Abstract
Aims
Insulin resistance and dyslipidaemia both increase cardiovascular risk in Type 1 diabetes. However, little data exist on the associations of insulin resistance to lipids in Type 1 diabetes. Our objective was to explore the associations between insulin resistance (assessed by glucose infusion rate) and lipids in people with Type 1 diabetes and determine whether adiposity and/or average glycaemia influence these associations.
Methods
Hyperinsulinaemic–euglycaemic clamp studies were performed in 60 subjects with Type 1 diabetes aged 12–19 years (age 15 ± 2 years, 57% female, duration of diabetes 6.3 ± 3.8 years, HbA1c 8.6 ± 1.5%) and 40 subjects with Type 1 diabetes aged 27–61 years (age 45 ± 9 years, 53% female, duration of diabetes 23 ± 8 years, HbA1c 7.5 ± 0.9%). Multiple linear regression models were fit to examine the association between glucose infusion rate and fasting lipid levels with adjustment for possible confounders.
Results
Lower glucose infusion rate was significantly associated with lower levels of HDL cholesterol in youths with Type 1 diabetes and with higher levels of triglycerides and higher triglyceride/HDL ratio in both youths and adults. The magnitude of the associations between glucose infusion rate and lipid levels translate into interquartile differences of 0.098 mmol/l for HDL cholesterol, 0.17 mmol/l for triglycerides and 1.06 for triglycerides/HDL in the adolescents and 0.20 mmol/l for triglycerides and 1.01 for triglycerides/HDL in the adults. The associations were attenuated and no longer statistically significant by adjustment for adiposity among adults, while adjustment for HbA1c had a small effect in youths and adults.
Conclusions
Lower insulin sensitivity is associated with a more atherogenic lipid profile in both youths and adults with Type 1 diabetes.
Keywords: insulin resistance, insulin sensitivity, lipids, Type 1 diabetes
Introduction
Type 1 diabetes is increasing in youths and presenting at younger ages [1], which implies a longer burden of disease and possibly an earlier onset of vascular complications. Although intensive glycaemic control can decrease vascular complications [2], cardiovascular disease remains the leading cause of death in Type 1 diabetes [3]. Dyslipidaemia is an important cardiovascular disease risk factor, in addition to glycaemic control, hypertension and others[3,4]. One such additional cardiovascular disease risk factor is insulin resistance [5]. However, direct measurement of insulin sensitivity in insulin-deficient patients with Type 1 diabetes is difficult and requires the hyperinsulinaemic–euglycaemic insulin clamp approach [6].
Studies using the hyperinsulinaemic–euglycaemic clamp have demonstrated decreased insulin sensitivity in patients with Type 1 diabetes when compared with people without diabetes [7,8]. Insulin resistance is associated with a more atherogenic lipid profile in non-diabetic subjects and people with Type 2 diabetes [9]. In the non-diabetic population, insulin resistance is an important component of accelerated atherosclerosis [10]. It is plausible that cardiovascular disease develops earlier in people with Type 1 diabetes with insulin resistance [11,12] and that one pathway by which insulin resistance increases cardiovascular disease is via a more atherogenic lipid profile. However, because of the challenges of measuring insulin sensitivity in people with Type 1 diabetes, little data exist on the association of insulin resistance to lipids in people with Type 1 diabetes.
The aim of this paper is to determine the associations between insulin resistance (as measured by lower glucose infusion rate in a hyperinsulinaemic–euglcyaemic clamp) and a standard fasting lipid profile in youths and adults with Type 1 diabetes who participated in clamp sub-studies of the SEARCH for Diabetes in Youths (SEARCH) Study [13] and the Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study [12]. We hypothesized that insulin resistance would be associated with a more atherogenic lipid profile (higher total cholesterol, triglycerides, LDL cholesterol and triglyceride/HDL ratio and lower HDL cholesterol) and that adiposity and hyperglycaemia (HbA1c) would attenuate this association.
Patients and methods
Study populations
Both the CACTI and SEARCH studies performed hyperinsulinaemic–euglycaemic clamps in a subset of subjects with Type 1 diabetes residing in Colorado, who were recruited from the larger study cohorts [12,13]. In the SEARCH study, 60 youths with Type 1 diabetes aged 12–19 years completed a clamp study, whereas in the CACTI study the 40 subjects with Type 1 diabetes were aged 27–61 years. All subjects with Type 1 diabetes in the clamp studies with complete data were included in the analysis. All clamp studies were performed in either the Pediatric or Adult Clinical Translational Research Centers at the University of Colorado Denver. All participants (or guardians) provided informed consent (and assent for subjects < 18 years) and the study was approved by the Colorado Multiple Institutional Review Board.
SEARCH clamp cohort
Screening included a history, physical examination, Tanner staging and fasting laboratory testing. Type 1 diabetes was defined by American Diabetes Association (ADA) criteria, plus the presence of glutamic acid decarboxylase (GAD), islet cell autoantibodies (ICA-2) or insulin autoantibodies (IAA), as well as insulin requirement. Inclusion criteria included Tanner stage > 1 and sedentary status (< 3 h of regular exercise/week) to minimize pubertal and training effects. Exclusions included resting blood pressure > 140/90 mmHg, haemoglobin < 9 mg/dl, serum creatinine > 1.5 mg/dl, HbA1c > 12%, smoking, medications affecting insulin resistance (oral or inhaled steroids, metformin, thiazolidinediones, atypical antipsychotics), anti-hypertensive medications, statins, pregnancy, breastfeeding, plans to alter exercise or diet during the study. The study day was preceded by 3 days of restricted physical activity and a fixed-macronutrient, weight-maintenance diet (55% carbohydrates, 30% fat, 15% protein). A 3-h hyperinsulinaemic–euglycaemic clamp (80 mU m−2 min−1 of insulin) was performed fasting from 09.00 to 12.00 h to estimate insulin sensitivity as previously described [8]. Body composition by dual energy X-ray absorptiometry was performed by standard methods as previously described [8].
CACTI cohort clamp
Inclusion criteria for the clamp sub-study included BMI between 18–40 kg/m2, blood pressure < 160/100 mmHg, HbA1c ≤ 9.5%, albumin excretion rate < 200 µg/min, triglycerides < 4.52 mmol/l and no proliferative retinopathy. Type 1 diabetes was defined as insulin therapy within 1 year of diagnosis and current insulin therapy, diagnosed before age 30 years, and/or with positive antibodies or a provider diagnosis of Type 1 diabetes, and diabetes duration ≥ 10 years [12]. Subjects were maintained on a provided diet with standardized macronutrient composition (50% carbohydrate, 30% fat, 20% protein) for 3 days prior to their study day. Body composition measures were performed by dual energy X-ray absorptiometry. A three-stage hyperinsulinaemic–euglycaemic clamp was then initiated and continued for the next 4.5 h using the method of DeFronzo et al. [6]. Briefly, a primed continuous infusion of insulin was administered at 4 mU m−2 min−1 for 1.5 h, 8 mU m−2 min−1 for 1.5 h and then 40 mU m−2 min−1 for the final 1.5 h. A variable infusion of 20% dextrose was infused to maintain blood glucose at ~90 mg/dl.
Comparison of clamp protocols
Subjects were instructed to take their last long-acting insulin injections at least 12 h prior to admission between 16.00 and 18.00 h, and subcutaneous insulin was discontinued at dinner and subjects were maintained overnight on intravenous regular insulin with adjustments by a standard protocol to maintain near euglycaemia until starting the clamp in the morning. Fasting blood was collected for laboratory analyses at the University of Colorado Denver Clinical—Translational Research Center Laboratory.
Differences existed in the clamp protocols for these two groups. For example, while the overnight glycaemic goals and study protocols to achieve near euglycaemia were similar, during the SEARCH clamp a higher insulin infusion rate of 80 mU m−2 min−1 was used because of highly insulin-resistant pubertal subjects secondary to the physiologic insulin resistance of puberty [8], whereas the highest dose in the CACTI study was 40 mU m−2 min−1. Additionally, the CACTI study included two stages with lower dose insulin infusions (4 and 8 mU m−2 min−1 for 90 min each). Both protocols achieved a hyperinsulinaemic–euglycaemic steady-state in the final 30 min of the clamp and glucose infusion rate (mg/kg of fat-free mass/min) was measured. Although this difference in insulin infusion rates may have resulted in differences in absolute levels of glucose infusion rate between the studies, they should not have influenced the strength of the association of glucose infusion rate to lipids within or between studies. SIclamp was defined as glucose infusion rate (mg/kg fat-free mass/min)/[delta insulin from baseline to end of clamp (µU/ml)] × [mean glucose concentration in final 30 min of clamp (mg/dl)] [14].
Measurement of lipid profiles
Fasting plasma samples were obtained prior to initiation of the clamp for measurement of total cholesterol, HDL cholesterol and triglycerides in the same core laboratory for both groups. Measurements of total cholesterol, HDL cholesterol and triglycerides were performed enzymatically on a Hitachi 917 autoanalyser (Boehringer Mannheim Diagnostics, Indianapolis, IN, USA). LDL cholesterol levels were calculated by the Friedewald equation for individuals with triglyceride levels less than 4.52 mmol/l [15] and by Lipid Research Clinics Beta Quantification [16] for those with triglycerides ≥ 4.52 mmol/l.
Other variables
Weight and height were measured using standard methods and BMI, defined as weight (kg) divided by height (m2) was calculated. Minimal waist circumference was measured. HbA1c levels were measured by (Diabetes Control and Complications Trial calibrated) ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical methods
Analyses were stratified according to study group (SEARCH and CACTI). Separate multiple linear regression models were developed for each lipid outcome (total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol and triglyderide/HDL ratio). To investigate the associations of glucose infusion rate to lipids while adjusting for potential confounders, the following strategy was used: (Basic Model 1) adjustment for demographic factors of age, sex, race/ethnicity. A separate model included diabetes duration instead of age. Adjustment for Tanner stage (SEARCH only) was also explored. Next (Model 2a), we adjusted for adiposity (waist circumference), in addition to demographic factors. Next, (Model 2b) we adjusted for glycaemia (HbA1c), in addition to demographic factors. In CACTI, we adjusted for statin use (Model 2c), in addition to demographic factors. Finally, we adjusted for both adiposity and glycaemia (Model 3, waist circumference and A1c), in addition to demographic factors. The role of confounding by adiposity or hyperglycaemia was evaluated by removing waist circumference and HbA1c individually from Model 3 and determining the % change in the β-coefficient for the relationship between glucose infusion rate and each lipid outcome.
For statistical analyses, SAS 9.2 (SAS Institute, Cary, NC, USA) was used. P-values < 0.05 were considered statistically significant and, a priori, no corrections were made for multiple comparisons.
Results
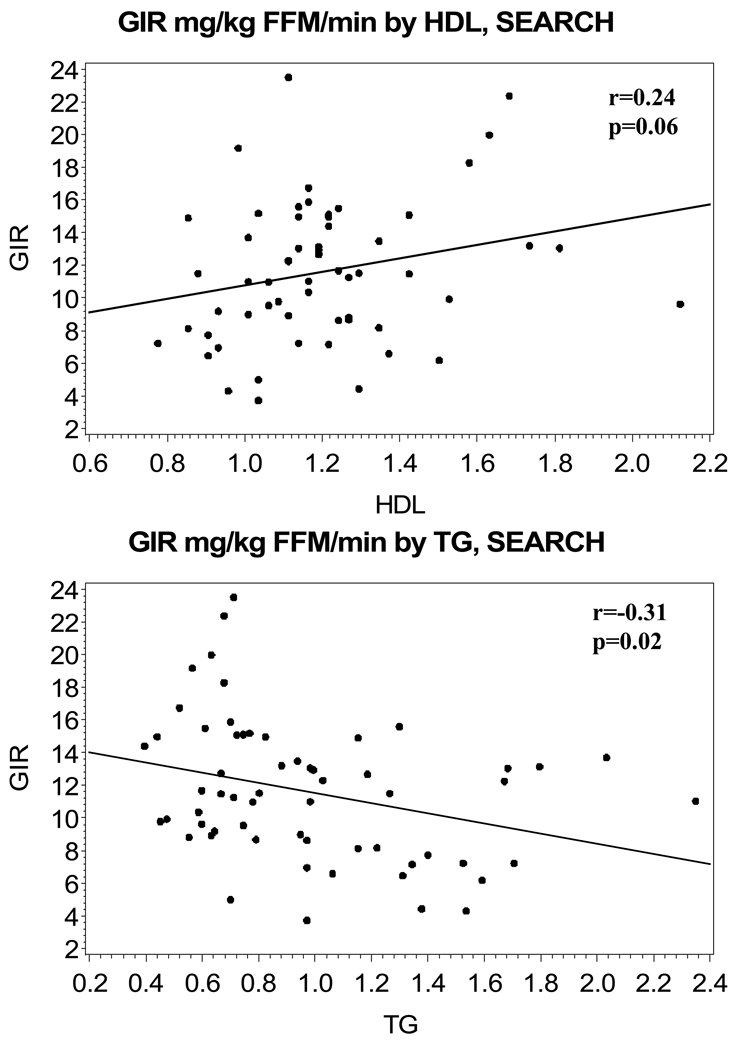
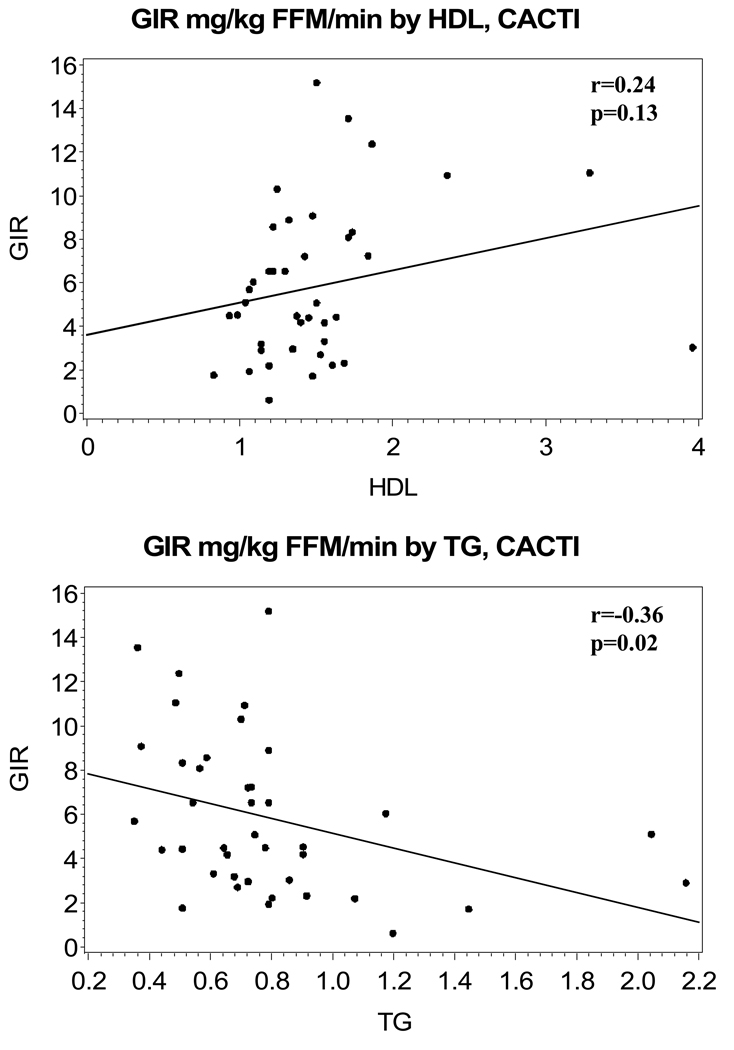
Characteristics of the study subjects are shown in Table 1, stratified by study group. The mean age of subjects in the SEARCH group was 15 ± 2 years and, for subjects in the CACTI group, was 45 ± 9 years, with subjects in the SEARCH group having a shorter diabetes duration. In SEARCH, 50% of subjects reported using insulin pumps, whereas in CACTI pump use was reported in 38% of subjects. The steady-state glucose infusion rate was higher in the SEARCH than the CACTI group. Lipid measures were similar in the two cohorts and, on average, at American Diabetes Association goals, although none of the youths were on lipid-lowering medications and 60% of the adults were on statins (no fibrate use reported). As expected (and attributable to different inclusion criteria), the adolescent cohort had a higher mean HbA1c than the adults (8.6 ± 1.5 vs. 7.5 ± 0.9%). In SEARCH, 28% of subjects had a BMI > 85%th percentile (BMI z-score 0.49 ± 0.91) and, in CACTI, 28% of the subjects had a BMI > 30 kg/m2. Mean overnight glucose concentrations were similar in the two groups (8.00 ± 2.67 mmol/l in SEARCH and 6.94 ± 2.61 mmol/l in CACTI). SIclamp was 17.4 ± 12.9 dl/(min × kg fat-free mass)/µU/ml in SEARCH and 7.6 ± 4.2 dl/(min × kg fat-free mass)/µU/ml in SEARCH. The unadjusted association of glucose infusion rate to HDL and triglycerides were similar in SEARCH (r = 0.24, P = 0.06 for HDL and r = −0.31, P = 0.02 for triglycerides) and CACTI (r = 0.24, P = 0.13 for HDL and r = −0.36, P = 0.02 for triglycerides) and are shown in Figs 1a–d. The partial coefficients for the association of glucose infusion rate to HDL and triglycerides were similar when adjusted for change in insulin concentration or for mean glucose concentration in the final 30 min (data not shown).
Table 1.
Characteristics of study subjects
| SEARCH n = 60 |
CACTI n = 40 |
|
|---|---|---|
| Age, years* | 15 ± 2 (12–19) | 45 ± 9 (27–61) |
| Sex, % female | 57% | 53% |
| Menopause (%) | NA | 19% |
| Race/ethnicity, % NHW | 85% | 95% |
| Type 1 diabetes duration, years | 6.3 ± 3.8 | 23 ± 8 |
| Tanner Stage, n (%) 2 3 4 5 |
3 (5%) 9 (15%) 16 (27%) 32 (53%) |
NA |
| GIR, mg/kg FFM/min† | 11.5 (8.7–14.7) | 4.8 (3.0–8.2) |
| SIclamp, dl/(min × kg FFM)/µU/ml | 17.4 ± 12.9 | 7.6 ± 4.2 |
| Body fat, % | 17.6 ± 12.1 | 28.6 ± 7.5 |
| BMI, kg/m2* | 22.6 ± 4.7 (17–36) | 27.0 ± 4.4 (19–36) |
| BMI z-score | 0.49 ± 0.91 | NA |
| Waist, cm | 76 ± 12 | 88 ± 12 |
| Total cholesterol, mmol/l* | 3.82 ± 0.81 (2.62–7.07) | 3.61 ± 0.84 (2.10–5.80) |
| HDL cholesterol, mmol/l* | 1.20 ± 0.25 (1.14–4.84) | 1.50 ± 0.58 (0.83–3.96) |
| Triglycerides, mmol/l* | 0.98 ± 0.43 (0.40–2.35) | 0.79 ± 0.38 (0.35–2.16) |
| LDL cholesterol, mmol/l* | 2.17 ± 0.67 (1.14–4.84) | 1.75 ± 0.64 (0.74–3.08) |
| Triglycerides/HDL* | 4.54 ± 2.41 (1.48–11.54) | 3.11 ± 2.03 (0.78–10.37) |
| HbA1c, %* | 8.6 ± 1.5 (5.9–11.9) | 7.5 ± 0.9 (5.9–9.1) |
| Fasting glucose, mmol/l | 7.71 ± 2.50 | 6.44 ± 2.19 |
| Fasting insulin, µU/ml | 30 ± 22 | 32 ± 28 |
| FFA, µmol/l | 502 ± 292 | 531 ± 218 |
| On statins, % | 0% | 60% |
Means ± sd or count and frequency. No P-values are shown as no hypotheses were tested for between-group differences for these variables.
Ranges shown.
Median and IQ range.
CACTI, Coronary Artery Calcification in Type 1 Diabetes; FFM, fat-free mass; GIR, glucose infusion rate; NA, not available; NHW, non-Hispanic White; SEARCH, SEARCH for Diabetes in Youths
Figure 1.
Unadjusted association of glucose infusion rate (mg/kg fat-free mass/min) to (a) HDL, SEARCH for Diabetes in Youths (SEARCH), (b) triglycerides, SEARCH, (c) HDL, Coronary Artery Calcification in Type 1 Diabetes (CACTI) and (d) triglycerides, CACTI.
Associations between glucose infusion rate and lipid levels in youths with Type 1 diabetes (Table 2)
Table 2.
Association of glucose infusion rate (GIR) to lipids (HDL cholesterol, triglycerides and triglycerides/HDL) in the SEARCH for Diabetes in Youths (SEARCH) group
| HDL cholesterol | Triglycerides | Triglycerides/HDL | |
|---|---|---|---|
| Model 1: GIR plus age, sex, race/ethnicity | 0.016 ± 0.008* 0.037† |
−0.028 ± 0.013 0.039 |
−0.177 ± 0.073 0.019 |
| Model 2a: Model 1 plus waist circumference | 0.016 ± 0.008 0.059 |
−0.028 ± 0.014 0.046 |
−0.178 ± 0.078 0.03 |
| Model 2b: Model 1 plus HbA1c | 0.018 ± 0.008 0.03 |
−0.024 ± 0.013 0.08 |
−0.169 ± 0.075 0.03 |
| Model 3: Model 1 plus waist, HbA1c | 0.017 ± 0.008 0.046 |
−0.025 ± 0.014 0.09 |
−0.168 ± 0.081 0.041 |
β-coefficient ± se
P-value.
Lower glucose infusion rate (more insulin resistance) was associated with lower HDL cholesterol after adjustment for age, sex and race/ethnicity (Model 1). A similar association was seen with adjustment for diabetes duration and Tanner stage, each individually in place of age (data not shown). (Note: in forward selection models, age, diabetes duration and Tanner stage all had P > 0.2). Glucose infusion rate was associated with HDL cholesterol after additional adjustment for HbA1c (Model 2b) and with adjustment for both adiposity and glycaemia measures (Model 3). There was a similar, but not statistically significant association between glucose infusion rate and HDL cholesterol after adjustment for waist circumference (Model 2a).
Lower glucose infusion rate was associated with higher triglyceride levels after adjustment for age, sex and race/ethnicity (Model 1), as well as when diabetes duration and Tanner stage each individually replaced age (data not shown for diabetes duration and Tanner stage adjustments). After additional adjustment for waist circumference (Model 2a), glucose infusion rate remained significantly associated with trigylcerides. Once glycaemia (Model 2b) or both glycaemia and adiposity measures were included (Model 3), the association was no longer statistically significant.
Glucose infusion rate was inversely associated with triglycerides/HDL after adjustment for age, sex and race/ethnicity (Model 1), as well as when diabetes duration and Tanner stage each individually replaced age (data not shown). The association remained significant after additional adjustment for waist circumference (Model 2a), glycaemia (Model 2b) and when both glycaemia and adiposity measures were added to Model 1 (Model 3). No association between glucose infusion rate and total cholesterol or LDL cholesterol in youths with Type 1 diabetes was observed in multiple linear regression analysis (P > 0.39).
In all of the models, waist circumference and HbA1c were not significantly associated with HDL cholesterol, triglycerides or triglycerides/HDL. Removal of waist circumference from Model 3 resulted in a < 3% change in the magnitude of the β-coefficient for the association of glucose infusion rate to HDL cholesterol, trigylcerides and triglycerides/HDL and removal of HbA1c from Model 3 resulted in 10, 14 and 5% changes in the β-coefficient for the association of glucose infusion rate to HDL cholesterol, triglycerides and triglyceride/HDL, respectively. Based on Model 1, the difference between the 25th and 75th percentiles of glucose infusion rate would result in a predicted difference of 0.098 mmol/l in HDL cholesterol, 0.17 mmol/l in triglycerides and 1.06 mmol/l in triglyceride/HDL ratio.
Associations between glucose infusion rate and lipid levels in adults with Type 1 Diabetes (Table 3)
Table 3.
Association of glucose infusion rate (GIR) to lipids (HDL, triglycerides and triglycerides/HDL) in the Coronary Artery Calcification in Type 1 Diabetes (CACTI) group
| HDL | Triglycerides | Triglycerides/HDL | |
|---|---|---|---|
| Model 1: GIR plus age, sex, race/ethnicity | 0.030 ± 0.026* 0.25† |
−0.038 ± 0.017 0.037 |
−0.194 ± 0.091 0.04 |
| Model 2a: Model 1 plus waist circumference | 0.023 ± 0.029 0.42 |
−0.021 ± 0.018 0.25 |
−0.103 ± 0.096 0.29 |
| Model 2b: Model 2 plus HbA1c | 0.031 ± 0.026 0.24 |
−0.040 ± 0.017 0.026 |
−0.206 ± 0.090 0.028 |
| Model 2c: Model 1 plus statin use | 0.024 ± 0.029 0.40 |
−0.034 ± 0.020 0.09 |
−0.176 ± 0.102 0.09 |
| Model 3: Model 1 plus waist, HbA1c | 0.025 ± 0.029 0.40 |
−0.024 ± 0.018 0.20 |
−0.117 ± 0.094 0.23 |
β-coefficient ± se.
P-value.
In contrast to the SEARCH cohort, in the CACTI cohort glucose infusion rate was not significantly associated with HDL cholesterolyouths. In CACTI, there was no observed association between glucose infusion rate and total cholesterol or LDL cholesterol (P > 0.55), consistent with the findings in SEARCH youths (data not shown). An additional analysis performed to examine the association of glucose infusion rate to LDL cholesterol when subjects were stratified by dyslipidaemia status (defined either as on a statin or with LDL cholesterol ≥ 2.59 mmol/l) showed no association between LDL cholesterol and glucose infusion rate in either of the lipid groups.
Glucose infusion rate was inversely associated with triglycerides after adjustment for age, sex and race/ethnicity (Model 1) [as well as when diabetes duration replaced age (data not shown)]. The association of glucose infusion rate with triglycerides lost statistical significance after additional adjustment for waist circumference (Model 2a) or statin use (Model 2c). Glycaemia did not attenuate the association between glucose infusion rate and triglyceride levels (Model 2b); however, the association was attenuated when both glycaemia and adiposity measures were added to Model 1 (Model 3). Waist circumference was statistically significantly associated with triglycerides in Model 2a (P = 0.048), but not quite in Model 3 (P = 0.052). Removal of waist circumference from Model 3 resulted in a 40% increase in the magnitude of the β-coefficient for glucose infusion rate’s association to triglycerides, whereas removal of HbA1c resulted in a 12% decrease.
Similarly, glucose infusion rate was inversely associated with triglyceride/HDL ratio when adjusted for age, sex and race/ethnicity (Model 1) or HbA1c (Model 2b). The addition of waist circumference (Model 2a), statin use (Model 2c), or when both glycaemia and adiposity measures were added (Model 3), attenuated this association. Waist circumference was statistically significantly associated with triglycerides/HDL in Models 2a and 3 (P = 0.03 and P = 0.04, respectively). Removal of waist circumference from Model 3 resulted in a 43% increase in the magnitude of the β-coefficient for the association of glucose infusion rate to triglycerides/HDL, whereas removal of HbA1c resulted in a 13% decrease.
Based on Model 1, the difference between the 25th and 75th percentiles of glucose infusion rate would result in a predicted difference of 0.16 mmol/l in HDL cholesterol, 0.20 mmol/l in triglycerides and 1.01 in triglyceride/HDL ratio.
Discussion
We found that glucose infusion rate is inversely associated with triglycerides and triglyceride/HDL ratio in both youths and adults with Type 1 diabetes and glucose infusion rate is also positively associated with HDL cholesterol in youths. Although we were unable to detect a statistically significant association between glucose infusion rate and HDL cholesterol in adults, the direction and magnitude of the association in adults was similar to the association in youths. The magnitude of the association between glucose infusion rate and lipid levels translate into predicted interquartile differences of 0.098 mmol/l for HDL cholesterol, 0.17 mmol/l for triglycerides and 1.06 for triglycerides/HDL in the adolescents and 0.20 mmol/l for triglycerides and 1.01 for triglycerides/HDL in adults. Therefore, interventions to improve insulin sensitivity in people with Type 1 diabetes could translate into a less atherogenic lipid profile with expected improvement in cardiovascular health.
We also hypothesized that the association between glucose infusion rate and a more atherogenic lipid profile would be attenuated by adjustment for hyperglycaemia and adiposity. Waist circumference was significantly associated with triglyerides and borderline with triglycerides/HDL in adults and significantly influenced the association of glucose infusion rate to HDL cholesterol, triglycerides and triglycerides/HDL. Waist circumference was not significantly associated with lipid levels in youths in multiple linear regression and therefore the effect of adjustment for waist circumference on the association of glucose infusion rate to HDL cholesterol, triglycerides or triglycerides/HDL was more modest in youths than in adults. Glycaemia was not significantly associated with HDL cholesterol, triglycerides or triglycerides/HDL in either youths or adults and had a modest effect on the association of glucose infusion rate to HDL cholesterol and triglycerides in youths and to triglycerides and triglycerides/HDL in adults. In these groups, youths had greater variability in glycaemia than adults, while adults had greater variability in waist circumference than youths. No differences existed in lipids based on statin status in the CACTI clamp group, as previously reported [17]. One explanation is that subjects receiving statin treatment were being treated because of previous lipid abnormalities.
In general, the associations of glucose infusion rate to lipids were consistent between adolescents and adults with Type 1 diabetes. The interquartile differences in glucose infusion rate had a relatively greater effect on HDL cholesterol, triglycerides and the triglyceride/HDL ratio in adults as compared with adolescents. Although glucose infusion rate was not significantly associated with HDL cholesterol in adults, the β-coefficient was larger than in youths and the larger variance in HDL cholesterol in adults suggests that this lack of statistical significance could be related to insufficient power. One possible interpretation is that insulin resistance has a larger contribution to lipids in adults than in adolescents with Type 1 diabetes.
The effects of glycaemia and adiposity on the associations between glucose infusion rate and lipid levels were somewhat different in youths and adults. One explanation of these differences is that the youths, as compared with the adults, had worse glycaemic control. This was in part attributable to different HbA1c inclusion criteria, but it is also consistent with the literature in which adolescents generally have a higher mean HbA1c than adults [18,19]. Conversely, while adults have better glycaemic control, they are more likely to be obese.
Historically, obesity was uncommon in Type 1 diabetes, but autoimmune diabetes does not confer protection from the recent obesigenic milieu. Rates of obesity at presentation with Type 1 diabetes have been increasing in children similar to non-diabetic youths [20]. For example, in the full SEARCH study, 37% of females and 32% of males with Type 1 diabetes were either overweight or obese [20]. Obesity has a well-established link to insulin resistance in general [5,10] and this is also the case in people with Type 1 diabetes [21–23]. Furthermore, obesity has been associated consistently with a more atherogenic lipid profile in people with Type 1 diabetes [4]. Our data suggest that, as in non-diabetic individuals, insulin resistance is a mechanism by which abdominal adiposity could lead to a more atherogenic lipid profile in people with Type 1 diabetes. Interestingly, we also explored BMI (and BMI z-score in the adolescents) in place of waist circumference, but it was less strongly associated with glucose infusion rate and lipids (data not shown).
Previous clamp studies in Type 1 diabetes have reported a positive association between glycaemia and insulin resistance [21–23]; however, many of these studies were performed before currently available treatment options, including basal–bolus insulin and continuous subcutaneous insulin infusion. The association between glycaemia and insulin resistance could be different in Type 1 diabetes subjects with relatively improved glycaemic control. Previous studies in Type 1 diabetes consistently report a correlation between HbA1c and a more atherogenic lipid profile [4]. For example, the Diabetes Control and Complications Trial reported that total cholesterol, LDL cholesterol and triglycerides increased with elevated HbA1c, but HDL cholesterol was not correlated [24]. Also, a recent publication of longitudinal lipid data from adolescents in the UK reported a significant positive association of glycaemia to total cholesterol, triglycerides, LDL and non-HDL [25]. Insulin resistance is one possible mechanism by which hyperglycaemia could lead to a more atherogenic lipid profile in people with Type 1 diabetes.
While both hyperglycaemia and adiposity contribute to dyslipidaemia in Type 1 diabetes, intensification of glycaemic control may also have an adverse effect on adiposity and the lipid profile. Specifically, the Diabetes Control and Complications Trial study reported that an increase in BMI was associated with a worsening in lipids (and blood pressure) in the intensive vs. the conventionally treated groups [26]. Furthermore, among Diabetes Control and Complications Trial subjects who were intensively treated, those with a family history of Type 2 diabetes had greater central weight gain and dyslipidaemia [27]. Our data expand on the previous literature by investigating the association between directly measured glucose infusion rate and fasting lipid profile in 100 subjects with Type 1 diabetes from 12–19 and 27–61 years of age and whether glycaemia and adiposity attenuate this association. In addition to glycaemia and adiposity, factors such as diet, exercise (both controlled for in these studies over the short term) and genetic factors undoubtedly play a role in both insulin resistance and dyslipidaemia. Also, these data are cross-sectional and cause and effect cannot be firmly established; for instance, obesity and hyperglycaemia may be independently associated with dyslipidaemia.
Our data have limitations that must be considered. First, these were convenience samples and therefore the generalizability of these findings to other populations is uncertain; however, it is unlikely that this has influenced the associations between glucose infusion rate and lipid levels observed in our study. Also, a larger sample might have detected a statistically significant smaller association; however, our study had 80% power to determine whether 7–10% of the variance in HDL cholesterol is attributable to glucose infusion rate in analysis adjusted for five variables; it is unlikely that an amount smaller than this is clinically relevant. Additionally, we did not correct for multiple comparisons and, although many of the reported associations were of borderline significance, they were in the same direction. Finally, extrapolation of the data to more physically active youths is uncertain.
In conclusion, we found that glucose infusion rate is inversely associated with triglycerides and triglycerides/HDL across the lifespan and positively associated with HDL cholesterol in youths with Type 1 diabetes. The associations were attenuated by adjustment for adiposity among adults, while adjustment for HbA1c had a modest effect in youths and adults. Further investigation of insulin resistance, its determinants, its relationship to cardiovascular disease risk (specifically a more atherogenic lipid profile) and its potential as a therapeutic target is of importance to the care of adolescents and adults with Type 1 diabetes.
Acknowledgements
Support for the CACTI study was provided by the NIH NHLBI grant R01 HL61753, HL79611 and DERC Clinical Investigation Core P30 DK57516. Support for the SEARCH Clamp study was provided by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK059184-03S1 and R01 DK059184). The study was performed at the Adult and Pediatric CTRC at UCD supported by the NIH-M01-RR00051 and at the Barbara Davis Center for Childhood Diabetes. DMM was supported by K23 DK075360. JKS-B was supported by American Diabetes Association Takeda postdoctoral fellowship 7-09-CVD-06.
Abbreviations
- CACTI
Coronary Artery Calcification in Type 1 Diabetes
- SEARCH
SEARCH for Diabetes in Youths
Footnotes
Competing interests
DMM is the recipient of a research grant from Merck for a clinical trial of lipid-lowering medications in youths with Type 1 diabetes. All other authors have nothing to declare.
The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.EURODIAB ACE Study Group. Variation and trends in incidence of childhood diabetes in Europe. Lancet. 2000;355:873–876. [PubMed] [Google Scholar]
- 2.Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, Miller R, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years' duration: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Pittsburgh Epidemiology of Diabetes Complications Experience (1983–2005) Arch Intern Med. 2009;169:1307–1316. doi: 10.1001/archinternmed.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–126. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 4.Maahs DM, Wadwa RP, Bishop F, Daniels SR, Rewers M, Klingensmith GJ. Dyslipidemia in youths with diabetes: to treat or not to treat? J Pediatr. 2008;153:458–465. doi: 10.1016/j.jpeds.2008.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 7.Perseghin G, Lattuada G, Danna M, Sereni LP, Maffi P, De CF, et al. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2003;285:E1174–E1181. doi: 10.1152/ajpendo.00279.2003. [DOI] [PubMed] [Google Scholar]
- 8.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95:513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline KL, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- 12.Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, et al. Effect of Type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance?: The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes. 2003;52:2833–2839. doi: 10.2337/diabetes.52.11.2833. [DOI] [PubMed] [Google Scholar]
- 13.Liese AD, D'Agostino RB, Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, et al. The burden of diabetes mellitus among US youths: prevalence estimates from the SEARCH for Diabetes in Youths Study. Pediatrics. 2006;118:1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 14.Perseghin G, Caumo A, Sereni LP, Battezzati A, Luzi L. Fasting blood sample-based assessment of insulin sensitivity in kidney-pancreas-transplanted patients. Diabetes Care. 2002;25:2207–2211. doi: 10.2337/diacare.25.12.2207. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–503. [PubMed] [Google Scholar]
- 16.Hainline A, Jr, Miller DT, Mather A. The Coronary Drug Project. Role and methods of the central laboratory. Control Clin Trials. 1983;4:377–387. doi: 10.1016/0197-2456(83)90023-5. [DOI] [PubMed] [Google Scholar]
- 17.Maahs DM, Hokanson JE, Wang H, Kinney GL, Snell-Bergeon JK, East A, et al. Lipoprotein subfraction cholesterol distribution is pro-atherogenic in women with Type 1 diabetes and insulin resistance. Diabetes. 2010;59:1771–1779. doi: 10.2337/db09-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 19.de Beaufort CE, Swift PG, Skinner CT, Aanstoot HJ, Aman J, Cameron F, et al. Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidoere Study Group on Childhood Diabetes. Diabetes Care. 2007;30:2245–2250. doi: 10.2337/dc07-0475. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence JM, Liese AD, Liu L, Dabelea D, Anderson A, Imperatore G, et al. Weight-loss practices and weight-related issues among youths with type 1 or type 2 diabetes. Diabetes Care. 2008;31:2251–2257. doi: 10.2337/dc08-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–219. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 22.Yki-Jarvinen H, Koivisto VA. Natural course of insulin resistance in type I diabetes. N Engl J Med. 1986;315:224–230. doi: 10.1056/NEJM198607243150404. [DOI] [PubMed] [Google Scholar]
- 23.Szadkowska A, Pietrzak I, Mianowska B, Bodalska-Lipinska J, Keenan HA, Toporowska-Kowalska E, et al. Insulin sensitivity in Type 1 diabetic children and adolescents. Diabet Med. 2008;25:282–288. doi: 10.1111/j.1464-5491.2007.02357.x. [DOI] [PubMed] [Google Scholar]
- 24.The DCCT Research Group. Lipid and lipoprotein levels in patients with IDDM diabetes control and complication. Trial experience. Diabetes Care. 1992;15:886–894. doi: 10.2337/diacare.15.7.886. [DOI] [PubMed] [Google Scholar]
- 25.Loredana MM, Neil DR, Toby PA, Acerini CL, Barrett TG, Cooper JD, et al. Prevalence of abnormal lipid profiles and the relationship with the development of microalbuminuria in adolescents with type 1 diabetes. Diabetes Care. 2009;32:658–663. doi: 10.2337/dc08-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the Diabetes Control and Complications Trial. J Am Med Assoc. 1998;280:140–146. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purnell JQ, Dev RK, Steffes MW, Cleary PA, Palmer JP, Hirsch IB, et al. Relationship of family history of type 2 diabetes, hypoglycemia, and autoantibodies to weight gain and lipids with intensive and conventional therapy in the Diabetes Control and Complications Trial. Diabetes. 2003;52:2623–2629. doi: 10.2337/diabetes.52.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]