Abstract
In immature wild savannah baboons (Papio cynocephalus), we observed symptoms consistent with copper (Cu) deficiency and, more specifically, with a disorder referred to as white monkey syndrome (WMS) in laboratory primates. The objectives of this study were to characterize this pathology and test three hypotheses – that Cu deficiency may have been induced by zinc (Zn) toxicity, that it may have been induced by molybdenum (Mo) toxicity, and that cumulative rainfall during the perinatal period and particularly during gestation is an ecological factor distinguishing infants afflicted with WMS from non-WMS infants. During 2001–09, we observed 22 instances of WMS out of a total 377 live-births in the study population. Visible symptoms exhibited by WMS infants included whitening of the animal’s fur and/or impaired mobility characterized by an apparent “stiffening” of the hindlimbs. Occurrence of WMS did not vary significantly by gender. However, among individuals that survived at least 180 days, WMS males had a significantly lower survivorship probability than non-WMS males. Zn/Cu ratios assessed from hair samples of adult female baboons were higher in females who had produced at least one WMS offspring relative to females who had not had a WMS offspring. This was true even when the hair sample was collected long after the birth of the female’s afflicted infant. We consider this potentially indicative of a robust tendency for low Cu levels induced by elevated Zn intake in some individuals. No significant differences of Mo/Cu ratios were observed. Cumulative rainfall during gestation (~179 days) was 50% lower for WMS infants relative to non-WMS infants. In contrast, rainfall for the two classes of infants did not differ in the 180 days prior to conception or in the 180 days following birth. This finding highlights the importance of prenatal ecological conditions in healthy fetal development with regard to WMS.
Keywords: Amboseli, copper deficiency, Papio cynocephalus, white monkey syndrome, wild baboons, zinc toxicity
INTRODUCTION
Trace minerals are naturally-occurring elements essential in minute concentrations for optimal growth and development of living organisms. Deleterious effects are often observed with either excessive or deficient intake, and imbalances are considered risk factors for several diseases in a wide range of species. In particular, extensive research on human and nonhuman subjects has focused on the causes and consequences of deficiencies in the trace element copper (Cu). Cu is required for numerous cellular processes, including mitochondrial respiration, antioxidant defense, neurotransmitter synthesis, connective tissue formation, and tissue pigmentation [reviewed in Culotta & Gitlin, 2000]. Cu deficiencies are associated with symptoms including immune system dysfunction, anemia, pigmentation loss in the skin and hair, gait difficulty, lower limb parathesias, and failure to thrive [reviewed in Culotta & Gitlin, 2000].
Both genetic and environmental factors can lead to Cu deficiency. For example, Menkes disease is an x-linked recessive disorder in humans resulting from disruptions to the body’s pathways for Cu absorption and transport; severity of symptoms varies among patients and can include hypopigmentation, anemia, neurological defects, connective tissue defects, and distinctively brittle hair [reviewed in Cox et al., 2002; de Bie et al., 2007; Vonk et al., 2008]. Cu deficiency may also be acquired due to environmental factors either (1) directly as a result of inadequacies in an organism’s habitat and/or nutrition or (2) indirectly as a result of Cu’s susceptibility to transport or bioavailability interference from other trace elements, notably zinc (Zn) and molybdenum (Mo) in the circumstances of the current study [reviewed in McDowell, 2003]. Consequently, elevated levels of one or more of these other elements may result in a secondary Cu deficiency.
In nonhuman primates, acquired Cu deficiency induced by Zn toxicity has been associated with a disorder known as white monkey syndrome (WMS). In documented cases of Zn/Cu imbalances in rhesus macaques (Macaca mulatta) [Obeck, 1978] and baboons (Papio spp.) [Frost et al., 2004], afflicted individuals were juveniles characterized by alopecia, dehydration, emaciation, cachexia, dermatitis, diarrhea, and whitening of hair, skin, and mucous membranes. Researchers attributed the occurrence of these symptoms to elevated Zn intake originating from the galvanized cages in which the animals were housed. Prolonged, untreated exposure to the toxic Zn levels resulted in death; symptoms were occasionally reversible if animals were relocated to non-toxic enclosures. Similar pigmentation loss in hair was also observed in a population of captive squirrel monkeys (Saimiri sciureus) housed in outdoor pens with galvanized metal fencing and roofs at the Sabana Seca Field Station of the Caribbean Primate Research Center, University of Puerto Rico. However, no tests were performed to confirm the conditions were directly attributable to Zn/Cu imbalances (Matthew Kessler, personal communication, 3 Feb 2011).
Here we report the occurrence of symptoms consistent with WMS and, more broadly, with Cu deficiency, in wild savannah baboons (Papio cynocephalus). Using long-term observational data available through the Amboseli Baboon Research Project (ABRP), we offer novel documentation and insight into the characterization of this pathology in wild primates. Specifically, we tested two hypotheses regarding the probable source of this pathology. First, we evaluated whether Cu deficiency was induced by Zn toxicity (as reported by Obeck [1978] and Frost and colleagues [2004]) by analyzing the Zn/Cu ratio in hair samples obtained from a subset of individuals within our study population. Second, because previous research suggested Cu deficiency in east African wildlife may be secondary to toxic Mo concentrations in volcanic soils [Maskall & Thornton, 1991, 1996], we also analyzed the Mo/Cu ratio in the same hair samples. For both hypotheses, we predicted that mineral ratios would be higher in individuals associated with WMS (reflecting high Zn or Mo concentration relative to the concentration of Cu) compared to the ratios in individuals with no WMS association. Finally, because (1) rain in the semi-arid Amboseli ecosystem is a key environmental variable influencing the nutritional and reproductive status of baboons [e.g. Alberts et al., 2005; Beehner et al., 2006] and (2) gestation is a vulnerable time to mineral imbalances [e.g. Adogwa et al., 1999; Barone et al., 1998; Gambling & McArdle, 2004], we tested the hypothesis that rainfall in the perinatal period contributes to WMS. Specifically, we predicted that infants afflicted with WMS experienced lower rainfall regimes during gestation than did non-WMS infants.
METHODS
All project protocols complied with regulations in Kenya (Republic of Kenya Research Permits NCST/5/002/R/776 to J.A. and NCST/5/002/R/777 to S.C.A.) and in the United States (Princeton University IACUC 1649), and adhered to the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates.
We observed a population of wild savannah baboons living in the Amboseli basin, a semi-arid short-grass savannah located in east Africa at the northwestern base of Mt. Kilimanjaro. Alberts and colleagues [2005] provide a thorough description of the Amboseli study site and the broader ecological region. Review of the area’s volcanic geochemistry and clay mineralogy are described by Hay and colleagues [1995] and Stoessell & Hay [1978]. Maskall & Thornton [1996] provide an overview of the trace mineral content of soils in Amboseli; elevated Mo concentration is of particular relevance.
Savannah baboons are a highly social species obligated to group-living. Many aspects of their sociality have been studied extensively, including patterns characteristic of typical infant care and development [e.g. Altmann, 1980; Altmann et al., 1981; Rhine et al., 1985]. The species is omnivorous but seasonally available seeds, leaves, pods, and fruits comprise the bulk of their diet [e.g. Alberts et al., 2005; Altmann, 1998; Norton et al., 1987].
All baboons within the study population were individually identifiable by ABRP field researchers, and each of the 5 social groups monitored by ABRP was the focus of detailed observations several days each week. Consequently, conception dates, ages, and the onset of visible pathological symptoms of individuals born into the study population were typically accurate to within a few days. Complete details on monitoring effort and data collection protocols can be accessed online (http://www.princeton.edu/~baboon/).
For this study, we used data on demography, pathology, and rainfall from 2001–09, a time period during which we standardized recording of observational data on infants/juveniles with regard to whitening of the fur and/or impaired hindlimb mobility. Any time a baboon showed pathological symptoms (e.g. malaise, diarrhea, limping in the absence of a wound), field observers recorded the animal’s ID, date and time of the observation, and a description of the animal’s condition. All pathologies were rechecked regularly to record any changes in the animal’s status. We queried this complete dataset of recorded pathologies to extract the subset of observations used in this study that unambiguously described individuals with whitening fur and/or impaired hindlimb mobility. “Ambiguous” WMS instances (N=4; fur color that was lighter than typical but deviation was within the known variability in the coat color of “healthy” individuals) were not included in analyses as either WMS or non-WMS. Rainfall values (mm) were measured daily from a rain gauge located centrally within the Amboseli basin at the ABRP base camp.
Adult hair samples were collected during 2006–09 from female baboons darted using a blowpipe to inject a syringe containing Telazol™ (tiletamine hydrochloride and zolazepam); for details, see Altmann et al. [1993] and Sapolsky & Altmann [1991]. In order to avoid even unlikely complications affecting the viability of a fetus or dependent offspring, we selected individuals that, at the time of darting, were not past the first trimester of pregnancy and did not have a young, nursing infant. A small quantity of hair (≤0.25 g) was cut from the distal flank of the animal’s hind leg; to ensure that the sample included the full length of the hair shaft, we cut the hair as close to the animal’s skin as possible.
Infant and young juvenile baboons were not darted. However, one hair sample was opportunistically collected post-mortem from an infant baboon who had exhibited WMS symptoms when first observed at approximately 2 days. The infant was 11 days when she died and her corpse was carried by her mother for several days post death; this behavior is commonly observed in the immediate aftermath of a newborn’s death [Altmann, 1980]. Field observers were able to retrieve the body 3 days after the infant’s death when the mother left it unattended.
Hair samples were analyzed for total metal content (Cu, Zn, Mo) by mass spectrometry. Samples were dried and reduced to powder in liquid nitrogen using a mortar and pestle. Hair samples were then digested using a microwave-assisted procedure (trace metal grade nitric acid, 200°C, 20 min) with a Mars 5 digester [CEM Corporation, Matthews, NC, USA]. The solution was collected, diluted, spiked with two internal standards ([In] = [Sc] = 2 ppb) and analyzed for its element concentration with an Inductively Coupled Plasma-Mass Spectrometer (ICP-MS, Element 2, Thermo Finnigan, Bremen, Germany) at medium resolution.
Statistics
We evaluated the potential influence of gender on the occurrence of WMS by performing a chi-square analysis in STASTISTICA v.5 (StatSoft Inc., 1995) where expected WMS occurrences were derived from observed births per gender-class. One non-WMS individual born in the study period was excluded from this analysis because it died before researchers observed its gender. To test whether survivorship differed between WMS and non-WMS individuals, we used Kaplan-Meier survival analysis with Mantel Cox log rank test in SPSS 17.0 (SPSS Inc., 2008); due to challenges in consistently discerning WMS in very young baboons, survivorship analysis was focused on individuals who survived at least 180 days, an age when coat color changes and extent of independent locomotion makes WMS identification reliable. We performed a one-way ANOVA in SPSS 17.0 to analyze variation in Zn/Cu and Mo/Cu ratios for adult females categorized by whether any of their offspring born within the 9-year study period were afflicted with WMS. Females categorized as mothers of WMS offspring were not necessarily caring for their WMS offspring at the time their hair was sampled, and they may have borne non-WMS offspring at some point as well. To further probe variability in Zn/Cu ratios between females, we paired each female who had had a WMS offspring with a female who not had a WMS offspring. We minimized, to the extent possible, the time difference in the date of hair sample collection for paired females (median 5 days, range 0–105 days). We performed a paired-samples t-test using SPSS 17.0 to examine whether Zn/Cu ratios differed significantly between these two categories of females. Lastly, we performed a series of one-way ANOVAs in SPSS 17.0 to test for differences in rainfall during the 180 days prior to conception, gestation (N=373 infants, mean 179 days ± 0.3 SE, range 156–194 days), and 180 days post-birth of WMS infants vs. non-WMS infants.
RESULTS
Of the 377 infants born in the study population during 2001–09, researchers observed 22 (5.8%) instances of unambiguous WMS. Occurrences of WMS did not differ significantly by sex (males: N=9, 40.9%; females: N=13, 59.1%; χ2=1.03, df=1, P=0.31) compared to the sex ratio of non-WMS infants born during the study window. For 17 of 22 individuals afflicted with WMS, detailed data existed on the first date on which the pathology was observed. For these 17 individuals, mean age of observed symptom onset was 245 days (± 42.2 SE, range 2–731 days). Fig. 1 shows pictures taken of the youngest documented case of WMS in our study; the photographed individual was born with white fur and was described as suffering from poor/weak health, alopecia [Novak & Meyer, 2009], and difficulty using her hindlimbs to cling ventrally to her mother. Careful visual observation confirmed that the infant had normal eye pigmentation and was therefore not a “true” albino.
Fig. 1.
Photos in left column depict an infant with white monkey syndrome (WMS); images are of the youngest documented case of WMS within the Amboseli baboon population. For comparison, photos in right column depict “healthy” infants (no observed pathologies, including WMS) of comparable age. Note that the individual featured in the top right was from a captive Papio spp. population (photo of an individual in the same position from study population was not available).
Eleven of the WMS infants died prior to the end of the study period; probable cause of death was pathology in 4 of these cases and predation in 7. Mean age of death for all WMS infants that did not survive was 709 days (± 145.6 SE, range 11–1597 days); mean age of death for those who died of pathology was 577 days (± 190.9 SE, range 11–820 days). Among individuals that survived to at least 180 days of age, male WMS infants had a significantly lower survivorship probability than non-WMS males (N=154; Mantel Cox log rank, χ2=8.68, df=1, P=0.003). We did not observe significant differences in survivorship for WMS vs. non-WMS females (N=145; Mantel Cox log rank, χ2=0.01, df=1, P=0.92) or for both sexes combined (N=299; Mantel Cox log rank, χ2=3.26, df=1, P=0.07).
Three mothers had 2 offspring that developed WMS during the 9-year study period; all other mothers of individuals with WMS (N=16) only had 1 afflicted offspring. All but one mother of WMS offspring (N=18) also had ≥1 non-WMS infant (range 2–6 infants).
Zn/Cu and Mo/Cu Concentrations in Hair Samples
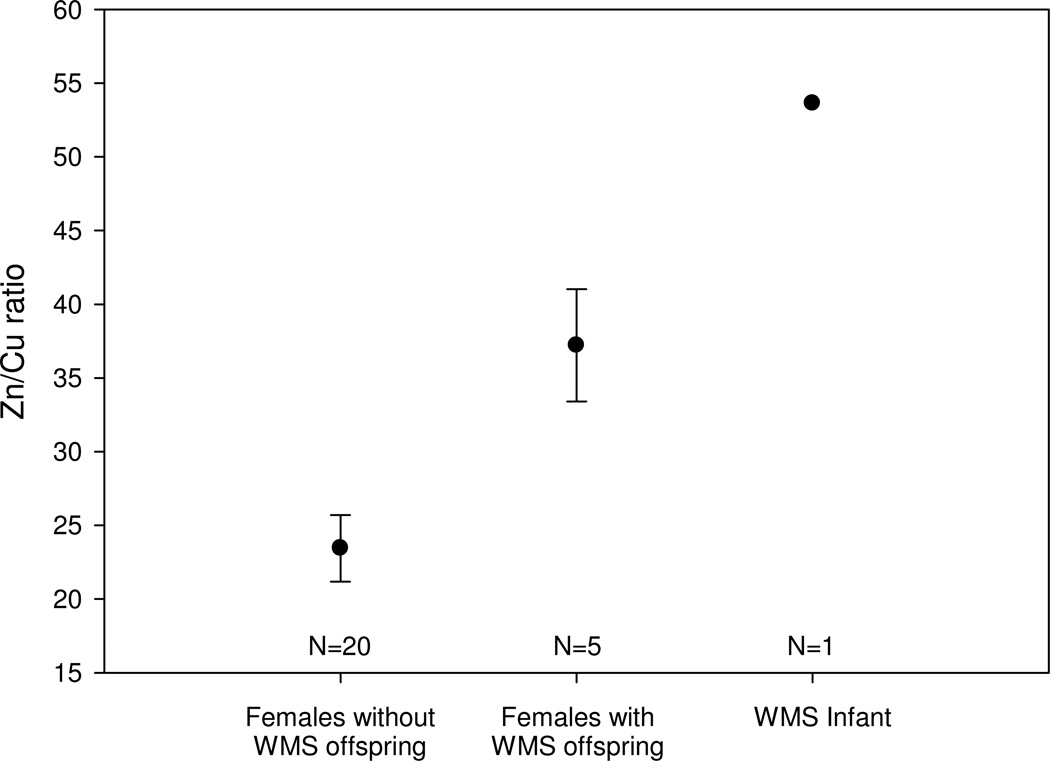
Hair of the WMS infant had the highest Zn/Cu ratio (53.6) of any individual we sampled, followed by females with WMS offspring (mean ± SE: 37.2 ± 3.81), and finally females without WMS offspring (23.4 ± 2.27; Fig. 2). Hair of the WMS infant also had the highest Mo/Cu ratio (0.17), however mean values were nearly identical for hair of females who had had WMS offspring (0.06 ± 0.01) and females without WMS offspring (0.07 ± 0.00). Zn/CU ratios differed significantly between females with WMS offspring and females without WMS offspring (one-way ANOVA, F=7.80, df=24, P=0.01). In contrast, the comparable model predicting Mo/Cu ratios was not significant (one-way ANOVA, F=0.78, df=24, P=0.39).
Fig. 2.
Zn/Cu ratio in analyzed hair samples for adult female baboons without white monkey syndrome (WMS) offspring (N=20), female baboons with WMS offspring (N =5), and an infant baboon with WMS (N =1). Points and error bars represent mean ± SE. Zn/CU ratios differed significantly between females with WMS offspring and females without WMS offspring (one-way ANOVA, F=7.80, df=24, P=0.01).
To further probe variability in Zn/Cu values between females, we paired each female with WMS offspring with a female without WMS offspring (see Methods for pairing details). Consistent with the results from the one-way ANOVA, we found significantly higher Zn/Cu levels in females with WMS offspring (mean ± SE = 37.2 ± 3.81) relative to females without WMS offspring (24.9 ± 5.67; t=5.25, df=4, P=0.006).
Table I provides the trace mineral concentrations (Cu, Zn, and Mo) and ratios (Zn/Cu and Zn/Mo) in hair samples of individual baboons in each of the 3 categories evaluated: an infant baboon with WMS, adult female baboons with WMS offspring, and adult female baboons without WMS offspring.
Table I.
Trace Mineral Concentrations (Cu, Zn, and Mo) and Ratios (Zn/Cu and Zn/Mo) in Hair Samples of Baboons.
| Category, individual's name | Cu (mg/kg) | Zn (mg/kg) | Mo (mg/kg) | Zn/Cu | Mo/Cu |
|---|---|---|---|---|---|
| *WMS infant | |||||
| IDA | 2.2 | 117.3 | 0.4 | 53.64 | 0.17 |
| Adult female with *WMS offspring | |||||
| RWA | 3.5 | 165.8 | 0.2 | 47.25 | 0.07 |
| YAI | 3.8 | 169.1 | 0.3 | 44.02 | 0.07 |
| VET | 4.0 | 149.1 | 0.3 | 37.27 | 0.08 |
| HON | 6.1 | 176.7 | 0.3 | 28.88 | 0.05 |
| LOL | 4.7 | 134.7 | 0.3 | 28.66 | 0.06 |
| Adult female without *WMS offspring | |||||
| OXY | 4.0 | 172.5 | 0.3 | 42.88 | 0.08 |
| LAO | 4.5 | 176.7 | 0.3 | 39.19 | 0.06 |
| VOT | 4.5 | 157.8 | 0.3 | 35.40 | 0.07 |
| VIN | 2.5 | 88.1 | 0.3 | 34.57 | 0.10 |
| WIP | 6.8 | 222.0 | 0.3 | 32.44 | 0.05 |
| ABB | 5.5 | 168.1 | 0.5 | 30.55 | 0.10 |
| NUT | 4.6 | 137.1 | 0.5 | 30.04 | 0.11 |
| KOL | 6.4 | 159.7 | 0.4 | 24.80 | 0.05 |
| NAP | 3.0 | 72.4 | 0.1 | 24.39 | 0.04 |
| LYM | 7.6 | 169.7 | 0.3 | 22.34 | 0.04 |
| OPH | 4.4 | 92.4 | 0.2 | 21.16 | 0.05 |
| EVA | 3.5 | 65.5 | 0.2 | 18.88 | 0.05 |
| FAX | 3.7 | 66.9 | 0.2 | 17.91 | 0.05 |
| KIW | 3.8 | 65.1 | 0.2 | 17.21 | 0.04 |
| WON | 4.0 | 68.5 | 0.2 | 17.11 | 0.04 |
| SCE | 4.7 | 79.6 | 0.1 | 17.10 | 0.03 |
| DUN | 15.0 | 242.9 | 0.4 | 16.15 | 0.03 |
| WEN | 6.2 | 7.1 | 0.3 | 11.49 | 0.05 |
| OCT | 17.0 | 154.0 | 0.4 | 9.03 | 0.03 |
| NIK | 12.1 | 74.1 | 0.5 | 6.15 | 0.04 |
WMS: White monkey syndrome
Perinatal Ecological Conditions (Cumulative Rainfall)
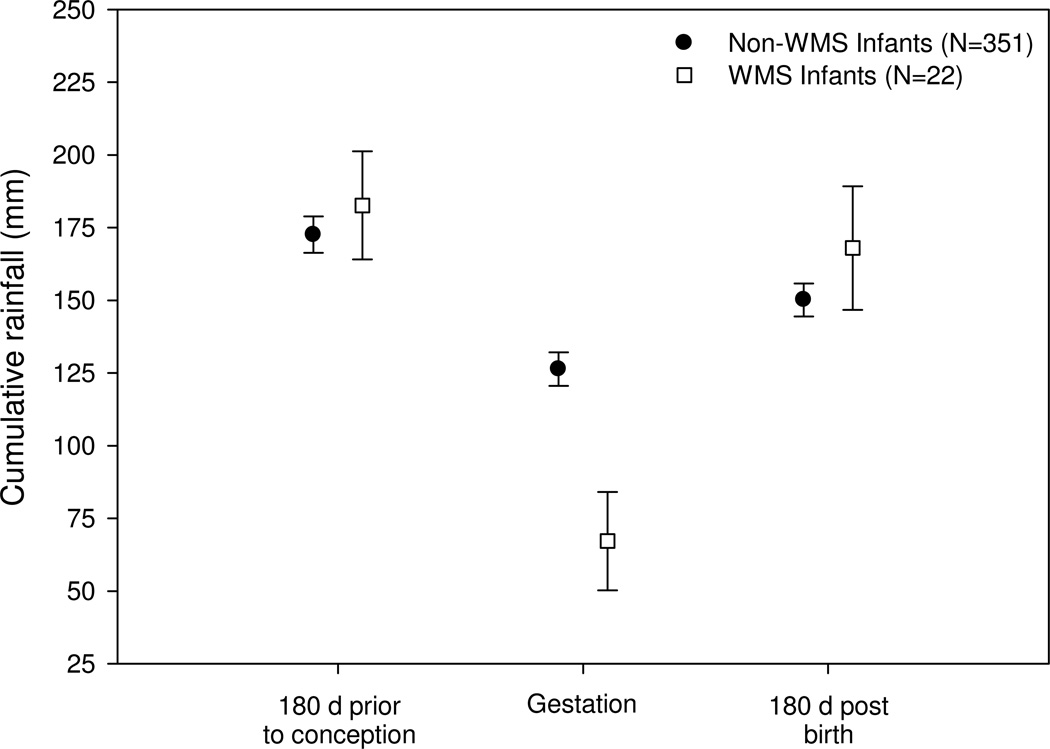
We evaluated variability in ecological conditions predisposing an infant to WMS by comparing cumulative rainfall during 3 time periods: 180 days pre-conception, gestation (~179 days), and 180 days post-birth. Cumulative rainfall differed significantly between WMS infants and non-WMS infants during gestation (one-way ANOVA, F=6.34, df=372, P=0.01) but not in the 180 days prior to conception (one-way ANOVA, F=0.16, df=372, P=0.69) nor in the 180 days post-birth (one-way ANOVA, F=0.57, df=372, P=0.45; Fig. 3).
Fig. 3.
Cumulative rainfall (mm) during the 180 days prior to conception, gestation (~179 days), and 180 days post-birth for white monkey syndrome (WMS) infants (N=22) and non-WMS infants (N=351) born in the study population during 2001–09. Cumulative rainfall differed significantly between WMS infants and non-WMS infants during gestation (one-way ANOVA, F=6.34, df=372, P=0.01) but not in the 180 days prior to conception (one-way ANOVA, F=0.16, df=372, P=0.69) nor in the 180 days post-birth (one-way ANOVA, F=0.57, df=372, P=0.45). Points and error bars represent mean ± SE.
DISCUSSION
Copper is a trace mineral so important for proper growth and development that deficiencies significantly compromise the health and survivorship of afflicted individuals. Our results characterize a pathology in wild savannah baboons that is consistent with symptoms of a disorder known as WMS in laboratory primates and, more broadly, with Cu deficiency as described in a range of species, including humans. We used field observations of physical symptoms and complemented them with a laboratory assessment of trace mineral concentrations in hair samples. Our approach thus balanced long-term, non-invasive monitoring with quantitative measures of trace mineral levels in a subset of individuals from our study population for whom samples were available and therefore could be evaluated retrospectively.
Hair analysis has been widely used for trace mineral studies in both humans and non-humans [e.g. Aydin, 2008; Bencko, 1995; Combs, 1987; Holmes et al., 2003; Kim & Mahan, 2001; Marriott et al., 1986]. This tissue source has a number of advantages including: (1) the stability of hair as a biological material, which facilitates storage and transport; (2) the high concentrations of trace minerals usually found in hair samples, and the resulting high probability of detection, compared to concentrations in blood and urine; and (3) the capacity of hair to accumulate minerals and thus serve as a biomarker of mineral availability during extended time periods rather than a reflection of short-term environmental challenges and nutritional assessment [reviewed in Hambridge, 1982]. Despite these advantages, hair mineral testing can be sensitive to sampling procedures, variability in the age and sex of sampled individuals, conditions at time of collection (e.g. seasonal effects), and exogenous contamination [reviewed in Bozsai, 1992; Combs et al., 1982; Hambridge, 1982; Marriott et al., 1986]. Further, hair analyses performed in commercial laboratories potentially yield inconsistent and unreliable results [e.g. Barrett, 1985; Seidel et al., 2001]. We minimized these potential problems by using consistent field methods for specimen collection, by focusing our sampling and analysis on one age-sex class, and by adhering to a single, calibrated laboratory protocol implemented at Princeton University by the authors.
Several cases of WMS have been previously documented in the Amboseli baboon population [Altmann, 1980; Altmann, 1998; Altmann et al., 1981]. However, prior monitoring was of a much smaller study population, incidents were too infrequent to produce a sample size adequate for characterization until recently, and no earlier work associated the pathology with potential trace mineral deficiencies. In all cases, symptoms described herein are consistent with those listed in these prior accounts.
One aspect of the pathology that is particularly striking is the extent to which observations of symptoms were limited to young baboons. Mean age of symptom onset (approximately 8 months) coincides with the age at which the natal pelage of most infants transitions from black to golden brown/yellow [Altmann et al., 1981]. Individuals at this age are still largely dependent on their mothers for nutrition, protection, and long-distance transportation; dietary independence and increasing exploratory behavior are achieved later in life when individuals are between 12–18 months [Altmann, 1980; Altmann, 1998; Rhine et al., 1985]. Unweaned infant and juvenile mammals are generally more susceptible than adults to Cu deficiencies resulting from the low mineral content of milk [Blood et al., 1983; Salih et al. 1987] coupled with elevated Cu requirements relative to the requirements of individuals in older age-classes [Mills, 1966; Sakai et al., 2000]. When milk is inadequate to provide sufficient Cu intake, the onset of clinical symptoms coincides with depletion of fetal Cu stores [Blood et al., 1983]. The extent to which fetal Cu stores can buffer subsequent deficiency – and thus the duration following parturition before symptoms of deficiency are evident – can vary with the mother’s Cu intake during pregnancy. Cu may be preferentially allocated to the developing fetus by mothers on a diet marginally Cu deficient [Xin et al., 1993], however chronically low Cu intake during gestation can result in deficiency in both mother and offspring [Barone et al., 1998]. Inadequate Cu nutrition of the mother during gestation can have a major impact on fetal [Keen et al., 1998] and subsequent offspring growth and development [Barone et al., 1998; Blood et al., 1983; Gambling & McArdle 2004; Prohaska & Bailey, 1993; Prohaska & Brokate, 2002].
Cu deficiency has been documented in a variety of species in the wild [e.g. Flynn et al., 1977; Shen et al., 2010], including some primate species [e.g. Rode et al., 2003; Rode et al., 2006]. Deficiencies are commonly identified by apparent dietary inadequacies, either through low Cu intake or high intake of minerals that interfere with Cu absorption such as Mo and Zn [reviewed in McDowell, 2003]. Trace mineral concentration in forage is influenced both by the mineral content of soils and species-characteristic variation between plants in mineral uptake. Maskall & Thornton [1996] suggest that soils of Amboseli may contain toxic levels of Mo, consistent with geochemical analyses of volcanic soils in other savannah ecosystems of east Africa [Maskall & Thornton, 1991]. However, we found no differences in hair Mo/Cu ratios between females with WMS offspring and females without WMS offspring. This finding does not support the prediction, based on Maskall & Thornton [1991, 1996], that high Mo concentration in volcanic soils of east Africa is a potential source of subsequent Cu deficiency in this species. In contrast, our results that hair Zn/Cu ratios differed significantly between the same two categories of females is consistent with previous research linking Zn intake with Cu deficiency in general [reviewed in McDowell, 2003] and in laboratory primates afflicted with WMS specifically [Obeck, 1978; Frost et al., 2004]. It further suggests that Zn may be at toxic levels for baboons in this region’s soils or vegetation. Further research is needed to understand the underlying factors that contribute to Cu deficiency in this ecosystem, in particular the spatial-temporal bioavailability of Cu itself as well as other trace minerals that can induce Cu deficiency.
Infants that developed WMS experienced half the rainfall during gestation, on average, that non-WMS infants experienced. This supports our hypothesis that rainfall in the prenatal period distinguishes WMS and non-WMS individuals, and highlights the importance of ecological conditions in healthy fetal development with regard to WMS. In particular, environmental challenges that the fetus experiences may be detrimental even when buffered by favorable conditions prior to conception, and may also prove irreversible even under favorable postnatal conditions. Because rainfall is unlikely to have a direct effect on an animal’s trace mineral concentration, the suggested association between relative mineral concentrations in the baboon hair and rainfall probably results from an indirect effect propagated by environmental factors that influence trace mineral bioavailability. One plausible explanation is that soil moisture affects the trace mineral concentration in plants and soil [Howard et al., 1962; Shuman, 1980]. Particularly in tropical savannah ecosystems, highly seasonal rainfall patterns can result in either severe depletion of elements or accumulation to toxic levels [reviewed in Dissanayake & Chandrajith, 1999]. Thus, animals may be exposed to variable concentrations of trace minerals under different rainfall conditions even when making the same food-selection decisions. Of particular relevance to savannah habitats like Amboseli, Dharani and colleagues [2007] found that Cu concentrations in Acacia xanthophloea were significantly higher in samples collected during wet season than dry season months. A. xanthophloea is one of two dominant tree species in our study site and baboons within this population rely heavily upon the species as food resource, consuming its leaves, bark, sap, flowers, and pods [Altmann, 1998].
Our results also reveal intriguing inter-individual variability in Zn/Cu ratios within the study population. Higher Zn/Cu ratios are expected in Cu deficient individuals and our analyses of hair from a single WMS infant supports findings in previous research that Cu deficiency induced by Zn toxicity underlies WMS symptoms [Frost et al., 2004; Obeck, 1978]. Because individuals afflicted with WMS in our population were almost exclusively nursing offspring, a strong correlation in trace mineral toxicity/deficiency between mothers and infants is expected. This prediction is supported by our results of higher Zn/Cu ratios in females who had a WMS offspring during the study period relative to females who did not. It is noteworthy that mothers of WMS infants had higher Zn/Cu ratios despite the fact that the hair samples we analyzed from these mothers were not collected near the time of the WMS offspring’s birth (a reflection of our post-hoc analyses of hair samples from the subset of animals targeted as darting candidates for other ABRP research objectives). This suggests the possibility of stable individual differences in vulnerability to low Cu levels induced by Zn toxicity, which may then conditionally result in offspring pathology. Whether genetic variation within the population influences a proclivity to Cu deficiency – and how this is affected by ecological conditions during gestation – is an important unanswered question. The extent to which genetic and environmental factors together affect Cu deficiency is not known but may explain why (1) many females with WMS offspring also had non-WMS offspring and (2) some females with relatively high Zn/Cu ratios did not have WMS offspring.
One of the major limitations we faced in this study was an inability to detect subclinical Cu deficiency. Although extreme cases of deficiency were readily identifiable, some individuals may have had low Cu levels in the absence of whitening fur and/or impaired hindlimb mobility. Consequently, we may not have visually identified all individuals that experienced Cu deficiency. However, our findings that hair sample analysis may provide a reliable biomarker for baboons susceptible to low Cu levels suggest that broader application of this technique to include more individuals may reveal important patterns of Cu limitation in this population. More generally, given the known impact Cu deficiency can have on immune system function [reviewed in Bonham et al., 2002] and the effects of mineral availability on population densities [McNaughton, 1988; Milewski, 2000; Oates et al., 1990], such additional studies would offer substantial contribution to the understanding of nutritional limitations on population dynamics in wild animals.
ACKNOWLEDGMENTS
We are grateful to the government of the Republic of Kenya, to the Kenya Wildlife Services, the staff and wardens of Amboseli National Park, and the local community of the Amboseli region. Tremendous thanks go to ABRP researchers for their contributions to data collection and dedication in the field: R. Mututua, S. Sayialel, and J.K. Warutere. We also thank: M. Akinyi, C. Fitzpatrick, N. Learn, L. Roerish, J. Stroud, and J. Tung for their invaluable assistance. We benefited from A. Williams’ preliminary research on WMS in the Amboseli baboon population; her contribution enhanced our understanding of this pathology in the region. Lastly, we thank F. Morel for greatly contributing to the study’s success by making his laboratory available for mineral analysis and by offering helpful suggestions during numerous discussions with the authors, and D.C. Dunbar and M.J. Kessler for their comments on an earlier version of the manuscript. Financial support was provided by American Society of Primatologists (to A.C.M.), Animal Behavior Society (to A.C.M.), International Primatological Society (to A.C.M.), NSF (IBN-0322613 to J.A. and S.C.A. and BCS-0851750 to A.C.M.), and Sigma Xi (to A.C.M.).
REFERENCES
- Adogwa AO, Alleyne T, Mohammed A. Degenerative changes in the cerebral cortex in swayback disease lambs. Alzheimer’s Disease Review. 1999;4:19–22. [Google Scholar]
- Alberts SC, Hollister-Smith JA, Mututua RS, Sayialel SN, Muruthi PM, Warutere JK, Altmann J. Brockman DK, van Schaik CP. Seasonality in primates: Studies of living and extinct human and non-human primates. Cambridge: Cambridge University Press; 2005. Seasonality and long term change in a savanna environment; pp. 157–195. [Google Scholar]
- Altmann J. Baboon mothers and infants. Chicago: University of Chicago Press; 1980. [Google Scholar]
- Altmann J, Altmann S, Hausfater G. Physical maturation and age estimates of yellow baboons, Papio cynocephalus, in Amboseli National Park, Kenya. American Journal of Primatology. 1981;1:389–399. doi: 10.1002/ajp.1350010404. [DOI] [PubMed] [Google Scholar]
- Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM. Body size and fatness of free-living baboons reflect food availability and activity levels. American Journal of Primatology. 1993;30:149–161. doi: 10.1002/ajp.1350300207. [DOI] [PubMed] [Google Scholar]
- Altmann SA. Foraging for survival: Yearling baboons in Africa. Chicago: University of Chicago Press; 1998. [Google Scholar]
- Aydin I. Comparison of dry, wet and microwave digestion procedures for the determination of chemical elements in wool samples in Turkey using ICP-OES techniques. Microchemical Journal. 2008;90:82–87. [Google Scholar]
- Barone A, Ebesh O, Harper RG, Wapnir RA. Placental copper transport in rats: Effects of elevated dietary zinc on fetal copper, iron and metallothionein. The Journal of Nutrition. 1998;128:1037–1041. doi: 10.1093/jn/128.6.1037. [DOI] [PubMed] [Google Scholar]
- Barrett S. Commercial hair analysis: Science or scam? The Journal of the American Medical Association. 1985;254:1041–1045. [PubMed] [Google Scholar]
- Beehner JC, Onderdonk DA, Alberts SC, Altmann J. The ecology of conception and pregnancy in wild baboons. Behavioral Ecology. 2006;17:741–750. [Google Scholar]
- Bencko V. Use of human hair as a biomarker in the assessment of exposure to pollutants in occupational and environmental settings. Toxicology. 1995;101:29–39. doi: 10.1016/0300-483x(95)03018-b. [DOI] [PubMed] [Google Scholar]
- Blood DC, Radostits OM, Henderson JA. Veterinary Medicine. 6th ed. London: Baillière Tindall; 1983. [Google Scholar]
- Bonham M, O’Connor JM, Hannigan BM, Strain JJ. The immune system as a physiological indicator of marginal copper status? British Journal of Nutrition. 2002;87:393–403. doi: 10.1079/BJNBJN2002558. [DOI] [PubMed] [Google Scholar]
- Bozsai G. Quality control and assurance in hair analysis. Microchemical Journal. 1992;46:159–166. [Google Scholar]
- Combs DK. Hair analysis as an indicator of mineral status of livestock. Journal of Animal Science. 1987;65:1753–1758. doi: 10.2527/jas1987.6561753x. [DOI] [PubMed] [Google Scholar]
- Combs DK, Goodrich RD, Meiske JC. Mineral concentrations in hair as indicators of mineral status: A review. Journal of Animal Science. 1982;54:391–398. doi: 10.2527/jas1982.542391x. [DOI] [PubMed] [Google Scholar]
- Cox DW, Cullen LM, Forbes JR. Genetic susceptibility to heavy metals in the environment. In: Sarkar B, editor. Heavy metals in the environment. New York: Marcel Dekker, Inc; 2002. pp. 549–586. [Google Scholar]
- Culotta VC, Gitlin JD. Disorders of copper transport. In: Scriver CR, Sly WS, Childs B, Beaudet AL, Valle D, Kinzler KW, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 2000. pp. 3105–3126. [Google Scholar]
- de Bie P, Muller P, Wijmenga C, Klomp LWJ. Molecular parthogenesis of Wilson and Menkes disease: Correlation of mutations with molecular defects and disease phenotypes. Journal of Medical Genetics. 2007;44:673–688. doi: 10.1136/jmg.2007.052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharani N, Onyari JM, Maina DM, Mavuti KM. The distribution of Cu and Pb levels in soils and Acacia xanthophloea Benth. from Lake Nakuru National Park Kenya. Bulletin of Environmental Contamination and Toxicology. 2007;79:172–177. doi: 10.1007/s00128-007-9138-2. [DOI] [PubMed] [Google Scholar]
- Dissanayake CB, Chandrajith R. Medical geochemistry of tropical environments. Earth-Science Reviews. 1999;47:219–258. [Google Scholar]
- Flynn A, Franzmann AW, Arneson PD, Oldemeyer JL. Indications of copper deficiency in a subpopulation of Alaskan moose. The Journal of Nutrition. 1977;107:1182–1189. doi: 10.1093/jn/107.7.1182. [DOI] [PubMed] [Google Scholar]
- Frost PA, Hubbard GB, Dammann MJ, Snider CL, Moore CM, Hodara VL, Giavedoni LD, Rohwer R, Mahaney MC, Butler TM, Cummins LB, McDonald TJ, Nathanielsz PW, Schlabritz-Loutsevitch NE. White monkey syndrome in infant baboons (Papio species) Journal of Medical Primatology. 2004;33:197–213. doi: 10.1111/j.1600-0684.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Gambling L, McArdle HJ. Iron, copper and fetal development. Proceedings of the Nutrition Society. 2004;63:553–562. doi: 10.1079/pns2004385. [DOI] [PubMed] [Google Scholar]
- Hambridge KM. Hair analyses: Worthless for vitamins, limited for minerals. The American Journal of Clinical Nutrition. 1982;36:943–949. doi: 10.1093/ajcn/36.5.943. [DOI] [PubMed] [Google Scholar]
- Hay RL, Hughes RE, Kyser TK, Glass HD, Liu J. Magnesium-rich clays of the meerschaum mines in the Amboseli basin, Tanzania and Kenya. Clays and Clay Minerals. 1995;43:455–466. [Google Scholar]
- Holmes AS, Blaxill MF, Haley BE. Reduced levels of mercury in first baby haircuts of autistic children. International Journal of Toxicology. 2003;22:277–285. doi: 10.1080/10915810305120. [DOI] [PubMed] [Google Scholar]
- Howard DA, Burdin ML, Lampkin GH. Variation in mineral and crude-protein content of pastures at Muguga in the Kenya highlands. The Journal of Agricultural Science. 1962;59:251–256. [Google Scholar]
- Keen CL, Uriu-Hare JY, Hawk SN, Jankowski MA, Daston GP, Kwid-Uribe CL, Rucker RB. Effect of copper deficiency on prenatal development and pregnancy outcome. The American Journal of Clinical Nutrition. 1998;67S:1003S–1011S. doi: 10.1093/ajcn/67.5.1003S. [DOI] [PubMed] [Google Scholar]
- Kim YY, Mahan DC. Effect of dietary selenium source, level, and pig hair color on various selenium indices. Journal of Animal Science. 2001;79:949–955. doi: 10.2527/2001.794949x. [DOI] [PubMed] [Google Scholar]
- Marriott BM, Smith JC, Jr, Jacobs RM, Lee Jones AO, Rawlins RG, Kessler MJ. Hair mineral content as an indicator of mineral intake in rhesus monkeys (Macaca mulatta) In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques: History, behavior and biology. New York: State University of New York Press; 1986. pp. 219–231. [Google Scholar]
- Maskall J, Thornton I. Trace element geochemistry of soils and plants in Kenyan conservation areas and implications for wildlife nutrition. Environmental Geochemistry and Health. 1991;13:93–107. doi: 10.1007/BF01734300. [DOI] [PubMed] [Google Scholar]
- Maskall J, Thornton I. The distribution of trace and major elements in Kenyan soil profiles and implications for wildlife nutrition. In: Appleton JD, Fuge R, McCall GJH, editors. Environmental geochemistry and health with special reference to developing countries. Geological Society Special Publication No. 113. London: The Geological Society; 1996. pp. 47–63. [Google Scholar]
- McDowell LR. Minerals in animal and human nutrition. 2nd ed. London: Elsevier Press; 2003. [Google Scholar]
- McNaughton SJ. Mineral nutrition and spatial concentrations of African ungulates. Nature. 1988;334:343–345. doi: 10.1038/334343a0. [DOI] [PubMed] [Google Scholar]
- Milewski A. Iodine as a possible controlling nutrient for elephant populations. Pachyderm. 2000;28:78–90. [Google Scholar]
- Mills CF. Trace element deficiency in livestock in Europe. World Review of Animal Production. 1966;2:51–57. [Google Scholar]
- Norton GW, Rhine RJ, Wynn GW, Wynn RD. Baboon diet: A five-year study of stability and variability in the plant feeding and habitat of the yellow baboons (Papio cynocephalus) of Mikumi National Park, Tanzania. Folia Primatologica. 1987;48:78–120. doi: 10.1159/000156287. [DOI] [PubMed] [Google Scholar]
- Novak MA, Meyer JS. Alopecia: Possible causes and treatments, particularly in captive nonhuman primates. Comparative Medicine. 2009;59:18–26. [PMC free article] [PubMed] [Google Scholar]
- Oates JP, Whitesides GH, Davies AG, Waterman PG, Green SM, Dasilva GL, Mole S. Determinants of variation in tropical forest primate biomass: new evidence from West Africa. Ecology. 1990;71:328–343. [Google Scholar]
- Obeck DK. Galvanized caging as a potential factor in the development of the “fading infant” or “white monkey” syndrome. Laboratory Animal Science. 1978;28:698–704. [PubMed] [Google Scholar]
- Prohaska JR, Bailey WR. Persistent regional changes in brain copper, cuproenzymes and catecholamines following perinatal copper deficiency in mice. The Journal of Nutrition. 1993;123:1226–1234. doi: 10.1093/jn/123.7.1226. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Brokate B. The timing of perinatal copper deficiency in mice influences offspring survival. The Journal of Nutrition. 2002;132:3142–3145. doi: 10.1093/jn/131.10.3142. [DOI] [PubMed] [Google Scholar]
- Rhine RJ, Norton GW, Wynn GM, Wynn RD. Weaning of free-ranging infant baboons (Papio cynocephalus) as indicated by one-zero and instantaneous sampling of feeding. International Journal of Primatology. 1985;6:491–499. [Google Scholar]
- Rode KD, Chapman CA, Chapman LJ, McDowell LR. Mineral resource availability and consumption by colobus in Kibale National Park, Uganda. International Journal of Primatology. 2003;24:541–573. [Google Scholar]
- Rode KD, Chapman CA, McDowell LR, Stickler C. Nutritional correlates of population density across habitats and logging intensities in redtail monkeys (Cercopithecus ascanius) Biotropica. 2006;38:625–634. [Google Scholar]
- Sakai T, Wariishi M, Nishiyama K. Changes in trace element concentration in hair of growing children. Biological Trace Element Research. 2000;77:43–51. doi: 10.1385/BTER:77:1:43. [DOI] [PubMed] [Google Scholar]
- Salih Y, McDowell LR, Hentges JF, Mason RM, Jr, Wilcox CJ. Mineral content of milk, colostrum, and serum as affected by physiological state and mineral supplementation. Journal of Dairy Science. 1987;70:608–612. doi: 10.3168/jds.S0022-0302(87)80048-6. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Altmann J. Incidence of hypercortisolism and dexamathasone resistance increases with age among wild baboons. Biological Psychiatry. 1991;30:1008–1016. doi: 10.1016/0006-3223(91)90121-2. [DOI] [PubMed] [Google Scholar]
- Seidel S, Kreutzer R, Smith D, McNeel S, Gilliss D. Assessment of commercial laboratories performing hair mineral analysis. The Journal of the American Medical Association. 2001;285:67–72. doi: 10.1001/jama.285.1.67. [DOI] [PubMed] [Google Scholar]
- Shen X, Li X, Zhang R. Studies of “unsteady gait disease” of the Tibetan gazelle (Procapra picticaudata) Journal of Wildlife Diseases. 2010;46:560–563. doi: 10.7589/0090-3558-46.2.560. [DOI] [PubMed] [Google Scholar]
- Shuman LM. Effects of soil temperature, moisture, and air-drying on extractable manganese, iron, copper, and zinc. Soil Science. 1980;130:336–343. [Google Scholar]
- Stoessell RK, Hay RL. The geochemical origin of sepiolite and kerolite at Amboseli, Kenya. Contributions to Mineralogy and Petrology. 1978;65:255–267. [Google Scholar]
- Vonk WIM, Wijmenga C, van de Sluis B. Relevance of animal models for understanding mammalian copper homeostasis. The American Journal of Clinical Nutrition. 2008;88S:840S–845S. doi: 10.1093/ajcn/88.3.840S. [DOI] [PubMed] [Google Scholar]
- Xin Z, Waterman DF, Hemken RW, Harmon RK. Copper status and requirement during the dry period and early lactation in multiparous Holstein cows. Journal of Dairy Science. 1993;76:2711–2716. doi: 10.3168/jds.S0022-0302(93)77607-9. [DOI] [PubMed] [Google Scholar]