Abstract
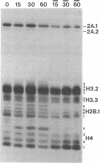
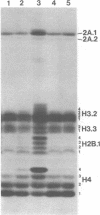
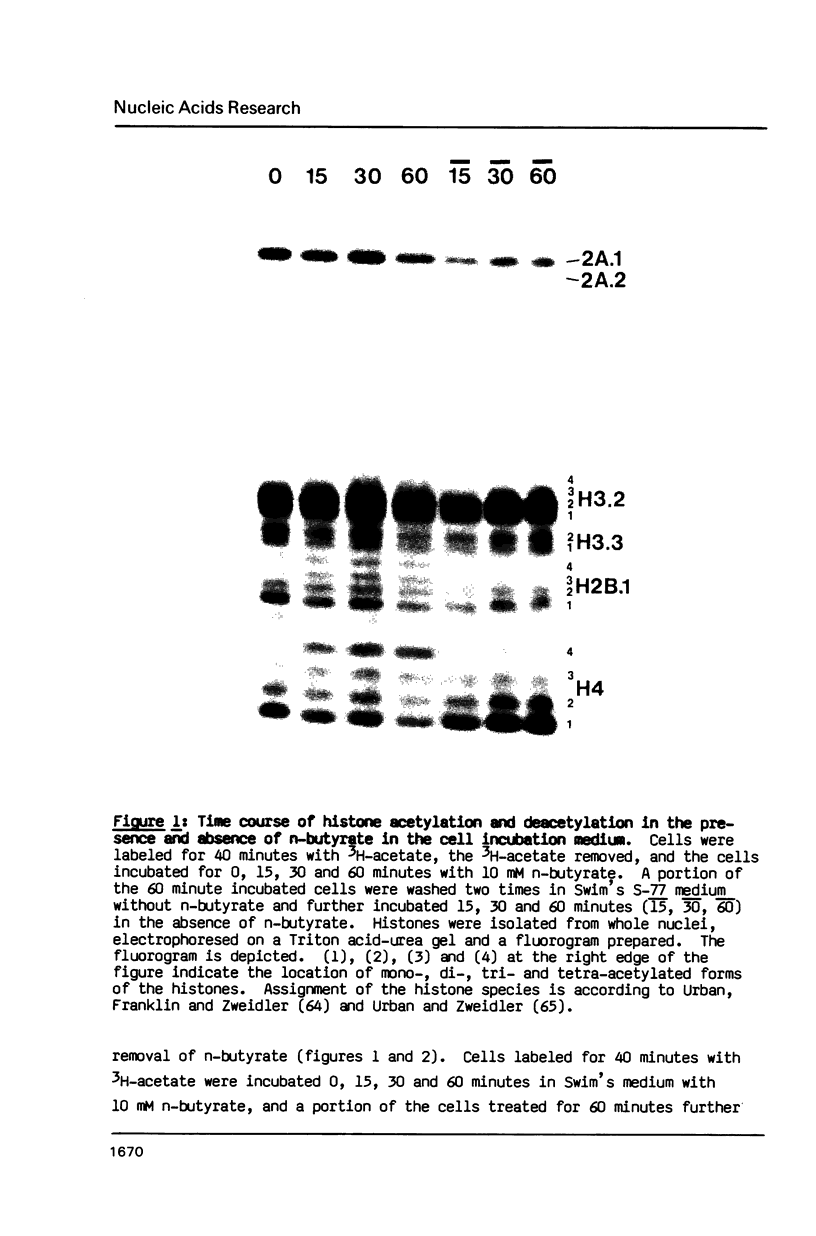
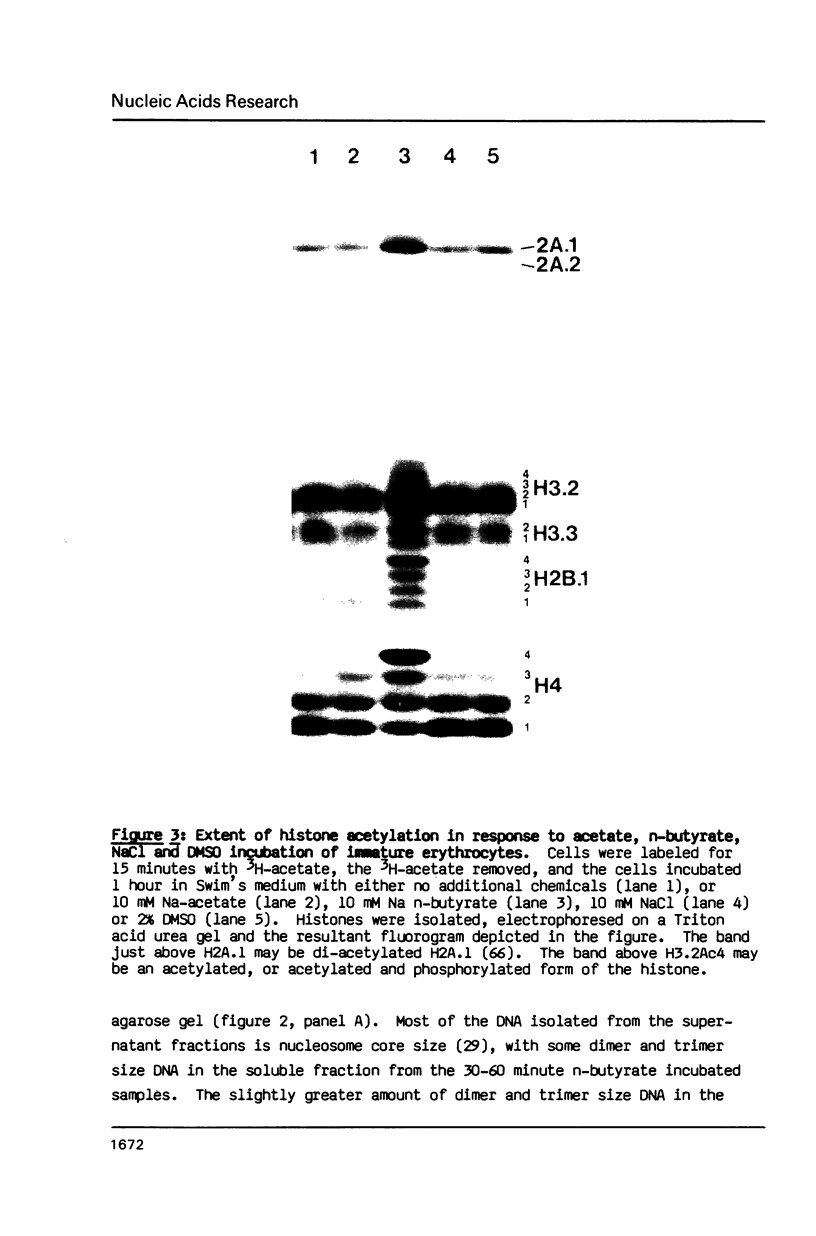
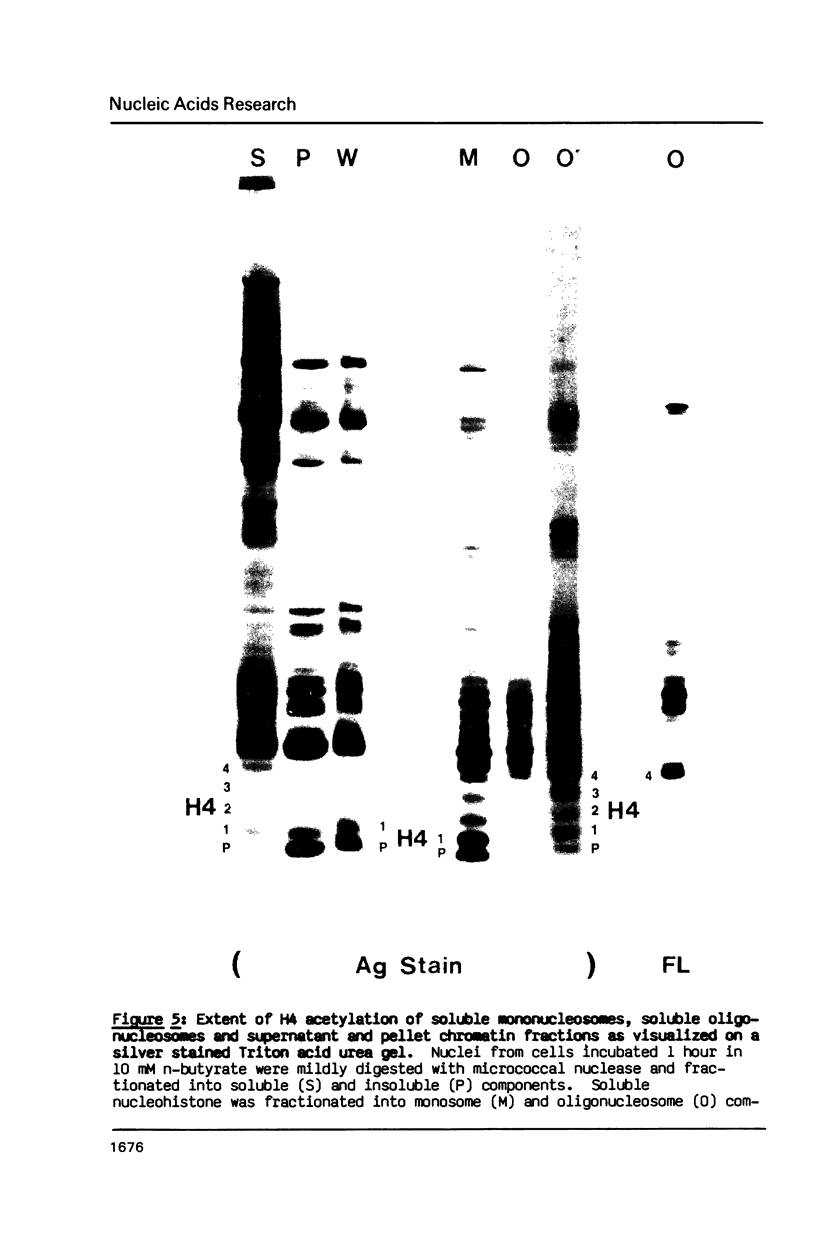
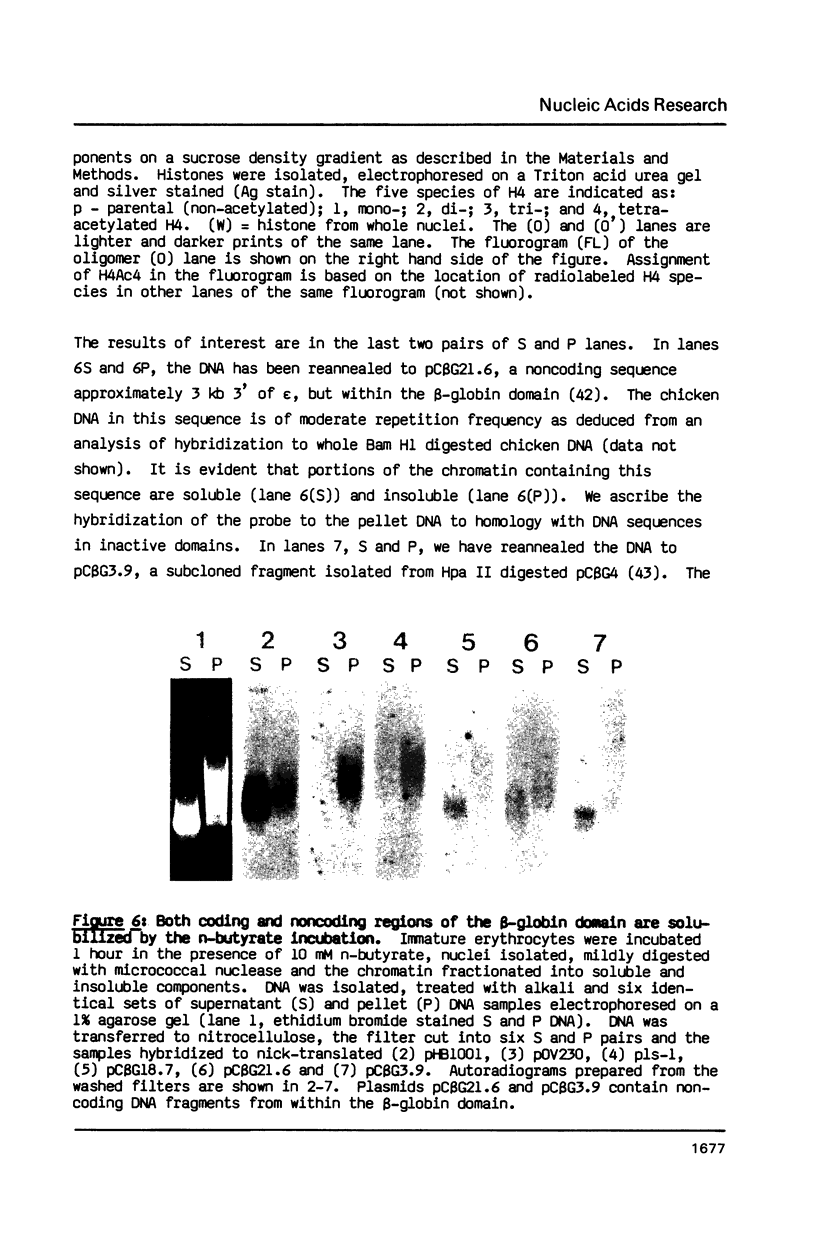
A 60 minute exposure of chicken immature erythrocytes to n-butyrate shifts actively acetylated and deacetylated histones to hypermodified forms. Micrococcal nuclease digestion of nuclei from n-butyrate treated cells and subsequent fractionation of the chromatin releases 40-45% of the adult beta-globin (beta A) nucleohistone into a soluble fraction. This is an eleven fold enrichment over the soluble chromatin from untreated cells (Ferenz and Nelson (1985) Nucleic Acids Res. 13, 1977-1995). The enhanced beta A chromatin solubility and induced histone hyperacetylation are coincident. Removal of n-butyrate from the cell incubation medium allows rapid histone deacetylation and a striking reduction in beta A chromatin solubility. Chromatin from cells incubated in the absence of n-butyrate, or in medium containing 10 mM NaCl or 2% dimethylsulfoxide, does not exhibit histone hyperacetylation, or the acquired solubility of beta A chromatin. We show that the H4 histone co-isolated with the beta A DNA is in a hyperacetylated state and present evidence that the n-butyrate incubation increases the solubility of both coding and noncoding chromatin regions in the beta-globin domain.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., FAULKNER R., MIRSKY A. E. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 May;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Annunziato A. T., Seale R. L. Histone deacetylation is required for the maturation of newly replicated chromatin. J Biol Chem. 1983 Oct 25;258(20):12675–12684. [PubMed] [Google Scholar]
- Belikoff E., Wong L. J., Alberts B. M. Extensive purification of histone acetylase A, the major histone N-acetyl transferase activity detected in mammalian cell nuclei. J Biol Chem. 1980 Dec 10;255(23):11448–11453. [PubMed] [Google Scholar]
- Berlowitz L., Pallotta D. Acetylation of nuclear protein in the heterochromatin and euchromatin of mealy bugs. Exp Cell Res. 1972 Mar;71(1):45–48. doi: 10.1016/0014-4827(72)90261-3. [DOI] [PubMed] [Google Scholar]
- Bode J., Henco K., Wingender E. Modulation of the nucleosome structure by histone acetylation. Eur J Biochem. 1980 Sep;110(1):143–152. doi: 10.1111/j.1432-1033.1980.tb04849.x. [DOI] [PubMed] [Google Scholar]
- Boffa L. C., Gruss R. J., Allfrey V. G. Manifold effects of sodium butyrate on nuclear function. Selective and reversible inhibition of phosphorylation of histones H1 and H2A and impaired methylation of lysine and arginine residues in nuclear protein fractions. J Biol Chem. 1981 Sep 25;256(18):9612–9621. [PubMed] [Google Scholar]
- Boffa L. C., Vidali G., Mann R. S., Allfrey V. G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J Biol Chem. 1978 May 25;253(10):3364–3366. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brotherton T. W., Covault J., Shires A., Chalkley R. Only a small fraction of avian erythrocyte histone is involved in ongoing acetylation. Nucleic Acids Res. 1981 Oct 10;9(19):5061–5073. doi: 10.1093/nar/9.19.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido E. P. Partial characterization of a histone acetyltransferase from trout testis. Can J Biochem. 1975 Jul;53(7):796–803. doi: 10.1139/o75-107. [DOI] [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Christensen M. E., Dixon G. H. Hyperacetylation of histone H4 correlates with the terminal, transcriptionally inactive stages of spermatogenesis in rainbow trout. Dev Biol. 1982 Oct;93(2):404–415. doi: 10.1016/0012-1606(82)90127-0. [DOI] [PubMed] [Google Scholar]
- Christensen M. E., Rattner J. B., Dixon G. H. Hyperacetylation of histone H4 promotes chromatin decondensation prior to histone replacement by protamines during spermatogenesis in rainbow trout. Nucleic Acids Res. 1984 Jun 11;12(11):4575–4592. doi: 10.1093/nar/12.11.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens L. S., Alberts B. M. Accessibility of newly synthesized chromatin to histone acetylase. J Biol Chem. 1982 Apr 10;257(7):3945–3949. [PubMed] [Google Scholar]
- Estepa I., Pestaña A. Isolation and partial characterization of three histone-specific acetyltransferases from Artemia. Eur J Biochem. 1983 May 2;132(2):249–254. doi: 10.1111/j.1432-1033.1983.tb07356.x. [DOI] [PubMed] [Google Scholar]
- Ferenz C. R., Nelson D. A. N-Butyrate incubation of immature chicken erythrocytes preferentially enhances the solubility of beta A chromatin. Nucleic Acids Res. 1985 Mar 25;13(6):1977–1995. doi: 10.1093/nar/13.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R. F., Moyer M. P., Bishop J. M., Moyer R. C., Waite M. R. Transformation parameters induced in chick cells by incubation in media of altered NaCl concentration. Virology. 1981 Jun;111(2):427–439. doi: 10.1016/0042-6822(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Hay C. W., Candido E. P. Histone deacetylase from HeLa cells: properties of the high molecular weight complex. Biochemistry. 1983 Dec 20;22(26):6175–6180. doi: 10.1021/bi00295a021. [DOI] [PubMed] [Google Scholar]
- Hay C. W., Candido E. P. Histone deacetylase. Association with a nuclease resistant, high molecular weight fraction of HeLa cell chromatin. J Biol Chem. 1983 Mar 25;258(6):3726–3734. [PubMed] [Google Scholar]
- Kelner D. N., McCarty K. S., Sr Porcine liver nuclear histone acetyltransferase. Partial purification and basic properties. J Biol Chem. 1984 Mar 25;259(6):3413–3419. [PubMed] [Google Scholar]
- Landes G. M., Villeponteau B., Pribyl T. M., Martinson H. G. Hemoglobin switching in chickens. Is the switch initiated post-transcriptionally? J Biol Chem. 1982 Sep 25;257(18):11008–11014. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Libby P. R. Calf liver nuclear N-acetyltransferases. Purification and properties of two enzymes with both spermidine acetyltransferase and histone acetyltransferase activities. J Biol Chem. 1978 Jan 10;253(1):233–237. [PubMed] [Google Scholar]
- Libby P. R. Histone acetylation and hormone action. Early effects of aldosterone on histone acetylation in rat kidney. Biochem J. 1973 Aug;134(4):907–912. doi: 10.1042/bj1340907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. R. Histone acetylation and hormone action. Early effects of oestradiol-17beta on histone acetylation in rat uterus. Biochem J. 1972 Dec;130(3):663–669. doi: 10.1042/bj1300663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. R. Rat liver nuclear N-acetyltransferases: separation of two enzymes with both histone and spermidine acetyltransferase activity. Arch Biochem Biophys. 1980 Aug;203(1):384–389. doi: 10.1016/0003-9861(80)90190-3. [DOI] [PubMed] [Google Scholar]
- Louie A. J., Candido E. P., Dixon G. H. Enzymatic modifications and their possible roles in regulating the binding of basic proteins to DNA and in controlling chromosomal structure. Cold Spring Harb Symp Quant Biol. 1974;38:803–819. doi: 10.1101/sqb.1974.038.01.084. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Nickol J. M., Felsenfeld G., Rau D. C. Histone hyperacetylation has little effect on the higher order folding of chromatin. Nucleic Acids Res. 1983 Jun 25;11(12):4065–4075. doi: 10.1093/nar/11.12.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds L., O'Malley B. W., Nisbet A. D., Fothergill J. E., Givol D., Fields S., Robertson M., Brownlee G. G. Sequence of chicken ovalbumin mRNA. Nature. 1978 Jun 29;273(5665):723–728. doi: 10.1038/273723a0. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Perry M., Sealy L., Chalkley R. DNAse I preferentially digests chromatin containing hyperacetylated histones. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1346–1353. doi: 10.1016/0006-291x(78)90337-6. [DOI] [PubMed] [Google Scholar]
- Nelson D., Perry M. E., Chalkley R. A correlation between nucleosome spacer region susceptibility to DNase I and histone acetylation. Nucleic Acids Res. 1979 Feb;6(2):561–574. doi: 10.1093/nar/6.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva R., Mezquita C. Histone H4 hyperacetylation and rapid turnover of its acetyl groups in transcriptionally inactive rooster testis spermatids. Nucleic Acids Res. 1982 Dec 20;10(24):8049–8059. doi: 10.1093/nar/10.24.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T., Terayama H., Takaku F., Nakao K. Hydrocortisone effect upon the phytohemagglutin-stimulated acetylation of histones in human lymphocytes. Biochim Biophys Acta. 1969 Mar 18;179(1):214–220. doi: 10.1016/0005-2787(69)90138-5. [DOI] [PubMed] [Google Scholar]
- Pantazis P., Bonner W. M. Butyrate-induced histone hyperacetylation in human and mouse cells: estimation of putative sites of histone acetylation in vivo. J Cell Biochem. 1982;20(3):225–235. doi: 10.1002/jcb.240200303. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Perry M., Chalkley R. Histone acetylation increases the solubility of chromatin and occurs sequentially over most of the chromatin. A novel model for the biological role of histone acetylation. J Biol Chem. 1982 Jul 10;257(13):7336–7347. [PubMed] [Google Scholar]
- Perry M., Chalkley R. The effect of histone hyperacetylation on the nuclease sensitivity and the solubility of chromatin. J Biol Chem. 1981 Apr 10;256(7):3313–3318. [PubMed] [Google Scholar]
- Pogo B. G., Allfrey V. G., Mirsky A. E. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci U S A. 1966 Apr;55(4):805–812. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Candido E. P. Turnover of histone acetyl groups in cultured cells is inhibited by sodium butyrate. FEBS Lett. 1978 Jul 1;91(1):117–120. doi: 10.1016/0014-5793(78)80030-1. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Selective synthesis and modification of nuclear proteins during maturation of avian erythroid cells. Arch Biochem Biophys. 1976 May;174(1):273–290. doi: 10.1016/0003-9861(76)90346-5. [DOI] [PubMed] [Google Scholar]
- Sanders L. A., Schechter N. M., McCarty K. S. A comparative study of histone acetylation, histone deacetylation, and ribonucleic acid synthesis in avian reticulocytes and erythrocytes. Biochemistry. 1973 Feb 27;12(5):783–791. doi: 10.1021/bi00729a001. [DOI] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. DNA associated with hyperacetylated histone is preferentially digested by DNase I. Nucleic Acids Res. 1978 Jun;5(6):1863–1876. doi: 10.1093/nar/5.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978 May;14(1):115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Simpson R. T. Structure of chromatin containing extensively acetylated H3 and H4. Cell. 1978 Apr;13(4):691–699. doi: 10.1016/0092-8674(78)90219-2. [DOI] [PubMed] [Google Scholar]
- Sippel A. E., Land H., Lindenmaier W., Nguyen-Huu M. C., Wurtz T., Timmis K. N., Giesecke K., Schütz G. Cloning of chicken lysozyme structural gene sequences synthesized in vitro. Nucleic Acids Res. 1978 Sep;5(9):3275–3294. doi: 10.1093/nar/5.9.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sures I., Gallwitz D. Histone-specific acetyltransferases from calf thymus. Isolation, properties, and substrate specificity of three different enzymes. Biochemistry. 1980 Mar 4;19(5):943–951. doi: 10.1021/bi00546a019. [DOI] [PubMed] [Google Scholar]
- Urban M. K., Franklin S. G., Zweidler A. Isolation and characterization of the histone variants in chicken erythrocytes. Biochemistry. 1979 Sep 4;18(18):3952–3960. doi: 10.1021/bi00585a017. [DOI] [PubMed] [Google Scholar]
- Urban M. K., Zweidler A. Changes in nucleosomal core histone variants during chicken development and maturation. Dev Biol. 1983 Feb;95(2):421–428. doi: 10.1016/0012-1606(83)90043-x. [DOI] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Bradbury E. M., Allfrey V. G. Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A. 1978 May;75(5):2239–2243. doi: 10.1073/pnas.75.5.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeponteau B., Landes G. M., Pankratz M. J., Martinson H. G. The chicken beta globin gene region. Delineation of transcription units and developmental regulation of interspersed DNA repeats. J Biol Chem. 1982 Sep 25;257(18):11015–11023. [PubMed] [Google Scholar]
- Villeponteau B., Lundell M., Martinson H. Torsional stress promotes the DNAase I sensitivity of active genes. Cell. 1984 Dec;39(3 Pt 2):469–478. doi: 10.1016/0092-8674(84)90454-9. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Galeazzi D., Schulman H. Acetylation and calcium-dependent phosphorylation of histone H3 in nuclei from butyrate-treated HeLa cells. J Biol Chem. 1983 Jan 25;258(2):1299–1304. [PubMed] [Google Scholar]
- Wiktorowicz J. E., Bonner J. Studies on histone acetyltransferase. Partial purification and basic properties. J Biol Chem. 1982 Nov 10;257(21):12893–12900. [PubMed] [Google Scholar]
- Wood W. I., Felsenfeld G. Chromatin structure of the chicken beta-globin gene region. Sensitivity to DNase I, micrococcal nuclease, and DNase II. J Biol Chem. 1982 Jul 10;257(13):7730–7736. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Yukioka M., Sasaki S., Qi S. L., Inoue A. Two species of histone acetyltransferase in rat liver nuclei. J Biol Chem. 1984 Jul 10;259(13):8372–8377. [PubMed] [Google Scholar]