CO2 is a critical signaling molecule in a variety of biological processes. The transcription factor Flo8 is identified as a key regulator of CO2 sensing, which governs CO2-induced phenotypic transitions in Candida albicans. These findings provide new insights into the understanding of CO2 sensing in pathogenic fungi.
Abstract
Physiological levels of CO2 have a profound impact on prominent biological attributes of the major fungal pathogen of humans, Candida albicans. Elevated CO2 induces filamentous growth and promotes white-to-opaque switching. However, the underlying molecular mechanisms of CO2 sensing in C. albicans are insufficiently understood. Here we identify the transcription factor Flo8 as a key regulator of CO2-induced morphogenesis in C. albicans by screening a gene null mutant library. We show that Flo8 is required for CO2-induced white-to-opaque switching, as well as for filamentous growth. Ectopic expression of FLO8 hypersensitizes C. albicans cells to the elevated CO2 levels. Furthermore, we demonstrate that CO2 signaling in C. albicans involves two pathways: the already reported cAMP/protein kinase A and another major one that is unidentified. The two pathways converge on the transcription factor Flo8, which is the master regulator of CO2 sensing in C. albicans and plays a critical role in regulation of white-to-opaque switching and filamentous growth. Our findings provide new insights into the understanding of CO2 sensing in pathogenic fungi that have important implications for higher organisms.
INTRODUCTION
To improve fitness, organisms must have the ability to sense and adapt to their natural environments. Carbon dioxide (CO2) is a key signal for most living organisms. Not only is this molecule a product of cellular respiration, but it is also involved in the regulation of many biological processes (Hetherington and Raven, 2005; Bahn and Muhlschlegel, 2006; Sharabi et al., 2009b). For instance, blood-feeding mosquitoes use environmental CO2 as a cue for prey-seeking behavior (Gillies and Wilkes, 1969), whereas fruit flies detect CO2 as an alarmone to elicit avoidance behavior (Turner and Ray, 2009). In animals, CO2/bicarbonate activates sperm maturation and motility (Esposito et al., 2004). Recent studies suggest that elevated CO2 levels modulate lifespan in Caenorhabditis elegans and Drosophila melanogaster (Sharabi et al., 2009a; Poon et al., 2010). The CO2 concentration in humans (4.5–30.0%) is >100 times higher than that in air (0.03%; Levitt and Bond, 1970), and it is now well established that CO2 regulates the expression of virulence determinants such as filamentation or capsule biosynthesis in fungal pathogens of humans (Mardon et al., 1969; Granger et al., 1985; Bahn et al., 2005; Bahn and Muhlschlegel, 2006; Klengel et al., 2005; Huang et al., 2006).
We previously showed that a high level of CO2 also promotes Candida albicans white-to-opaque switching, thus facilitating mating (Huang et al., 2009). White and opaque cells differ in many features, including colony and cellular appearances, mating competence, and virulence (Anderson and Soll, 1987; Kvaal et al., 1997, 1999; Miller and Johnson, 2002; Soll et al., 2003; Soll, 2004). To mate, C. albicans must undergo homozygosis at the mating type–like locus MTL and then switch from the white to the opaque form (Miller and Johnson, 2002).
CO2-induced filamentation in C. albicans involves the fungal adenylyl cyclase Cyr1 (also named Cdc35; Klengel et al., 2005; Hall et al., 2010); however, CO2-induced promotion of white-to-opaque switching is independent of this enzyme, as we recently showed (Huang et al., 2009). In fact, the CYR1-null mutant showed white-to-opaque switching frequencies that were similar to those of the wild-type control when exposed to high levels of CO2 (20%; Huang et al., 2009). Recently we found that the Ras1-Cyr1 cAMP/protein kinase A (PKA) pathway is the major pathway regulating N-acetylglucosamine (GlcNAc)–induced white-to-opaque switching (Huang et al., 2010). Of interest, CO2 and GlcNAc showed a synergistic effect in promoting opaque cell formation (Huang et al., 2010), suggesting that the major pathways involved in CO2- and GlcNAc-induced white-to-opaque switching are different.
In spite of the importance of CO2 in morphogenesis and pathogenesis, it is unclear how CO2 is sensed and how the signal is transduced in C. albicans. In this study, we identify the transcription factor Flo8 as a master regulator of CO2-induced morphogenesis in C. albicans by screening a null mutant library for nearly 200 genes with combined analysis of white-to-opaque switching and filamentous growth. Flo8, a LisH domain–containing protein, has been reported to be essential for filamentous growth and virulence in C. albicans (Cao et al., 2006). Here we find that Flo8 is required for CO2-induced white-to-opaque switching, as well as for filamentous growth, via different downstream regulators. Further experiments demonstrate that Wor1 and Wor2 function downstream of Flo8 and are essential for CO2-induced white-to-opaque switching. Efg1 and Hgc1 are required for CO2-induced filamentous growth. We also provide evidence indicating that the adenylyl cyclase Cyr1 is not essential for the activating function of Flo8, suggesting the presence of a major, Cyr1-independent, CO2-sensing pathway.
RESULTS
Roles of adenylyl cyclase and high-affinity cyclical AMP phosphodiesterase in CO2-regulated white-to-opaque switching
The cells of the cyr1/cyr1 mutant have no detectable cAMP levels (Rocha et al., 2001), whereas the cells of the mutant of PDE2 gene, encoding the high-affinity cyclical AMP phosphodiesterase gene in C. albicans, show constitutively high levels of cAMP (Jung and Stateva, 2003). These two mutants respectively represent the inactivated and activated states of cAMP signaling. The switching frequencies can be easily quantified because of the bistable feature of the white-to-opaque transition (Slutsky et al., 1987; Huang et al., 2006). Therefore the CO2-induced responses can be indicated by the increased fold of switching frequencies even in the mutants with varied basal levels of switching ability.
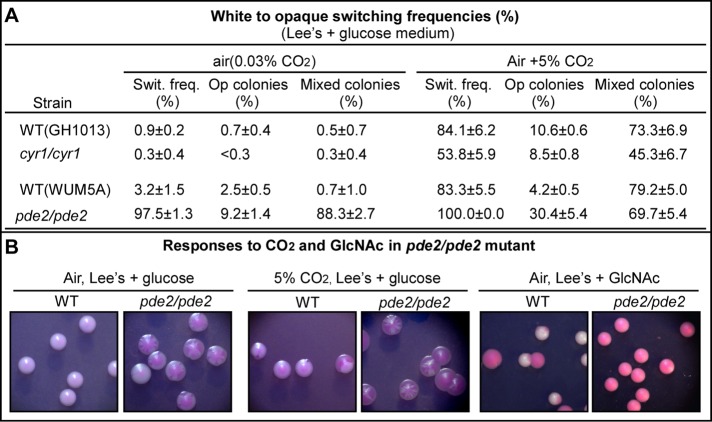
To evaluate the role of cAMP signaling in CO2 sensing, we first tested the responses to 5% CO2 in the cyr1/cyr1 mutant by using white-to-opaque phenotypic switching assay. Here the switching frequency was defined as the percentage of the colonies containing cells of alternative phenotype. The switching frequencies are shown in Figure 1A. Consistent with our previous study (Huang et al., 2009), the white-to-opaque switching frequency of the cyr1/cyr1 mutant in 5% CO2 (53.8 ± 5.9%) was 179 times higher than in air (0.3 ± 0.4%), although it was lower than for the wild-type (WT) control in 5% CO2 (84.1 ± 6.2%). According to our previous study, the frequencies of the cyr1/cyr1 mutant in 1 and 20% CO2 were 0.3 ± 0.3 and 97.6 ± 0.8%, respectively (Huang et al., 2009). These results suggest that cAMP is not required for high levels of CO2-induced opaque cell formation but is required for low levels of CO2-induced efficient switch. To further evaluate the roles of cAMP signaling in CO2-induced response, we examined the switching ability of the pde2/pde2 mutant, in which the cAMP level was constitutively high (Jung and Stateva, 2003). We found that the white-to-opaque switching frequency of the pde2/pde2 mutant was almost 100% both in air and in 5% CO2. Noticeably, although 5% CO2 increased the homogeneous opaque colony formation in the pde2/pde2 mutant, the percentage of mixed or sectored colonies remained very high (69.7 ± 5.4%; Figure 1, A and B). Consistent with our previous results, we found that the pde2/pde2 mutant formed 100% homogeneous opaque colonies on nutrient agar containing GlcNAc, which has been proved to stimulate opaque cell formation primarily via the cAMP pathway (Huang et al., 2010; Figure 1B). These results indicate that constitutive activation of cAMP signal by deletion of PDE2 gene hypersensitizes C. albicans cells to GlcNAc but not to the elevated levels of CO2. On the basis of the data presented here and our previous reports (Huang et al., 2009, 2010), we suggest that whereas the cAMP signaling plays a critical role in white-to-opaque switching, it is not the major pathway for high CO2 concentration–induced white-to-opaque switching in C. albicans.
FIGURE 1:
Effect of activation and inactivation of cAMP signaling on CO2-induced white-to-opaque switching. (A) Switching frequencies (Swit. freq.) of the cyr1/cyr1 (inactivation of cAMP signaling), pde2/pde2 (activation of cAMP signaling) mutants, and their corresponding reference strains (WT) in air and 5% CO2. The cells were grown on Lee's plus glucose agar plates with phloxin B at 25°C for 7 d. <, no colonies containing cells of alternative phenotype were observed. (B) Examples of colonies formed by the WT and pde2/pde2 mutant on glucose agar in air, glucose agar in 5% CO2, and GlcNAc agar in air. The cells were grown on agar plates with phloxine B at 25°C for 7 d.
Screen for the CO2-sensing regulators
We were interested to know what pathways or key regulators predominantly control CO2-regulated C. albicans morphogenesis. To this end, we screened a library containing 197 null mutants for transcription factors, kinases, and other proteins by using the white-to-opaque switching assay.
Most of the mutants we used were heterozygous at the MTL locus and not switching competent. The MTL locus is on chromosome 5, which can be reduced to monosomy in sorbose-containing medium and then become homozygous at the MTL locus through chromosome duplication (Janbon et al., 1998; Magee and Magee, 2000). To convert the mutants to MTL-homozygous cells, we first grew the mutant cells in liquid YPS medium (1% yeast extract, 2% peptone, and 10% sorbose) at 37°C for 48 h. The reported method for identification of MTL-homozygous isolates is laborious and inefficient (Magee and Magee, 2000). Genomic DNA has to be extracted from each isolate and then be used for PCR of MTLa and MTLα genes. Given the synergistic effect of GlcNAc and CO2 on induction of opaque cell formation (Huang et al., 2010), we hypothesized that most MTL-homozygous cells resulting from sorbose treatment would grow as opaque colonies on GlcNAc-containing medium in 5% CO2. This method was proved to be very efficient. We found that most of the mutants (95.5%) formed opaque colonies on GlcNAc medium in 5% CO2. Four examples are shown in Supplemental Figure S1A. To confirm that the opaque isolates were homozygous at MTL, we randomly selected 10 mutants for MTL locus PCR. They were all MTLa or α homozygous (Supplemental Figure S1B, lanes 2–11). An MTLa/α reference strain served as control for PCR (Supplemental Figure S1B, lane 1). A few strains of the library failed to form opaque colonies either because they failed to grow in YPS or GlcNAc media or underwent constitutive filamentous growth (such as sfl1/sfl1 and tup1/tup1mutants). The wor1/wor1 mutant, which is locked in white phase under all conditions (Huang et al., 2006), served as the negative control.
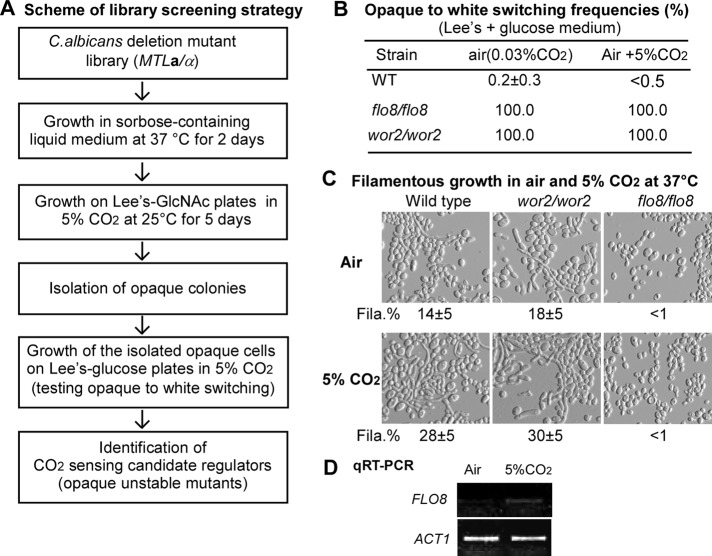
CO2 not only induces opaque cell formation, but it also stabilizes the opaque phenotype (Huang et al., 2009). The induction of white-to-opaque switching requires relatively high levels of CO2, and the efficiencies vary greatly in different natural strains and mutants, whereas the maintenance of the opaque phenotype needs very low levels of CO2 (<1%; Huang et al., 2009, and unpublished data). We hypothesized that the opaque cells of the CO2-sensing candidate gene mutants should be unstable even when cultured in high levels of CO2 in the absence of GlcNAc (since GlcNAc stabilized the opaque phenotype via a pathway different from that of CO2). We therefore replated the opaque cells of the mutants initially grown on GlcNAc medium onto Lee's medium plus glucose plates and incubated in 5% CO2. The opaque-to-white switching frequencies of the majority were <4% (Supplemental Table S1). We noted that the mutants of orf19.3809, orf19.1032, and 19.2088 showed the switching frequencies of 4.1, 9.4, and 4.2%, respectively. Surprisingly, the wor2/wor2 and flo8/flo8 mutants completely converted to white phase (frequency, 100%; Supplemental Table S1). To confirm these results, we performed the opaque-to-white switching experiments in the wor2/wor2 and flo8/flo8 mutants by using a standard method (Huang et al., 2009). The switching frequencies of the two mutants were 100% in either air or 5% CO2 on glucose medium, whereas the frequency of the WT control was <1% under both culture conditions (Figure 2B). These data indicated that Wor2 and Flo8 might be key candidates of CO2-sensing regulators.
FIGURE 2:
A library screening identified the transcription factor Flo8 as a master regulator of CO2-induced morphogenesis in C. albicans. (A) Scheme of library screening strategy. To revive the library strains from glycerol stock at −80°C, the cells were first grown on YPD plates for 2 d at 30°C. (B) Opaque-to-white switching frequencies (given as percentages) of the flo8/flo8 and wor2/wor2 mutants on Lee's plus glucose agar plates in air and in 5% CO2. The cells were grown on agar plates with phloxin B at 25°C for 6 d. The flo8/flo8 and wor2/wor2 mutants, isolated from the screening in A, were MTLa/a at the mating type–like locus. The wild-type strain (MTLa/a) was used as the control. <, no colonies containing cells of alternative phenotype were observed. (C) Filamentous growth of the flo8/flo8 and wor2/wor2 mutants (MTLa/α) on Lee's plus glucose agar plates in air and in 5% CO2. Two thousand cells of each strain in 3 μl of double-distilled H2O were dropped and grown on agar plates at 37°C for 2 d. The wild-type strain (166 in Table S1, MTLa/α) was used as the control. Fila.%, percentage of filamentous cells. (D) Semiquantitative reverse transcription (RT)-PCR. Two thousand cells of a wild-type strain were dropped onto Lee's glucose plates and cultured in air and in 5% CO2 for 48 h at 37°C. Total RNA was extracted and used for quantitative RT-PCR (25 cycles).
The transcription factor Flo8 carries a conserved LisH domain, which is found in many eukaryotic proteins with a wide range of functions. In C. albicans, Flo8 is essential for virulence and controls filamentous growth under a wide variety of culture conditions, including Lee's medium, serum, and GlcNAc induction (Cao et al., 2006). Wor2 is a zinc finger transcription factor, which is essential for maintenance of the opaque state in glucose medium (Zordan et al., 2007). To ensure the roles of Flo8 and Wor2 in the CO2-sensing process, we examined whether these two transcription factors were required for CO2-induced filamentous growth on Lee's plus glucose plates. The flo8/flo8 mutant failed to undergo filamentous growth both in air and in 5% CO2, whereas the wor2/wor2 mutant and the WT control formed filamentous colonies under both conditions (unpublished data). In addition, CO2 induced more wrinkled morphologies in the last two strains. The cellular phenotypes are shown in Figure 2C. The ratios of filamentous cells of the WT and wor2/wor2 mutant increased from 14 ± 5 to 28 ± 5% and from 18 ± 5 to 30 ± 5%, respectively, whereas the majority of flo8/flo8 mutant cells exhibited no filamentous growth. These results suggest that Wor2 is not required for CO2-mediated filamentous growth but is required for white-to-opaque switching. Semiquantitative reverse transcription PCR demonstrated that 5% CO2 obviously increased FLO8 expression in a WT strain, suggesting that the expression of FLO8 is regulated by the concentration of environmental CO2 (Figure 2D).
As mentioned, the opaque phase of orf19.3809, orf19.1032, and 19.2088 mutants was relatively unstable in 5% CO2, but we found that their white-to-opaque switching frequencies were remarkably increased when cultured under the same condition (unpublished data). These results suggest that the three genes are not essential for CO2-induced responses and can be excluded as CO2-sensing regulators.
Flo8 is required for CO2-induced white-to-opaque switching and efficient mating in glucose medium
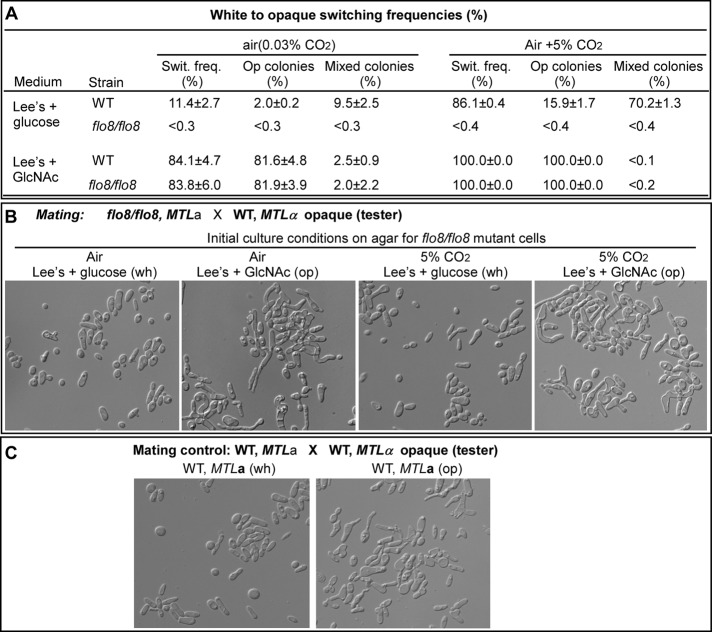
To investigate the role of Flo8 in the white-to-opaque switching process, we plated the cells of flo8/flo8 mutant and the WT onto glucose medium and GlcNAc medium. No opaque or opaque-sectored colonies of the flo8/flo8 mutant were observed on glucose-medium plates both in air (switching frequency, <0.3%) and in 5% CO2 (switching frequency, <0.4%), whereas the switching frequencies of the WT were 11.4 ± 2.7% in air and 86.1 ± 0.4% in 5% CO2 (Figure 3A). On GlcNAc medium in air, the switching frequencies of the flo8/flo8 mutant and the WT were 83.8 ± 6.0 and 84.1 ± 4.7%, respectively. When cultured on GlcNAc medium in 5% CO2, both the mutant and the WT formed 100% opaque colonies (Figure 3A). These results demonstrate that Flo8 is essential for CO2-induced opaque cell formation but is not required for GlcNAc-stimulated switching, which has been shown to play a role mainly via the Ras1-cAMP pathway (Huang et al., 2010).
FIGURE 3:
White-to-opaque switching of the WT and flo8/flo8 mutant in air and in 5% CO2. (A) White-to-opaque switching frequencies. The cells were grown on agar plates with phloxin B at 25°C for 6 d. The strains used were MTLa/a at the mating type–like locus. For switching on GlcNAc agar, aged cells (5 d old in liquid Lee's glucose medium) were used for plating. <, no colonies containing cells of alternative phenotype were observed. (B) Mating of the flo8/flo8 mutant. The mating experiments were performed in liquid glucose medium at 25°C for 24 h. The flo8/flo8 cells were collected from typical colonies on agar plates cultured under different conditions as indicated. The tester was GH1349, an MTLα/α strain, in the opaque state. (C) Mating of the wild type in white and opaque states. This experiment was used as a control experiment to B. Opaque and white cells of the wild-type MTLa/a strain grown on glucose agar were used for this experiment. The tester was GH1349 in the opaque state.
Only opaque cells can undergo efficient mating in C. albicans (Miller and Johnson, 2002). To further confirm that the flo8/flo8 mutant (MTLa/a) was capable of forming bona fide opaque cells on GlcNAc medium, we tested the mating ability of the representative colonies collected from glucose-medium plates and GlcNAc-medium plates. The mating responses were indicated by the formation of “shmoos” and “conjugation tubes.” As shown in Figure 3B, the flo8/flo8 mutant cells from GlcNAc-medium plates cultured both in air and in 5% CO2 could undergo efficient mating with an MTLα opaque tester, whereas the cells from glucose medium showed poor mating ability under both culture conditions. The mating control experiments of the WT white and opaque cells are shown in Figure 3C. As expected, the WT opaque, but not white, underwent efficient mating. These data demonstrate that the flo8/flo8 mutant has the basal level of switching ability and formed bona fide opaque colonies on GlcNAc medium plates.
Ectopic expression of FLO8 hypersensitizes C. albicans cells to elevated CO2 levels
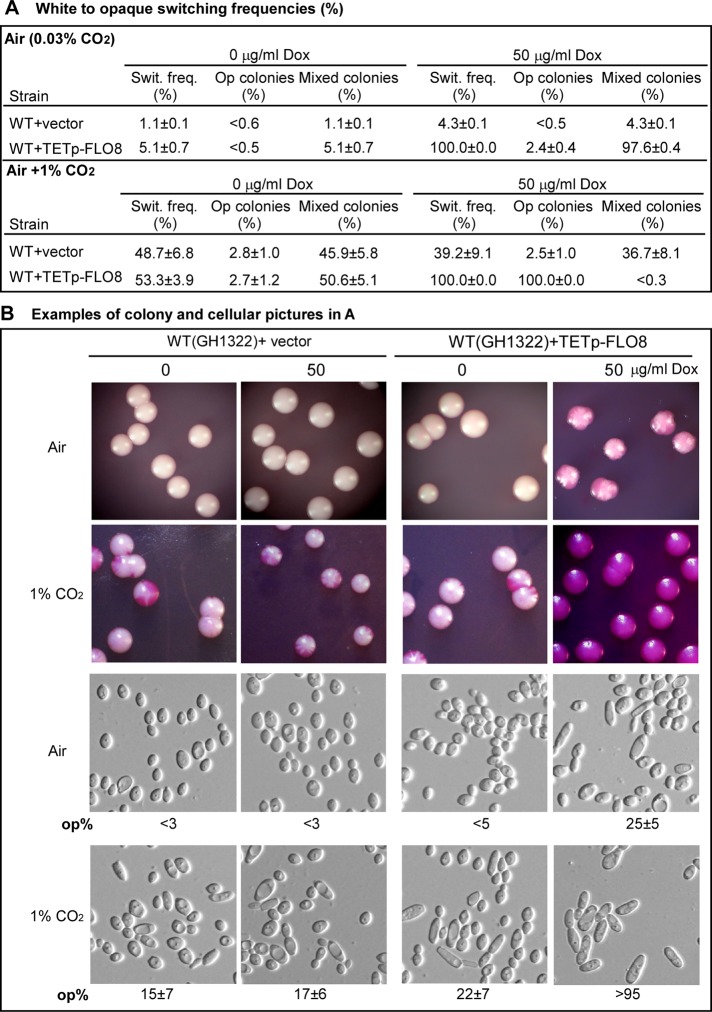
We showed that deletion of FLO8 blocked CO2-induced opaque cell formation and filamentous development. Given that Flo8 is the key CO2-sensing regulator, we reasoned that overexpression of FLO8 in a wild-type strain should increase the sensitivity to elevated CO2 levels. To test this hypothesis, we first examined the white-to-opaque switching frequencies of the strains WT+vector and WT+TETp-FLO8 in air and in 1% CO2. Consistent with our previous study, the WT+vector control showed low switching frequencies (<5%) in air either under the noninducing condition (without doxycycline) or under the inducing condition (with 50 μg/ml doxycycline). At 1% CO2, the switching frequencies of the WT+vector control were increased to 48.7 ± 6.8 and 39.2 ± 9.1% in the absence and presence of 50 μg/ml doxycycline, respectively. Under the noninducing condition, the switching frequency of the WT+TETp-FLO8 overexpression strain was similar as that of the WT+vector control either in air or in 1% CO2. Under the inducing condition, the switching frequency of the strain WT+TETp-FLO8 was increased to 100% both in air and in 1% CO2. Remarkably, when cultured in air under the inducing condition, the strain WT+TETp-FLO8 mainly formed highly opaque-sectored colonies (97.6 ± 0.4%), whereas it formed 100% homogeneous opaque colonies in 1% CO2 (Figure 4, A and B). The cellular phenotypes of a representative plate of each strain and the percentages of opaque cells are also shown in Figure 4B. Although the switching frequencies of the strain WT+TETp-FLO8 under inducing condition both in air and in 1% CO2 were 100%, the percentage of opaque cells of a representative plate in CO2 (>95%) was much higher than that in air (20 ± 5%). These results suggest that overexpression of FLO8 leads to the hypersensitivity of C. albicans cells to elevated CO2 levels.
FIGURE 4:
Overexpression of FLO8 stimulates white-to-opaque switching in 1% CO2. (A) White-to-opaque switching frequencies in air and in 1% CO2. The cells were grown on glucose agar with phloxin B at 25°C for 6 d. The WT strain used was WUM5A, an MTLα/α strain derived from the clinically isolated strain WO-1. <, no colonies containing cells of alternative phenotype were observed. (B) Examples of colony and cellular phenotypes in A. Cells collected from a representative plate of each strain were mixed and imaged. Op%, percentage of opaque cells.
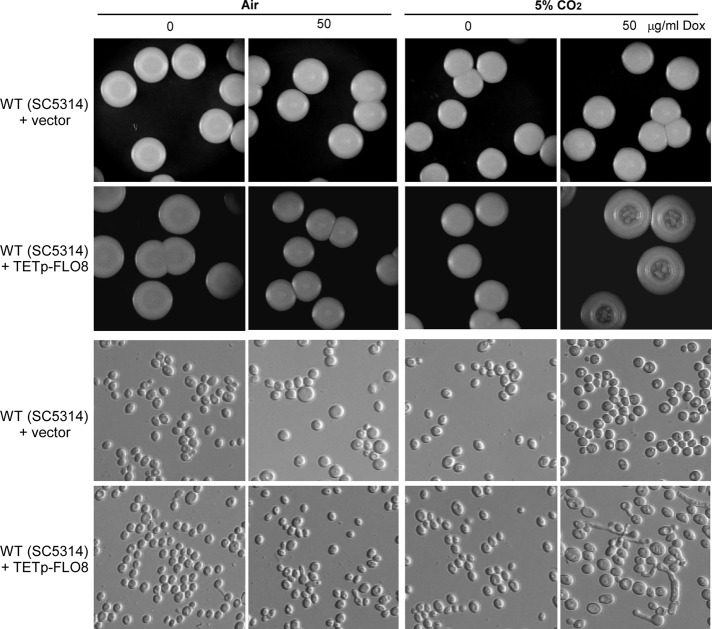
To further prove that overexpression of FLO8 contributes to the increase of sensitivity to elevated CO2 levels in C. albicans, we introduced the plasmid pNIM-FLO8 (TETp-FLO8) into a natural MTLa/α strain SC5314 and examined the effect of overexpressing FLO8 on filamentous development. The WT (SC5314) carrying the empty vector or TETp-FLO8 underwent robust filamentous growth at 37ºC either in air or in elevated levels of CO2, which would skew the examination. We did the experiments at 25ºC, a temperature not conducive to filamentous growth, which allowed us to examine the effect of overexpressing FLO8. The WT+vector control formed only smooth colonies containing yeast cells in air and in 5% CO2 under either noninducing condition or inducing condition. The WT+TETp-FLO8 strain also developed smooth colonies in air under both inducing and noninducing conditions. In 5% CO2, whereas the WT+TETp-FLO8 strain primarily formed smooth colonies under noninducing condition, it indeed grew as rough and wrinkled colonies containing both yeast and filamentous cells under inducing condition (Figure 5). The cellular morphology confirmed that the colonies formed by the WT+TETp-FLO8 strain under inducing condition in 5% CO2 contained filamentous cells (Figure 5). These data indicate that overexpression of FLO8 also promotes CO2-induced filamentous growth besides opaque cell formation.
FIGURE 5:
Overexpression of FLO8 induces filamentous growth at 25°C in 5% CO2. The cells were grown on Lee's plus glucose agar in air and in 5% CO2 at 25°C for 5 d. The WT strain was SC5314, a clinically isolated MTLa/α strain. The cellular phenotypes of a representative colony under each culture condition are also shown.
Flo8-promoted filamentous growth partially bypasses cAMP signaling
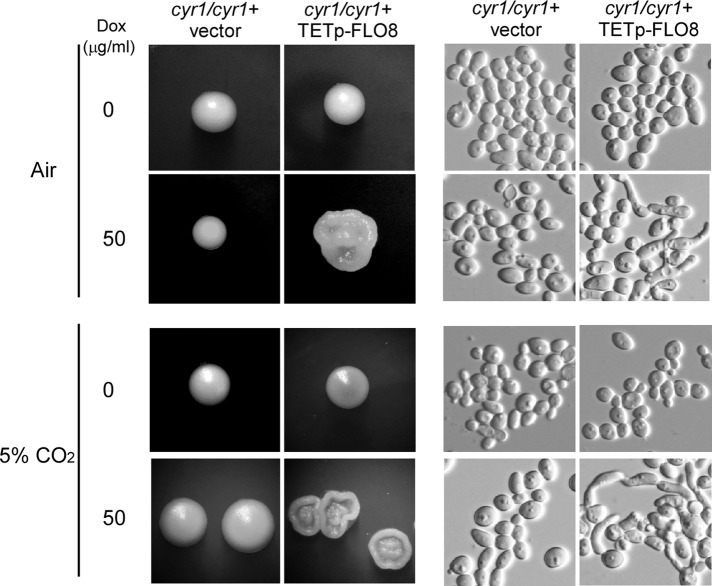
cAMP signaling has been reported to be required for CO2-induced filamentous growth in C. albicans (Klengel et al., 2005). C. albicans Flo8 is a Saccharomyces cerevisiae Flo8 homologue, which is a target of the cAMP/PKA pathway required for pseudohyphal development (Liu et al., 1996; Pan and Heitman, 1999; Cao et al., 2006). By physical interaction with Efg1, a transcription factor regulated by the catalytic subunit of cAMP-dependent protein kinase Tpk2 (Bockmuhl and Ernst, 2001), C. albicans Flo8 has been indicated to function downstream of the cAMP/PKA pathway and regulate hyphal development (Cao et al., 2006). To test whether Flo8-mediated CO2 sensing is dependent on the cAMP signaling, we overexpressed FLO8 in WT and a cyr1/cyr1 mutant and examined the effect of CO2 on filamentous growth. Because the filamentous growth ability of the cyr1/cyr1 mutant is very poor, we did this experiment at 37ºC for 5 d. Compared to the WT+vector control, the WT+TETp-FLO8 strain underwent more robust filamentous growth under inducing condition either in air or in 5% CO2 (unpublished data). As expected, the cyr1/cyr1+vector strain grew as smooth yeast colonies under all conditions. Although the cyr1/cyr1+TETp-FLO8 overexpression strain grew as yeast colonies under noninducing condition, it formed obviously wrinkled filamentous colonies under inducing condition both in air and in 5% CO2 (Figure 6). Of note, under inducing conditions, the filamentous growth of the overexpression mutant was more evident in 5% CO2 than in air. The cellular morphology showed that overexpression of FLO8 indeed induced filamentous growth (including elongated cells, hyphae, and pseudohyphae) in the cyr1/cyr1 mutant (Figure 6). To verify the effect of overexpressing FLO8 in CO2, we did the experiments by using a different method. To avoid secondary effects, we dropped 3 μl of double-distilled H2O containing 2000 cells of the strains on Lee's plus glucose and cultured them for 2 d at 37 ºC (rather than 5 d). The cellular phenotypes and the percentage of filamentous cells of the solid cultures are shown in Supplemental Figure S2. The ratio of filamentous cells of the FLO8 overexpression strains in 5% CO2 was obviously higher than that in air. The filamentous growth of the cyr1/cyr1+TETp-FLO8 strain in air might represent the effect induced by low concentration of CO2 in air or the metabolically produced CO2 inside the colony. These results suggest that Flo8 mediates CO2-induced filamentous growth and at least partially bypasses Cyr1 or the cAMP signal.
FIGURE 6:
Upstream pathways of Flo8 in CO2 sensing and morphogenesis regulation. The adenylyl cyclase Cyr1 (cAMP) was not required for Flo8-induced filamentous growth. The cells were grown on Lee's plus glucose agar with or without 50 μg/ml doxycycline. The plates were incubated in air or in 5% CO2 at 37°C for 5 d before acquiring the images. The cellular phenotypes of a representative colony under each culture condition are also shown.
Roles of Efg1 and Hgc1
The opaque phase of the efg1/efg1 and hgc1/hgc1 mutants were stable in air as well as in 5% CO2, suggesting that Efg1 and Hgc1were not involved in CO2-induced opaque cell formation. Cao et al. (2006) reported that Flo8 physically interacts with Efg1, and the two proteins play a similar role in regulation of filamentous growth. Hgc1 is a G1 cyclin-related protein and is required for hyphal growth (Zheng and Wang, 2004). The expression of HGC1 is regulated by Flo8. Northern blot and microarray analysis indicated that deletion of FLO8 blocked expression of HGC1 in C. albicans (Cao et al., 2006). To test whether these two genes were involved in CO2-induced filamentous growth, we cultured the efg1/efg1 and hgc1/hgc1 mutants on Lee's glucose medium in air and in 5% CO2. The WT and the flo8/flo8 mutant were used as positive and negative controls, respectively. In contrast to the WT reference strain, the flo8/flo8, efg1/efg1, and hgc1/hgc1 mutants could not undergo invasive (Supplemental Figure S3A) and filamentous (Supplemental Figure S3B) growth either in air or in 5% CO2. Overexpression of EFG1 in the flo8/flo8 mutant had no effect on filamentous growth either in air or in 5% CO2 (unpublished data), suggesting that Flo8 was required for the function of the Flo8–Efg1 complex. However, overexpression of HGC1 in the flo8/flo8 mutant induced filamentous growth both in air and in 5% CO2 (Supplemental Figure S4), suggesting that Hgc1 is downstream of Flo8.
Roles of Wor1 and Wor2
Although Wor1 and Wor2 were not required for CO2-induced filamentous growth, they were required for the white-to-opaque transition. The CO2 signal must be finally passed to the master regulator Wor1 to induce opaque cell formation. Overexpression of WOR1 in the flo8/flo8 mutant promoted white-to-opaque switching in air or in 5% CO2 (unpublished data), suggesting that Wor1 functioned downstream of Flo8. Overexpression of WOR2 in the flo8/flo8 mutant had no notable effect on switching (unpublished data), suggesting that Flo8 and Wor2 might function in different pathways.
DISCUSSION
CO2 is a ubiquitous molecule and an important environmental cue to all living organisms. The concentration of CO2 in the human host is much higher than that in the ambient atmosphere (Levitt and Bond, 1970). To better survive and colonize in the host, C. albicans must be capable of sensing and responding to ever-changing CO2 levels. In this study, we set out to explore the molecular mechanisms of CO2 sensing and how the CO2 signaling is transduced in C. albicans. Taking advantage of the unstable feature of C. albicans chromosome 5 in sorbose-containing medium and the synergistic effect of GlcNAc and CO2 on induction of opaque cell formation (Janbon et al., 1998; Huang et al., 2010), we developed a simple and efficient method to convert MTL-heterozygous into MTL-homozygous strains. This method is very useful for studies of white-to-opaque switching and mating, since the majority of gene mutants are made in the MTLa/α background strain SC5314 and its derivatives. By screening the null-mutant library homozygous for MTL, we identified the transcription factor Flo8 as a key regulator of CO2-induced morphogenesis in this fungal pathogen. Flo8 was initially characterized as a critical filamentous growth regulator (Cao et al., 2006). Here we showed that Flo8 governs white-to-opaque switching, as well as filamentous development, in response to elevated levels of CO2 via distinct downstream regulators. Hyphal-specific regulators Efg1 and Hgc1 mediate CO2-induced filamentous growth, and opaque-specific regulators Wor1 and Wor2 are required for CO2-induced white-opaque switching.
We showed that the transcription factor Flo8 plays a central role in CO2 sensing and morphogenesis in C. albicans. First, deletion of the FLO8 gene specifically blocked white-to-opaque switching induced by CO2 but had no effect on GlcNAc induction. These data suggest that Flo8 is not a general regulator required for opaque cell formation under all circumstances but is a component specifically essential for CO2 induction. Second, overexpression of the FLO8 gene sensitized the cells to the elevated levels of CO2 and induced mass conversion of white to opaque. Of interest, we also observed an increase of the white-to-opaque switching frequency of the FLO8 overexpression strain in air. This hypersensitivity to CO2 could be an effect resulting from accumulated metabolic CO2 inside colonies. In a recent study, Hall et al. demonstrated that there is an accumulation of metabolic CO2 inside a fungal biomass, and the CO2 released by metabolism is sufficient to induce the growth of the nce103/nce103 mutant, which cannot grow under circumstances of low CO2 levels as in the ambient atmosphere (Klengel et al., 2005; Huang et al., 2009; Hall et al., 2010). When the colonies initially formed, the quantity of CO2 released by the cells was very low and diffused into the air. Therefore the cells of the WT+TETp-FLO8 colonies initially maintained in white form in air even under the inducing condition. With the growth of the colonies, metabolic CO2 accumulated inside the colonies and then signaled the cells of the WT+TETp-FLO8 strain to switch to opaque, which resulted in the highly opaque–sectored phenotype observed later. Third, the effect of deletion and overexpression of FLO8 on filamentous growth further confirmed that Flo8 plays a critical role in CO2 sensing. Fourth, the cAMP/PKA pathway, which primarily mediates GlcNAc induction in white-to-opaque switching and filamentous growth, plays a minor role in CO2 sensing (this study; Cassone et al., 1985; Huang et al., 2010). We propose that there must exist a distinct unidentified pathway predominantly responsible for CO2 sensing and regulation of Flo8 in C. albicans (Figure 7). Of interest, the existence of a cAMP-independent, CO2-sensing pathway in C. albicans was also recently reported (Cottier et al., 2012). In this study, the authors show that the transcriptional regulator Rca1 induces the expression of the carbonic anhydrase Nce103 in response to low environmental CO2 concentration independent of the cAMP/PKA pathway. Given that Flo8 and Rca1 are both transcriptional regulators under the control of a yet-uncharacterized CO2-sensing pathway, one could hypothesize the existence of a common pathway resulting in the control of different outcomes like the white-to-opaque switch and control of NCE103 expression.
FIGURE 7:
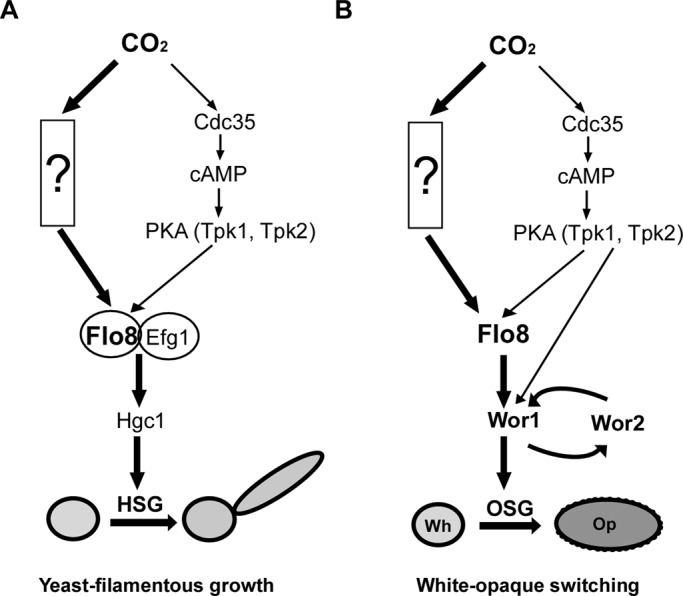
Models of CO2 mediated morphogenesis in C. albicans. The unidentified pathway represents the major pathway involved in CO2-induced responses. The cAMP signaling and the unidentified pathway converge on the key regulator Flo8 in the regulation of white-to-opaque switching, whereas the two pathways converge on the Flo8–Efg1 complex in the regulation of filamentous growth. HSG, hyphal-specific genes; OSG, opaque-specific genes. (A) A model of CO2-induced filamentation. (B) A model of CO2-induced white-to-opaque switching. Wor1 contains a conserved potential PKA phosphorylation site and has been proven to function downstream of the cAMP pathway.
According to our results in this study and previous reports (Huang et al., 2009, 2010), several lines of evidence further demonstrate that the unidentified pathway is independent of cAMP signaling. First, deletion of the adenylyl cyclase gene CYR1 blocks cAMP synthesis but not cell growth (Rocha et al., 2001), suggesting that cAMP is not essential for the survival of C. albicans. However, deletion of NCE103, a gene encoding the carbonic anhydrase catalyzing the conversion of CO2 into HCO3−, blocks cell growth in air (Rocha et al., 2001; Huang et al., 2009), suggesting that CO2 is an essential molecule for C. albicans growth. Therefore at least some biological processes (e.g., cell growth) in which CO2 is involved are independent of cAMP signaling in C. albicans. Second, Cyr1 is not required for CO2-regulated white-to-opaque switching and stabilization of the opaque phenotype (Huang et al., 2009; Supplemental Table S1 and Figure 1). Third, constitutive activation of the cAMP signal by deletion of the high-affinity cAMP phosphodiesterase gene PDE2 did not sensitize the cells to the elevated levels of CO2, whereas the mutant indeed became hypersensitive to the cAMP pathway activator GlcNAc (Figure 1; Huang et al., 2010).
Previous studies indicated that Flo8 functions downstream of the cAMP/PKA pathway in both C. albicans and S. cerevisiae (Pan and Heitman, 1999; Cao et al., 2006). Klengel et al. (2005) showed that CO2 directly activates the adenylyl cyclase and then stimulates hyphal growth in C. albicans. By sequence analysis, we found three putative PKA phosphorylation sites at the C-terminal of C. albicans Flo8: KKES (513–516), KRKS (603–606), and KKES (649–652). However, the experiment on the ectopic expression of FLO8 in the cyr1/cyr1 mutant proves that Flo8 regulates CO2-induced morphogenesis at least partially bypassing cAMP signaling (Figure 6). It is also notable that the filamentous growth of the cyr1/cyr1+TETp-FLO8 strain was not as robust as that of the WT+TETp-FLO8 strain. This might be attributed to the slow cell growth or partially to the regulation of cAMP signaling. On the basis of these and other results (Klengel et al., 2005; Huang et al., 2009, 2010; Hall et al., 2010), we propose a model in which both the major unidentified pathway and cAMP signaling are involved in CO2-sensing regulation. The two pathways converge on Flo8 or the Flo8–Efg1 complex, which then plays a key role in morphogenesis (Figure 7). The two pathways may function together and fine tune the CO2 response under different circumstances.
Another host environmental cue, GlcNAc, also induces both filamentous growth and opaque cell formation predominantly via the cAMP/PKA pathway (Huang et al., 2010). CO2 and GlcNAc have a synergistic effect on induction of opaque formation (Huang et al., 2010). In this study, we proved that Flo8 is required for CO2-regulated white-to-opaque switching but not for GlcNAc-regulated switching. These data suggest that CO2 and GlcNAc activate opaque cell formation via distinct major pathways. CO2 activates Flo8 primarily via an unidentified pathway, whereas GlcNAc directly stimulates Wor1 by phosphorylation through the cAMP/PKA pathway (Huang et al., 2010).
Both Efg1 and Hgc1 are important filamentous growth regulators. Efg1 coordinates with Flo8 by physical interaction to regulate filamentous growth (Cao et al., 2006). Deletion of EFG1 blocks CO2-induced filamentous growth, suggesting that Efg1 is essential for the function of the Efg1–Flo8 complex. Recently Nobile et al. (2012) found that Efg1 binds to the promoter region of the FLO8 gene in C. albicans, suggesting that Efg1 could play a role in the regulation of FLO8 expression at the transcriptional level. Transcriptional regulation of HGC1 indicates that Hgc1 functions downstream of the Flo8–Efg1 complex. Induction of filamentous growth by ectopic expression of HGC1 in the flo8/flo8 mutant confirms this hypothesis.
In this study, we also isolated the wor2/wor2 mutant, which shows the unstable opaque phenotype at high levels of CO2. We excluded the transcription factor Wor2 as a central CO2-sensing regulator because the wor2/wor2 mutant indeed responded to the elevated levels of CO2 when tested in a filamentous growth experiment (Figure 2C). Deletion of the master regulator WOR1 gene locks C. albicans in white phase either in air or in 5% CO2. Overexpression of WOR1 in the flo8/flo8 mutant promotes opaque cell formation (unpublished data). Although Wor1 and Wor2 are not directly involved in CO2 sensing in C. albicans, they may function downstream of the central regulator Flo8 to specifically control CO2-induced white-to-opaque switching. Because we do not have direct evidence showing that CO2 binds to the transcription factor Flo8, it is also possible that Flo8 responds to other changes induced by high levels of CO2 in the cell (such as reduced pH levels and changes in metabolic profiles).
On the basis of our results and previous reports (Cao et al., 2006), we propose that CO2 activates Flo8 via the cAMP/PKA and another, unidentified pathway (Figure 7). In the context of different external environments and internal genetic backgrounds, the activated Flo8 then delivers a signal to a distinct regulatory gene circuits to induce filamentous growth or white-to-opaque switching. Our study provides an example in which the same environmental factor regulates two different phenotypic switching systems via the same upstream pathways and distinct downstream regulatory circuits.
Conclusion
We set out to elucidate the molecular mechanisms of CO2 sensing by using the pathogenic fungus C. albicans as a model. Taking advantage of the bistable feature of white-to-opaque transition and the fact that CO2 and GlcNAc regulate this biological process via distinct pathways, we identified the transcription factor Flo8 as a master regulator of CO2 sensing in C. albicans. Flo8 controls CO2-induced white-to-opaque switching, as well as yeast filamentous growth transition, via distinct downstream factors. Further analysis suggests that there are two pathways involved in CO2-induced morphogenesis: the major, unidentified pathway and the minor, cAMP/PKA pathway. CO2 signal is transduced via the two pathways, which converge on Flo8. As mentioned, Flo8 is a transcription factor containing a conserved LisH domain that has been found in a wide variety of eukaryotic organisms, ranging from yeast to human (Reiner et al., 1993; Neer et al., 1994; Cao et al., 2006). Therefore our findings will provide new insights into the understanding of CO2 sensing in pathogenic fungi, as well as in higher organisms.
MATERIALS AND METHODS
Strains and plasmids
The strains used in this study are listed in Supplemental Tables S1 and S2. The original MTLa/α strains (001–167) listed in Supplemental Table S2 were the transcription factor (TF)–knockout set generated by Homann et al. (2009) and obtained from the Fungal Genetics Stock Center (University of Missouri, Kansas City, Kansas City, MO). The MTLa/α strains, including the TF set and others collected from the Candida research community, were converted to MTL-homozygous strains as described in the text (Figure 2A). The strains were first grown in liquid YPS medium in a test tube at 37°C for 48 h. The cells were diluted and replated onto Lee's GlcNAc plates for further growth in 5% CO2. After incubation for 5 d, opaque colonies were isolated for further experiments.
The FLO8 open reading frame was amplified by PCR from SC5314 genomic DNA and digested with BamHI and SalI. The primers for PCR were FLO8F (5-TCATTTGTCGACATGGTTCCCAACACAACTAAAC-3) and FLO8R (5-TCATTTGGATCCTAATCGCCATTTTCAATTGGATC-3). The GFP fragment of the plasmid pNIM1 was replaced with the digested PCR product of FLO8, generating the overexpression plasmid pNIM-FLO8 (TETp-FLO8; Park and Morschhauser, 2005).
White-to-opaque switching assay
White-to-opaque switching experiments were performed as previously described (Huang et al., 2009, 2010), with slight modification. Briefly, the original white or opaque colonies were first plated or streaked on agar contain Lee's glucose or Lee's GlcNAc medium (Lee et al., 1975; Huang et al., 2010). Modified Lee's medium with 1.25% (wt/vol) glucose as carbon source is referred to as Lee's glucose. Modified Lee's medium with GlcNAc 1.25% (wt/vol) as carbon source is referred to as Lee's GlcNAc medium. The homogeneous white or opaque colonies were then picked and replated onto glucose- or GlcNAc-medium plates. After incubation for 5–7 d in air or CO2 at 25°C, the colonies exhibiting different phenotypes were counted, and the cellular morphologies of several representative colonies were examined to ensure their phenotypes.
Mating assay
The mating experiments in Figure 3 were performed in Lee's glucose liquid medium. To obtain opaque colonies, we first grew the tester MTLα/α strain (GH1349) in 5% CO2. The isolated opaque colonies were streaked on Lee's glucose plates and incubated for 5 d. The experimental cell samples of the flo8/flo8 mutant (MTLa/a) were collected from the same set of plates presented in Figure 3A. The experimental white and opaque cell samples of the WT were collected from Lee's glucose plates. A total of 1 × 107 of MTLa/a cells and 1 × 107 of MTLa/α cells were mixed in 100 μl of Lee's glucose medium in a 1.5-ml centrifuge tube. Before acquiring the cellular images, the tubes containing a and α cell mixture were incubated at 25°C for 24 h with gentle shaking.
Filamentous growth assay
The cells were grown on YPD (1% yeast extract, 2% peptone, and 2% glucose) agar for 1–2 d before plating onto Lee's glucose agar for filamentous development. The plates were incubated at 25 or 37°C for 5–7 d as indicated in the text.
Supplementary Material
Acknowledgments
We are indebted to David Soll, Joachim Morschhäuser, Alexander Johnson, Haoping Liu, Gerald Fink, Yue Wang, Daniel Kornitzer, and James Konopka for generous gifts of plasmids and strains. This project is supported by a grant from the 100 Talent Program of the Chinese Academy of Sciences and Chinese National Natural Science Foundation Grant 31170086 to G.H.
Abbreviations used:
- cAMP
cyclic adenosine monophosphate
- GlcNAc
N-acetylglucosamine
- MTL
mating type–like locus
- PKA
protein kinase A
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E12-02-0094) on May 23, 2012.
REFERENCES
- Anderson JM, Soll DR. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987;169:5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn YS, Cox GM, Perfect JR, Heitman J. Carbonic anhydrase and CO2 sensing during Cryptococcus neoformans growth, differentiation, and virulence. Curr Biol. 2005;15:2013–2020. doi: 10.1016/j.cub.2005.09.047. [DOI] [PubMed] [Google Scholar]
- Bahn YS, Muhlschlegel FA. CO2 sensing in fungi and beyond. Curr Opin Microbiol. 2006;9:572–578. doi: 10.1016/j.mib.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Bockmuhl DP, Ernst JF. A potential phosphorylation site for an A-type kinase in the Efg1 regulator protein contributes to hyphal morphogenesis of Candida albicans. Genetics. 2001;157:1523–1530. doi: 10.1093/genetics/157.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Lane S, Raniga PP, Lu Y, Zhou Z, Ramon K, Chen J, Liu H. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol Biol Cell. 2006;17:295–307. doi: 10.1091/mbc.E05-06-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassone A, Sullivan PA, Shepherd MG. N-Acetyl-D-glucosamine-induced morphogenesis in Candida albicans. Microbiologica. 1985;8:85–99. [PubMed] [Google Scholar]
- Cottier F, et al. The bZIP transcription factor Rca1p is a central regulator of a novel CO2 sensing pathway in yeast. PLoS Pathog. 2012;8:e1002485. doi: 10.1371/journal.ppat.1002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MT, Wilkes TJ. A comparison of the range of attraction of animal baits and of carbon dioxide for some West African mosquitoes. Bull Entomol Res. 1969;59:441–456. doi: 10.1017/s0007485300003412. [DOI] [PubMed] [Google Scholar]
- Granger DL, Perfect JR, Durack DT. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985;76:508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, et al. CO2 acts as a signalling molecule in populations of the fungal pathogen Candida albicans. PLoS Pathog. 2010;6:e1001193. doi: 10.1371/journal.ppat.1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Raven JA. The biology of carbon dioxide. Curr Biol. 2005;15:R406–R410. doi: 10.1016/j.cub.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Homann OR, Dea J, Noble SM, Johnson AD. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 2009;5:e1000783. doi: 10.1371/journal.pgen.1000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Srikantha T, Sahni N, Yi S, Soll DR. CO2 regulates white-to-opaque switching in Candida albicans. Curr Biol. 2009;19:330–334. doi: 10.1016/j.cub.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Wang H, Chou S, Nie X, Chen J, Liu H. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci USA. 2006;103:12813–12818. doi: 10.1073/pnas.0605270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Yi S, Sahni N, Daniels KJ, Srikantha T, Soll DR. N-Acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS Pathog. 2010;6:e1000806. doi: 10.1371/journal.ppat.1000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbon G, Sherman F, Rustchenko E. Monosomy of a specific chromosome determines L-sorbose utilization: a novel regulatory mechanism in Candida albicans. Proc Natl Acad Sci USA. 1998;95:5150–5155. doi: 10.1073/pnas.95.9.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WH, Stateva LI. The cAMP phosphodiesterase encoded by CaPDE2 is required for hyphal development in Candida albicans. Microbiology. 2003;149:2961–2976. doi: 10.1099/mic.0.26517-0. [DOI] [PubMed] [Google Scholar]
- Klengel T, et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaal C, Lachke SA, Srikantha T, Daniels K, McCoy J, Soll DR. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect Immun. 1999;67:6652–6662. doi: 10.1128/iai.67.12.6652-6662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaal CA, Srikantha T, Soll DR. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect Immun. 1997;65:4468–4475. doi: 10.1128/iai.65.11.4468-4475.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Buckley HR, Campbell CC. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia. 1975;13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Levitt MD, Bond JH., Jr Volume, composition, and source of intestinal gas. Gastroenterology. 1970;59:921–929. [PubMed] [Google Scholar]
- Liu H, Styles CA, Fink GR. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee BB, Magee PT. Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science. 2000;289:310–313. doi: 10.1126/science.289.5477.310. [DOI] [PubMed] [Google Scholar]
- Mardon D, Balish E, Phillips AW. Control of dimorphism in a biochemical variant of Candida albicans. J Bacteriol. 1969;100:701–707. doi: 10.1128/jb.100.2.701-707.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MG, Johnson AD. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell. 2002;110:293–302. doi: 10.1016/s0092-8674(02)00837-1. [DOI] [PubMed] [Google Scholar]
- Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell. 2012;148:126–138. doi: 10.1016/j.cell.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Heitman J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4874–4887. doi: 10.1128/mcb.19.7.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YN, Morschhauser J. Tetracycline-inducible gene expression and gene deletion in Candida albicans. Eukaryot Cell. 2005;4:1328–1342. doi: 10.1128/EC.4.8.1328-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon PC, Kuo TH, Linford NJ, Roman G, Pletcher SD. Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. 2010;8:e1000356. doi: 10.1371/journal.pbio.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Rocha CR, Schroppel K, Harcus D, Marcil A, Dignard D, Taylor BN, Thomas DY, Whiteway M, Leberer E. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell. 2001;12:3631–3643. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabi K, Hurwitz A, Simon AJ, Beitel GJ, Morimoto RI, Rechavi G, Sznajder JI, Gruenbaum Y. Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009a;106:4024–4029. doi: 10.1073/pnas.0900309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharabi K, Lecuona E, Helenius IT, Beitel GJ, Sznajder JI, Gruenbaum Y. Sensing, physiological effects and molecular response to elevated CO2 levels in eukaryotes. J Cell Mol Med. 2009b;13:4304–4318. doi: 10.1111/j.1582-4934.2009.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. “White-opaque transition”: a second high-frequency switching system in Candida albicans. J Bacteriol. 1987;169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll DR. Mating-type locus homozygosis, phenotypic switching and mating: a unique sequence of dependencies in Candida albicans. Bioessays. 2004;26:10–20. doi: 10.1002/bies.10379. [DOI] [PubMed] [Google Scholar]
- Soll DR, Lockhart SR, Zhao R. Relationship between switching and mating in Candida albicans. Eukaryot Cell. 2003;2:390–397. doi: 10.1128/EC.2.3.390-397.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- Zheng X, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23:1845–1856. doi: 10.1038/sj.emboj.7600195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan RE, Miller MG, Galgoczy DJ, Tuch BB, Johnson AD. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 2007;5:e256. doi: 10.1371/journal.pbio.0050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.