar]
- 9.Nogami K, Lapan KA, Zhou Q, Wakabayashi H, Fay PJ. Identification of a factor Xa-interactive site within residues 337–372 of the factor VIII heavy chain. J Biol Chem. 2004;279(16):15763–15771. doi: 10.1074/jbc.M400568200. [DOI] [PubMed] [Google Scholar]
- 10.Hill-Eubanks DC, Lollar P. von Willebrand factor is a cofactor for thrombin-catalyzed cleavage of the factor VIII light chain. J Biol Chem. 1990;265(29):17854–17858. [PubMed] [Google Scholar]
- 11.Lawson JH, Kalafatis M, Stram S, Mann KG. A model for the tissue factor pathway to thrombin. I. An empirical study. J Biol Chem. 1994;269(37):23357–23366. [PubMed] [Google Scholar]
- 12.van 't Veer C, Golden NJ, Kalafatis M, Mann KG. Inhibitory mechanism of the protein C pathway on tissue factor-induced thrombin generation. Synergistic effect in combination with tissue factor pathway inhibitor. J Biol Chem. 1997;272(12):7983–7994. doi: 10.1074/jbc.272.12.7983. [DOI] [PubMed] [Google Scholar]
- 13.Kumar H, Healey JF, Lollar P, Durrani MJ. Determination of free sulfhydryls in human factor VIII. J Thromb Haemost. 2003;1(Suppl 1):P1067. [Google Scholar]
- 14.Bajaj SP, Rapaport SI, Prodanos C. A simplified procedure for purification of human prothrombin, factor IX and factor X. Prep Biochem. 1981;11(4):397–412. doi: 10.1080/00327488108065531. [DOI] [PubMed] [Google Scholar]
- 15.van 't Veer C, Mann KG. Regulation of tissue factor initiated thrombin generation by the stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin- III, and heparin cofactor-II. J Biol Chem. 1997;272(7):4367–4377. doi: 10.1074/jbc.272.7.4367. [DOI] [PubMed] [Google Scholar]
- 16.Katzmann JA, Nesheim ME, Hibbard LS, Mann KG. Isolation of functional human coagulation factor V by using a hybridoma antibody. Proc Natl Acad Sci U S A. 1981;78(1):162–166. doi: 10.1073/pnas.78.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffith MJ, Noyes CM, Church FC. Reactive site peptide structural similarity between heparin cofactor II and antithrombin III. J Biol Chem. 1985;260(4):2218–2225. [PubMed] [Google Scholar]
- 18.Higgins DL, Mann KG. The interaction of bovine factor V and factor V-derived peptides with phospholipid vesicles. J Biol Chem. 1983;258(10):6503–6508. [PubMed] [Google Scholar]
- 19.Cawthern KM, van 't Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91(12):4581–4592. [PubMed] [Google Scholar]
- 20.Butenas S, Parhami-Seren B, Gissel MT, Gomperts ED, Fass DN, Mann KG. Potency and mass of factor VIII in FVIII products. Haemophilia. 2008 doi: 10.1111/j.1365-2516.2008.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fay PJ, Smudzin TM. Intersubunit fluorescence energy transfer in human factor VIII. J Biol Chem. 1989;264(24):14005–14010. [PubMed] [Google Scholar]
- 22.Leyte A, Verbeet MP, Brodniewicz-Proba T, van Mourik JA, Mertens K. The interaction between human blood-coagulation factor VIII and von Willebrand factor. Characterization of a high-affinity binding site on factor VIII. Biochem J. 1989;257(3):679–683. doi: 10.1042/bj2570679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vlot AJ, Koppelman SJ, van den Berg MH, Bouma BN, Sixma JJ. The affinity and stoichiometry of binding of human factor VIII to von Willebrand factor. Blood. 1995;85(11):3150–3157. [PubMed] [Google Scholar]
- 24.Kaufman RJ. Biological regulation of factor VIII activity. Annu Rev Med. 1992;43:325–339. doi: 10.1146/annurev.me.43.020192.001545. [DOI] [PubMed] [Google Scholar]
- 25.Butenas S, van 't Veer C, Mann KG. Evaluation of the initiation phase of blood coagulation using ultrasensitive assays for serine proteases. J Biol Chem. 1997;272(34):21527–21533. doi: 10.1074/jbc.272.34.21527. [DOI] [PubMed] [Google Scholar]
- 26.Lollar P, Knutson GJ, Fass DN. Activation of porcine factor VIII:C by thrombin and factor Xa. Biochemistry. 1985;24(27):8056–8064. doi: 10.1021/bi00348a033. [DOI] [PubMed] [Google Scholar]
- 27.Rand MD, Lock JB, van 't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88(9):3432–3445. [PubMed] [Google Scholar]