Abstract
Purpose
Although the etiology of breast cancer is multifactorial, oxidative stress plays an important role in carcinogenesis. In this study, manganese superoxide dismutase (MnSOD) gene polymorphism and activity were evaluated in benign and breast cancer tissue.
Methods
One hundred and one females were enrolled in this study, 65 who were histopathologically diagnosed with breast cancer and 46 who were benign patients. MnSOD enzyme activity was determined using an indirect competitive inhibition assay and MnSOD gene polymorphism using poly merase chain reaction and agarose gel electrophoresis.
Results
MnSOD enzymatic activity (79.83±42.14) was lower in breast cancer tissue compared to benign tumors (236.18±46.37). At the same time, MnSOD enzymatic activity among Ala/Val patients was significantly lower in breast cancer tissue (39.19±7.33) than in Val/Val malignant breast tumors tissue (96.9±22.9). MnSOD enzymatic activity was significantly lower in Val/Val cancer tissue (96.9±22.9) than in benign tissue (255.44±42.7).
Conclusion
Breast cancer tumors contain less MnSOD than benign breast samples. Patients with Ala/Val polymorphism had reduced MnSOD activity compared to patients with Val/Val breast cancer. Ala/Val gene polymorphism may be a risk factor associated with more advanced breast cancer stage. MnSOD gene polymorphism Ala/Val may be a risk factor associated with more advanced breast cancer stage, and reduction of MnSOD activity may be a mechanism of the progression of benign to malignant tumors. Further investigations are needed to evaluate the role of MnSOD in breast cancer progression.
Keywords: Benign, Breast, Cancer, MnSOD, Polymorphism
INTRODUCTION
Manganese superoxide dismutase (MnSOD) is a metalloenzyme that converts superoxide to hydrogen peroxide and quenches the free radicals generated by the electron transport chain [1]. MnSOD plays a key role in protecting cells against oxidative damage [2] and regulating cellular concentrations of reactive oxygen species (ROS). Altered levels of this crucial enzyme play a significant role in disease development including cancer [3] by activating mitogen-activated protein kinase, which stimulates cell proliferation, and enhancing tumor cell migration [3-9]. Several studies have shown that MnSOD levels were significantly increased in tumor cells compared to controls [9], while other studies showed that serum superoxide and catalase activities were significantly decreased in patients with breast cancer compared to controls [10]. Forced overexpression of MnSOD enzyme inhibits malignant transformation and suppresses tumor growth in a variety of cancer cells both in vitro and in vivo [11-14]. Although MnSOD levels are reduced prior to cancer initiation [15], the exact cellular mechanism by which MnSOD suppresses the malignant phenotype is not yet clear.
The MnSOD gene (locus 6q25) contains the C47T single nucleotide protein, which results in an Ala16Val amino acid substitution [16]. A genetic variant of MnSOD was identified as a T to C substitution in the mitochondrial targeting sequence that changes the amino acid from valine (GTT) to alanine (GCT), leading to structural alteration of the enzyme conformation [17]. This alteration may affect the cellular allocation of MnSOD within the mitochondria; therefore, MnSOD leaves the mitochondria without defense against superoxide radicals [16]. The alanine (Ala) allele of MnSOD allows for efficient transport into the mitochondrial matrix and generates 30% to 40% more active MnSOD protein than the valine (Val) form of the enzyme [18]. The Val allele is partially arrested in the inner mitochondrial membrane, leading to decreased formation of active MnSOD within the mitochondrial matrix [16,17].
Studies have shown a controversial association between MnSOD gene polymorphism and breast cancer, and Mitrunen et al. [6] and Cai et al. [8] reported that the Ala allele increased the risk of breast cancer. On the contrary, Qiu et al. [19] and Eras-Erdogan et al. [20] reported no association. Therefore, it would be interesting to study the correlation between MnSOD gene polymorphism and its enzymatic activity in benign and malignant human breast tissue, which may clarify the role of MnSOD in breast cancer progression.
METHODS
Sixty-five females attending King Hussein Medical City (Amman) in 2008, Hamzah Hospital (Amman) in 2010 to 2011, and Al-Basheer Hospital in 2011 (Amman) were enrolled in this study. All females were histopathologically diagnosed with malignant or benign breast cancer. All breast cancer cases were staged from I to IV depending on the combination of Tumor, Node, Metastasis classification according to the National Cancer Institute and National Institutes of Health (USA) and revised by the American Joint Committee on Cancer staging system for breast cancer [21].
Seventeen control females were histopathologically proven free of disease. All participants provided informed consent and were interviewed personally by a professional technician. Data were collected about the participants' medical history and cancer grade and stage before the samples were collected. Biopsies were collected via fine needle aspiration or surgical biopsy by an oncologist.
MnSOD biochemical activity of the breast biopsies was measured from breast tissue homogenate using a modified protocol that was kindly provided by Spitz and Oberley [22]. Breast biopsies were washed in saline immediately after excision to remove excess blood cells, placed in Eppendorf tubes (Promega, Madison, USA) and stored at -60℃. Biopsies were minced on ice in 300 µL of diethylenetriaminepentaacetic acid buffer, homogenized five times for 7 seconds, burst on ice using an Ultra-Turrax Homogenizer (IKA Labortechnik, Staufen, Germany), and centrifuged at 1,000 rpm for 3 minutes. The tissue homogenates were then placed in Eppendorf tubes and stored at -60℃ for protein analysis. Protein concentrations (µg/mL) were determined according to the Lowry method. MnSOD enzymatic activity was then determined using an indirect competitive inhibition assay based on the principle that O2 will reduce an indicator substrate nitroblue tetrazolium (NBT) and superoxide dismutase (SOD) activity will inhibit the reduction rate in a competitive fashion. SOD-mediated inhibition (% inhibition) of the indicator substrate reduction was plotted as a function of the quantity of protein added to the reaction to construct an inhibition curve. The amount of cellular protein that caused 50% maximum inhibition was then calculated and defined as one unit of SOD activity [22].
Dilutions from each homogenate sample or CuZnSOD standard were prepared prior to the assay in 0.05 M phosphate buffer pH 7.8 such that each 100-µL volume added to the assay contained increasing quantities of protein (1-500 µg) of protein for each sample homogenate or (2-500 ng) of protein for the CuZnSOD standard. Inactivation of CuZnSOD by NaCN was determined by incubation of the assay tubes containing 100 µL of sample or standard mixed with 800 µL of final assay solution for at least 45 minutes at room temperature. Xanthine oxidase solution (100 µL) was then added to initiate the reaction starting with the blank tube (containing 100 µL of phosphate buffer instead of sample) to check for xanthine oxidase concentration and make sure that the preparation produced the desired NBT reduction rate (ideally 0.02 abs/min). The reaction was incubated for 1 minute at room temperature and the absorbance at 560 nm was read every 15 seconds for 2.5 minutes. The rates of abs/min for the blank and the samples were used in calculating percent inhibition. An inhibition curve was established for each sample and standard by plotting of the percent inhibition (%) versus protein quantities (µg), while the maximum inhibition was determined for each curve and used to calculate MnSOD activity units defined as the amount of protein that inhibited NBT reduction by 50% of the maximum inhibition. Finally, units of total MnSOD activity per milligram of protein were calculated for the tissue homogenate samples by taking 1,000 µg of protein per unit.
Genomic DNA was extracted from all blood samples using a Wizard Genomic DNA Purification Kit (Promega, Madison, USA) and stored at -60℃ until use. MnSOD gene polymorphism was determined as reported previously [8] using the polymerase chain reaction (PCR) and restriction fragment length polymorphisms method. The PCR product was digested with the NgoMIV restriction enzyme and separated on a 4% agarose gel. The genotypes were determined as heterozygous Ala/Val (18 bp, 89 bp, and 107 bp) bands, homozygous Val/Val (107 bp) band and homozygous Ala/Ala (18 bp and 89 bp).
Statistical analysis was carried out using the SPSS version 10.05 (SPSS Inc., Chicago, USA). Means and standard errors of the mean (SE) were calculated and compared among study groups using analysis of variance.
RESULTS
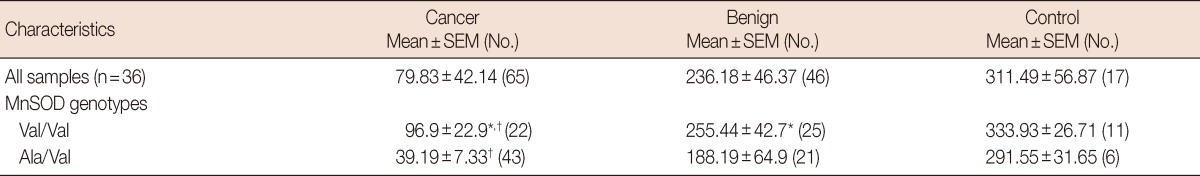
MnSOD concentrations from the breast tissue homogenates were determined from 65 breast cancer biopsies, 46 benign tissue samples, and 17 controls. MnSOD biochemical activity from benign tissue (236.18±46.37) was higher but not statistically different (p=0.087) from that of cancer tissue (79.83±42.14) as shown in Table 1.
Table 1.
MnSOD biochemical activity (Unit/mg) among breast cancer and benign biopsies stratified by MnSOD genotypes and its association with both benign and breast cancer risk
MnSOD=manganese superoxide; SEM=standard error of the mean.
*p≤0.05 values were calculated for breast cancer, normal vs. benign patients; †p≤0.05 values were calculated for Val/Val genotype versus Ala/Val genotype differences among each group.
The association among MnSOD biochemical activity in patients with breast cancer, those with benign tissue, and MnSOD genotype was evaluated. There were significant differences in MnSOD activity between the patients with breast cancer (96.9±22.9) and those with benign tissue (255.44±42.7) with Val/Val genotype (p=0.045) as presented in Table 1. Patients with breast cancer who had the Val/Val genotype had significantly higher MnSOD activity (96.19±22.9) than patients with breast cancer who had the Ala/Val genotype (39.19±7.33) (p=0.005). No statistical analysis was conducted for the Ala/Ala genotype due to the absence of this genotype in patients with either breast cancer or benign tissue.
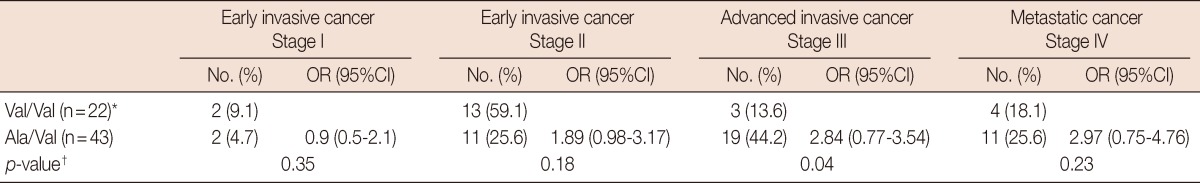
Table 2 shows that the highest frequency (44.2%) of the Ala/Val genotype was associated with advanced invasive cancer (stage III), the while highest frequency of the Val/Val genotype (59.1%) was seen in early cancer (stage II).
Table 2.
Frequency of MnSOD (Val/Val) and (Ala/Val) genotypes among breast cancer females
MnSOD=manganese superoxide dismutase; OR (95%CI)=odds ratio and 95% confidence interval for MnSOD Ala/Val compared Val/Val as a reference group. *Reference group; †p-values were calculated for Val/Val vs. Ala/Val differences.
DISCUSSION
Malignant breast cancer and benign disorders are common diseases among women that cause serious physical and emotional problems for patients and their families. Evidence indicates that mitochondrial oxidative stress may be involved in the development of breast cancer primarily through MnSOD, the major site of ROS production [23], which decreases in metastatic esophageal [24], glioblastoma [25], and other cancers.
To our knowledge, this is the first study that measures MnSOD enzyme activity in human representative mammalian tissues with malignant and benign characteristics. Studies have used human erythrocytes [23], human mammalian cell lines [9], fixed breast tissue samples [26], and human hepatoma cell lines [23]. This study showed that MnSOD activity was lower in cancerous tissues than in benign tissues with the Val/Val phenotype (Table 1), supporting that the MnSOD gene is a tumor suppressor gene [26]. The mechanism responsible for this reduction remains unclear, although it may be attributed to an epigenetic silencing effect of MnSOD [27].
Interestingly, there was a statistically significant difference in MnSOD activity between Val/Val and Ala/Val MnSOD gene polymorphism among cancerous tissue samples only. The Alavariant genotype confers lower MnSOD activity than the Valvariant, which supports the hypothesis described elsewhere [17] that the transport of the Val-variant of MnSOD may be partially arrested in the inner mitochondrial membrane, while the Ala-variant of the MnSOD successfully targets the mitochondria. Ala/Val disrupts the proper targets on MnSOD from the cytosol to the mitochondrial matrix, where it acts on highly reactive superoxide radicals to dismutate it to hydrogen peroxide. Therefore, the change in the level of highly reactive superoxide radicals and SOD in the mitochondria modulates the molecular mechanism of apoptosis, cellular adhesion, and cell proliferation and, thus, plays a key role in cancer development [28]. Our results also provide additional evidence that MnSOD polymorphism is an important modifier of oxidative stress since MnSOD is the primary antioxidant in mitochon dria, which converts superoxide radicals into hydrogen peroxide. Thus, inefficient MnSOD activity among Ala/Val could leave mitochondria without their full defense against superoxide radicals [16], which could lead to oxidative damage and, consequently, cancer advanced metastasis [5].
Patients with the Ala/Val genotype variant conferred low levels of MnSOD activity and were at increased risk of advanced cancer stage compared to the Val/Val variant. The highest frequency of the Val/Ala variant was seen in advanced invasive stage III (Table 2), while the highest frequency of the Val/Val variant was seen in early invasive stage II. These results were similar to those observed by Kucukgergin et al. [29]. Furthermore, these results supported that such oxidative stress could be involved in the development and progression of breast cancer since benign tumors have higher MnSOD levels than do malignant tumors, so if MnSOD activity decreased in benign tumors, oxidative damage would accumulate and, consequently, benign tumors might progress into malignant tumors.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Holley AK, Dhar SK, Xu Y, St Clair DK. Manganese superoxide dismutase: beyond life and death. Amino Acids. 2012;42:139–158. doi: 10.1007/s00726-010-0600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gago-Dominguez M, Jiang X, Castelao JE. Lipid peroxidation, oxidative stress genes and dietary factors in breast cancer protection: a hypothesis. Breast Cancer Res. 2007;9:201. doi: 10.1186/bcr1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol. 2004;14:259–266. doi: 10.1016/j.semradonc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Wang LI, Miller DP, Sai Y, Liu G, Su L, Wain JC, et al. Manganese superoxide dismutase alanine-to-valine polymorphism at codon 16 and lung cancer risk. J Natl Cancer Inst. 2001;93:1818–1821. doi: 10.1093/jnci/93.23.1818. [DOI] [PubMed] [Google Scholar]
- 6.Mitrunen K, Sillanpää P, Kataja V, Eskelinen M, Kosma VM, Benhamou S, et al. Association between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis. 2001;22:827–829. doi: 10.1093/carcin/22.5.827. [DOI] [PubMed] [Google Scholar]
- 7.Millikan RC, Player J, de Cotret AR, Moorman P, Pittman G, Vannappagari V, et al. Manganese superoxide dismutase Ala-9Val polymorphism and risk of breast cancer in a population-based case-control study of African Americans and whites. Breast Cancer Res. 2004;6:R264–R274. doi: 10.1186/bcr786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Q, Shu XO, Wen W, Cheng JR, Dai Q, Gao YT, et al. Genetic polymorphism in the manganese superoxide dismutase gene, antioxidant intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Breast Cancer Res. 2004;6:R647–R655. doi: 10.1186/bcr929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhopadhyay S, Das SK, Mukherjee S. Expression of Mn-superoxide dismutase gene in nontumorigenic and tumorigenic human mammary epithelial cells. J Biomed Biotechnol. 2004;2004:195–202. doi: 10.1155/S1110724304401016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Negahdar M, Djalali M, Abtahi H, Sadeghi MR, Aghvami T, Javadi E, et al. Blood superoxide dismutase and catalase activities in women affected with breast cancer. Iran J Public Health. 2005;34:39–43. [Google Scholar]
- 11.Amstad PA, Liu H, Ichimiya M, Berezesky IK, Trump BF. Manganese superoxide dismutase expression inhibits soft agar growth in JB6 clone41 mouse epidermal cells. Carcinogenesis. 1997;18:479–484. doi: 10.1093/carcin/18.3.479. [DOI] [PubMed] [Google Scholar]
- 12.Lam EW, Zwacka R, Seftor EA, Nieva DR, Davidson BL, Engelhardt JF, et al. Effects of antioxidant enzyme overexpression on the invasive phenotype of hamster cheek pouch carcinoma cells. Free Radic Biol Med. 1999;27:572–579. doi: 10.1016/s0891-5849(99)00109-4. [DOI] [PubMed] [Google Scholar]
- 13.Li JJ, Colburn NH, Oberley LW. Maspin gene expression in tumor suppression induced by overexpressing manganese-containing superoxide dismutase cDNA in human breast cancer cells. Carcinogenesis. 1998;19:833–839. doi: 10.1093/carcin/19.5.833. [DOI] [PubMed] [Google Scholar]
- 14.Oberley LW, Oberley TD. Role of antioxidant enzymes in the cancer phenotype. In: Massaro D, Clerch LB, editors. Oxygen, Gene Expression, and Cellular Function. New York: M. Dekker; 1997. pp. 279–307. [Google Scholar]
- 15.Zhao Y, Chaiswing L, Velez JM, Batinic-Haberle I, Colburn NH, Oberley TD, et al. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 2005;65:3745–3750. doi: 10.1158/0008-5472.CAN-04-3835. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblum JS, Gilula NB, Lerner RA. On signal sequence polymorphisms and diseases of distribution. Proc Natl Acad Sci U S A. 1996;93:4471–4473. doi: 10.1073/pnas.93.9.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimoda-Matsubayashi S, Matsumine H, Kobayashi T, Nakagawa-Hattori Y, Shimizu Y, Mizuno Y. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson's disease. Biochem Biophys Res Commun. 1996;226:561–565. doi: 10.1006/bbrc.1996.1394. [DOI] [PubMed] [Google Scholar]
- 18.Sutton A, Khoury H, Prip-Buus C, Cepanec C, Pessayre D, Degoul F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003;13:145–157. doi: 10.1097/01.fpc.0000054067.64000.8f. [DOI] [PubMed] [Google Scholar]
- 19.Qiu LX, Yao L, Mao C, Chen B, Zhan P, Yuan H, et al. Lack of associa tion between MnSOD Val16Ala polymorphism and breast cancer risk: a meta-analysis involving 58,448 subjects. Breast Cancer Res Treat. 2010;123:543–547. doi: 10.1007/s10549-010-0777-3. [DOI] [PubMed] [Google Scholar]
- 20.Eras-Erdogan N, Akbas E, Senli H, Kul S, Colak T. Relationship between polymorphism in the manganese superoxide dismutase gene and breast cancer. Mutat Res. 2009;680:7–11. doi: 10.1016/j.mrgentox.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Atoum MF, Hourani HM, Shoter A, Al-Raheem SN, Al Muhrib TK. TNM staging and classification (familial and nonfamilial) of breast cancer in Jordanian females. Indian J Cancer. 2010;47:194–198. doi: 10.4103/0019-509X.63022. [DOI] [PubMed] [Google Scholar]
- 22.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 23.Tamimi RM, Hankinson SE, Spiegelman D, Colditz GA, Hunter DJ. Manganese superoxide dismutase polymorphism, plasma antioxidants, cigarette smoking, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:989–996. [PubMed] [Google Scholar]
- 24.Sun GG, Wang YD, Chen LQ, Wang SJ, Liu GL, Yu XR, et al. Novel cancer suppressor gene for esophageal cancer: manganese superoxide dismutase. Dis Esophagus. doi: 10.1111/j.1442-2050.2010.01149.x. In press. [DOI] [PubMed] [Google Scholar]
- 25.Park CK, Jung JH, Moon MJ, Kim YY, Kim JH, Park SH, et al. Tissue expression of manganese superoxide dismutase is a candidate prognostic marker for glioblastoma. Oncology. 2009;77:178–181. doi: 10.1159/000231888. [DOI] [PubMed] [Google Scholar]
- 26.Baker AM, Oberley LW, Cohen MB. Expression of antioxidant enzymes in human prostatic adenocarcinoma. Prostate. 1997;32:229–233. doi: 10.1002/(sici)1097-0045(19970901)32:4<229::aid-pros1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Hitchler MJ, Oberley LW, Domann FE. Epigenetic silencing of SOD2 by histone modifications in human breast cancer cells. Free Radic Biol Med. 2008;45:1573–1580. doi: 10.1016/j.freeradbiomed.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bag A, Bag N. Target sequence polymorphism of human manganese superoxide dismutase gene and its association with cancer risk: a review. Cancer Epidemiol Biomarkers Prev. 2008;17:3298–3305. doi: 10.1158/1055-9965.EPI-08-0235. [DOI] [PubMed] [Google Scholar]
- 29.Kucukgergin C, Sanli O, Tefik T, Aydin M, Ozcan F, Seckin S. Increased risk of advanced prostate cancer associated with MnSOD Ala-9-Val gene polymorphism. Mol Biol Rep. 2012;39:193–198. doi: 10.1007/s11033-011-0725-2. [DOI] [PubMed] [Google Scholar]