Abstract
Cell culture studies and animal models point to an important role of oxidative/nitrosative stress in the pathogenesis of cerebral ammonia toxicity. However, it is unknown whether oxidative/nitrosative stress in the brain is also characteristic of hepatic encephalopathy (HE) in humans. We therefore analyzed post mortem cortical brain tissue samples from patients with cirrhosis dying with or without HE in comparison with brains from patients without liver disease. Significantly elevated levels of protein tyrosine-nitrated proteins, heat shock protein-27, and 8-hydroxyguanosine as a marker for RNA oxidation were found in the cerebral cortex of HE patients, but not of patients with cirrhosis but without HE. Glutamine synthetase (GS) activity was significantly decreased, whereas GS protein expression was not significantly affected. Protein expression of the glutamate/aspartate cotransporter was up-regulated in HE, whereas protein expression of neuronal and inducible nitric oxide synthases, manganese-dependent and copper/zinc-dependent superoxide dismutase, and glial glutamate transporter-1 were not significantly increased.
Conclusion
These data indicate that HE in patients with cirrhosis is associated with oxidative/nitrosative stress, protein tyrosine nitration, and RNA oxidation, suggesting a role of oxidative stress in the pathogenesis of HE in patients with cirrhosis.
Hepatic encephalopathy (HE) defines a neuropsychiatric manifestation of chronic or acute liver disease with disturbances of psychomotoric, intellectual, cognitive, emotional/affective, behavioral, and fine motor functions of varying severity (see Häussinger and Blei1 for a review). HE in cirrhosis has been reported to reflect the clinical manifestation of low-grade cerebral edema,2,3 as shown by in vivo studies using magnetic resonance spectroscopy2–4 and quantitative water imaging5 of the brain in vivo from patients with cirrhosis.
Ammonia, which is considered a key toxin in the pathogenesis of HE, can induce astrocyte swelling in vitro.6–8 Because astrocyte swelling is also induced by inflammatory cytokines, hyponatremia, or benzodiazepines, the action of long-known and heterogeneous precipitating factors of HE may integrate at the level of astrocyte swelling.2,3 In cultured rat astrocytes, ammonia, tumor necrosis factor-α, diazepam, and hypo-osmotic cell swelling produce an oxidative/nitrosative stress response9–12 through activation of nicotinamide adenine dinucleotide phosphate-oxidase.8 A self-amplifying signaling loop between astrocyte swelling and oxidative stress has been proposed, because astrocyte swelling produces reactive oxygen species, and reactive oxygen species trigger astrocyte swelling.11,13 The ammonia-induced, tumor necrosis factor α–induced, and swelling-induced oxidative/nitrosative stress response has multiple effects on astrocyte function, such as modulation of gene transcription through Zn++ mobilization with the consequence of up-regulation of the peripheral benzodiazepine receptor. 14 Other consequences of the reactive oxygen species/RNS formation are protein tyrosine nitration (PTN)10 and RNA oxidation through formation of 8-hydroxyguanosine. 15 These covalent modifications of astrocytic proteins and astrocytic and neuronal RNA occur not only in vitro in response to ammonia,10,15 benzodiazepines, 11,15 inflammatory cytokines,15 and experimental, hypo-osmotic astrocyte swelling,12,15 but also in rat brain in vivo following ammonia, diazepam, or lipopolysaccharide intoxication or portocaval shunting.10,11,15 Astrocytes located near the blood–brain barrier exhibit especially high levels of PTN, with potential consequences for blood–brain barrier permeability.10 RNA oxidation in neurons involves RNA species contained in NOVA-2–positive RNA granules along the dendrites and in postsynaptic regions.15 These findings suggest that HE-associated oxidative stress modifies RNA species, which are involved in local synaptic protein synthesis16 and therefore may impair synaptic plasticity and memory formation. 15 Because RNA oxidation in other systems can impair translational efficacy and accuracy,17,18 such effects may underly the multiple and inhomogeneous derangements of neurotransmitter/receptor systems in HE1 and the disturbances of oscillatory networks in the brain of HE patients, as shown in magnet encephalography studies on the human brain in vivo.19
Although cell culture studies in vitro and rodent models in vivo suggest an important role of oxidative/nitrosative stress in the pathogenesis of ammonia toxicity, it is unknown whether oxidative stress is also involved in the pathogenesis of HE in humans with cirrhosis. Furthermore, no data are available on the occurrence of PTN and RNA oxidation in the brains of humans with cirrhosis and HE. Whereas noninvasive nuclear magnetic resonance spectroscopy (NMR) and tracer techniques allow to pick up brain water, several cerebral metabolites and receptor expression in humans in vivo, no in vivo techniques on the detection of oxidative modifications of brain constituents in humans are available. We therefore analyzed human post mortem cortical brain samples from patients with cirrhosis dying with or without HE in comparison to brain samples from patients without evidence for preexisting liver disease. The analysis shows that PTN and RNA oxidation are significantly increased in HE, which shows for the first time that oxidative/nitrosative stress is a hallmark of HE in patients with cirrhosis.
Patients and Methods
Detailed information about the materials used in this study can be found in the Supporting Information.
Post Mortem Human Brain Tissue
Post mortem human brain tissue was obtained from autopsies of eight control subjects and eight patients with liver cirrhosis and accompanying HE. Controls were free from hepatic or neurological disorders at the time of death. Informed written consent was given either by the patients or by their relatives or had been included in the body donor program of the Department of Anatomy, University of Düsseldorf, Germany. Table 1 summarizes the causes of death, sex, age, and post mortem delay (duration from estimated time of death until autopsy) of patients included as controls in this study. Samples from 7/8 control and 7/8 HE brains have also been used in a recent study on multireceptor autoradiography in HE.20 A detailed description of patient histories can be found in the Supporting Information. In addition to these brain samples from European patients, brain samples from four controls without cirrhosis and four patients with cirrhosis but without HE were analyzed. These brain samples were obtained from the Australian Brain Donor Programs NSW Tissue Resource Centre, which is supported by the University of Sydney, National Health and Medical Research Council of Australia, Schizophrenia Research Institute, National Institute of Alcohol Abuse and Alcoholism and NSW Department of Health.
Table 1.
Characteristics of Control Patients and Patients With Cirrhosis With or Without HE
| Patient No. | Sex | Age (years) | Diagnosis | Post Mortem Delay (hours) |
|---|---|---|---|---|
| European patients | ||||
| Controls without cirrhosis | ||||
| 1 | M | 64 | Respiratory insufficiency, pulmonary embolism | 5 |
| 2 | F | 76 | Myocardial infarction | 24 |
| 3 | F | 63 | Cardiac insufficiency | 8 |
| 4 | F | 63 | Suicide by suffocation | 23 |
| 5 | M | 79 | Pancreatic carcinoma | 9 |
| 6 | M | 44 | Bronchial carcinoma, circulatory failure | 25 |
| 7 | F | 66 | Myocardial infarction | 18 |
| 8 | M | 61 | Pancreatic carcinoma | 12–14 |
| Patients with cirrhosis and HE | ||||
| 1 | M | 68 | Hepatitis B virus cirrhosis | <12 |
| 2 | F | 45 | Alcoholic cirrhosis, TIPS | 17 |
| 3 | M | 36 | Alcoholic cirrhosis, TIPS | 6 |
| 4 | M | 69 | Alcoholic cirrhosis | <12 |
| 5 | M | 63 | Alcoholic cirrhosis | 13.5 |
| 6 | M | 57 | Alcoholic cirrhosis | 9 |
| 7 | F | 57 | Alcoholic cirrhosis, TIPS | 12 |
| 8 | M | 61 | Hepatitis C virus cirrhosis, hepatocellular carcinoma | <24 |
| Australian patients | ||||
| Controls without cirrhosis | ||||
| 1 | M | 60 | Gastrointestinal stomach tumor | 25 |
| 2 | F | 71 | Adenocarcinoma of the pancreas | 16 |
| 3 | M | 46 | Acute myocardial infarction | 29 |
| 4 | F | 49 | Arrhythmogenic right ventricular dysplasia | 15 |
| Cirrhotic Patients without cirrhosis and HE | ||||
| 1 | M | 52 | Alcohol intoxication | 35 |
| 2 | M | 37 | Acute alcohol poisoning | 17 |
| 3 | M | 50 | Massive hemorrhage from esophageal varices | 26.5 |
| 4 | M | 58 | Ischemic heart disease | 20 |
HE patients were clinically diagnosed at the Clinic for Gastroenterology, Hepatology, and Infectiology of the Heinrich-Heine-University, Düsseldorf, Germany, or at the Department of Internal Medicine of the University Hospital of Patras, Greece. In addition, four brain samples of control patients and four brain samples of patients with cirrhosis without HE were obtained from the Australian Brain Donor Programs NSW Tissue Resource Centre, which is supported by the University of Sydney, National Health and Medical Research Council of Australia, Shizophrenia Research Institute, National Institute of Alcohol Abuse and Alcoholism and NSW Department of Health.
Abbreviations: F, female; M, male; TIPS, transjugular intrahepatic portosystemic stent shunt.
Controls from the European and Australian cohorts differed significantly with regard to glutamine synthetase (GS) activity and expression level of glutamate/aspartate cotransporter (GLAST), copper/zinc dependent superoxide dismutase, and NO2Tyr. Therefore, samples from Australia were treated as a separate cohort. All brain samples included in this study were taken from the intersection parietal to occipital cortex area. Proportion of white matter was kept minimal in all brain samples.
Western Blot Analysis
Western blot analysis was performed as described in the Supporting Information and previously.14
Detection of Oxidized RNA
Oxidized RNA was detected as described in the Supporting Information and previously.15
Densitometric Quantification
Chemiluminescence of western and northwestern blots was collected by digital image acquisition and densitometric analysis. Expression levels of proteins are given relative to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
GS Activity and Tyrosine Nitration
GS activity was determined using γ-glutamyl transferase reaction as described.21 Immunoprecipitation of nitrated proteins was performed as described.10
Statistical Analysis
Data are presented as the mean ± standard error of the mean. Descriptive statistics were performed by using a two-tailed Student t test for unpaired data. Correlation analysis was performed using Pearson correlation. P < 0.05 was considered statistically significant.
Results
Markers for Oxidative Stress Are Increased in the Cerebral Cortex of HE Patients
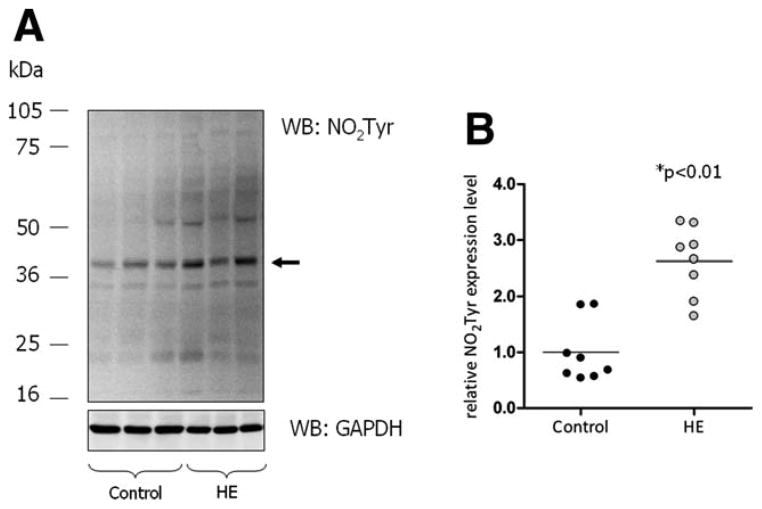
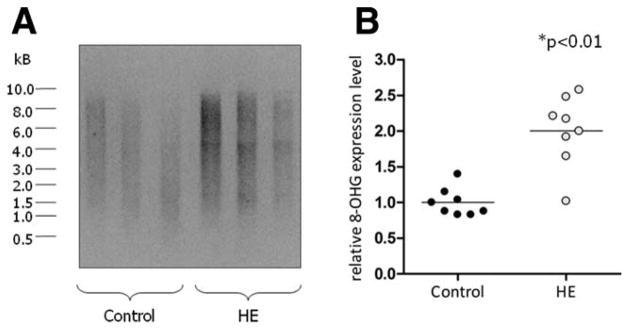
As shown recently, ammonia exposure induces oxidative/nitrosative stress in rat astrocyte cultures and mouse brain slices in vitro.8–10 In addition, acute ammonia intoxication of rats in vivo induces oxidative stress in the brain.22 Consequences of this oxidative/nitrosative stress response are PTN10 and oxidation of RNA, as evidenced by increased 8-hydroxyguanosine formation.15 Whether such alterations also occur in the brain of patients with hepatic encephalopathy is unknown. Therefore, oxidative stress markers were analyzed in post mortem human brain tissue. As shown by western blot analysis in Fig. 1, PTN of individual proteins within the molecular mass range between 20 and 75 kDa was significantly increased in brain samples from HE patients as compared with controls. Furthermore, total RNA extracted from the cerebral cortex of HE patients showed about two-fold higher levels of oxidized RNA as revealed by northwestern blot analysis when compared with control patients (Fig. 2). These data suggest the presence of oxidative stress in the brain from patients with cirrhosis and HE.
Fig. 1.
PTN in the cerebral cortex from control and HE patients. Protein extracts prepared from the cerebral cortex were analyzed for tyrosine- nitrated proteins by western blot analysis. GAPDH served as a loading control. (A) Western blots from cerebral cortex from three representative control and three HE patients, respectively. The arrow indicates the band used for densitometric quantification. (B) Densitometric quantification of western blot results. Expression of tyrosine- nitrated proteins is given relative to GAPDH expression. Each case included in this study is shown separately in the vertical scatter plot; bars represent the mean values. *Significantly different compared with the control group.
Fig. 2.
Levels of oxidized RNA in the cerebral cortex from control and HE patients. Purified RNA from the cerebral cortex of control and HE patients was analyzed for the presence of 8-hydroxyguanosine (8-OHG) by northwestern blot analysis. (A) Northwestern blot results taken from three representative control and HE patients, respectively. (B) Densitometric quantification of northwestern blot results. Each case included in this study is shown separately in the vertical scatter plot; bars represent the mean values. *Significantly different compared with the control group.
In line with this and as shown by western blot analysis, protein expression of the small heat shock protein-27 (Hsp27), a well-established and frequently used marker for oxidative stress23 was significantly and about four-fold up-regulated in the cerebral cortex of HE patients compared with controls (Fig. 3A,B). On the other hand, protein expression levels of neuronal nitric oxide synthase (nNOS) and of antioxidative enzymes, such as copper/zinc-dependent and manganese-dependent superoxide dismutase were not significantly increased in brains from HE patients when compared with the control group (Fig. 3A,B). Up-regulation of the inducible nitric oxide synthase (iNOS) has been reported in ammonia-treated astrocytes in vitro10 and in portocaval anastomized rats in vivo.24 However, in all cortical brain samples from controls and HE patients iNOS protein was not detectable (for representative blots, see Fig. 3C).
Fig. 3.
Expression levels of oxidative stress-related proteins in the cerebral cortex of control and HE patients. Protein extracts prepared from the cerebral cortex were analyzed for expression levels of nNOS, Hsp27, copper/zinc-dependent superoxide dismutase (CuZnSOD), manganese-dependent superoxide dismutase (MnSOD), and iNOS by western blot analysis. GAPDH served as a loading control. (A) Western blot results taken from three representative control and HE patients. (B) Densitometric quantification of western blot results. Protein expression levels are given relative to GAPDH expression in the individual sample. Each case is shown separately on the vertical scatter plot; bars represent the mean values. *Significantly different compared with the control group. n.s., not significant. (C) Western blot analysis of iNOS expression in the cerebral cortex from control and HE patients. Representative western blot samples (3/8) are shown for both investigational groups. Human hepatoma cells (HepG2) treated with proinflammatory cytokines (tumor necrosis factor-α [10 ng/mL], interferon-γ, interleukin-1β [100 U/mL each, 24 hours]) served as a positive control for iNOS expression.
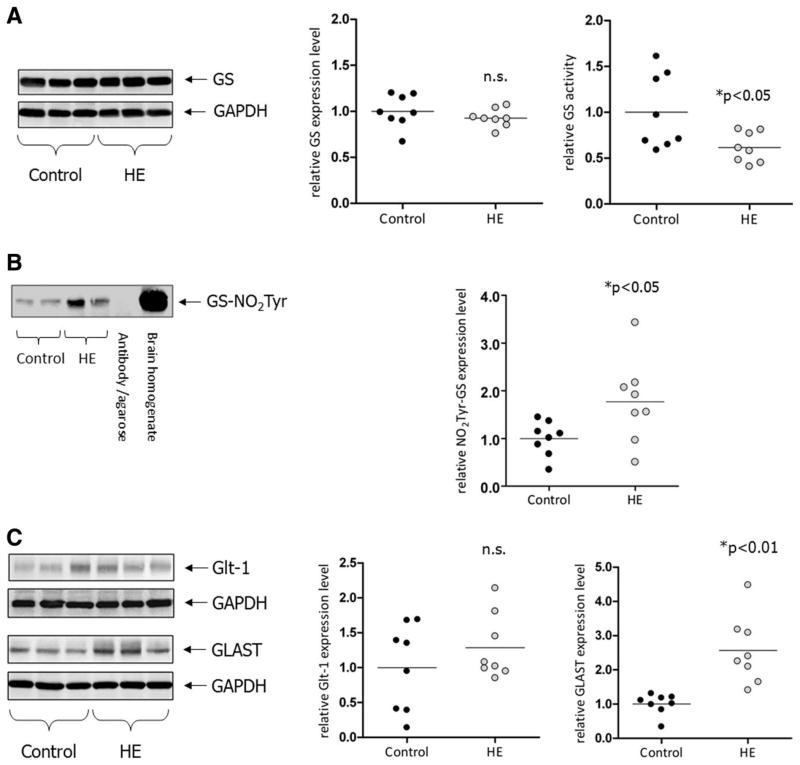
Astrocytic GS was identified as one target of PTN in response to ammonia,10 and further studies have shown that nitration of tyrosine residues in the adenosine triphosphate–binding pocket, similar to mutations in this region of the enzyme are associated with a loss of enzymatic activity of GS.25,26 By this means, ammonia intoxication lowers GS activity in the brain without affecting the amount of GS protein.10 As shown in Fig. 4A, also in the cerebral cortex from HE patients GS activity, but not the GS protein level was significantly decreased. This was accompanied by a significantly increased PTN of GS (Fig. 4B). A decrease of GS activity had also been described in rats after portocaval anastomosis.27
Fig. 4.
(A) Glutamine synthetase and glutamate transporter expression in cerebral cortex from control patients and patients with cirrhosis and HE. Protein lysates prepared from the cerebral cortex were analyzed by western blot analysis for expression of GS at the level of GS protein and enzymatic activity. Expression of GS protein is given relative to GAPDH expression. GS activity was measured as described in Patients and Methods. *Significantly different compared with the control group. n.s., not significant. (B) Detection of tyrosine-nitrated GS in cerebro-cortical protein lysates. Anti-3′-nitrotyrosine immunoprecipitates were analyzed by western blot analysis for the presence of GS (GS-NO2Tyr). As for control, precipitation was performed without addition of protein lysate (antibody/agarose control). Total lysate from human brain served as a positive control for detection of GS. Expression levels were quantified densitometrically and are shown separately for each case on the vertical scatter plot; bars represent the mean values. *Significantly different compared with the control group. (C) Protein lysates were prepared from the cerebral cortex and analyzed by western blot analysis for expression of GLAST and Glt-1. Expression of transporter proteins is given relative to GAPDH expression. Western blots from three representative control and HE patients are shown. GAPDH served as a loading control. Expression levels were quantified densitometrically and are shown separately for each case on the vertical scatter plot; bars represent the mean values. *Significantly different compared with the control group.
Protein Expression Level of Glial Glutamate Transporters in HE
Ammonia toxicity to rat astrocytes and rat brain and the associated oxidative/nitrosative stress response strongly depend on the activation of N-methyl-D-aspartic acid receptors,10,28 which play an important role in the glutamatergic neurotransmission and ammonia-induced excitotoxicity.29 As shown recently, ammonia and cell swelling induce glutamate release from astrocytes.30,31 Glutamate homeostasis in the synaptic cleft is maintained by astrocytic glutamate reuptake facilitated by GLAST and the glial glutamate transporter-1 (Glt-1).32 Experiments in long-term portocaval-shunted animals suggest a selective cerebral up-regulation of glial glutamate transporters.33 As shown in Fig. 4C, GLAST protein expression was significantly up-regulated in the cerebral cortex of HE patients compared with controls, whereas the mild increase of Glt-1 expression did not reach significance (Fig. 4C).
HE but Not Cirrhosis Triggers Oxidative Stress in the Cerebral Cortex
In order to determine whether increased cerebral oxidative stress is a feature of HE or of cirrhosis, brain samples from four patients with cirrhosis without HE were analyzed in comparison with four controls without cirrhosis. These eight brain samples were obtained from the Australian Brain Donor Programs NSW Tissue Resource Centre (see Table 1 for patient characteristics). As shown in Fig. 5A–E and Supporting Fig. 1, no significant differences with respect to tyrosine nitration of cerebral proteins, levels of RNA oxidation, GLAST and Hsp27 expression or GS activity were detectable in brain samples from patients with cirrhosis without HE compared with controls. These findings indicate that oxidative/nitrosative stress is a hallmark of HE, but not of cirrhosis itself.
Fig. 5.
Expression levels of oxidative/nitrosative stress markers, GS, and GLAST in the cerebral cortex of control patients and patients with cirrhosis without HE. Data were obtained from four Australian control patients and four Australian patients with cirrhosis without HE. Protein extracts prepared from the cerebral cortex were analyzed for expression level of (A) tyrosine-nitrated proteins (NO2Tyr), (C) Hsp27, and (D) GLAST by western blot analysis. GAPDH served as a loading control. Expression levels were quantified by densitometric analysis and are given relative to GAPDH expression. (B) Oxidized RNA was detected by northwestern blot analysis in total RNA extracts prepared from the cerebral cortex. (E) Activity of GS was determined as described in the Materials and Methods. Each control patient and patient with cirrhosis included in this study is shown separately in the vertical scatter plot; bars represent the mean values. n.s., not significant.
Correlational Analysis
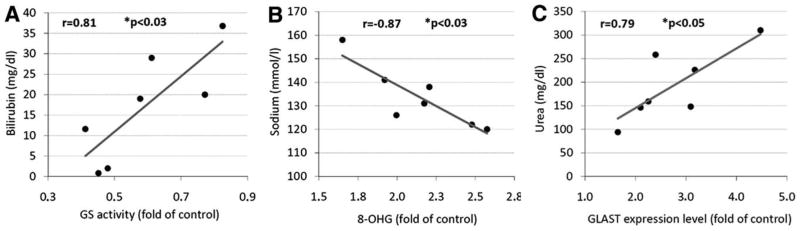
The expression of oxidative stress markers, glutamate transporters, and GS in post mortem brain tissue was correlated to in vivo laboratory data, which were obtained during the 24-hour period before death. Such data were available in six to seven patients from the European cohort (Table 2). Results of this analysis are given in Supporting Table 1. Highly significant correlations were found between serum bilirubin concentration and GS activity (Fig. 6A), the serum sodium concentration and the extent of RNA oxidation (Fig. 6B), and serum urea concentration and GLAST expression (Fig. 6C). Neither plasma ammonia nor C-reactive protein in serum or the blood leukocyte count correlated with oxidative stress parameters in post mortem brain cortex (Supporting Table 1).
Table 2.
Blood Test Results of European Patients with Cirrhosis and HE
| Patient No. | Sodium (135–145 mmol/L) | Urea (18–55 mg/dL) | Creatinine (<1.2 mg/dL) | Albumin (3.3–4.7 g/dL) | Bilirubin (<1.0 mg/dL) | Leukocytes (4.0–11.0 × 1,000/μL) | C-Reactive Protein (<0.5 mg/dL) | Quick (70–130%) | Ammonia (<60 μmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 131 | 146 | 2.6 | 3.1 | 19.0 | — | — | — | — |
| 2 | 122 | 258 | 2.9 | 2.4 | 0.8 | 30 | 14.9 | 37 | 97 |
| 3 | 138 | 148 | 1.5 | 1.8 | 20.0 | 22.9 | 4.6 | 10 | 154 |
| 4 | — | — | — | — | — | — | — | — | — |
| 5 | 120 | 226 | 7.9 | 1.8 | 29.0 | 2.5 | 1.0 | 13 | 148 |
| 6 | 158 | 310 | 5 | 2.0 | 36.8 | 27.3 | 1.6 | 29 | 86 |
| 7 | 126 | 94 | 2.4 | 2.1 | 11.6 | 15.8 | 5.8 | 26 | 48 |
| 8 | 141 | 159 | 4.1 | — | 1.98 | 2.9 | 1.1 | 59 | 343 |
Laboratory parameters were measured on the day of (patient 8) or the day before death (patients 1, 2, 3, 5, 6, and 7). Reference values for each parameter are included in brackets.
Fig. 6.
Correlational analysis of post mortem brain parameters and in vivo laboratory data. Laboratory data were obtained within 24 hours before death. Laboratory data were available for six to seven out of the eight HE patients. Statistically significant correlations (P < 0.05) were found between (A) serum bilirubin and GS activity in the cerebral cortex, (B) serum sodium concentration and the extent of RNA oxidation in the brain (8-hydroxyguanosine [8-OHG] immunoreactivity), and (C) serum urea and the expression level of GLAST.
Discussion
Cell culture studies and animal experiments suggest that the induction of oxidative/nitrosative stress is closely related to the pathogenesis of cerebral ammonia toxicity8–10,14,22 due to chemical modifications of proteins 10 and RNA15 as well as effects on cerebral microcirculation, 34 zinc homeostasis,14 and gene transcription. 35,36 However, it is unknown whether oxidative/nitrosative stress and its sequelae are also found in the brain of patients with cirrhosis with HE. This important question was addressed in the present study using post mortem brain tissue obtained at autopsy from eight European patients suffering from liver cirrhosis and accompanying HE and from eight European control patients without a record of hepatic, neurological, or psychiatric disease. In addition, brains from four Australian control patients and from four Australian patients with cirrhosis without HE could be analyzed. Both the European and Australian cohorts were analyzed separately in order to rule out population and sample handling differences. Several lines of evidence indicate for the first time an increased oxidative/nitrosative stress in the brains of HE patients. First, the expression of Hsp27 was found to be increased four- to five-fold in the brains of HE patients. Hsp27 is known to confer resistance toward oxidative stress37 and is frequently used as marker for tissue exposure to oxidative stress.23 In line with this, up-regulation of Hsp27 also occurs in ischemia/reperfusion injury and perilesional ischemic brain regions38 and multiple sclerosis. 39 Furthermore, brain samples from HE patients showed significantly increased levels of tyrosine-nitrated proteins and of oxidized RNA, both parameters being well-established surrogate markers for oxidative stress.40,41 All these changes were not found in brain samples from patients with cirrhosis without HE, indicating that oxidative/nitrosative stress is a feature of HE, but not of cirrhosis. The decrease of GS activity, but not GS protein in brains from HE patients reflects the known inactivating tyrosine nitration of the enzyme.21 GS inactivation through nitration was shown recently to occur in ammonia-intoxicated rat brain10 and endotoxin-treated rat liver,25 in the brains of epilepsia42 and HE patients (present study), and after in vitro treatment of GS with peroxynitrite. 21 Here, nitration involves tyrosine residues close to the adenosine triphosphate–binding pocket of the enzyme, triggering activity loss of the enzyme,25 as it is also found in genetic defects affecting this region.26 In the setting of HE, down-regulation of GS activity may reflect a beneficial adaptation toward hyperammonemia, because astrocytic glutamine accumulation was identified as one trigger of low-grade cerebral edema in patients with cirrhosis and HE.2 It should be emphasized that the increase in protein tyrosine nitration and RNA oxidation in the brain of HE patients was highly discriminative between HE patients and controls: most of the HE brains showed RNA oxidation and PTN levels, which were above the highest values found in the control group. This contrasts the results obtained in a recent study using 14 out of the 16 brains of the present study for multireceptor autoradiography. 20 Here, the HE cases did not build a homogeneous group, but differed concerning direction and intensity of binding site density divergence.20 Thus, neurotransmitter alterations may be less likely primary key factors underlying HE pathogenesis than RNA oxidation and protein tyrosine nitration.
Expression levels of superoxide dismutase isoenzymes and of neuronal and iNOS were not significantly altered in the brain from HE patients when compared with controls. This does not contradict the strong increase in tyrosine-nitrated proteins in HE, because nNOS is also regulated by Ca2+ and peroxynitrite-dependent and independent pathways of tyrosine nitration are known.11,43 Chronic hyperammonemia was reported to down-regulate nNOS in rat cerebellum, 44 whereas no effect on nNOS and iNOS was reported in the cerebellum of rats with thioacetamide induced45 and up-regulation of iNOS and nNOS was reported in rats with portocaval anastomosis.24,46 Similarly, opposing results regarding superoxide dismutase expression in rat brain have been reported. Whereas thioacetamide-induced fulminant hepatic failure is accompanied by an up-regulation of manganese-dependent superoxide dismutase,47 down-regulation of Cu/Zn-dependent superoxide dismutase was reported after portocaval anastomosis.36
Experiments with cultured rat astrocytes suggest that overstimulation of N-methyl-D-aspartic acid receptors plays an important role in triggering the oxidative/nitrosative stress response.10 Astrocyte swelling and ammonia trigger astrocytic glutamate release,30,31 thereby amplifying N-methyl-D-aspartic acid receptor activation.10,12 Glutamate reuptake is accomplished by the astrocytic glutamate transporters GLAST and Glt-1. A significant up-regulation of GLAST was found in the brains of HE patients, but not of patients with cirrhosis without HE. GLAST up-regulation was reported in long-term portocaval shunted rats,33 whereas ammonia intoxication for only a few days triggered down-regulation of GLAST protein and messenger RNA in rat astrocytes.48,49 The latter may be a consequence of GLAST messenger RNA oxidation, which was recently described in acutely ammonia intoxicated rats.15
Although the correlations between in vivo laboratory data and post mortem brain characteristics need to be interpreted with caution due to the small sample size, a few observations merit comment. There was a significant correlation between serum sodium concentration and the extent of cerebral RNA oxidation, indicating that hypo-osmolarity and RNA oxidation are somehow interlinked. In line with this, hypo-osmotic exposure of rat astrocyte cultures and brain slices induces RNA oxidation,15 and there is good evidence that HE in patients with cirrhosis reflects the clinical manifestation of low-grade cerebral edema, which can exacerbate in response to hyponatremia.2 However, it is also possible that hyponatremia (hypo-osmolarity) is not the cause of HE, but rather its consequence through swelling of hypothalamic structures promoting inadaequate vasopressin secretion.50 In any case, the correlation depicted in Fig. 6A corroborates clinical data on the relationship between hyponatremia and HE severity. 2,51 Interestingly, neither plasma ammonia nor markers of infection (leukocyte count, C-reactive protein) showed any relationship to oxidative/nitrosative brain modification. This finding may be explained by the multifactorial pathogenesis of low-grade cerebral edema and consequently generation of oxidative/nitrosative stress in HE11 and corroborates the view that HE in patients with cirrhosis is not simply ammonia intoxication or a pure response to systemic inflammation. In line with this, no increased expression of iNOS was detectable in the brains of patients with cirrhosis and HE. No significant relationship was found between RNA oxidation and PTN in the brain, although both processes are consequences of oxidative stress. Differences between both processes may relate to different nitric oxide requirements and kinetics of repair and elimination mechanism of nitrated proteins and oxidized RNA, respectively.
In conclusion, the present study shows for the first time the involvement of oxidative/nitrosative stress in the pathobiology of HE in patients with cirrhosis. Oxidative modifications of cerebral proteins and RNA are found in the cerebral cortex of HE patients, but not of patients with cirrhosis without HE. This may shed a new light on the pathogenesis of HE and offer potential new treatment options.
Acknowledgments
We thank the Australian Brain Donor Programs NSW Tissue Resource Centre, Sydney, for tissue support. Expert technical assistance was provided by Torsten Janssen.
This Study was Supported by Deutsche Forschungsgemeinschaft through Sonderforschungsbereich 575 “Experimentelle Hepatologle” (Düsseldorf ).
Abbreviations
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GLAST
glutamate/aspartate cotransporter
- Glt-1
glial glutamate transporter-1
- GS
glutamine synthetase
- HE
hepatic encephalopathy
- Hsp27
heat shock protein- 27
- iNOS
inducible nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- PTN
protein tyrosine nitration
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Häussinger D, Blei AT, Reichen J, Rizzetto M. Hepatic encephalopathy. In: Rodes J, Benhamou JP, Blei AT, Reichen J, Rizzetto M, editors. The Oxford Textbook of Hepatology. Oxford: Blackwell; 2007. pp. 728–760. [Google Scholar]
- 2.Häussinger D, Laubenberger J, vom Dahl S, Ernst T, Bayer S, Langer M, et al. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology. 1994;107:1475–1480. doi: 10.1016/0016-5085(94)90552-5. [DOI] [PubMed] [Google Scholar]
- 3.Häussinger D, Kircheis G, Fischer R, Schliess F, vom Dahl S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema? J Hepatol. 2000;32:1035–1038. doi: 10.1016/s0168-8278(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 4.Cordoba J, Sanpedro F, Alonso J, Rovira A. 1H magnetic resonance in the study of hepatic encephalopathy in humans. Metab Brain Dis. 2002;17:415–429. doi: 10.1023/a:1021926405944. [DOI] [PubMed] [Google Scholar]
- 5.Shah NJ, Neeb H, Kircheis G, Engels P, Häussinger D, Zilles K. Quantitative cerebral water content mapping in hepatic encephalopathy. Neuroimage. 2008;41:706–717. doi: 10.1016/j.neuroimage.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 6.Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorios JH, Neary JT. Ammonia-induced astrocyte swelling in primary culture. Neurochem Res. 1991;16:833–836. doi: 10.1007/BF00965694. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez-Leefmans FJ, Herrera-Perez JJ, Marquez MS, Blanco VM. Simultaneous measurement of water volume and pH in single cells using BCECF and fluorescence imaging microscopy. Biophys J. 2006;90:608–618. doi: 10.1529/biophysj.105.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinehr R, Görg B, Becker S, Qvartskhava N, Bidmon HJ, Selbach O, et al. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured and vital brain slices. Glia. 2007;55:758–771. doi: 10.1002/glia.20504. [DOI] [PubMed] [Google Scholar]
- 9.Murthy CR, Rama Rao KV, Bai G, Norenberg MD. Ammonia-induced production of free radicals in primary cultures of rat astrocytes. J Neurosci Res. 2001;66:282–288. doi: 10.1002/jnr.1222. [DOI] [PubMed] [Google Scholar]
- 10.Schliess F, Görg B, Fischer R, Desjardins P, Bidmon HJ, Herrmann A, et al. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat. FASEB J. 2002;16:739–741. doi: 10.1096/fj.01-0862fje. [DOI] [PubMed] [Google Scholar]
- 11.Häussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. 2008;57:1156–1165. doi: 10.1136/gut.2007.122176. [DOI] [PubMed] [Google Scholar]
- 12.Schliess F, Foster N, Görg B, Reinehr R, Häussinger D. Hypoosmotic swelling increases protein tyrosine nitration in cultured rat astrocytes. Glia. 2004;47:21–29. doi: 10.1002/glia.20019. [DOI] [PubMed] [Google Scholar]
- 13.Schliess F, Görg B, Häussinger D. Pathogenetic interplay between osmotic and oxidative stress: the hepatic encephalopathy paradigm. Biol Chem. 2006;387:1363–1370. doi: 10.1515/BC.2006.171. [DOI] [PubMed] [Google Scholar]
- 14.Kruczek C, Görg B, Keitel V, Pirev E, Kroncke KD, Schliess F, et al. Hypoosmotic swelling affects zinc homeostasis in cultured rat astrocytes. Glia. 2009;57:79–92. doi: 10.1002/glia.20737. [DOI] [PubMed] [Google Scholar]
- 15.Görg B, Qvartskhava N, Keitel V, Bidmon HJ, Selbach O, Schliess F, et al. Ammonia induces RNA oxidation in cultured astrocytes and brain in vivo. Hepatology. 2008;48:567–79. doi: 10.1002/hep.22345. [DOI] [PubMed] [Google Scholar]
- 16.Martin KC, Barad M, Kandel ER. Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol. 2000;10:587–592. doi: 10.1016/s0959-4388(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 17.Ding Q, Markesbery WR, Chen Q, Li F, Keller JN. Ribosome dysfunction is an early event in Alzheimer’s disease. J Neurosci. 2005;25:9171–9175. doi: 10.1523/JNEUROSCI.3040-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka M, Chock PB, Stadtman ER. Oxidized messenger RNA induces translation errors. Proc Natl Acad Sci USA. 2007;104:66–71. doi: 10.1073/pnas.0609737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timmermann L, Gross J, Butz M, Kircheis G, Häussinger D, Schnitzler A. Mini-asterixis in hepatic encephalopathy induced by pathologic thalamo- motor-cortical coupling. Neurology. 2003;61:689–692. doi: 10.1212/01.wnl.0000078816.05164.b1. [DOI] [PubMed] [Google Scholar]
- 20.Palomero-Gallagher N, Bidmon H, Cremer M, Schleicher A, Kircheis G, Reifenberger G, et al. Neurotransmitter receptor imbalances in motor cortex and basal ganglia in hepatic encephalopathy. Cell Physiol Biochem. 2009;24:291–306. doi: 10.1159/000233254. [DOI] [PubMed] [Google Scholar]
- 21.Görg B, Qvartskhava N, Voss P, Grune T, Häussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 22.Kosenko E, Kaminsky Y, Kaminsky A, Valencia M, Lee L, Hermenegildo C, et al. Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Radic Res. 1997;27:637–644. doi: 10.3109/10715769709097867. [DOI] [PubMed] [Google Scholar]
- 23.Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- 24.Suarez I, Bodega G, Arilla E, Felipo V, Fernandez B. The expression of nNOS, iNOS and nitrotyrosine is increased in the rat cerebral cortex in experimental hepatic encephalopathy. Neuropathol Appl Neurobiol. 2006;32:594–604. doi: 10.1111/j.1365-2990.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- 25.Görg B, Wettstein M, Metzger S, Schliess F, Häussinger D. Lipopolysaccharide- induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. HEPATOLOGY. 2005;41:1065–1073. doi: 10.1002/hep.20662. [DOI] [PubMed] [Google Scholar]
- 26.Häberle J, Görg B, Rutsch F, Schmidt E, Toutain A, Benoist J, et al. Congenital glutamine deficiency with glutamine synthetase mutations. N Engl J Med. 2005;353:1926–1933. doi: 10.1056/NEJMoa050456. [DOI] [PubMed] [Google Scholar]
- 27.Girard G, Giguere JF, Butterworth RF. Region-selective reductions in activities of glutamine synthetase in rat brain following portacaval anastomosis. Metab Brain Dis. 1993;8:207–215. doi: 10.1007/BF01001062. [DOI] [PubMed] [Google Scholar]
- 28.Kosenko E, Kaminski Y, Lopata O, Muravyov N, Felipo V. Blocking NMDA receptors prevents the oxidative stress induced by acute ammonia intoxication. Free Radic Biol Med. 1999;26:1369–1374. doi: 10.1016/s0891-5849(98)00339-6. [DOI] [PubMed] [Google Scholar]
- 29.Hermenegildo C, Marcaida G, Montoliu C, Grisolia S, Minana MD, Felipo V. NMDA receptor antagonists prevent acute ammonia toxicity in mice. Neurochem Res. 1996;21:1237–1244. doi: 10.1007/BF02532401. [DOI] [PubMed] [Google Scholar]
- 30.Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling- induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose C, Kresse W, Kettenmann H. Acute insult of ammonia leads to calcium-dependent glutamate release from cultured astrocytes, an effect of pH. J Biol Chem. 2005;280:20937–20944. doi: 10.1074/jbc.M412448200. [DOI] [PubMed] [Google Scholar]
- 32.Bezzi P, Vesce S, Panzarasa P, Volterra A. Astrocytes as active participants of glutamatergic function and regulators of its homeostasis. Adv Exp Med Biol. 1999;468:69–80. doi: 10.1007/978-1-4615-4685-6_6. [DOI] [PubMed] [Google Scholar]
- 33.Suarez I, Bodega G, Fernandez B. Modulation of glutamate transporters (GLAST, GLT-1 and EAAC1) in the rat cerebellum following portocaval anastomosis. Brain Res. 2000;859:293–302. doi: 10.1016/s0006-8993(00)01993-4. [DOI] [PubMed] [Google Scholar]
- 34.Master S, Gottstein J, Blei AT. Cerebral blood flow and the development of ammonia-induced brain edema in rats after portacaval anastomosis. Hepatology. 1999;30:876–880. doi: 10.1002/hep.510300428. [DOI] [PubMed] [Google Scholar]
- 35.Warskulat U, Kreuels S, Muller HW, Häussinger D. Identification of osmosensitive and ammonia-regulated genes in rat astrocytes by Northern blotting and differential display reverse transcriptase-polymerase chain reaction. J Hepatol. 2001;35:358–366. doi: 10.1016/s0168-8278(01)00149-0. [DOI] [PubMed] [Google Scholar]
- 36.Song G, Dhodda VK, Blei AT, Dempsey RJ, Rao VLR. GeneChip analysis shows altered mRNA expression of transcripts of neurotransmitter and signal transduction pathways in the cerebral cortex of portacaval shunted rats. J Neurosci Res. 2002;68:730–737. doi: 10.1002/jnr.10268. [DOI] [PubMed] [Google Scholar]
- 37.Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, et al. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28:13038–13055. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popp A, Jaenisch N, Witte OW, Frahm C. Identification of ischemic regions in a rat model of stroke. PLoS One. 2009;4:e4764. doi: 10.1371/journal.pone.0004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aquino DA, Capello E, Weisstein J, Sanders V, Lopez C, Tourtellotte WW, et al. Multiple sclerosis: altered expression of 70- and 27-kDa heat shock proteins in lesions and myelin. J Neuropathol Exp Neurol. 1997;56:664–672. [PubMed] [Google Scholar]
- 40.Li Z, Wu J, Deleo CJ. RNA damage and surveillance under oxidative stress. IUBMB Life. 2006;58:581–588. doi: 10.1080/15216540600946456. [DOI] [PubMed] [Google Scholar]
- 41.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 42.Bidmon H, Görg B, Palomero-Gallagher N, Schleicher A, Häussinger D, Speckmann EJ, et al. Glutamine synthetase becomes nitrated and its activity is reduced during repetitive seizure activity in the pentylentetrazole model of epilepsy. Epilepsia. 2008;49:1733–1748. doi: 10.1111/j.1528-1167.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 43.Hurst JK. Whence nitrotyrosine? J Clin Invest. 2002;109:1287–1289. doi: 10.1172/JCI15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Mlili N, Rodrigo R, Naghizadeh B, Cauli O, Felipo V. Chronic hyperammonemia reduces the activity of neuronal nitric oxide synthase in cerebellum by altering its localization and increasing its phosphorylation by calcium-calmodulin kinase II. J Neurochem. 2008;106:1440–1449. doi: 10.1111/j.1471-4159.2008.05495.x. [DOI] [PubMed] [Google Scholar]
- 45.Hernandez R, Martinez-Lara E, Del Moral ML, Blanco S, Canuelo A, Siles E, et al. Upregulation of endothelial nitric oxide synthase maintains nitric oxide production in the cerebellum of thioacetamide cirrhotic rats. Neuroscience. 2004;126:879–887. doi: 10.1016/j.neuroscience.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Rao VL, Audet RM, Butterworth RF. Increased neuronal nitric oxide synthase expression in brain following portacaval anastomosis. Brain Res. 1997;765:169–172. doi: 10.1016/s0006-8993(97)00652-5. [DOI] [PubMed] [Google Scholar]
- 47.Reddy PVB, Murthy CRK, Reddanna P. Fulminant hepatic failure induced oxidative stress in nonsynaptic mitochondria of cerebral cortex in rats. Neurosci Lett. 2004;368:15–20. doi: 10.1016/j.neulet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 48.Chan H, Hazell AS, Desjardins P, Butterworth RF. Effects of ammonia on glutamate transporter (GLAST) protein and mRNA in cultured rat cortical astrocytes. Neurochem Int. 2000;37:243–248. doi: 10.1016/s0197-0186(00)00026-7. [DOI] [PubMed] [Google Scholar]
- 49.Zhou BG, Norenberg MD. Ammonia downregulates GLAST mRNA glutamate transporter in rat astrocyte cultures. Neurosci Lett. 1999;276:145–148. doi: 10.1016/s0304-3940(99)00816-2. [DOI] [PubMed] [Google Scholar]
- 50.Huang DY, Boini KM, Lang PA, Grahammer F, Duszenko M, Heller-Stilb B, et al. Impaired ability to increase water excretion in mice lacking the taurine transporter gene TAUT. Pflugers Arch. 2006;451:668–677. doi: 10.1007/s00424-005-1499-y. [DOI] [PubMed] [Google Scholar]
- 51.Guevara M, Baccaro ME, Torre A, Gomez-Anson B, Rios J, Torres F, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. 2009;104:1382–1389. doi: 10.1038/ajg.2009.293. [DOI] [PubMed] [Google Scholar]