Abstract
BACKGROUND:
Coronary artery disease (CAD) occurs at an earlier age in South Asians compared with other ethnic groups. Infection and inflammation show a positive association with the disease.
OBJECTIVE:
To investigate the association of infection and inflammatory markers with premature CAD in the Indian Atherosclerosis Research Study population.
METHODS:
Antibody titres for Chlamydia pneumoniae, cytomegalovirus (CMV), Helicobacter pylori, herpes simplex virus and levels of interleukin-6 (IL-6), high-sensitivity C-reactive protein (hsCRP), fibrinogen and secretory phospholipase A2, were measured in 866 individuals (433 CAD patients and matched controls). All individuals were followed-up for recurrent cardiac events for four years. ANOVA was used to study the association of infection and inflammation with CAD.
RESULTS:
The present study found that the odds of CAD occurrence was 2.42 (95% CI 1.26 to 4.64; P<0.008), with all four infections and increased in the presence of hsCRP (OR 4.67 [95% CI 1.43 to 15.25]); P=0.011). Only anti-CMV antibody levels were a significant risk factor for CAD occurrence (OR 2.23 [95% CI 1.20 to 4.15]; P=0.011) and recurrent cardiac events (OR 1.94 [95% CI 0.85 to 4.45]; P=0.015). Mean values of the inflammatory biomarkers IL-6 (P=0.035), fibrinogen (P=0.014), hsCRP (P=0.010) and secretory phospholipase A2 (P=0.002) increased with CMV antibody levels. Incorporating hsCRP and IL-6 in the risk prediction models significantly increased the OR to 2.56 (95% CI 1.16 to 5.63; P=0.019) with a c statistic of 0.826.
CONCLUSIONS:
Pathogen burden, especially CMV infection in combination with inflammatory markers, is a significant predictor of CAD risk in the young Indian population.
Keywords: Coronary artery disease, C-reactive protein, Cytomegalovirus, Inflammatory markers, Pathogen burden
Coronary artery disease (CAD) is a major cause of morbidity and mortality worldwide. Asian Indians, especially the young and working population (1,2), are reported to have three times higher rates of incidence and prevalence of CAD when compared with western populations (3,4), leading to a greater social and economic impact (3,5,6). Various factors, such as metabolic syndrome, diabetes, diet, unique dyslipidemia (high triglycerides, low high-density lipoprotein cholesterol levels) and possible genetic predisposition are believed to contribute to this rising epidemic in India (3).
Atherosclerosis, the underlying cause of acute coronary syndrome, is a chronic inflammatory disease with multifactorial etiology (7). The contribution of infection to induction and progression of the disease has been surrounded by controversy. Although evidence for the role of infection comes from several epidemiological studies demonstrating an association between antibodies to pathogens including cytomegalovirus (CMV), Chlamydia pneumonia, Helicobacter pylori, hepatitis A, herpes simplex virus (HSV) and periodontal bacteria with atherosclerosis (8–11), other studies are less consistent (12). Pathogen burden (ie, the cumulative burden of infection rather than infection by any single pathogen) has been linked to the progression of CAD and its complications (10,13,14).
Chronic infection can cause proatherosclerotic effects by increasing the expression of inflammatory cytokines, chemokines and cellular adhesion molecules, as well as smooth muscle cell proliferation and migration (15). Infection may also trigger vascular damage by molecular mimicry wherein pathogenic antigens trigger an immune response cross-reacting with self-peptides expressed on host tissues (13,15,16).
Pathogen burden is reported to be higher in individuals of Indian origin (17). However, the association between pathogen burden in combination with inflammatory markers and CAD is not well defined in this population. It is possible that novel risk factors contribute to the high CAD incidence seen in Indians, which cannot be adequately explained by conventional risk factors alone (3,5). Therefore, the aim of the present study was to examine the association of increasing pathogen burden in combination with novel inflammatory markers with CAD in Indians. We also studied the association between antibody levels and individual pathogens with CAD and recurrent cardiac events.
METHODS
Indian Atherosclerosis Research Study
The study population was from the ongoing Indian Atherosclerosis Research Study (IARS), which is investigating the molecular basis of atherothrombosis in Asian Indian families (18). The present analysis was performed in an age- and sex-matched population of 866 individuals (433 CAD patients and 433 asymptomatic individuals) from phase 1 of IARS comprising of 744 CAD patients and 1502 asymptomatic individuals enrolled from 2004 to 2007. The patients were confirmed for CAD by coronary angiography and were admitted to participating hospitals in Bangalore and Mumbai, India, for coronary artery bypass graft or percutaneous coronary intervention. Subjects who were apparently healthy, with normal electrocardiogram and no clinical symptoms related to CAD, were enrolled as asymptomatic individuals. Periodic follow-up by telephone was performed for all IARS participants for three to five years. During follow-up, fatal myocardial infarction or nonfatal ischemic events requiring hospitalization and treatment, based on medical records from the hospitals, were categorized as recurring events. Because the study was designed to examine CAD risk in young Asian Indians, patients were selected based on predefined inclusion/exclusion criteria, with an age at onset of CAD of ≤60 years for men and ≤65 years for women. Participants with a history of any major illness, defined according to WHO criteria, or having any concomitant infection or primary myocardial disease/congenital heart disease or an organ transplant at the time of enrollment, were excluded from the study. Information regarding demographics, life style, anthropometrics, and medical history of diabetes, hypertension and medication were recorded for each participant. A fasting glucose level >6.67 mmol/L was considered to be diabetic and, systolic/diastolic blood pressure of >140/90 mmHg was considered to be hypertensive. The present study was conducted following the guidelines of the Indian Council of Medical Research and the Declaration of Helsinki on human clinical research. An institutional ethics committee approved the IARS study and voluntary, signed informed consent was obtained from all participants
Lipid assays
Total cholesterol, triglycerides and high-density lipoprotein cholesterol (Bayer Diagnostics, Randox Laboratories, Dade-Behring Ltd, United Kingdom), were determined using the Cobas Fara II Clinical Chemistry auto analyzer, (F Hoffman La Roche Ltd, Switzerland), following manufacturer’s instructions. Serum low-density lipoprotein cholesterol was calculated using Friedwald’s formula (19). Commercial controls for the lipids were purchased from Randox Laboratories and Orion Diagnostica (Finland) for apolipoproteins, while normal, pooled in-house serum was used as batch controls.
Antibody titres for common pathogens
Commercially available ELISA kits were used to determine the antibodies to pathogens. Pooled normal human serum was used as an internal control in every set of experiments and seropositive levels were defined following manufacturer’s instructions as follows:
CMV: Immunoglobulin (Ig) G levels were estimated for whole CMV antigens (Adaltis, Italy); an antibody index >0.5 IU/mL was defined as seropositive.
C pneumoniae: Serum IgG levels were estimated for purified antigen of C pneumoniae (Calbiotech, USA); an antibody index >0.9 IU/mL was defined as seropositive
H pylori: Serum IgG levels were estimated for inactivated H pylori antigens (Medical Biological Service, Italy); an antibody index >20 IU/mL was defined as seropositive
HSV: Serum IgG levels were estimated for purified antigen HSV-1 (Calbiotech, USA); an antibody index >1.1 IU/mL was defined as seropositive.
Levels of inflammatory markers interleukin 6 (IL-6) (R&D systems, Germany), high-sensitivity C-reactive protein (hsCRP) (Immune Diagnostic, USA), secretory phospholipase A2 (sPLA2) (Cayman Chemicals, USA) and soluble intercellular adhesion molecule (R&D systems, Germany), were estimated using ELISA as per manufacturer’s protocol. Fibrinogen levels were measured on the ACL 300 automated coagulation analyzer (IL Systems, Italy) using clotting assays. The interassay coefficient of variation ranged between 3.8% and 13.6% for all assays.
Statistical analyses
Prevalence of infection, pathogen burden and the association between infection, inflammation and CAD analyses were performed using an age- and sex-matched subgroup of 866 individuals (n=433 each for CAD patients and controls) from the IARS phase I study comprising 2318 participants. The IARS cohort was followed-up every year for any possible change in disease status and at the end of the fourth follow-up in 2009; 91 of the 773 affected individuals experienced recurrent events. This subgroup was analyzed to identify the role of pathogens in CAD recurrence.
The quantitative variables were normalized by log transformation before analysis. Association between categorical variables was analyzed using χ2 tests. Logistic regression analysis was used to identify the contributing variables and the associated risk in terms of OR and 95% CIs. The association between pathogen burden and CAD was assessed in terms of increased risk (OR) for seropositivity to multiple infections, using seropositivity to a single infection as the reference. Additive effects of inflammatory markers on the OR were analyzed by including individual markers in the model after adjusting for risk factors. Antibodies to pathogens were categorized into four quartiles for the association studies. The effect of higher antibody levels on CAD occurrence was assessed in terms of increased risk from the first quartile to the fourth quartile. CMV antibodies and levels of inflammatory markers were used as continuous variables to assess their combined effect on CAD. The models were adjusted for other confounding factors and traditional risk factors including age, sex, body mass index (BMI), waist to hip ratio, hypertension, diabetes, hyperlipidemia and statin use. Predictive probabilities were used in the ROC analysis to obtain area under curve (AUC) and c statistics to assess the contribution of additional biomarkers and model selection. Mean levels of inflammatory markers in the first and fourth quartiles of antipathogen antibodies were compared using multivariate ANOVA followed by a post hoc Student’s t test. SPSS version 17.0 (IBM Corporation, USA) and DTREG version 9.6 (Phillip H Sherrod, USA) statistical packages were used in the analysis. P<0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
The mean age of the CAD patient and control groups was 52.6 years, with 68% males and 32% females. The conventional risk factors, including hypertension, diabetes, current smoking and statin use, were higher in the patient population while the lipid levels were lower, likely due to the fact that 69% were using statins (Table 1). The IARS population was mostly urban (96%) and literate (99%) (data not shown).
TABLE 1.
Baseline characteristics of patients and controls
| Variable | Controls (n=433) | CAD patients (n=433) |
|---|---|---|
| Age*, years | 52.59±1.10 | 52.59±0.84 |
| Sex, male/female, % | 67.9/32.1 | 67.9/32.1 |
| Body mass index*, kg/m2 | 26.77±0.47 | 25.47±0.36 |
| Waist to hip ratio* | 0.94±0.01 | 0.94±0.01 |
| Hypertension, % | 30.3 | 50.5 |
| SBP*, mmHg | 127.95±0.812 | 127.50±0.858 |
| DBP*, mmHg | 83.58±0.461 | 81.46±0.428 |
| Diabetes, % | 23.9 | 45.5 |
| Current smoking, % | 21.1 | 41.3 |
| Statin use, % | 4.9 | 69.9 |
| Total cholesterol†, mmol/L | 1.54±0.01 (4.74) | 1.35±0.01 (3.99) |
| Triglycerides†, mmol/L | 0.38±0.02 (1.69) | 0.46±0.02 (1.74) |
| HDL†, mmol/L | 0.016±0.01 (1.05) | −0.06±0.01 (0.967) |
| LDL†, mmol/L | 1.04±0.01 (2.90) | 0.71±0.02 (2.19) |
| Cytomegalovirus, % | 97.4 | 98.1 |
| Chlamydia pneumoniae, % | 25.6 | 26.9 |
| Herpes simplex virus, % | 49.5 | 55.5 |
| Helicobacter pylori, % | 62.8 | 71.2 |
| hsCRP†, μg/mL | 0.71±0.11 (2.04) | 0.59±0.09 (1.80) |
| sPLA2†, μg/mL | 7.95±0.04 (2.84) | 8.08±0.05 3.23)‡ |
| Interleukin 6†, pg/mL | 1.21±0.06 (3.37) | 1.09±0.06 (2.97) |
| Fibrinogen†, mmol/mL | −2.21±0.01 (0.112) | −2.15±0.01 (0.120) |
Mean ± SD;
Log-transformed mean ± SEM (retransformed mean);
P<0.05. CAD Coronary artery disease; DBP Diastolic blood pressure; HDL High density lipoprotein cholesterol; hsCRP High-sensitivity C-reactive protein; LDL Low-density lipoprotein cholesterol; SBP Systolic blood pressure; sICAM Soluble intercellular adhesion molecule; sPLA2 Secretory phospholipase A2. Significance was determined by Student’s t test for variables expressed as means ± SEM and by the χ2 test for variables expressed as percentages
Prevalence of seropositivity and pathogen burden
Seropositivity was 97.7% for CMV infection, 67.0% for H pylori, 52.5% for HSV and 26.2% for C pneumoniae (Figure 1). The proportion of patients with CAD showed a strong association with increased pathogen burden: 44.4% for a single infection, 48.5% for two, 55.3% for three and 66.0% for all four infections (Figure 1).
Figure 1).
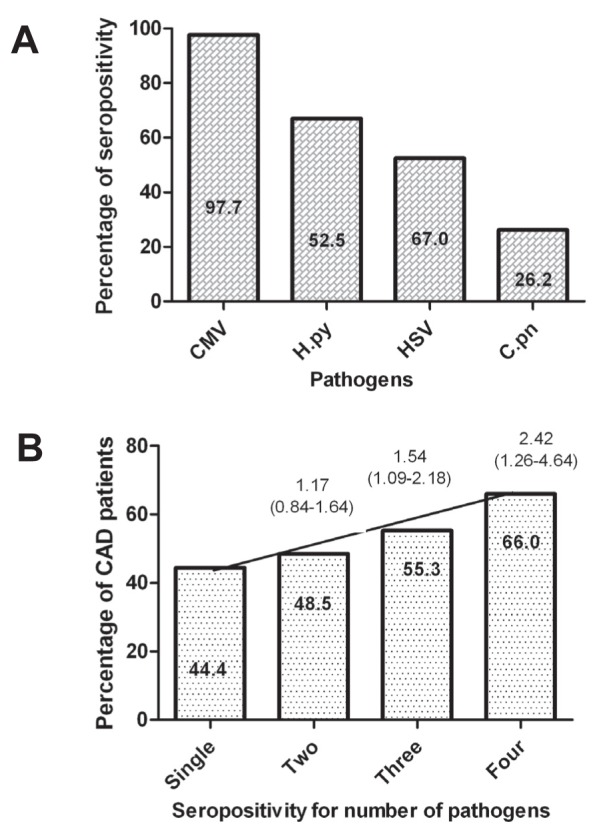
Prevalence of seropositivity (A) and pathogen burden (B) in the Indian Atherosclerosis Study (IARS) population. Seropositivity to single infection, any two, three and all four infections was analyzed for the coronary artery disease patient population. OR was calculated using single infection as reference. CAD Coronary artery disease; CMV Cytomegalovirus; C.pn Chlamydia pneumoniae; H.py Helicobacter pylori; HSV Herpes simplex virus.
Association of pathogen burden and inflammatory markers with CAD
Pathogen burden was found to be significantly associated with increased risk of CAD. The risk of occurrence of the disease was two times higher in the presence of all four infections (OR 2.19 [95% CI 1.03 to 4.67); P=0.042) after adjusting for established risk factors including age, sex, BMI, waist to hip ratio, hypertension, diabetes and hyperlipidemia. The association of pathogen burden and CAD in terms of OR amplified four times when analyzed in the presence of hsCRP (OR4.67 [95%CI 1.43 to 15.25]; P=0.011) after adjustment for risk factor (Table 2). The risk association of pathogen burden with CAD was not found to change in the presence of other markers of inflammation such as sPLA2 (OR 2.12 [95% CI 1.02 to 4.49]; P=0.048), fibrinogen (OR 2.05 [95% CI 0.97 to 4.30]; P=0.062) and IL-6 (OR 2.09 [95% CI 0.98 to 4.46]; P=0.057).
TABLE 2.
Combined effects of seropositivity and levels of inflammatory markers on the OR for coronary artery disease occurrence
|
Seropositivity for
|
|||
|---|---|---|---|
| Model | 2 pathogens | 3 pathogens | 4 pathogens |
| Pathogen burden (unadjusted) | 1.17 (0.84–1.64); 0.344 | 1.54 (1.09–2.18); 0.014 | 2.42 (1.26–4.64); 0.008 |
| Pathogen burden (adjusted for risk factors) | 1.02 (0.79–1.43); 0.908 | 1.48 (1.01–2.04); 0.057 | 2.19 (1.03–4.67); 0.042 |
| Pathogen burden + hsCRP | 1.02 (0.59–1.74); 0.946 | 2.07 (1.17–3.67); 0.013 | 4.67 (1.43–15.25); 0.011 |
| Pathogen burden + sPLA2 | 1.03 (0.69–1.51); 0.894 | 1.46 (0.98–2.19); 0.061 | 2.12 (1.02–4.49); 0.048 |
| Pathogen burden + IL-6 | 0.98 (0.67–1.49); 0.921 | 1.44 (0.96–2.15); 0.076 | 2.09 (0.84–4.02); 0.057 |
| Pathogen burden + fibrinogen | 0.98 (0.67–1.45); 0.945 | 1.41 (0.95–2.05); 0.091 | 2.05 (0.98–4.46); 0.062 |
Data presented as OR (95% CI); P. Risk factors include age, sex, body mass index, waist to hip ratio, hypertension, diabetes, smoking and hyperlipidemia. Seropositivity for single infection was taken as reference in the logistic regression analysis. OR calculations in the presence of inflammatory markers were adjusted for the risk factors. hsCRP High-sensitivity C-reactive protein; IL-6 Interleukin 6; sPLA2 Secretory phospholipase A2
Association of antibody levels to individual pathogens and CAD
The association between individual pathogen and CAD was studied to understand the contribution of each infectious agent. A positive association was observed with anti-CMV antibody levels (OR 1.62 [95% CI 1.10 to 2.39]; P=0.014), with CAD in the relatively young Indian population (Table 3). Adjustment for classical risk factors, such as age, sex, BMI, waist to hip ratio, hypertension, diabetes, hypercholesterolemia, statin use and smoking did not alter this relationship (OR 2.23 [95% CI 1.20 to 4.15]; P=0.011) (Table 3). An association between CAD and antibody levels to C pneumoniae was also observed (OR 2.76 [95% CI 0.87 to 8.75]), but it was not found to be significant (P=0.085). No significant relationship was observed between H pylori and HSV infection in the present study. These data suggest that CMV infection maybe an important CAD risk factor in the Asian Indian population.
TABLE 3.
OR for coronary artery disease occurrence with high levels of antibodies to pathogen (quartiles)
|
OR* (95%CI)
|
||||
|---|---|---|---|---|
| Pathogen | Unadjusted | P | Adjusted | P |
| CMV | 1.62 (1.10–2.39) | 0.014† | 2.23 (1.20–4.15) | 0.011† |
| HSV | 1.43 (0.75–2.73) | 0.280 | 0.59 (0.17–2.03) | 0.402 |
| C pneumoniae | 1.35 (0.64–2.82) | 0.425 | 2.76 (0.87–8.75) | 0.085 |
| H pylori | 0.70 (0.40–1.23) | 0.220 | 1.05 (0.46–2.42) | 0.905 |
OR was determined using the first quartile as reference. Logistic regression analysis was performed in the age- and sex-matched subpopulation (n=866). Risk factors include age, sex, body mass index, waist to hip ratio, hypertension, diabetes, hyperlipidemia smoking and statin use;
Statistically significant. CMV Cytomegalovirus, C pneumonia Chlamydia pneumonia; H pylori Helicobacter pylori; HSV Herpes simplex virus.
Association of antibody levels with individual pathogens and recurrence of cardiac events
The role of CMV infection in recurrent cardiac events was studied in a four-year follow-up of the study population. Among the 744 CAD patients, 91 experienced recurrent cardiac events. CMV antibody levels in the fourth quartile were significantly associated with recurrent cardiac events (OR 2.13 [95% CI 1.09 to 4.16]; P=0.024) compared with the first quartile (Table 4). Fatal events in patients (14 of 21 patients [66.7%]) showed significantly higher CMV antibody levels (P<0.005) compared with nonfatal events (21 of 66 patients [31.8%]), suggesting that CMV infection could be an important mediator of pathological complications. A new cardiac event was observed in 24 asymptomatic individuals during the follow-up, all of whom were seropositive for CMV; however, extensive statistical analysis was not performed due to the small number of patients.
TABLE 4.
Risk of recurrent events in individuals with high antibody levels to pathogens
|
OR (95% CI)
|
||||
|---|---|---|---|---|
| Pathogen | Unadjusted | P | Adjusted | P |
| CMV | 1.86 (0.87–3.96) | 0.007 | 1.94 (0.85–4.43) | 0.015 |
| HSV | 1.54 (0.54–4.38) | 0.413 | 2.19 (0.67–7.19) | 0.194 |
| C pneumoniae | 1.93 (0.72–5.14) | 0.191 | 1.82 (0.63–5.28) | 0.271 |
| H pylori | 1.61 (0.44–5.96) | 0.413 | 2.19 (0.67–7.19) | 0.194 |
Recurrent cardiac event was reported for 91 patients. Relative risk of recurrent cardiac event was calculated based on the antibody levels to pathogen in the first and fourth quartiles using the first quartile as the reference. CMV Cytomegalovirus; C pneumoniae Chlamydia pneumoniae; H pylori Helicobacter pylori; HSV Herpes simplex virus
CMV infection inflammatory status and its association with CAD
Because infections induce secretion of proatherogenic inflammatory and procoagulant markers (15), the association of serum levels of inflammatory biomarkers with CMV infection and their concurrent effect on CAD risk was studied. Significantly higher mean levels of IL-6 (P=0.035), fibrinogen (P=0.014), hsCRP (P=0.010), sPLA2 (P=0.002) and soluble intercellular adhesion molecule (P=0.003) were found in the fourth quartile of CMV antibody levels compared with the first quartile (Table 5).
TABLE 5.
Mean of values of inflammatory markers in the first and fourth quartiles of anticytomegalovirus (CMV) antibodies
| Anti-CMV IgG quartiles (Q) |
Inflammatory markers
|
||||
|---|---|---|---|---|---|
| sPLA2, pg/mL | sICAM, pg/mL | Fibrinogen, mmol/mL | IL-6, pg/mL | hsCRP, μg/mL | |
| Q1 | 7.77±0.04 (2368.47) | 5.28±0.02 (197.74) | −2.30±0.01 (0.106) | 0.94±0.06 (2.56) | 0.26±0.08 (1.30) |
| Q4 | 7.93±0.03 (2798.95) | 5.39±0.22 (220.52) | −2.21±0.01 (0.110) | 1.16±0.05 (3.18) | 0.54±0.07 (1.73) |
| P | 0.002* | 0.003* | 0.014* | 0.035* | 0.010* |
Data presented as Ln mean ± standard error (retransformed mean). Univariate analysis was performed to determine the differences in the mean levels of inflammatory markers in first and fourth quartile of antibody levels to pathogens.
Statististically significant. hsCRP High-sensitivity C-reactive protein; IL-6 Interleukin 6; sICAM Soluble intercellular adhesion molecule; sPLA2 Secretory phospholipase A2
The risk of elevated CMV antibodies was subsequently examined in the presence of inflammatory markers after adjusting for the established risk factors. A ROC analysis was performed and obtained AUC (c statistics) to study the discriminatory power of the CMV antibodies in the presence of other inflammatory markers. In these models, the increase in OR and AUC values were evaluated by defining the anti-CMV antibody levels and inflammatory markers as continuous variables. The relative risk of CAD occurrence for anti-CMV antibodies defined as a continuous variable was 1.61 (95% CI 1.03 to 2.53; P=0.038), with an AUC of 0.777 the risk remained in the same range with the addition of inflammatory markers except for hsCRP when it increased to 2.11 (95% CI 1.12 to 3.95; P=0.021) with an AUC of 0.772. Addition of IL-6 to this model increased the accuracy of prediction with an AUC of 0.826 (P<0.001) and the risk to 2.560 (95% CI 1.164 to 5.629; P=0.019), suggesting an important role for CMV infection and inflammation in CAD susceptibility in this population (Table 6).
TABLE 6.
OR and c statistics for logistic regression models predicting the risk of occurrence of coronary artery disease associated with cytomegalovirus (CMV) antibody titres in the presence of other inflammatory markers
| Model |
Logistic regression
|
C statistics
|
||
|---|---|---|---|---|
| OR (95% CI) | P | |||
| CMV | 1.61 (1.03–2.53) | 0.038 | 0.777 | 0.016 |
| CMV + hsCRP | 2.11 (1.12–3.95) | 0.021 | 0.772 | <0.001 |
| CMV + fibrinogen | 1.56 (0.99–2.46) | 0.050 | 0.782 | <0.001 |
| CMV + sPLA2 | 1.53 (0.97–2.42) | 0.069 | 0.774 | 0.017 |
| CMV + IL6 | 1.34 (0.74–2.42) | 0.335 | 0.790 | <0.001 |
| CMV + hsCRP + fibrinogen | 2.16 (1.15–4.06) | 0.017 | 0.774 | <0.001 |
| CMV + hsCRP+ sPLA2 | 2.14 (1.13–4.04) | 0.019 | 0.766 | <0.001 |
| CMV + hsCRP + IL-6 | 2.56 (1.16–5.63) | 0.019 | 0.826 | <0.001 |
Logistic regression was computed using continuous variables. All above models are adjusted for age, sex, body mass index, waist to hip ratio, hypertension, diabetes, smoking and hyperlipidemia. AUC Area under the curve; hsCRP High-sensitivity C-reactive protein; IL-6 Interleukin 6; sPLA2 Secretory phospholipase A2
DISCUSSION
In the present study, we found a significant association between pathogen burden and CMV seropositivity with CAD in the Asian Indian population, which is known to have higher risk for early development of the disease (20,21). Serostatus was used as an indicator of previous infection with these pathogens. We also report that, in the presence of inflammatory markers, the risk association between burden of infection and CAD is augmented. Our results suggest that the levels of markers, such as hsCRP, IL-6, fibrinogen and sPLA2, show an association with anti-CMV antibody levels and the presence of these markers and, along with CMV infection, may enhance the susceptibility of the individual to cardiac events.
We found a high seropositivity for CMV (97.7%) in our study population compared with 50% to 85% in the western population (22–24). However, the seropositivity was lower for C pneumoniae in our population (26.2% versus 40% to 80% in the western population [25–27]). The burden of infection showed a positive association with CAD, with the highest RR observed in the group with seropositivity for all the four pathogens studied, suggesting that pathogen burden is an important risk factor in the Asian Indian population. In a multiethnic study of atherosclerosis, Moyses Szkloa et al (28), studied the cross sectional relationship between pathogen burden and subclinical atherosclerosis in a random sample of 1056 multiethnic individuals but failed to find an infectious etiology for subclinical atherosclerosis. A higher prevalence of infection in our population and the fact that we examined the clinical manifestation of the disease – not subclinical atherosclerosis – may explain the difference in the results
Pathogens can cause an increase in expression of proatherogenic markers such as acute phase proteins, chemokines, cytokines and adhesion molecules (15). We chose CRP, sPLA2, IL-6 and fibrinogen as a few inflammatory markers to study the effect of these markers on infection associated risk of CAD. Interestingly, the risk association with seropositivity increased twofold in the presence of hsCRP, which has been extensively reported to have an association with cardiovascular diseases (29,30). Although the ORs remained significant with the other three markers, we did not observe an increase in the RR. Thus, pathogen burden appears to be an important factor in determining the predisposition to CAD in Asian Indians and the addition of hsCRP levels can improve the identification of high-risk individuals in this population.
CMV seropositivity contributed independently to CAD risk as well as recurrent cardiac events in patients in this population. Several epidemiological studies have reported twofold or higher ORs for the association between CMV antibodies and CAD occurrence (22,31). Increasing titres of CMV antibodies have been shown to be associated with severity of atherosclerosis, indicating a virus-mediated pathogenic mechanism for CAD (32,33). This association appears most pronounced with coronary restenosis following angioplasty, graft rejection and transplantation (24,34). High levels of anti-CMV antibodies are known to be associated with reactivation of a latent infection, a detrimental condition for atherosclerosis (35). In our study, the basal level of CMV antibodies in CAD patients showed a direct association with mortality with significantly higher CMV antibodies in patients reporting a fatal event, suggesting that it could be one of the markers for the pathological complications.
C pneumoniae and H pylori serostatus were not associated with CAD in Indians. Our results are in agreement with a meta-analysis of 15 prospective studies; a pooled covariate adjusted OR of 1.15 (95% CI 0.97 to 1.36; P>0.1) was reported for the association of C pneumoniae IgG antibodies and CAD (12). The absence of risk association between CAD and C pneumoniae antibodies may be explained by the fact that we measured only IgG antibodies and not IgA, which has been reported to be a better marker for recent exposure to infection in Indian and European populations (23,27,36). Clinical reports on the association of CAD and H pylori infection have also been inconsistent. These contradictory reports may, in part, be explained by the different genotypes of H pylori among which only the cytotoxin-associated gene-A (Cag A)-positive virulent strains show a disease association (37,38). In our study, we could not establish any association between H pylori infection and CAD, probably due to the estimation of antibody titres for H pylori antigens and not specifically the CagA-positive strain.
Several mechanisms have been proposed to associate CMV infection with cardiovascular diseases. CMV can directly infect vessels, and its presence may induce smooth muscle cell proliferation, expression of inflammatory cytokines, increased uptake of lipoproteins and increased procoagulant activity of endothelial cells (15,39). Expression of several genes involved in immune and inflammatory responses were found to be altered on CMV infection in normal mice (15,40). Increased release of inflammatory cytokines in turn is believed to stimulate the release of sPLA2 from vascular smooth muscle cells (41), which is also associated with the risk of coronary events (42,43).
It is also suggested that individuals seropositive for CMV, with a subclinical inflammatory profile as estimated by CRP levels, are more susceptible to atherosclerosis compared with those without inflammation (44). We observed a direct association between CMV antibody levels and mean levels of IL-6, fibrinogen, sPLA2 and hsCRP, suggesting a subclinical inflammatory profile for these individuals. Although the levels of the four inflammatory markers were higher in the highest quartile of CMV antibodies, the RR association of CMV antibodies increased only in the presence of hsCRP reiterating the importance of hsCRP in subclinical inflammation. The biomarkers IL-6, fibrinogen and sPLA2 increased the risk as well as the discriminatory power of CMV antibodies when included along with hsCRP, suggesting that a multi-marker-based model including CMV antibodies and inflammation may improve the prediction of CAD risk in this population.
It has also been reported that CMV reactivation may accelerate the process of atherogenesis (15,39). During the process of reactivation, cross-reacting human peptides may be presented by the virus due to molecular mimicry resulting in hyperactivation of immune system (16,45). Thus, CMV reactivation seems to be important in the physiological harm caused by the virus. The factors that can cause viral reactivation include stress, age, immunosuppression and coinfection, which may influence the risk association of CMV with CAD. Given the high CMV seropositivity in the Indian population, along with higher stress associated with the working class, the possibility of reactivation and CMV-induced pathogenesis may be an important risk factor for this population.
CONCLUSION
We report a high prevalence of CMV infection in a large Asian Indian population, and elevated CMV antibody titres are an important CAD marker for incident and recurrent cardiac events. Our results also suggest that it is not the presence of antibodies to the pathogen alone, but overall titres that influence CAD risk. Estimation of specific markers of CMV reactivation may serve as a better marker for risk prediction compared with total anti-CMV antibody levels.
The strengths of our study include its large Asian Indian population size, measurement of covariates and adjustment for the potential confounding factors. A limitation of the study includes the case control design, wherein the blood samples were collected after the CAD event, which limits the causal interpretation of the relationship between CMV titres, increase in the levels of inflammatory markers and CAD risk. Future studies will be directed toward including a larger case control population and estimating titres of specific antibodies to other clinically relevant pathogenic antigens, and longer follow-up to study the association among infection, inflammation and recurrent cardiac events
Acknowledgments
The authors gratefully acknowledge the support of the trustees of Thrombosis Research Institute, London and Bangalore, Tata Social Welfare Trust, India (TSWT/IG/SNB/JP/Sdm) and Department of Biotechnology, Ministry of Science and Technology, Government of India (BT/01/CDE/08/07). The sponsors did not participate in the design, conduct, sample collection analysis and interpretation of the data or in the preparation, review or approval of the manuscript. The authors acknowledge the contribution of Mr K Karthik and Mr S Niladri for the estimation of inflammatory biomarkers and Dr Usha Narayan for editorial assistance. The authors thank all investigators, staff, administrative teams and participants of the IARS from Narayana Hrudayalaya, Bangalore and Asian Heart Centre, Mumbai for their contribution. The authors are grateful to the patients, their family members and the unaffected subjects for participating in the study.
Footnotes
DISCLOSURE: The authors have no financial disclosures or conflicts of interest to declare.
AUTHOR CONTRIBUTIONS: LM and VSR equally contributed to preparation of the manuscript. The experiments were designed by VSR. The data analysis for this manuscript was performed by SH, LM and RN. LM drafted the manuscript, which was approved by all authors
FUNDING: Tata Social Welfare Trust, India (TSWT/IG/SNB/JP/Sdm) and Department of Biotechnology, Ministry of Science and Technology, Government of India (BT/01/CDE/08/07).
REFERENCES
- 1.Enas EA. Coronary artery disease epidemic in Indians: A cause for alarm and call for action. J Indian Med Assoc. 2000;98:694–5. 697–702. [PubMed] [Google Scholar]
- 2.Gupta R. Recent trends in coronary heart disease epidemiology in India. Indian Heart J. 2008;60:B4–18. [PubMed] [Google Scholar]
- 3.Enas EA, Singh V, Munjal YP, et al. Reducing the burden of coronary artery disease in India: Challenges and opportunities. Indian Heart J. 2008;60:161–75. [PubMed] [Google Scholar]
- 4.Hansa G, Bhargava K, Bansal M, Tandon S, Kasliwal RR. Carotid intima-media thickness and coronary artery disease: An Indian perspective. Asian Cardiovasc Thorac Ann. 2003;11:217–21. doi: 10.1177/021849230301100308. [DOI] [PubMed] [Google Scholar]
- 5.Goyal P, Kalek SC, Chaudhry R, Chauhan S, Shah N. Association of common chronic infections with coronary artery disease in patients without any conventional risk factors. Indian J Med Res. 2007;125:129–36. [PubMed] [Google Scholar]
- 6.Gupta R. Secondary prevention of coronary artery disease in urban Indian primary care. Int J Cardiol. 2009;135:184–6. doi: 10.1016/j.ijcard.2008.03.047. [DOI] [PubMed] [Google Scholar]
- 7.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 8.Huittinen T, Leinonen M, Tenkanen L, et al. Autoimmunity to human heat shock protein 60, Chlamydia pneumoniae infection, and inflammation in predicting coronary risk. Arterioscler Thromb Vasc Biol. 2002;22:431–7. doi: 10.1161/hq0302.104512. [DOI] [PubMed] [Google Scholar]
- 9.Oshima T, Ozono R, Yano Y, et al. Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J Am Coll Cardiol. 2005;45:1219–22. doi: 10.1016/j.jacc.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Zhu J, Quyyumi AA, Norman JE, et al. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000;85:140–6. doi: 10.1016/s0002-9149(99)00653-0. [DOI] [PubMed] [Google Scholar]
- 11.J Shanker KVV. Role of periodontal infection in cardiovascular disease: A current perspective. Arch Med Sci. 2009;5:125–34. [Google Scholar]
- 12.Danesh JPW, Walker M, Lennon L, et al. Chlamydia pneumoniae IgG titres and coronary heart disease: Prospective study and meta-analysis. BMJ. 2000;321:208–12. doi: 10.1136/bmj.321.7255.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein SE. The multiple mechanisms by which infection may contribute to atherosclerosis development and course. Circ Res. 2002;90:2–4. [PubMed] [Google Scholar]
- 14.Zhu J, Nieto FJ, Horne BD, et al. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation. 2001;103:45–51. doi: 10.1161/01.cir.103.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Epstein SE, Zhu J, Najafi AH, Burnett MS. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation. 2009;119:3133–41. doi: 10.1161/CIRCULATIONAHA.109.849455. [DOI] [PubMed] [Google Scholar]
- 16.Albert LJ, Inman RD. Molecular mimicry and autoimmunity. N Engl J Med. 1999;341:2068–74. doi: 10.1056/NEJM199912303412707. [DOI] [PubMed] [Google Scholar]
- 17.Zaidi AK, Awasthi S, deSilva HJ. Burden of infectious diseases in South Asia. BMJ. 2004;328:811–5. doi: 10.1136/bmj.328.7443.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shanker J, Maitra A, Rao VS, et al. Rationale, design & preliminary findings of the Indian Atherosclerosis Research Study. Indian Heart J. 2011;62:286–95. [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Enas EA, Mohan V, Deepa M, et al. The metabolic syndrome and dyslipidemia among Asian Indians: A population with high rates of diabetes and premature coronary artery disease. J Cardiometab Syndr. 2007;2:267–75. doi: 10.1111/j.1559-4564.2007.07392.x. [DOI] [PubMed] [Google Scholar]
- 21.Jayasinghe SR, Jayasinghe SH. Variant metabolic risk factor profile leading to premature coronary disease: Time to define the syndrome of accelerated atherocoronary metabolic syndrome in Asian Indians. Singapore Med J. 2009;50:949–55. [PubMed] [Google Scholar]
- 22.Haider AW, Wilson PW, Larson MG, et al. The association of seropositivity to Helicobacter pylori, Chlamydia pneumoniae, and cytomegalovirus with risk of cardiovascular disease: A prospective study. J Am Coll Cardiol. 2002;40:1408–13. doi: 10.1016/s0735-1097(02)02272-6. [DOI] [PubMed] [Google Scholar]
- 23.Smieja M, Gnarpe J, Lonn E, et al. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2003;107:251–7. doi: 10.1161/01.cir.0000044940.65226.1f. [DOI] [PubMed] [Google Scholar]
- 24.Zhou YF, Leon MB, Waclawiw MA, et al. Association between prior cytomegalovirus infection and the risk of restenosis after coronary atherectomy. N Engl J Med. 1996;335:624–30. doi: 10.1056/NEJM199608293350903. [DOI] [PubMed] [Google Scholar]
- 25.Guech-Ongey M, Brenner H, Twardella D, Rothenbacher D. Chlamydia pneumoniae, heat shock proteins 60 and risk of secondary cardiovascular events in patients with coronary heart disease under special consideration of diabetes: A prospective study. BMC Cardiovasc Disord. 2006;6:17. doi: 10.1186/1471-2261-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jha HC, Srivastava P, Sarkar R, Prasad J, Mittal A. Chlamydia pneumoniae IgA and elevated level of IL-6 may synergize to accelerate coronary artery disease. J Cardiol. 2008;52:140–5. doi: 10.1016/j.jjcc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Jha HC, Prasad J, Mittal A. High immunoglobulin A seropositivity for combined Chlamydia pneumoniae, Helicobacter pylori infection, and high-sensitivity C-reactive protein in coronary artery disease patients in India can serve as atherosclerotic marker. Heart Vessels. 2008;23:390–6. doi: 10.1007/s00380-008-1062-9. [DOI] [PubMed] [Google Scholar]
- 28.Moyses Szkloa JD, Tsaic MY, Cushmand M, et al. Individual pathogens, pathogen burden and markers of subclinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Med. 2009;10:747–51. doi: 10.2459/JCM.0b013e32832cacab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Silvertown JD. Inflammation, C-reactive protein, and atherothrombosis. J Periodontol. 2008;79:1544–51. doi: 10.1902/jop.2008.080249. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Kundsin RB, Stampfer MJ, Poulin S, Hennekens CH. Prospective study of Chlamydia pneumoniae IgG seropositivity and risks of future myocardial infarction. Circulation. 1999;99:1161–4. doi: 10.1161/01.cir.99.9.1161. [DOI] [PubMed] [Google Scholar]
- 32.Degre M. Has cytomegalovirus infection any role in the development of atherosclerosis? Clin Microbiol Infect. 2002;8:191–5. doi: 10.1046/j.1469-0691.2002.00407.x. [DOI] [PubMed] [Google Scholar]
- 33.Sorlie PD, Nieto FJ, Adam E, et al. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: The atherosclerosis risk in communities (ARIC) study. Arch Intern Med. 2000;160:2027–32. doi: 10.1001/archinte.160.13.2027. [DOI] [PubMed] [Google Scholar]
- 34.Manegold C, Alwazzeh M, Jablonowski H, et al. Prior cytomegalovirus infection and the risk of restenosis after percutaneous transluminal coronary balloon angioplasty. Circulation. 1999;99:1290–4. doi: 10.1161/01.cir.99.10.1290. [DOI] [PubMed] [Google Scholar]
- 35.Eryol NK, Kilic H, Gul A, et al. Are the high levels of cytomegalovirus antibodies a determinant in the development of coronary artery disease? Int Heart J. 2005;46:205–9. doi: 10.1536/ihj.46.205. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher A, Lerkerod AB, Seljeflot I, et al. Chlamydia pneumoniae serology: Importance of methodology in patients with coronary heart disease and healthy individuals. J Clin Microbiol. 2001;39:1859–64. doi: 10.1128/JCM.39.5.1859-1864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pasceri V, Cammarota G, Patti G, et al. Association of virulent Helicobacter pylori strains with ischemic heart disease. Circulation. 1998;97:1675–9. doi: 10.1161/01.cir.97.17.1675. [DOI] [PubMed] [Google Scholar]
- 38.Pietroiusti A, Diomedi M, Silvestrini M, et al. Cytotoxin-associated gene A-positive Helicobacter pylori strains are associated with atherosclerotic stroke. Circulation. 2002;106:580–4. doi: 10.1161/01.cir.0000023894.10871.2f. [DOI] [PubMed] [Google Scholar]
- 39.Stassen FR, Vega-Cordova X, Vliegen I, Bruggeman CA. Immune activation following cytomegalovirus infection: More important than direct viral effects in cardiovascular disease? J Clin Virol. 2006;35:349–53. doi: 10.1016/j.jcv.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Burnett MS, Durrani S, Stabile E, et al. Murine cytomegalovirus infection increases aortic expression of proatherosclerotic genes. Circulation. 2004;109:893–7. doi: 10.1161/01.CIR.0000112585.47513.45. [DOI] [PubMed] [Google Scholar]
- 41.Menschikowski M, Rosner-Schiering A, Eckey R, et al. Expression of secretory group IIA phospholipase A(2) in relation to the presence of microbial agents, macrophage infiltrates, and transcripts of proinflammatory cytokines in human aortic tissues. Arterioscler Thromb Vasc Biol. 2000;20:751–62. doi: 10.1161/01.atv.20.3.751. [DOI] [PubMed] [Google Scholar]
- 42.Mallat Z, Steg PG, Benessiano J, et al. Circulating secretory phospholipase A2 activity predicts recurrent events in patients with severe acute coronary syndromes. J Am Coll Cardiol. 2005;46:1249–57. doi: 10.1016/j.jacc.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 43.Kugiyama K, Ota Y, Takazoe K, et al. Circulating levels of secretory type II phospholipase A(2) predict coronary events in patients with coronary artery disease. Circulation. 1999;100:1280–4. doi: 10.1161/01.cir.100.12.1280. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, Quyyumi AA, Norman JE, Csako G, Epstein SE. Cytomegalovirus in the pathogenesis of atherosclerosis: The role of inflammation as reflected by elevated C-reactive protein levels. J Am Coll Cardiol. 1999;34:1738–43. doi: 10.1016/s0735-1097(99)00410-6. [DOI] [PubMed] [Google Scholar]
- 45.Reinhardt B, Mertens T, Mayr-Beyrle U, et al. HCMV infection of human vascular smooth muscle cells leads to enhanced expression of functionally intact PDGF beta-receptor. Cardiovasc Res. 2005;67:151–60. doi: 10.1016/j.cardiores.2005.03.012. [DOI] [PubMed] [Google Scholar]


