Abstract
Background
Human papillomavirus (HPV) is an oncogenic virus causing oropharyngeal cancers and resulting in a favorable outcome after the treatment. The role of HPV in oral cavity squamous cell carcinoma (OSCC) remains ambiguous.
Objective
This study aimed to examine the effect of HPV infection on disease control among patients with OSCC following radical surgery with radiation-based adjuvant therapy.
Patients and Method
We prospectively followed 173 patients with advanced OSCC (96% were stage III/IV) who had undergone radical surgery and adjuvant therapy between 2004 and 2006. They were followed between surgery and death or up to 60 months. Surgical specimens were examined using a PCR-based HPV blot test. The primary endpoints were the risk of relapse and the time to relapse; the secondary endpoints were disease-free survival, disease-specific survival, and overall survival.
Results
The prevalence of HPV-positive OSCC was 22%; HPV-16 (9%) and HPV-18 (7%) were the genotypes most commonly encountered. Solitary HPV-16 infection was a poor predictor of 5-year distant metastases (hazard ratio, 3.4; 95% confidence interval, 1.4–8.0; P = 0.005), disease-free survival (P = 0.037), disease-specific survival (P = 0.006), and overall survival (P = 0.010), whereas HPV-18 infection had no impact on 5-year outcomes. The rate of 5-year distant metastases was significantly higher in the HPV-16 or level IV/V metastasis group compared with both the extracapsular spread or tumor depth ≥11-mm group and patients without risk factors (P<0.001).
Conclusions
HPV infections in advanced OSCC patients are not uncommon and clinically relevant. Compared with HPV-16-negative advanced OSCC patients, those with a single HPV-16 infection are at higher risk of distant metastases and poor survival despite undergoing radiation-based adjuvant therapy and require a more aggressive adjuvant treatment and a more thorough follow-up.
Introduction
Human papillomavirus (HPV) is a well-known oncogenic virus often observed in patients who have had a favorable outcome after the treatment of oropharyngeal cancers [1]–[11]. The causative mechanism is unclear but may be partially related to the radiosensitivity of the primary tumor or the less aggressive nature of tumors that are small at presentation (i.e., primary tumor [T]: T1–T2) [1], [8], [9], [11]. HPV-positive tumors usually coincide with more regional lymph node (N) metastases (i.e., N2–N3) [4], [7]–[9], [11], though some patients with HPV-positive tumors show fewer nodal metastases [6]. Analyses of failure patterns are important for the post-treatment surveillance of early disease recurrence because the only chance for survival in patients with recurrent tumors is the early detection of lesions that can serve as the targets of salvage therapy. The majority of previous studies have focused on the correlations between HPV infection and various measures of survival; few studies have addressed the failure patterns at local, regional, and distant sites [8], [11].
However, the role of HPV in oral cavity squamous cell carcinoma (OSCC) remains ambiguous because of the relatively small number of recorded OSCC patients in comparison with the larger population of oropharyngeal cancer [3], [6], [9], [10], [12]–[16]. In southern Asia, OSCC is an endemic cancer with an etiology that is distinct from that seen in the United States and Europe. Generally, OSCC patients with resectable tumors, but without distant metastasis, undergo radical surgery as the primary treatment in southern Asia. In the case of advanced OSCC (T4 lesion, lymph node metastasis, margin status of ≤4 mm] or extracapsular spread [ECS]), postoperative radiotherapy (RT) or concomitant chemoradiation therapy (CCRT) is used for adjuvant therapy [17], [18].
Several questions about the role of HPV in advanced OSCC patients who require adjuvant therapy following radical surgery in southern Asia remain unanswered. For example, what is the incidence of HPV infections among OSCC patients? Are the clinical and biological behaviors of HPV in OSCC the same as those in oropharyngeal SCC? Are different treatment strategies and follow-up protocols appropriate in HPV-positive OSCC patients? Does HPV infection affect the outcomes of postoperative adjuvant therapy? To answer these questions, we studied a large cohort of patients with previously untreated OSCC who underwent radical surgery with or without adjuvant therapy; in particular, we focused on the impact of HPV infections on the outcomes of radiation-based adjuvant therapy for advanced OSCC. Accordingly, this study aimed to test the hypothesis that HPV infections among advanced OSCC patients are associated with a decreased risk of disease relapse, including local recurrence, neck recurrence, and distant metastasis, and therefore improve the rates of survival, including disease-free survival (DFS), disease-specific survival (DSS), and overall survival (OS).
Materials and Methods
Patients
The Institutional Review Board at Chang Gung Memorial Hospital approved this study, which complied with the Declaration of Helsinki. All participants provided written informed consent. The inclusion criteria were as follows: a histological diagnosis of OSCC, the presence of a previously untreated tumor scheduled for radical surgery with neck dissection (ND), the absence of other suspected distant metastatic lesions detected by imaging, and a willingness to undergo imaging-guided biopsy or exploratory surgery if necessary. The exclusion criteria included a refusal or inability to undergo radical surgery.
Between 2004 and 2006, 333 patients were prospectively included in this study. All patients consented to and participated in the long-term outcome survey program of the Head and Neck Oncology Group at the Chang Gung Memorial Hospital. All participants underwent an extensive presurgical evaluation that included a medical history and a complete physical examination, flexible fiberoptic pharyngoscopy, a complete blood count, routine blood biochemistry, CT or MRI scans of the head and neck, chest radiographs, bone scans, and liver ultrasonography. Cancer staging was performed according to the 2002 American Joint Committee on Cancer 6th edition staging criteria [19].
All patients underwent radical excision of the primary tumor with ≥1 cm gross safety margins (both peripheral and deep margins). Classic radical or modified NDs (level I–V) were performed in the patients with clinically positive lymph node disease. Supra-omohyoid NDs (level I–III) were performed in clinically node-negative patients. Most of the uncomplicated patients underwent surgery alone except for those who unexpectedly had close margins ≤4 mm and/or positive lymph nodes as identified by pathological examinations. In this study, the subjects who underwent adjuvant therapy were considered as advanced OSCC patients. The indications for postoperative RT (60–66 Gy) included pathological T4 tumor, a positive lymph node, or a close margin ≤4 mm. ECS or multiple lymph node metastases were the reasons for the administration of CCRT with 50 mg/m2 cisplatin biweekly plus 800 mg daily oral tegafur and 60 mg leucovorin, or 30 mg/m2 weekly cisplatin [17], [18]. In the present study, 173 (52%) of the 333 OSCC patients underwent radical surgery followed by adjuvant therapy for advanced OSCC for the reasons stated above.
Clinicopathologic Characteristics
Patient data were extracted from medical records and classified according to our previously identified risk factors for OSCC, which were described in detail elsewhere [20]. The clinical and pathologic characteristics of interest included sex, age of disease onset, alcohol drinking, betel quid chewing, cigarette smoking, tumor subsite, differentiation, pathological T-status, pathological N-status, pathological stage, ECS, level IV/V metastases, treatment mode, and patient status at the last follow-up. Tumor subsite was determined by direct oral inspection and confirmed by pathological examination. Local recurrence was defined as a positive biopsy in the area of the primary tumor after a radical surgery as determined by a negative post-treatment screen. A neck recurrence was defined as a positive cytology/biopsy in the cervical lymphatic region after primary surgery. An incident distant metastasis was identified through biopsy or by imaging, as verified by our tumor board.
HPV Detection
Excised tumor samples were collected during radical surgery. DNA was extracted from paraffin-embedded tumor samples using a Lab Turbo 48 automatic nucleic acid extraction system and a Lab Turbo Virus Mini Kit LVN500 (Taigen, Taipei, Taiwan). Finally, 50 µL of DNA solution was eluted, and 1 µL was used as the PCR template. HPV infection was diagnosed in subjects using PCR on the HPV L1 gene. HPV DNA was amplified with MY11/biotinylated GP6+ primers, which targeted the L1 region and produced a 192-bp DNA fragment. The PCR reaction volume was 25 µL, which included a 2-µL aliquot of purified DNA. In the positive cases, the HPV L1 gene was genotyped using an HPV Blot kit (EasyChip™, King Car Ltd., Yilan, Taiwan) that can differentiate the 39 HPV types (HPV 6, 11, 16, 18, 26, 31, 32, 33, 35, 37, 39, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 72, 74, 82, CP8061, CP8304, L1AE5, MM4, MM7, and MM8). HPV type-specific probes were immobilized on a nylon membrane, which was used for reverse blot hybridization to detect HPV DNA in a single assay. The HPV types were determined using a visual assessment protocol provided by the manufacturer [21]–[23].
Study Endpoints
The primary endpoint was time to disease relapse including local recurrence, neck recurrence, and distant metastasis. The secondary endpoints were DFS, DSS, and OS. DFS was calculated as the date of primary surgery to the date of disease relapse. DSS was calculated as the date of primary surgery to the date of death caused by a disease recurrence, and OS was defined as the time period between primary surgery and death caused by any reason.
Statistical Analysis
Follow-up visits continued until December 2011. All patients received follow-up examinations for at least 60 months after surgery or until death. The procedure used for selecting the optimal cutoff values for clinicopathological factors has been previously described (20). Five-year local control, neck control, distant metastasis, DFS, DSS, and OS rates were computed using the Kaplan-Meier method (log-rank test). Univariate and multivariate analyses were used to identify independent predictors of 5-year outcomes. Independent prognostic factors were identified using multivariate Cox regression analysis with a forward selection procedure. Statistical analyses were performed using the SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA). A two-sided P value <0.05 was considered statistically significant.
Results
Characterization of Patients
During the study period, we recruited 333 OSCC patients (316 males and 17 females; mean age at onset, 51 years; age range, 25–83 years). A total of 240 patients (72%) reported drinking alcohol, 284 (85%) reported chewing betel quid, and 290 (87%) reported smoking cigarettes. Nineteen patients (6%) underwent primary tumor excision only, and the other 314 (94%) underwent ND in addition to primary tumor excision. The results of the pathological staging were as follows: pT1 (15%); pT2 (41%); pT3 (15%); pT4 (29%); pNx (6%); pN0 (55%); pN1 (12%); pN2b (24%); and pN2c (3%). When pNx (in the absence of ND) was classified as pN0, the pathological stages were as follows: p-stage I (14%), p-stage II (29%), p-stage III (15%), and p-stage IV (43%). The 5-year control and survival rates for all of the OSCC patients were as follows: local control, 89%; neck control, 86%; distant metastases, 12%; DFS, 73%; DSS, 79%; and OS, 66%.
Among the 333 patients, 52% had advanced OSCC and underwent radical surgery followed by adjuvant therapy (RT, n = 81; CCRT, n = 92), whereas 48% were the uncomplicated OSCC patients who underwent surgery alone. The tumor aggressiveness that was observed in the advanced OSCC patients was distinctively different from that of the uncomplicated OSCC patients in terms of pathological T-status (pT3-4: 70% vs. 30%, P<0.001), pathological N-status (pN1-2: 72% vs. 4%, P<0.001), pathological stage (p-stage III-IV: 96% vs. 17%, P<0.001), and ECS (positive: 45% vs. 2%, P<0.001). The 5-year control/survival rates were significantly worse among the advanced OSCC patients compared with those of the uncomplicated OSCC patients: local control (84% vs. 93%, P = 0.003), neck control (81% vs. 92%, P = 0.007), distant metastases (22% vs. 2%, P<0.001), DFS (60% vs. 87%, P<0.001), DSS (65% vs. 93%, P<0.001), and OS (50% vs. 83%, P<0.001).
The median duration of follow-up for the advanced OSCC patients was 58 months (mean, 47 months; range, 2–95 months). At the time of the analysis, 81 of the 173 patients (47%) were alive, and 92 (53%) were dead (59 due to the primary cancer, 20 due to other cancers, and 13 due to non-cancer causes). Twenty-five patients (15%) developed local recurrences, 31 (18%) had neck recurrences, and 35 (20%) experienced distant metastases. A total of 47 patients (27%) exhibited local and/or neck recurrence, salvage therapy was performed in 29 individuals (62%) and 21 (72%) dead at the time of the analysis.
HPV Status, Disease Relapse, and Survival
The overall prevalence of HPV infection among the OSCC patients was 21.3% (n = 71), and the 3 most common genotypes, including single and multiple infections, were as follows: HPV-16 (9.6%, n = 26), HPV-18 (7.8%, n = 23), and HPV-52 (2.4%, n = 6). The proportion of HPV-positive cases among the advanced OSCC patients was similar to that among the uncomplicated OSCC patients (22.0% vs. 20.6%, P = 0.765). The distributions of HPV-16 (9.2% vs. 6.3%), HPV-18 (6.9% vs. 6.9%), and HPV-52 (0.6% vs. 3.1%) were similar in both groups (all P>0.05).
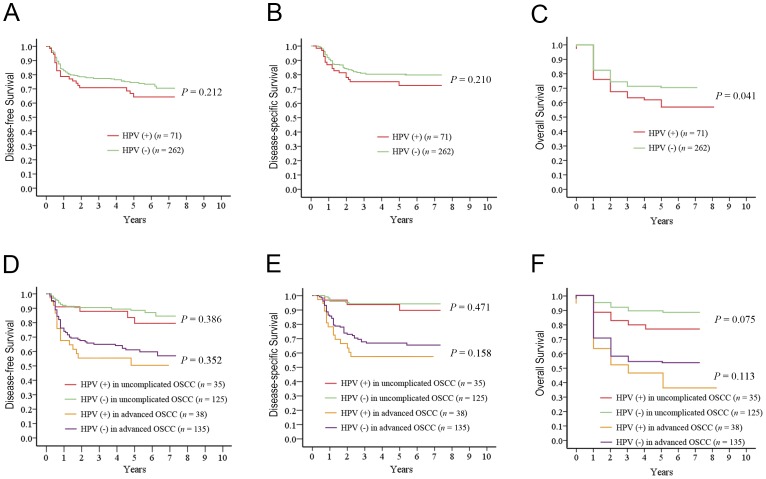
We calculated the 5-year DFS, DSS, and OS rates among the OSCC patients according to HPV status. Compared with the HPV-negative patients, those with HPV-positive tumors had similar rates of DFS (P = 0.212; Fig. 1A) and DSS (P = 0.210; Fig. 1B), but had a less favorable OS (P = 0.041; Fig. 1C) regardless of the treatment modality. When the entire cohort was further analyzed according to the need for treatment, the impact of HPV on 5-year DFS and DSS was not significant in both the uncomplicated and advanced groups (all P>0.05; Fig. 1D & Fig. 1E). Among the uncomplicated and advanced OSCC patients, the HPV-positive cases seemed to have a shorter time to death than those without detectable HPV, although these difference were not statistically significant (P = 0.075 & 0.112, respectively; Fig. 1F).
Figure 1. Five-year survivals by HPV status for patients with OSCC.
A, DFS by HPV status for the entire population; B, DSS by HPV status for the entire population; C, OS by HPV status for the entire population; D, DFS by HPV status for uncomplicated and advanced OSCC patients; E, DSS by HPV status for uncomplicated and advanced OSCC patients; F, OS by HPV status for uncomplicated and advanced OSCC patients.
To shed more light on the influence of the HPV genotype on the study endpoints, we further classified the study participants according to different HPV statuses (i.e., solitary HPV-infection, solitary HPV-18 infection, HPV-16 and/or HPV-18 infection, high-risk HPV infection, and HPV infection). The 5-year outcomes were calculated in both the advanced OSCC group and in the uncomplicated OSCC group. There were no significant differences in time-to-recurrence or time-to-death among the HPV subgroups in the uncomplicated OSCC group (all P>0.05; data not shown). Table 1 shows that solitary HPV-16 infection was associated with a significantly higher rate of distant metastases (56% vs. 19%, P = 0.007), lower DSS (37% vs. 68%, P = 0.025) and lower OS (25% vs. 53%, P = 0.028) in advanced OSCC patients. HPV-16 and/or HPV-18 infection was significantly related to an increased rate of distant metastases (43% vs. 18%, P = 0.031). In contrast, solitary HPV-18 infection, high-risk HPV infection, and HPV infection (Fig. 1) did not show a statistically significant association with 5-year control and survival rates.
Table 1. HPV statuses and 5-year control and survival rates in advanced OSCC patients (n = 173).
| Characteristics | Local control | P | Neck control | P | Distant metastases | P | Disease-free survival | P | Disease-specificsurvival | P | Overallsurvival | P |
| (n, %) | (5-yr %, n event) | (5-yr %, n event) | (5-yr %, n event) | (5-yr %, n event) | (5-yr %, n event) | (5-yr %, nevent) | ||||||
| Solitary HPV-16 | 0.774 | 0.726 | 0.007 | 0.058 | 0.025 | 0.028 | ||||||
| No (157, 91) | 84 (23) | 82 (28) | 19 (28) | 62 (61) | 68 (50) | 53 (80) | ||||||
| Yes (16, 9) | 87 (2) | 77 (3) | 56 (7) | 38 (9) | 37 (9) | 25 (12) | ||||||
| Solitary HPV-18 | 0.797 | 0.416 | 0.685 | 0.955 | 0.996 | 0.602 | ||||||
| No (161, 93) | 86 (23) | 81 (30) | 20 (32) | 60 (65) | 66 (55) | 46 (87) | ||||||
| Yes (12, 7) | 83 (2) | 92 (1) | 25 (3) | 58 (5) | 67 (4) | 58 (5) | ||||||
| HPV-16 and/or HPV18 | 0.378 | 0.634 | 0.031 | 0.125 | 0.057 | 0.088 | ||||||
| No (143, 83) | 86 (20) | 81 (27) | 18 (25) | 62 (55) | 69 (45) | 49 (73) | ||||||
| Yes (30, 17) | 83 (5) | 87 (4) | 43 (10) | 50 (15) | 53 (14) | 37 (19) | ||||||
| High-risk HPV | 0.361 | 0.653 | 0.147 | 0.188 | 0.078 | 0.073 | ||||||
| No (137, 79) | 86 (19) | 81 (26) | 18 (25) | 61 (53) | 69 (43) | 50 (69) | ||||||
| Yes (36, 21) | 83 (6) | 86 (5) | 28 (10) | 53 (17) | 56 (16) | 36 (23) | ||||||
| HPV | 0.512 | 0.528 | 0.230 | 0.352 | 0.158 | 0.113 | ||||||
| No (135, 78) | 86 (19) | 81 (26) | 19 (25) | 61 (53) | 68 (43) | 50 (68) | ||||||
| Yes (38, 22) | 84 (6) | 87 (5) | 27 (10) | 55 (17) | 58 (16) | 37 (24) |
HPV: human papillomavirus. OSCC: oral squamous cell carcinoma. n: patient number. W-M: Well-moderate.
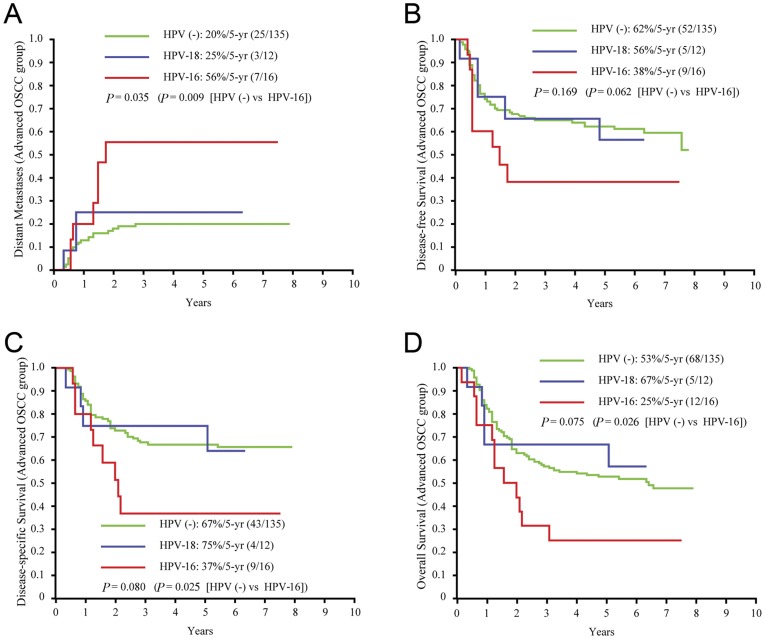
To further comparison, we further divided HPV infection into 3 subgroups according to genotype frequency (i.e., HPV-negative [n = 135], solitary HPV-16 infection [n = 16], and solitary HPV-18 infection [n = 12). Among the advanced OSCC patients, single HPV-16 infection was unrelated to local control or neck recurrence. Solitary HPV-16 infection was associated with a higher rate of distant metastases than the HPV-negative status (56% vs. 20%, P = 0.009; Fig. 2A). Despite postoperative adjuvant therapy, HPV-16 infection seemed to have a negative, although not statistically significant, impact on DFS compared with HPV-negative status (38% vs. 62%, P = 0.062; Fig. 2B). Moreover, advanced OSCC patients with HPV-16 infection had significantly lower DSS and OS rates than HPV-negative subjects (DSS: 37% vs. 67%, P = 0.025; OS: 25% vs. 53%, P = 0.026; Fig. 2C & Fig. 2D). Nevertheless, the differences in time-to-relapse and time-to-death between HPV-18 and HPV-negative cases were not statistically significant (Fig. 2).
Figure 2. Five-year outcomes by HPV subgroups for patients with advanced OSCC.
A, time to distant metastases; B, DFS; C, DSS; D, OS.
Table 2 demonstrates the clinical/pathological characteristics associated with lack of HPV, HPV-16, and HPV-18 infections among the advanced OSCC patients. The patients who were HPV-positive (with either HPV-16 or HPV-18) had a higher rate of poor differentiation than those who were HPV-negative (P = 0.013). The HPV-18 group seemed to have a slightly lower rate of pT3-4 than both the HPV-negative and HPV-16 groups (P = 0.069). The HPV-16 group seemed to have a higher rate of distant metastases than the HPV-negative and HPV-18 groups (P = 0.064). The remaining endpoints (i.e., local recurrence, neck recurrence, relapse, secondary primary tumors, death, and cause of death) did not differ significantly among the study groups (all P>0.05).
Table 2. Clinicopathological characteristics of patients with advanced OSCC according to their HPV status.
| Characteristic | All cases (n = 163)a | HPV (-) (n = 135) | Solitary HPV-16(n = 16) | Solitary HPV-18(n = 12) | P |
| n (%) | n (%) | n (%) | n (%) | ||
| Sex | 0.707 | ||||
| Male | 156 (96) | 129 (96) | 15 (94) | 12 (100.0) | |
| Female | 7 (4) | 6 (4) | 1 (6) | 0 | |
| Age of disease onset (years) | 0.921 | ||||
| ≤40 | 16 (10) | 13 (10) | 2 (13) | 1 (8) | |
| >40 | 147 (90) | 122 (90) | 14 (87) | 11 (92) | |
| Alcohol drinking | 0.715 | ||||
| No | 43 (26) | 37 (27) | 4 (25) | 2 (17) | |
| Yes | 120 (74) | 98 (73) | 12 (75) | 10 (83) | |
| Betel quid chewing | 0.669 | ||||
| No | 21 (13) | 18 (13) | 1 (6) | 2 (17) | |
| Yes | 142 (87) | 117 (87) | 15 (94) | 10 (83) | |
| Cigarette smoking | 0.550 | ||||
| No | 22 (14) | 20 (15) | 1 (6) | 1 (8) | |
| Yes | 141 (86) | 115 (85) | 15 (94) | 11 (92) | |
| Tumor subsite | 0.952 | ||||
| Tongue | 42 (26) | 34 (25) | 5 (31) | 3 (25) | |
| Mouth floor | 7 (4) | 6 (4) | 1 (6) | 0 | |
| Lip | 1 (1) | 1 (1) | 0 | 0 | |
| Buccal | 60 (37) | 50 (37) | 5 (31) | 5 (42) | |
| Gum | 35 (22) | 28 (21) | 5 (31) | 2 (17) | |
| Hard palate | 5 (3) | 4 (3) | 0 | 1 (8) | |
| Retromolar | 13 (8) | 12 (9) | 0 | 1 (8) | |
| Differentiation | 0.013 | ||||
| Well/moderate | 150 (92) | 128 (95) | 13 (81) | 9 (75) | |
| Poor | 13 (8) | 7 (5) | 3 (19) | 3 (25) | |
| Pathological T-status | 0.069 | ||||
| pT1-2 | 20 (31) | 37 (27) | 6 (38) | 7 (58) | |
| pT3-4 | 113 (69) | 98 (73) | 10 (62) | 5 (42) | |
| Pathological N-status | 0.901 | ||||
| pN0-1 | 77 (48) | 64 (48) | 8 (50) | 5 (42) | |
| pN2 | 85 (52) | 70 (52) | 8 (50) | 7 (58) | |
| Pathological stage | 0.832 | ||||
| p-stage I–III | 36 (22) | 31 (23) | 3 (19) | 2 (17) | |
| p-stage IV | 127 (78) | 104 (77) | 13 (81) | 10 (83) | |
| Extracapsular spread | 0.897 | ||||
| No | 88 (54) | 74 (55) | 8 (50) | 6 (50) | |
| Yes | 75 (46) | 61 (45) | 8 (50) | 6 (50) | |
| Level IV/V metastases | 0.563 | ||||
| No | 155 (95) | 128 (95) | 16 (100) | 11 (92) | |
| Yes | 8 (5) | 7 (5) | 0 | 1 (8) | |
| Treatment mode | 0.652 | ||||
| Surgery plus RT | 74 (45) | 62 (46) | 8 (50) | 4 (33) | |
| Surgery plus CCRT | 89 (55) | 73 (54) | 8 (50) | 8 (67) | |
| Local recurrence | 0.942 | ||||
| No | 141 (86) | 117 (87) | 14 (87) | 10 (83) | |
| Yes | 22 (14) | 18 (13) | 2 (13) | 2 (17) | |
| Neck recurrence | 0.645 | ||||
| No | 133 (82) | 109 (81) | 13 (81) | 11 (92) | |
| Yes | 30 (18) | 26 (19) | 3 (19) | 1 (8) | |
| Distant metastases | 0.064 | ||||
| No | 128 (78) | 110 (81) | 9 (56) | 9 (75) | |
| Yes | 35 (22) | 25 (19) | 7 (44) | 3 (25) | |
| Relapse | 0.392 | ||||
| No | 97 (59) | 83 (61) | 7 (44) | 7 (58) | |
| Yes | 66 (41) | 52 (39) | 9 (56) | 5 (42) | |
| Secondary primary tumors | 0.351 | ||||
| No | 124 (76) | 102 (76) | 11 (69) | 11 (92) | |
| Yes | 39 (24) | 33 (24) | 5 (31) | 1 (8) | |
| Death | 0.132 | ||||
| No | 78 (48) | 67 (50) | 4 (25) | 7 (58) | |
| Yes | 85 (52) | 68 (50) | 12 (75) | 5 (42) | |
| Cause of death | 0.316 | ||||
| Primary cancer | 56 (34) | 43 (32) | 9 (56) | 4 (33) | |
| Other cancers | 17 (10) | 16 (12) | 1 (6) | 0 (0) | |
| Noncancer causes | 12 (7) | 9 (7) | 2 (13) | 1 (8) |
OSCC: oral squamous cell carcinoma. HPV: human papillomavirus. n: number of patients. HPV (-): HPV-negative. T: primary tumor. N: regional lymph node. RT: radiotherapy. CCRT: concomitant chemoradiation therapy.
All cases comprised HPV (-), solitary HPV-16, and solitary HPV-18 patients.
Combining HPV-16 with Traditional Prognostic Factors in Advanced OSCC Patients
Compared with the HPV-16-negative patients using the univariate analysis, the advanced OSCC patients with solitary HPV-16 infections had a significantly higher rate of distant metastases (56% vs. 19%, P = 0.007) and markedly lower DSS and OS rates (DSS: 37% vs. 68%, P = 0.025; OS: 25% vs. 53%, P = 0.028). The local control and neck control status were similar in both groups (local control: 87% vs. 84%, P = 0.774; neck control: 77% vs. 82%, P = 0.726). The multivariate analyses of important risk factors, including solitary HPV-16 infection, pN2, level IV/V metastases, ECS, tumor depth ≥11 mm, and lymphatic invasion, are shown in Table 3. Solitary HPV-16 infection was a significant independent predictor of 5-year distant metastases (hazard ratio [HR], 3.4; 95% confidence interval [CI], 1.4–8.0; P = 0.005), DFS (HR, 2.1; 95% CI, 1.0–4.3; P = 0.037), DSS (HR, 2.8; 95% CI, 1.4–5.8; P = 0.006), and OS (HR, 2.3; 95% CI, 1.2–4.3; P = 0.010). Other independent risk factors for 5-year distant metastases included neck level IV/V metastases, ECS, and tumor depth ≥11 mm. Except for pN2, HPV-16 and the other risk factors could independently predict 5-year OS. HPV-16, pN2, and level IV/V metastases were independent predictors of 5-year DFS, whereas these risk factors and tumor depth ≥11 mm were important risk factors of 5-year DSS.
Table 3. Multivariate analyses of 5-year control and survival rates in advanced OSCC patients (n = 173).
| Risk factors | Local control | Neck control | Distant metastasis | Disease-free survival | Disease-specific survival | Overall survival |
| P, HR (95%CI) | P, HR (95%CI) | P, HR (95%CI) | P, HR (95%CI) | P, HR (95%CI) | P, HR (95%CI) | |
| Solitary HPV-16 (n = 16 ) | ns | ns | 0.005, 3.4 (1.4–8.0) | 0.037, 2.1 (1.0–4.3) | 0.006, 2.8 (1.4–5.8) | 0.010, 2.3 (1.2–4.3) |
| pN2 (n = 88) | ns | 0.011, 2.9 (1.3–6.7) | ns | 0.001, 2.5 (1.5–4.1) | <0.001, 3.2 (1.8–5.7) | ns |
| Level IV/V metastasis(n = 8 ) | ns | 0.026, 3.4 (1.2–10.2) | <0.001, 5.2 (2.1–13.3) | 0.024, 2.75 (1.1–6.5) | 0.042, 2.5 (1.0–6.0) | 0.034, 2.4 (1.1–5.6) |
| Extracapsular spread(n = 78) | ns | ns | <0.001, 4.7 (2.1–10.6) | ns | ns | 0.001, 2.2 (1.4–3.5) |
| Tumor depth ≥11 mm(n = 103) | ns | ns | 0.009, 3.1 (1.3–7.1) | ns | 0.001, 2.8 (1.5–5.2) | 0.012, 1.8 (1.1–2.8) |
| Lymphatic invasion(n = 13) | ns | ns | ns | ns | ns | 0.049, 1.9 (1.0–3.7) |
OSCC: oral squamous cell carcinoma. n: patient number. HR: hazard ratio. CI: confidence interval. HPV: human papillomavirus. ns, not significant. pN: pathological lymph node metastases.
Table 4 presents the demographic, clinical, pathological, and therapeutic characteristics of the 16 advanced OSCC patients who were infected with HPV-16. Eight of these patients underwent CCRT due to ECS (100%) and had significantly higher risks of distant metastases than those that underwent RT (75% vs. 13%, P = 0.041). Moreover, 75% of the 16 patients who were dead at the end of study had died of the following causes, by decreasing order of frequency: disease (56%), other cancers (6%), or other reasons (13%). Accordingly, the major impact of HPV-16 on 5-year survival was due to the failure of treatment at distant sites resulting in death; DSS in patients with distant metastases was significantly lower than that in patients without distant metastasis (22% vs. 100%, P = 0.003). Of note, none of the 16 patients had level IV/V metastases (Tables 1 and 3).
Table 4. Demographic, clinical, pathological, and therapeutic characteristics of the advanced OSCC patients with solitary HPV-16 infections.
| No. | Sex | Age (y) | Oral habit | Site | p-Stage | ECS | Level IV/V metastases | Tumor depth (mm) | Adjuvant therapy | Interval between primary surgery and relapse (mo) | ||||
| TR (mo) | NR (mo) | DM (mo) | Salvage | Outcomes (mo) | ||||||||||
| 1 | m | 58 | a, b, c | buccal | T3N1 | - | - | 1 | RT | DOO, 2 | ||||
| 2 | m | 58 | a, b, c | gum | T4N2b | + | - | 35 | CCRT | 7 | – | DOD, 7 | ||
| 3 | m | 51 | b, c | buccal | T4N1 | - | - | 25 | RT | 6 | – | DOD, 8 | ||
| 4 | m | 25 | a, b, c | gum | T4N2b | + | - | 12 | CCRT | 7 | – | DOD, 8 | ||
| 5 | m | 47 | a, b, c | tongue | T4N1 | - | - | 13 | RT | 5 | CCRT | DOD, 14 | ||
| 6 | m | 62 | a, b, c | buccal | T4N0 | - | - | 50 | RT | DOO, 15 | ||||
| 7 | m | 73 | a, c | tongue | T2N2b | + | - | 6 | CCRT | 7 | 8 | CCRT | DOD,15 | |
| 8 | m | 69 | b, c | buccal | T2N2b | + | - | 22 | CCRT | 18 | – | DOD, 19 | ||
| 9 | m | 31 | a, b, c | gum | T2N2b | + | - | 9 | CCRT | 7 | 18 | CCRT | DOD, 24 | |
| 10 | m | 47 | b, c | buccal | T2N1 | + | - | 14 | CCRT | 15 | 16 | – | DOD, 25 | |
| 11 | m | 60 | a, b, c | gum | T4N0 | - | - | 13 | RT | 21 | – | DOD, 26 | ||
| 12 | m | 47 | a, b, c | tongue | T4N0 | - | - | 12 | RT | DOC, 37 | ||||
| 13 | m | 69 | a, b, c | gum | T4N2c | + | - | 10 | CCRT | NER, 61 | ||||
| 14 | m | 45 | a, b, c | mouth floor | T2N2b | + | - | 2 | CCRT | NER, 70 | ||||
| 15 | f | 54 | B | tongue | T2N2b | - | - | 13 | RT | NER, 79 | ||||
| 16 | m | 47 | a, b, c | tongue | T3N0 | - | - | 24 | RT | NER, 90 | ||||
OSCC, oral squamous cell carcinoma; HPV, human papillomavirus; m, male; f, female; a, alcohol drinking; b, betel nut chewing; c, cigarette smoking; p, pathological; ECS, extracapsular spread; mo: month; TR, tumor recurrence; NR, neck recurrence; DM, distant metastases; RT, radiotherapy; CCRT, concomitant chemoradiation; DOO, died of other reason; DOC, died of other cancer; DOD, died of disease; NER, no evidence of recurrence.
Post Hoc Analyses for 5-year Distant Metastasis Rates in Advanced OSCC Patients
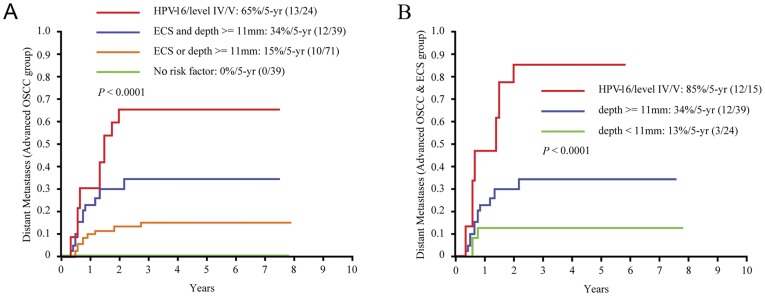
Using level IV/V metastases, solitary HPV-16 infection, ECS, and tumor depth ≥11 mm as independent predictors, we created four subgroups (5-year distant metastases: 83%, 56%, 38%, and 30%, respectively): an “HPV-16 or level IV/V metastases” group (n = 24), a “both ECS and tumor depth ≥11 mm” group (n = 39), an “ECS or tumor depth ≥11 mm” group (n = 71), and a “no risk factor” group (n = 39). The 5-year distant metastatic rate was higher in the HPV-16 or level IV/V group than in the ECS and tumor depth ≥11 mm group (P = 0.075), the ECS or tumor depth ≥11 mm group (P<0.001), and the no risk factor group (P<0.001) (Fig. 3A).
Figure 3. Five-year distant metastases in patients with advanced OSCC.
A, distant metastases by newly classified subgroups in the advanced OSCC patients; B, distant metastases according to the new risk stratification in advanced OSCC patients with ECS.
Among the 173 advanced OSCC patients, 78 had ECS diseases; these ECS patients were divided in 3 subgroups based on the occurrence of 5-year distant metastases (event/n): the HPV-16 (6/8) or level IV/V (6/7) group, the tumor depth ≥11 mm group (12/39), and the tumor depth <11 mm group (3/24). The 5-year distant metastasis rates of the ECS patients were higher in the HPV-16 or level IV/V group than in the tumor depth ≥11 mm group (P = 0.004, HR = 3.2, 95% CI = 1.4–7.3) and the tumor depth <11 mm group (P<0.001, HR = 9.3, 95% CI = 2.6–33.4) (Fig. 3B).
Discussion
The null hypothesis of the present study was rejected, that is, HPV infections did not decrease the risk of disease relapse and was not associated with a better survival among advanced OSCC patients undergoing radical surgery and radiation-based adjuvant therapy (Fig. 1 & Fig. 2). By contrast, advanced OSCC patients with a solitary HPV-16 infection were 3 times more likely to develop distant metastases and were 2–3 times more likely to die earlier (including those who died due to DFS, DSS, or OS) compared with HPV-negative patients (Table 3). Among the OSCC patients, including uncomplicated and advanced cases, the prevalence of HPV infection was 21.3%. The HPV-positive patients had similar rates of DFS and DSS, but a lower OS rate, compared with the HPV-negative patients (Fig. 1). Radical surgery seemed to be sufficiently effective for uncomplicated OSCC regardless of the HPV status, whereas radiation-based adjuvant therapy was unsatisfactory in treating HPV-16-positive advanced OSCC. In contrast to HPV-18 infection, HPV-16 infection had a negative impact on distant metastases, DFS, DSS, and OS despite immediately postoperative adjuvant therapy (Fig. 2).
Our findings are surprisingly different from the recent HPV-related outcome surveys in the field of head and neck cancers that have shown positive clinical impacts of HPV on DSS or OS [1]–[10], [13], [14], [24], [25]. Moreover, only a few studies have assessed failure patterns and time to relapse [1], [8], [9], especially with regard to cancers of the oral cavity [9]. This lack of attention to OSCC might be because some studies had relatively lower rates of detecting HPV (<10%), small samples, or included patients with cancers in different subsites (i.e., both the oral cavity and oropharynx) [3], [4], [10], [12]–[15], [24], [26]–[28]. For this reason, different treatment modalities were used (surgery vs. RT/CCRT), ultimately leading to different outcomes. Other possible explanations are that the enrolled patients came from different regions and were exposed to different carcinogens (e.g., betel quid chewing), different cultural norms (e.g., habitual oral sex behavior) and different genetic backgrounds (e.g., HLA typing) [29].
The evidence regarding the clinical impact of HPV in OSCC patients remains inconclusive [6], [12], [13], [28], [30]. HPV-seropositive heavy smokers or heavy drinkers are at a significantly higher risk of having OSCC than HPV-seronegative heavy smokers or drinkers [30]. Accordingly, it is reasonable that most of the HPV-positive OSCC patients smoke cigarettes and/or drink alcohol (Table 2). Based on Maxwell’s findings, cigarette smoking remarkably increases the risk of local recurrence and distant metastases among HPV-positive oropharyngeal cancer [31]. Smoking may induce genetic mutations, which facilitate the integration of HPV DNA into the host genome, and causes somatic gene errors. The sophisticated relationships among these oncogenic agents, including tobacco, smoking, and HPV, and tumor control are particularly difficult to clarify when most of the advanced OSCC patients within Taiwan have been exposed to tobacco and/or alcohol regardless of the HPV status. However, HPV-16 infection is an important, independent predictor of worse outcome among advanced OSCC patients, even those who underwent extensive operations and received adjuvant therapy. To our knowledge, longstanding betel quid chewing can damage the HPV-infected epithelia of the oral cavity and can potentially lead to a significant accumulation of chemicals, which may also influence the carcinogenic effect of HPV, and probably results in clinical and biological discriminations between HPV-positive OSCC and HPV-positive oropharyngeal cancer. Therefore, additional studies are needed to examine the significance of HPV infection in the presence or absence of betel nut chewing; this knowledge may be helpful to elucidate the genetic alterations and the molecular pathways that may underlie the observed survival differences.
HPV-18 infection is uncommon (<10% of all HPV infections) in oropharyngeal cancer [11] but is frequently found (32%, n = 12) in advanced OSCC. However, there was no difference in relapse or survival between patients with and without solitary HPV-18 infections. In this context, we further focused our study on HPV-16. Previous studies have reported that HPV-positive oropharyngeal cancers are associated with poorly differentiated histology, T1–T2 disease, N2–N3, and radiosensitivity [1]–[11]. By contrast, we observed that HPV-16-positive advanced OSCC cases have a similar status in terms of T-staging/N-staging and a remarkably higher incidence of distant metastases within two years of radical surgery (56%, 7/16) compared with HPV-negative cases. Even after adjuvant therapy, the advanced OSCC patients with solitary HPV-16 infection still had a relative higher risk of early distant metastases (Fig. 2A). We believe that the possible survival benefit of HPV-16 might be diminished by oral habits or reduced by surgery; however, early diagnosis and adequate radical surgery are still the most important measures in the control of OSCC tumors.
In addition to solitary HPV-16 infection, we further showed that level IV/V metastases, ECS, and tumor depth ≥11 mm are independent risk factors for 5-year distant metastases in advanced OSCC. Four subgroups of distant metastases were thus created (Fig. 3A). We previously demonstrated that OSCC patients with ECS have a higher potential for distant metastases than other groups of patients [32]. In this study, distant metastases were found in 6 of the 7 OSCC patients with level IV/V metastases. Among the HPV-16-positive advanced OSCC patients, six of 8 cases with ECS developed distant metastases (Table 3 and Fig. 3B). Accordingly, more intensive and specific treatments should be administered in OSCC patients with level IV/V metastases or HPV-16 with ECS, such as taxane-based chemotherapy regimens as an adjuvant strategy immediately following radical surgery; transitions to alternative, palliative treatments; biotherapy; and anti-angiogenesis strategies utilized during the postoperative recovery period [33], [34].
Several caveats of this study merit comment. First, a potential limitation of our report is the use of specific PCR assays for detecting the HPV L1 gene. Because PCR amplification of HPV DNA is a very sensitive technique, we ruled out the possibility of laboratory artifacts and the presence of environmental virions by performing all amplifications in duplicate and with the use of two different PCR assays. We acknowledge that reverse transcriptase-PCR assays for E6 and E7 transcripts may be more reliable for the detection of oncogenic HPV infections. Moreover, the lack of available tumor marker data (e.g., p16, p53, and epidermal growth factor receptor) does not allow us to draw any conclusion on the activity of viral oncogenes. To further characterize the possible mechanisms by which HPV-16 infections could be related to the risk of distant metastases and death, the measurement of E6 and E7 expression and HPV-associated biomarkers will be required in future studies.
In conclusion, HPV infection does not represent a favorable prognosticator in the OSCC patients who receive radical surgery regardless of radiation-based adjuvant therapy. Notably, the advanced OSCC patients with solitary HPV-16 infection require priority adjuvant treatment and follow-up due to increased risk of early distant metastases and death. Moreover, in particular, patients with level IV/V metastases or HPV-16 infection with ECS require a more intensive therapeutic protocol. Our findings suggest that different types of HPV infections present distinct clinical and biological challenges among advanced OSCC patients, and there is at least one such HPV-16 associated with unfavorable outcomes in individuals who have received conventional adjuvant treatment. The significance of HPV-16 infection should be further studied in future translational and clinical research.
Acknowledgments
We gratefully thank Mr. Rong-Gin Li and Miss Kwei-Hwei Chen for their technique support, and all members of the Head and Neck Oncology Group at Chang Gung Memorial Hospital who actively participated in OSCC collection.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grant DOH100-TD-C-111-006 (http://www.doh.gov.tw/) from the Department of Health, Taiwan and by grant CMRPG391431/391432 (http://www.cgmh.org.tw/) from the Chang Gung Memorial Hospital in Linkou, Taoyuan, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Friesland S, Mellin H, Munck-Wikland E, Nilsson A, Lindholm J, et al. Human papilloma virus (HPV) and p53 immunostaining in advanced tonsillar carcinoma–relation to radiotherapy response and survival. Anticancer Res. 2001;21:529–534. [PubMed] [Google Scholar]
- 3.Dahlgren L, Dahlstrand HM, Lindquist D, Högmo A, Björnestål L, et al. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int J Cancer. 2004;112:1015–1019. doi: 10.1002/ijc.20490. [DOI] [PubMed] [Google Scholar]
- 4.Na II, Kang HJ, Cho SY, Koh JS, Lee JK, et al. EGFR mutations and human papillomavirus in squamous cell carcinoma of tongue and tonsil. Eur J Cancer. 2007;43:520–526. doi: 10.1016/j.ejca.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Chien CY, Su CY, Fang FM, Huang HY, Chuang HC, et al. Lower prevalence but favorable survival for human papillomavirus-related squamous cell carcinoma of tonsil in Taiwan. Oral Oncol. 2008;44:174–179. doi: 10.1016/j.oraloncology.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Klozar J, Kratochvil V, Salakova M, Smahelova J, Vesela E, et al. HPV status and regional metastasis in the prognosis of oral and oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2008;265:S75–S82. doi: 10.1007/s00405-007-0557-9. [DOI] [PubMed] [Google Scholar]
- 7.Jo S, Juhasz A, Zhang K, Ruel C, Loera S, et al. Human papillomavirus infection as a prognostic factor in oropharyngeal squamous cell carcinomas treated in a prospective phase II clinical trial. Anticancer Res. 2009;29:1467–1474. [PMC free article] [PubMed] [Google Scholar]
- 8.Hong AM, Dobbins TA, Lee CS, Jones D, Harnett GB, et al. Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br J Cancer. 2010;103:1510–1517. doi: 10.1038/sj.bjc.6605944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laco J, Vosmikova H, Novakova V, Celakovsky P, Dolezalova H, et al. The role of high-risk human papillomavirus infection in oral and oropharyngeal squamous cell carcinoma in non-smoking and non-drinking patients: a clinicopathological and molecular study of 46 cases. Virchows Arch. 2011;458:179–187. doi: 10.1007/s00428-010-1037-y. [DOI] [PubMed] [Google Scholar]
- 10.Joo YH, Jung CK, Sun DI, Park JO, Cho KJ, et al. High-risk human papillomavirus and cervical lymph node metastasis in patients with oropharyngeal cancer. Head Neck. 2012;34:10–14. doi: 10.1002/hed.21697. [DOI] [PubMed] [Google Scholar]
- 11.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shima K, Kobayashi I, Saito I, Kiyoshima T, Matsuo K, et al. Incidence of human papillomavirus 16 and 18 infection and p53 mutation in patients with oral squamous cell carcinoma in Japan. Br J Oral Maxillofac Surg. 2000;38:445–450. doi: 10.1054/bjom.2000.0162. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz SR, Yueh B, McDougall JK, Daling JR, Schwartz SM. Human papillomavirus infection and survival in oral squamous cell cancer: a population-based study. Otolaryngol Head Neck Surg. 2001;125:1–9. doi: 10.1067/mhn.2001.116979. [DOI] [PubMed] [Google Scholar]
- 14.Sugiyama M, Bhawal UK, Kawamura M, Ishioka Y, Shigeishi H, et al. Human papillomavirus-16 in oral squamous cell carcinoma: clinical correlates and 5-year survival. Br J Oral Maxillofac Surg. 2007;45:116–122. doi: 10.1016/j.bjoms.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Machado J, Reis PP, Zhang T, Simpson C, Xu W, et al. Low prevalence of human papillomavirus in oral cavity carcinomas. Head Neck Oncol. 2010;2:6. doi: 10.1186/1758-3284-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Guily JL, Jacquard AC, Prétet JL, Haesebaert J, Beby-Defaux A, et al. Human papillomavirus genotype distribution in oropharynx and oral cavity cancer in France-The EDiTH VI study. J Clin Virol. 2011;51:100–104. doi: 10.1016/j.jcv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E, et al. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys. 1996;36:999–1004. doi: 10.1016/s0360-3016(96)00430-0. [DOI] [PubMed] [Google Scholar]
- 18.Wang HM, Wang CS, Chen JS, Chen IH, Liao CT, et al. Cisplatin, tegafur, and leucovorin: a moderately effective and minimally toxic outpatient neoadjuvant chemotherapy for locally advanced squamous cell carcinoma of the head and neck. Cancer. 2002;94:2989–2995. doi: 10.1002/cncr.10570. [DOI] [PubMed] [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM. AJCC cancer staging manual. 2002. 6th ed. New York: Springer-Verlag.
- 20.Liao CT, Chang JT, Wang HM, Ng SH, Hsueh C, et al. Analysis of risk factors predictive of local tumor control in oral cavity cancer. Ann Surg Oncol. 2008;15:915–922. doi: 10.1245/s10434-007-9761-5. [DOI] [PubMed] [Google Scholar]
- 21.Huang SL, Chao A, Hsueh S, Chao FY, Huang CC, et al. Comparison between the Hybrid Capture II test and an SPF1/GP6+ PCR-based assay for detection of human papillomavirus DNA in cervical swab samples. J Clin Microbiol. 2006;44:1733–1739. doi: 10.1128/JCM.44.5.1733-1739.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CY, Chen HC, Lin RW, You SL, You CM, et al. Quality assurance of genotyping array for detection and typing of human papillomavirus. J Virol Methods. 2007;140:1–9. doi: 10.1016/j.jviromet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Luo CW, Roan CH, Liu CJ. Human papillomaviruses in oral squamous cell carcinoma and pre-cancerous lesions detected by PCR-based gene-chip array. Int J Oral Maxillofac Surg. 2007;36:153–158. doi: 10.1016/j.ijom.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Ritchie JM, Smith EM, Summersgill KF, Hoffman HT, Wang D, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104:336–344. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 25.Zhao D, Xu QG, Chen XM, Fan MW. Human papillomavirus as an independent predictor in oral squamous cell cancer. Int J Oral Sci. 2009;1:119–125. doi: 10.4248/IJOS.09015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang XH, Lewis J, Foote R, Smith D, Kademani D. Prevalence and significance of human papillomavirus in oral tongue cancer: the Mayo Clinic experience. J Oral Maxillofac Surg. 2008;66:1875–1880. doi: 10.1016/j.joms.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Pathare SM, Gerstung M, Beerenwinkel N, Schäffer AA, Kannan S, et al. Clinicopathological and prognostic implications of genetic alterations in oral cancers. Oncol Lett. 2011;2:445–451. doi: 10.3892/ol.2011.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SY, Cho NH, Choi EC, Baek SJ, Kim WS, et al. Relevance of human papilloma virus (HPV) infection to carcinogenesis of oral tongue cancer. Int J Oral Maxillofac Surg. 2010;39:678–683. doi: 10.1016/j.ijom.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Herrero R, Castellsagué X, Pawlita M, Lissowska J, Kee F, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 30.Smith EM, Rubenstein LM, Haugen TH, Pawlita M, Turek LP. Complex etiology underlies risk and survival in head and neck cancer human papillomavirus, tobacco, and alcohol: a case for multifactor disease. J Oncol 2012: 571862 [Epub 2012 Jan 23] 2012. [DOI] [PMC free article] [PubMed]
- 31.Maxwell JH, Kumar B, Feng FY, Worden FP, Lee JS, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao CT, Wang HM, Chang JT, Ng SH, Hsueh C, et al. Analysis of risk factors for distant metastases in squamous cell carcinoma of the oral cavity. Cancer. 2007;110:1501–1508. doi: 10.1002/cncr.22959. [DOI] [PubMed] [Google Scholar]
- 33.Baghi M, Hambek M, May A, Radeloff A, Gstoettner W, et al. Adjuvant docetaxel, cisplatin and 5-fluorouracil (TPF) in locally advanced squamous cell carcinoma of the head and neck. Anticancer Res. 2006;26:559–563. [PubMed] [Google Scholar]
- 34.Rosenthal DI, Harris J, Forastiere AA, Weber RS, Ridge JA, et al. Early postoperative paclitaxel followed by concurrent paclitaxel and cisplatin with radiation therapy for patients with resected high-risk head and neck squamous cell carcinoma: Report of the Phase II trial RTOG 0024. J Clin Oncol. 2009;27:4727–4732. doi: 10.1200/JCO.2008.21.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]