Abstract
Background and Purpose
Interleukin-6 (IL-6) has been shown to have a neuroprotective effect in brain ischemic injury. However, its molecular mechanisms are still poorly understood. In this study, we investigated the neuroprotective role of the IL-6 receptor (IL-6R) by IL-6 in the reactive oxygen species defense system after transient focal cerebral ischemia (tFCI).
Methods
IL-6 was injected in mice before and after middle cerebral artery occlusion. Co-immunoprecipitation assays were performed for analysis of IL-6R association after tFCI. Primary mouse cerebral cortical neurons were transfected with small interfering RNA probes targeted to IL-6Rα or gp130 and were used for chromatin-immunoprecipitation assay, luciferase promoter assay, and cell viability assay. Reduction in infarct volumes by IL-6 was measured after tFCI.
Results
IL-6R was disrupted via a disassembly between IL-6Rα and gp130 associated by protein oxidation after reperfusion following tFCI. This suppressed phosphorylation of signal transducer and activator of transcription 3 (STAT3), and finally induced neuronal cell death via a decrease in manganese-superoxide dismutase (Mn-SOD). However, IL-6 injections prevented disruption of IL-6R against reperfusion after tFCI, consequently restoring activity of STAT3 through recovery of the binding of STAT3 to gp130. Moreover, IL-6 injections restored the transcriptional activity of the Mn-SOD promoter through recovery of the recruitment of STAT3 to the Mn-SOD promoter, and reduced infarct volume after tFCI.
Conclusions
This study demonstrates that IL-6 has a neuroprotective effect against cerebral ischemic injury through IL-6R-mediated STAT3 activation and Mn-SOD expression.
Keywords: IL-6, STAT3, Mn-SOD, cerebral ischemia, neuroprotection
Introduction
Many studies have demonstrated the neuroprotective effects of interleukin-6 (IL-6) in ischemic brain injuries,1–5 although a few studies suggest that IL-6 has an injurious effect.6 Continuous injection of IL-6 reduced neuronal loss after forebrain ischemia,4 and injection of the IL-6 receptor (IL-6R) antibody after middle cerebral artery occlusion (MCAO) increased cell death and infarct volume.3 However, the mechanism of neuroprotection by IL-6 in relation to IL-6R in cerebral ischemia has not been fully elucidated.
The action of IL-6 begins at the cell surface via its binding to IL-6R,7 which is composed of two major subunits, a ligand bindingα subunit (IL-6Rα) and a β subunit (gp130), which is a transmembrane glycoprotein with signal transduction capability.7 The binding of IL-6 to IL-6Rα induces homodimerization of gp130,7 and consequently recruits the Janus kinase (JAK) to the cytosolic part of gp130.7 Recruited JAK becomes phosphorylated and phosphorylates multiple tyrosine residues on the cytoplasmic part of gp130, thereby creating docking sites for signal transducer and activator of transcription 3 (STAT3).8 The recruited STAT3 is then phosphorylated at Y705 residues, and phosphorylated STAT3 (p-STAT3; Y705) dimerizes and translocates to the nucleus, where it binds to the promoter of target genes to regulate transcription.8
STAT3 is involved in neuroprotection against various brain injuries, including cerebral ischemia.3,9–11 After brain ischemia, superoxide anions and reactive oxygen species (ROS) are produced in mitochondria. These ROS induce the mitochondrial-dependent apoptotic pathways.12,13 These ROS, however, are removed by manganese-containing superoxide dismutase (Mn-SOD or SOD2), a primary cellular defense antioxidant enzyme specific to superoxide.14 STAT3 regulates transcription of the Mn-SOD gene in the mouse cerebral cortex and cortical neurons.11 In this study, we elucidated the molecular mechanism of neuroprotection by IL-6 in relation to IL-6R-mediated STAT3/ROS regulation in mouse ischemic brains.
Materials and Methods
Animals
All procedures in experiments with animals were performed in accordance with National Institutes of Health guidelines and were approved by Stanford University's Administrative Panel on Laboratory Animal Care. Male CD1 mice (30–35 grams, 2 months old) were purchased from Charles River Laboratories (Wilmington, MA). Heterozygous SOD2 knockout (KO) mutant mice (CD1/SV129 background, backcrossed with CD1 for more than 10 generations) and their wild-type (WT) littermates with an identical genetic background were used. Heterozygous copper/zinc-superoxide dismutase transgenic (SOD1 TG) mice (SOD1 TGHS/SF-218-3 strain with a CD1 background) and their WT littermates with an identical genetic background were also used.
Focal Cerebral Ischemia
Forty-five minutes of transient focal cerebral ischemia (tFCI) were induced by MCAO. CD1 mice were anesthetized with 1.5% isoflurane in 70% nitrous oxide and 30% oxygen using a face mask, and maintained with 1.5% isoflurane during surgery. Rectal temperature was kept at 37°C with a homeothermic blanket. An 11.0-mm 6-0 surgical monofilament nylon suture, blunted at the tip, was introduced into the left internal carotid artery through the external carotid artery stump. After 45 minutes of MCAO, the nylon suture was withdrawn, restoring blood flow. Sham-operated mice underwent the same procedure without occlusion of the vessels.
Statistical Analysis
All results were obtained from 3–5 independent experiments and are presented as mean ± S.E.M. Data are expressed using Student's t test. Differences were considered statistically significant at a P value <0.05.
Detailed descriptions of our methods appear in the Supplemental Material. These methods include, primary cortical neuron culture, oxygen-glucose deprivation (OGD) and reoxygenation, IL-6 treatments, co-immunoprecipitation (Co-IP) assay, immunofluorescence staining and confocal microscopy, detection of protein oxidation, small interfering RNA (siRNA) transfection, Western blot, chromatin immunoprecipitation (ChIP) assay, luciferase activity assay, determination of infarction, and cell death assay.
Results
IL-6 Increases p-STAT3 and Mn-SOD Expression in the Ischemic Brain and Cortical Neurons
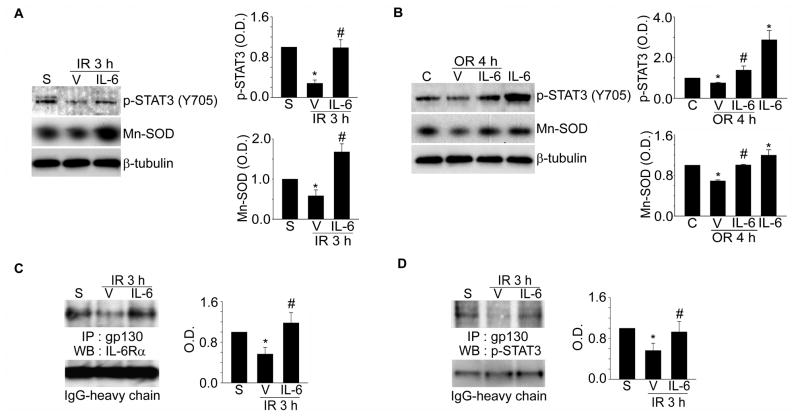
To investigate the neuroprotective effect of IL-6, two intracerebroventricular injections of IL-6 (50 ng in 2 μl of phosphate-buffered saline) were administered 30 minutes before and 15 minutes after MCAO in mice. We then examined the change in p-STAT3 and Mn-SOD expression caused by IL-6 in ischemic cerebral tissue. Injections of IL-6 significantly recovered p-STAT3 and Mn-SOD expression, which was reduced by 3 hours of reperfusion in the cerebral ischemic cortex (Figure 1A). The Mn-SOD mRNA level also recovered after these injections (data not shown). In addition, recovery of p-STAT3 and Mn-SOD expression (Figure 1B) and the Mn-SOD mRNA level (data not shown) was detected after treatment with IL-6 (50 ng/ml) in primary cortical neurons subjected to 2.5 hours of OGD/4 hours of reoxygenation.
Figure 1.
Increase in p-STAT3 (Y705) and Mn-SOD by IL-6 in mouse cerebral ischemic reperfusion in vivo and in vitro, and disruption of IL-6R after ischemic reperfusion. Western blot analysis of p-STAT3 (Y705), Mn-SOD, andβ-tubulin after 3 hours of reperfusion following tFCI with or without IL-6 injections (A) or after 4 hours of reoxygenation following OGD with or without IL-6 treatments in cerebral cortical neurons (B). Summary graphs depicting the band intensity of each Western blot. *P<0.05 vs. sham (A) or control (B), #P<0.05 vs. vehicle (n=4 per group). Co-IP assays for analysis of association between IL-6Rα and gp130 (C) or between gp130 and p-STAT3 (D) after 3 hours of reperfusion following tFCI with or without IL-6 injections. Summary graphs depicting the band intensity of all Co-IP data. *P<0.05 vs. sham, #P<0.05 vs. vehicle (n=4 per group). S indicates sham; IR, ischemic reperfusion; IP, immunoprecipitation; WB, Western blot; V, vehicle; C, control; OR, oxygen-glucose deprivation/reoxygenation; O.D., optical density.
Disruption of IL-6R by Ischemic Reperfusion in Mouse Cerebral Cortex and Cortical Neurons
To elucidate the neuroprotective role of IL-6R by IL-6 in cerebral ischemic injury, we first examined the conformational status of IL-6R using a Co-IP assay of ischemic mouse brain tissue. We found that there was an interaction between IL-6Rα and gp130 in IL-6R with sham surgery under normal physiological conditions in mouse brain cortices (Figure 1C). The specificity of each antibody used in this assay was confirmed using normal rabbit IgG as a negative control (Supplemental Figure 1A and 1B). Interestingly, this interaction dissociated within 3 hours of reperfusion after tFCI (Figure 1C). Association between IL-6Rα and gp130 subsequently causes phosphorylation of JAK, and phosphorylated JAK phosphorylates multiple tyrosine residues on the cytoplasmic part of gp130.8 However, dissociation between IL-6Rα and gp130 after reperfusion in cerebral ischemic injury caused suppression of JAK2 phosphorylation (Y1007/1008), and then blocked phosphorylation of multi-tyrosine residues on the cytosolic part of gp130 (Supplemental Figure 1C). This sequential disruption blocked recruitment of STAT3 to the cytosolic domain of gp130 (Figure 1D), and then finally blocked phosphorylation of STAT3 (Y705) (Supplemental Figure 1C). However, IL-6 injections restored the association between IL-6Rα and gp130 and reinstated recruitment and phosphorylation of STAT3 (Figure 1C and 1D). The dissociation of IL-6R after reperfusion following tFCI in vivo and the recovery of IL-6R association by IL-6 were also confirmed using a confocal microscope (Supplemental Figure 2). Moreover, the same disruption of IL-6R was revealed in cerebral cortical neurons within 4 hours of reoxygenation following 2.5 hours of OGD (Supplemental Figure 3A and 3B). This disruption of IL-6R suppressed recruitment of STAT3 to the cytosolic domain of gp130 (Supplemental Figure 3C), and treatment with IL-6 restored the assembly in the IL-6R component (Supplemental Figure 3). We found a continuous association between IL-6Rα and gp130 in the mouse cerebral cortex and cortical neurons under normal conditions (Figure 1C, Supplemental Figures 2 and 3). This continuous association led to the recruitment of STAT3 to the cytosolic domain of gp130 and phosphorylation of STAT3 (Y705), even under normal conditions (Figure 1D, Supplemental Figures 1C and 3C). These results explain why STAT3 had high activity, even in normal brain tissue and cortical neurons, in our previous report.11
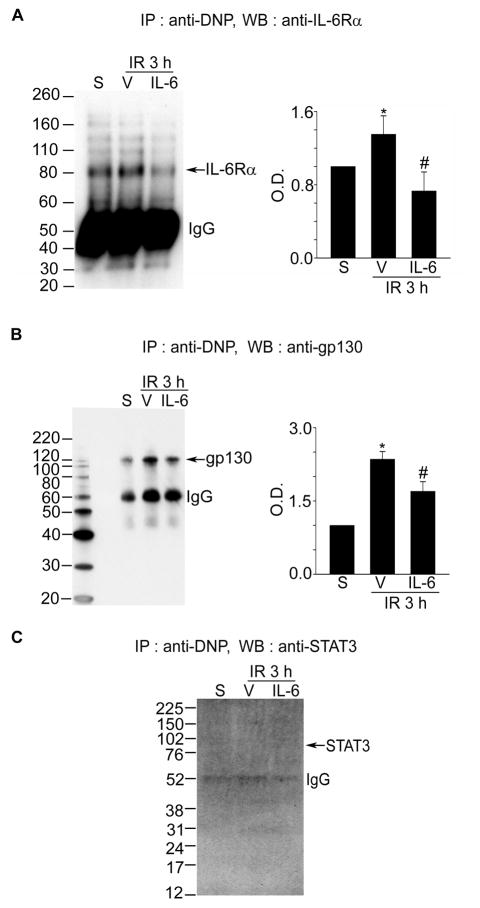
Oxidation of Each Component of IL-6R Caused by Reperfusion in the Mouse Cerebral Ischemic Cortex and Cortical Neurons
To clarify why the association between IL-6Rα and gp130 is blocked after reperfusion following tFCI, we examined oxidative injury caused directly by reperfusion in each component of IL-6R. Under exposure to ROS, proteins are processed to oxidative modification, which can lead to conformational changes in the proteins.15 We measured the quantification of proteins modified by oxygen free radicals using 2,4-dinitrophenyl, which specifically reacted with the oxidized carbonyl residue on the protein samples.16 To detect oxidation of IL-6Rα or gp130 after reperfusion following tFCI, the oxidized proteins were immunoprecipitated by an anti-2,4-dinitrophenyl antibody and then immunoblotted using IL-6Rα or gp130 antibodies. As shown in Figure 2, the oxidation level of IL-6Rα or gp130 was significantly increased after 3 hours of reperfusion in the cerebral ischemic cortex (Figure 2A and 2B). However, oxidation of STAT3 was not detected (Figure 2C). Interestingly, injections of IL-6 blocked oxidation of IL-6Rα and gp130 in the cerebral ischemic cortex. Also, the oxidation level of IL-6Rα or gp130 was significantly increased after 4 hours of reoxygenation in cortical neurons subjected to 2.5 hours of OGD, and this oxidation was blocked by IL-6 treatments (Supplemental Figure 4A and 4B).
Figure 2.
Oxidation of each component of IL-6R in mouse cerebral ischemic reperfusion. A–C, Co-IP assays for analysis of protein oxidation in the IL-6R components after 3 hours of reperfusion following tFCI with or without IL-6 injection. Summary graphs depicting the band intensity of all Co-IP data. *P<0.05 vs. sham, #P<0.05 vs. vehicle (n=4 per group). IR indicates ischemia reperfusion; S, sham; V, vehicle; IP, immunoprecipitation; DNP, 2,4-dinitrophenyl; WB, Western blot.
To confirm whether protein oxidation in each IL-6R component causes disassociation of IL-6R, we compared the level of disassociation of IL-6R caused by ischemic reperfusion between the SOD1 TG mice and the WT mice. The level of the disassociation between IL-6Rα and gp130 in the SOD1 TG mice was lower than in the WT mice (Supplemental Figure 4D). These results indicate that the acute influx of oxygen during reperfusion after tFCI induces the oxidation of IL-6Rα and gp130, and that these consequently cause disassociation of each component of IL-6R.
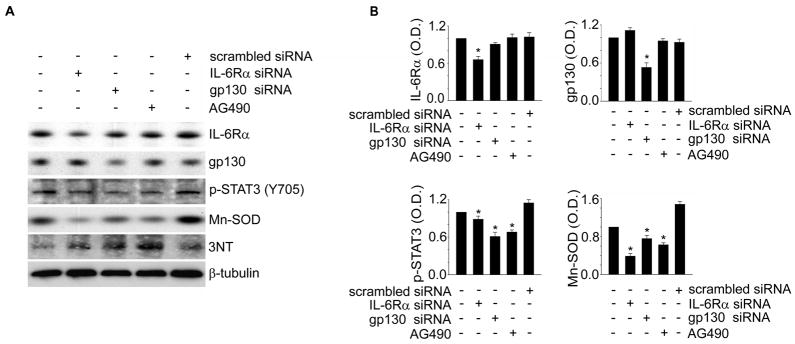
Gene Silencing of IL-6R Causes a Decrease in Mn-SOD Expression via STAT3 Inactivation in Cerebral Cortical Neurons
IL-6 injections restored IL-6R association, consequently leading to the recovery of p-STAT3 (Y705) and Mn-SOD levels after reperfusion in the cerebral ischemic cortex (Figure 1). These results indicate that IL-6R is an up-stream regulator in STAT3-mediated Mn-SOD expression in the mouse cerebral cortex. To clarify this, we applied the siRNA transfection system for knockdown of each component of IL-6R in mouse cerebral cortical neurons, and then examined STAT3 activity and the Mn-SOD level. As shown in Figure 3, the transfection efficiency of each specific siRNA was confirmed by quantification of the total protein level for IL-6Rα or gp130. Inhibition of IL-6Rα or gp130 by transfection with each specific siRNA in cerebral cortical neurons significantly reduced STAT3 phosphorylation and Mn-SOD expression. Reduction in Mn-SOD by STAT3 inhibition was also confirmed using the STAT3 inhibitor, AG490. Moreover, the level of 3-nitrotyrosine protein nitrosylation was increased in cortical neurons transfected with IL-6Rα- or gp130-specific siRNA. This result indicates that IL-6R is involved in the ROS regulation system via up-regulation of STAT3 and Mn-SOD.
Figure 3.
Reduction of p-STAT3 and Mn-SOD by IL-6R knockdown in mouse cerebral cortical neurons. A, Western blot analysis of IL-6Rα, gp130, p-STAT3 (Y705), Mn-SOD, 3NT, andβ-tubulin in cerebral cortical neurons transfected with IL-6Rα- or gp130-specific siRNA or treated with AG490 for 24 hours. B, Summary graphs depicting the band intensity of each Western blot. *P<0.05 (n=4 per group). 3NT indicates 3-nitrotyrosine; O.D., optical density.
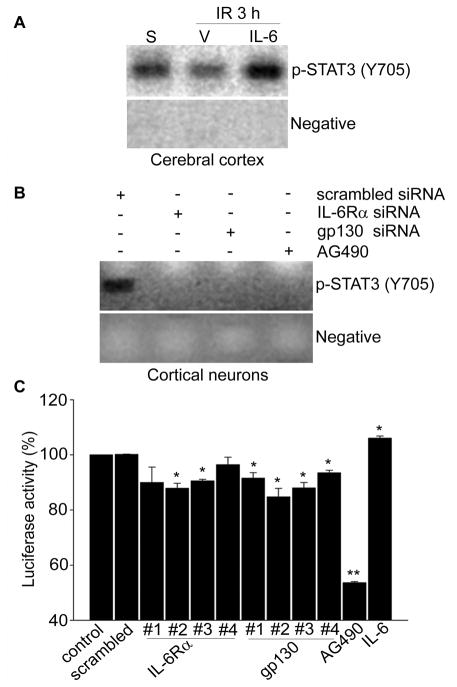
IL-6 Recovers STAT3 Recruitment to the Mn-SOD Promoter in Ischemic Reperfusion
To confirm whether IL-6R regulates STAT3-induced transcriptional activity of the Mn-SOD promoter, we first examined recruitment of STAT3 by IL-6 to the mouse Mn-SOD promoter using a ChIP assay. As shown in Figure 4A, STAT3 was strongly recruited to the Mn-SOD promoter in chromatin from sham-operated mouse brain cortices, and this recruitment was completely blocked after reperfusion following tFCI. However, IL-6 injections reinstated the recruitment of STAT3 into the Mn-SOD promoter. Moreover, IL-6Rα or gp130 inhibition by transfection with each specific siRNA in cerebral cortical neurons completely blocked the recruitment of STAT3 into the Mn-SOD promoter (Figure. 4B).
Figure 4.
IL-6R up-regulates transcriptional activity of Mn-SOD via STAT3 recruitment into the Mn-SOD promoter. ChIP assay for analysis of STAT3 recruitment into the mouse Mn-SOD promoter after 3 hours of reperfusion with or without IL-6 injection in the cerebral ischemic cortex (A) or in cerebral cortical neurons transfected with IL-6Rα- or gp130-specific siRNA for 24 hours (B). C, Luciferase assay for analysis of transcriptional activity of the Mn-SOD promoter in cerebral cortical neurons transfected with pGLu–Mn-SOD and IL-6Rα- or gp130-specific siRNA or treated with AG490 or IL-6 for 24 hours (n=4 per group). Data are mean± S.E.M. *P<0.05, **P<0.001 vs. control. IR indicates ischemic reperfusion; S, sham; V, vehicle.
Decreased Transcriptional Activity of the Mn-SOD Promoter by Knockdown of the IL-6R Component in Cerebral Cortical Neurons
Using a luciferase assay, we investigated transcriptional activity of the Mn-SOD promoter in cerebral cortical neurons transfected with IL-6Rα- or gp130-specific siRNA. As shown in Figure 4C, inhibition of IL-6Rα or gp130 by transfection with each specific siRNA significantly decreased luciferase activity in cortical neurons transfected with pGLu-Mn-SOD. In contrast, treatments with IL-6 significantly increased luciferase activity.
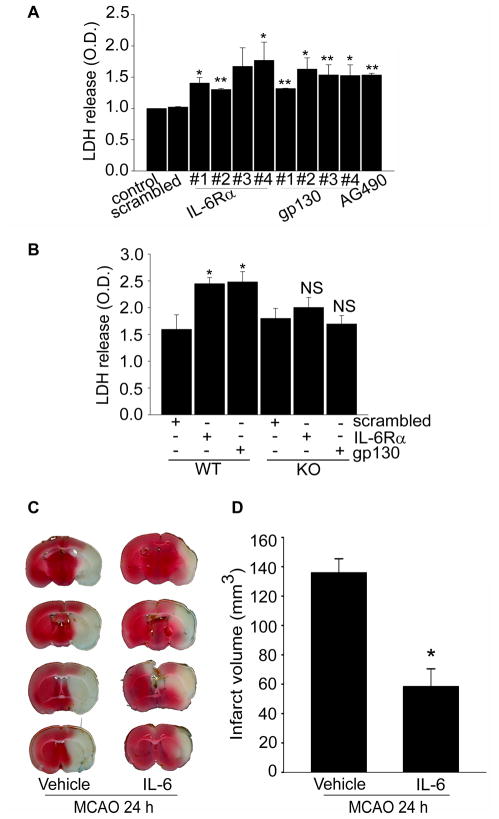
Cell Death by Knockdown of IL-6R in Cerebral Cortical Neurons
Our results indicate that IL-6R is the up-stream regulator in STAT3-mediated Mn-SOD expression in cerebral cortical neurons. This may indicate that sustaining IL-6R at active status is critical for neuronal survival against oxidative stress in cerebral ischemic injury. Thus, we measured cell death by IL-6R inhibition in cerebral cortical neurons. Lactate dehydrogenase (LDH) release significantly increased from cortical neurons transfected with IL-6Rα- or gp130-specific siRNA for 24 hours (Figure 5A). To confirm whether this cell death was caused by reduction in Mn-SOD, we measured cell death by IL-6R inhibition in cerebral cortical neurons from SOD2-/+ heterozygous KO mice. As shown in Figure 5B, there was no significant increase in LDH release in the cortical neurons transfected with IL-6Rα- or gp130-specific siRNA for 24 hours in the SOD2-/+ group, which expressed less Mn-SOD compared with the cortical neurons of WT mice. These results support Mn-SOD as the final target of IL-6R for neuronal survival via the ROS defense system in cerebral cortical neurons.
Figure 5.
Knockdown of IL-6R induces cell death in cerebral cortical neurons, and enhancement of IL-6R by IL-6 reduces infarct volumes in cerebral ischemic injury. A, Cell death assessed by LDH activity in a medium of cortical neurons transfected with IL-6Rα- or gp130-specific siRNA for 24 hours or treated with AG490 for 24 hours (n=4 per group). Data are mean± S.E.M. *P<0.05, **P<0.001 vs. control. B, LDH activity in a medium of cortical neurons transfected with IL-6Rα- or gp130-specific siRNA for 24 hours in SOD2 WT or SOD2-/+ KO mouse brains (n=4 per group). Data are mean ± S.E.M. *P<0.05 vs. control. C, 2,3,5-triphenyltetrazolium chloride staining for analysis of infarct volumes after 24 hours of reperfusion following MCAO with or without IL-6 injection in male mice. D, Summary graph showing the size of infarction volumes. *P<0.05 (n=9 per group). NS indicates not significant; O.D., optical density.
IL-6 Reduces Infarct Volume in the Ischemic Brain
To confirm the neuroprotective effect of IL-6 in vivo, we injected IL-6 into mouse brains before and after MCAO and measured infarction volume after 24 hours of reperfusion. The infarct volumes in the IL-6-treated mice were significantly smaller (P<0.05, n=9 per group) than in the vehicle-treated mice, using 2,3,5-triphenyltetrazolium chloride staining (Figure 5C and 5D).
Discussion
This study has clearly elucidated the mechanism of neuroprotection by IL-6 via the IL-6R-mediated ROS defense system in mouse cerebral cortex and cortical neurons. Injections of IL-6 restored the signal transduction of STAT3-mediated Mn-SOD expression via recovery of IL-6R association, which had been blocked by reperfusion after cerebral ischemia (Figures 1 and 4), and finally protected neuronal cells against oxidative stress (Figure 5). IL-6R was disrupted via a disassociation between IL-6Rα and gp130 caused by reperfusion after cerebral ischemia (Figure 1). This disruption was the result of the oxidation of each component of IL-6R by a direct and acute influx of oxygen during reperfusion after ischemia (Figure 2). However, in SOD1 TG mice, the disassociation between IL-6Rα and gp130 by reperfusion was attenuated compared with SOD1 WT mice (Supplemental Figure 4D). Injection of IL-6 saved each component of IL-6R from protein oxidation during reperfusion after ischemia (Figure 2). To explain why IL-6 blocks protein oxidation by reperfusion in cerebral ischemic injury, further study is needed. There are some reports that IL-6 up-regulates antioxidants.17,18 Thus, IL-6 may accelerate gene expression or antioxidant activity that can block protein oxidation against oxidative stress in the ischemic brain. Also, IL-6 may play a role as an antioxidant molecule in an indirect way. The direct binding of IL-6 to IL-6Rα at the cell surface may serve as a shield from an attack of acute oxygen influx.
Disruption of IL-6R by transfection with IL-6α- or gp130-specific siRNA in cerebral cortical neurons significantly increased superoxide radical production through Mn-SOD reduction (Figure 3). This was caused by suppression of the transcriptional activity of the Mn-SOD promoter via blockage of STAT3 recruitment to the Mn-SOD promoter (Figure 4). Moreover, disruption of IL-6R by transfection with IL-6α- or gp130-specific siRNA induced neuronal cell death (Figure 5A). This cell death was caused by blockage of the ROS defense system. In cortical neurons from SOD2-/+ KO mice, cell death by transfection with IL-6α- or gp130-specific siRNA was not increased compared with a significant increase in cortical neurons from the SOD2 WT mice (Figure 5B).
STAT3 is highly phosphorylated at Y705 in the mouse brain and in cortical neurons under physiological conditions, and p-STAT3 (Y705) up-regulates Mn-SOD expression.11 However, STAT3 immediately lost its activity after reperfusion in cerebral ischemic injury.11 Our present study explains why this happens. The disassembly between IL-6Rα and gp130 after ischemic reperfusion makes it impossible to recruit STAT3 into the cytosolic domain of gp130, and then STAT3 cannot be phosphorylated via sequential disruption of JAK2 phosphorylation (Y1007/1008) and phosphorylation of the cytosolic domain of gp130 (Figure 1C and 1D, Supplemental Figure 1C). However, injections of IL-6 restored STAT3 activity through recovery of IL-6R association against ischemic reperfusion (Figure 1). Also, enhancement of IL-6R by IL-6 recovered the pathway of STAT3-mediated Mn-SOD expression, and protected neuronal cells against ischemic reperfusion (Figures 1, 4, and 5). Injection of IL-6 significantly reduced infarct volume after 24 hours of reperfusion in the ischemic brain (Figure 5C and 5D), although the limitations of the IL-6 effect should be considered, since the protection afforded by it may be partially lost with longer reperfusion. Thus, we suggest that IL-6 may have therapeutic potential against oxidative stress such as cerebral ischemic reperfusion and stroke.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by grants PO1 NS014543, RO1 NS036147, and RO1 NS038653 from the National Institutes of Health, and by the James R. Doty Endowment (P.H.C.).
Footnotes
Disclosures
None.
References
- 1.Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Ali C, Nicole O, Docagne F, Lesne S, MacKenzie ET, Nouvelot A, et al. Ischemia-induced interleukin-6 as a potential endogenous neuroprotective cytokine against NMDA receptor-mediated excitotoxicity in the brain. J Cereb Blood Flow Metab. 2000;20:956–966. doi: 10.1097/00004647-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita T, Sawamoto K, Suzuki S, Suzuki N, Adachi K, Kawase T, et al. Blockade of interleukin-6 signaling aggravates ischemic cerebral damage in mice: possible involvement of Stat3 activation in the protection of neurons. J Neurochem. 2005;94:459–468. doi: 10.1111/j.1471-4159.2005.03227.x. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda S, Wen T-C, Morita F, Otsuka H, Igase K, Yoshimura H, et al. Interleukin-6 prevents ischemia-induced learning disability and neuronal and synaptic loss in gerbils. Neurosci Lett. 1996;204:109–112. doi: 10.1016/0304-3940(96)12340-5. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29:464–479. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- 6.Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL, et al. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS ONE. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shyu W-C, Lin S-Z, Chiang M-F, Chen D-C, Su C-Y, Wang H-J, et al. Secretoneurin promotes neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway in murine models of stroke. J Clin Invest. 2008;118:133–148. doi: 10.1172/JCI32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dziennis S, Jia T, Rønnekleiv OK, Hurn PD, Alkayed NJ. Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection. J Neurosci. 2007;27:7268–7274. doi: 10.1523/JNEUROSCI.1558-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci. 2009;29:7003–7014. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J Neurosci. 1999;19:RC39:1–6. doi: 10.1523/JNEUROSCI.19-22-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan PH. Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann N Y Acad Sci. 2005;1042:203–209. doi: 10.1196/annals.1338.022. [DOI] [PubMed] [Google Scholar]
- 14.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 15.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Powell SR. Decreased sensitivity associated with an altered formulation of a commercially available kit for detection of protein carbonyls. Free Radic Biol Med. 2010;49:119–121. doi: 10.1016/j.freeradbiomed.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penkowa M, Hidalgo J. IL-6 deficiency leads to reduced metallothionein-I+II expression and increased oxidative stress in the brain stem after 6-aminonicotinamide treatment. Exp Neurol. 2000;163:72–84. doi: 10.1006/exnr.2000.7383. [DOI] [PubMed] [Google Scholar]
- 18.Hernández J, Hidalgo J. Endotoxin and intracerebroventricular injection of IL-1 and IL-6 induce rat brain metallothionein-I and -II. Neurochem Int. 1998;32:369–373. doi: 10.1016/s0197-0186(97)00096-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.