Abstract
Purpose
Pathologic complete response (pCR) has been suggested as a surrogate prognostic indicator in breast cancer patients treated with neoadjuvant chemotherapy. We assessed whether the likelihood of pCR and survival is associated with the immunohistochemistry-based molecular subtypes.
Methods
We retrospectively analyzed the records of 276 patients with breast cancer who received neoadjuvant chemotherapy between January 2000 and January 2010. Patients were classified into four molecular subtypes based on the immunohistochemistry profiles of estrogen receptor, progesterone receptor, and HER2/neu. Logistic regression was used to analyze variables associated with pCR.
Results
The pCR was achieved in 45 patients (16.3%). The triple negative subtype was an independent predictive factor for pCR (odds ratio, 3.21; 95% confidence interval, 1.20-8.56; p=0.020), and the ERBB-2 subtype showed a trend for higher pCR rates (odds ratio, 3.03; 95% confidence interval, 0.93-9.89; p=0.067) compared with the luminal A subtype. In 99 patients with HER2/neu-positive breast cancer, pCR rates were higher in those who received trastuzumab (31.7%) than those treated with conventional chemotherapy regimens (17.2%, p=0.023). The pCR was significantly associated with prolonged progression-free survival (p=0.008). The triple negative subgroup had shorter progression-free survival (p=0.001) and overall survival (p=0.001) than the other subgroups.
Conclusion
We demonstrated that the triple negative and ERBB-2 subtypes are more likely to obtain pCR when neoadjuvant chemotherapy is given, compared to the luminal A subtype. Despite the high pCR rate, the triple negative subtype showed worse survival outcomes, paradoxically, primarily due to patients who had residual disease.
Keywords: Breast neoplasms, Molecular subtypes, Neoadjuvant therapy, Pathologic complete response
INTRODUCTION
Neoadjuvant chemotherapy (NACT) was initially used for inoperable locally advanced and inflammatory breast cancer [1,2]. Recently, NACT has become a standard treatment option for patients with primary operable disease when breast conservation is desired [3,4]. Although several NACT regimens have been investigated, survival benefit is not clear compared to adjuvant chemotherapy [3,4]. However, NACT has potential advantages over adjuvant chemotherapy because it downstages the tumor prior to surgery, thereby allowing breast conservation, and it enhances drug delivery due to the maintenance of intact vasculature in NACT [4,5].
However, not all patients with breast cancer benefit from NACT. There may be identifiable subgroups that benefit more from this treatment than other subgroups. Therefore, numerous surrogate endpoints have been investigated in the setting of NACT. Among these, pathologic complete response (pCR), defined by the absence of invasive residual tumor in the breast and lymph nodes, has been strongly suggested as a surrogate indicator of long-term prognosis. It has also been used as a primary endpoint in many clinical trials for neoadjuvant chemotherapy although there are still controversies surrounding this [4,6]. The above suggests that identification of the predictive factors for pCR might be important in addressing adequate therapeutic approaches.
Recent studies have used gene expression profiling to classify breast cancers into molecularly distinct subgroups, and have suggested that this categorization could be used for the prediction of prognosis [7,8]. In patients treated with NACT, molecular subtype by gene expression profiling could also effectively identify patients who are likely to achieve pCR [8]. However, this is not widely available in daily clinical practice. Thus, molecular subtyping based on clinically available immunohistochemical markers has been considered a more practical approach to identification of the corresponding subgroups based on gene expression profiling [9-12]. In this study, we therefore assessed whether the likelihood of pCR and survival are associated with the Immunohistochemistry (IHC)-based molecular subtypes in patients with breast cancer who received NACT.
METHODS
Patients
Between January 2000 and January 2010, 316 patients with breast cancer (diagnosed by core needle biopsy) were given NACT at Asan Medical Center (Seoul, Korea). There was no patient who had metastatic disease. Due to the lack of full immunohistochemical data, 40 patients were excluded; therefore, total 276 patients were included in this analysis. All clinicopathologic data were collected by review of medical records. This study was approved by Asan Medical Center Institutional Review Board (IRB number of our study is 2010-0630).
Staging and immunohistochemistry
Initial staging included consideration of medical history, physical examination, complete blood count, chemistry with electrolytes, chest X-ray, mammography, and breast ultrasound. Computed tomography (CT) of the chest and magnetic resonance imaging (MRI) of breast were performed if clinically indicated. After surgery, patients were staged according to the American Joint Committee on Cancer (AJCC) sixth edition [13].
Immunohistochemical assay was used to test for the expression of the estrogen receptor (ER), progesterone receptor (PR), and HER2/neu. The positivity of ER (1:30 dilution; Dinona, Seoul, Korea) and PR (1:100 dilution; Dinona) was defined by the Allred score when strong nuclear staining was observed in at least 3/8 of tumor cells examined [14]. Immunostaining for HER2/neu (1:250 dilution; Dako, Glostrup, Denmark) was considered positive in the case of strong (3+) membranous staining in at least 10% of tumor cells, or in the case of 2+ with unequivocal amplification by fluorescence in situ hybridization (FISH). According to the IHC features on core biopsies before NACT, patients were classified into the previously suggested IHC-based molecular subgroups as follows: luminal A (ER+ or PR+/HER2/neu-), luminal B (ER+ or PR+/HER2/neu+), ERBB-2 (ER- and PR-/HER2/neu+), and triple negative (ER-, PR- and HER2/neu-) [9,10].
Treatment and surveillance
The NACT regimen and the extent of surgical resection were at the discretion of the medical oncologist and surgeon in charge, and were based on response to NACT and patient preference. The greatest diameters of the breast and lymph node tumors were measured prior to each cycle of chemotherapy and surgery. Mammography, ultrasound, and MRI were performed during (after three or four cycles) and at the end of chemotherapy.
Statistical analysis
The primary end point was pCR which was defined as the absence of residual invasive tumor in the primary breast and lymph nodes at the time of surgery, regardless of the presence of ductal carcinoma in situ. The secondary end point was progression-free survival (PFS). Logistic regression was used to analyze variables associated with pCR, and the odds ratio (OR) and 95% confidence interval (CI) were presented. All variables were subjected to univariate analyses, and then the variables that were associated (p≤0.1) were analyzed in a stepwise multivariate logistic regression model. Hormone receptor and HER2/neu status were not included in the multivariate model to avoid multicollinearity, because of the correlation between these factors and IHC-based molecular subtypes. PFS was defined as the time from diagnosis to relapse, clinically inoperable disease or death from any cause, and was censored at the last follow-up visit. Overall survival (OS) was defined as the time from diagnosis to death from any cause, and was censored at the last follow-up visit for the patients who were still alive. Survival probabilities were estimated using the Kaplan-Meier method and compared using the log rank test. Chi-square or Fisher's exact test was used to analyze the categorical variables. A two-sided p-value<0.05 was considered statistically significant. SPSS version 18.0 (SPSS Inc., Chicago, USA) was used for statistical analysis.
RESULTS
Patient characteristics
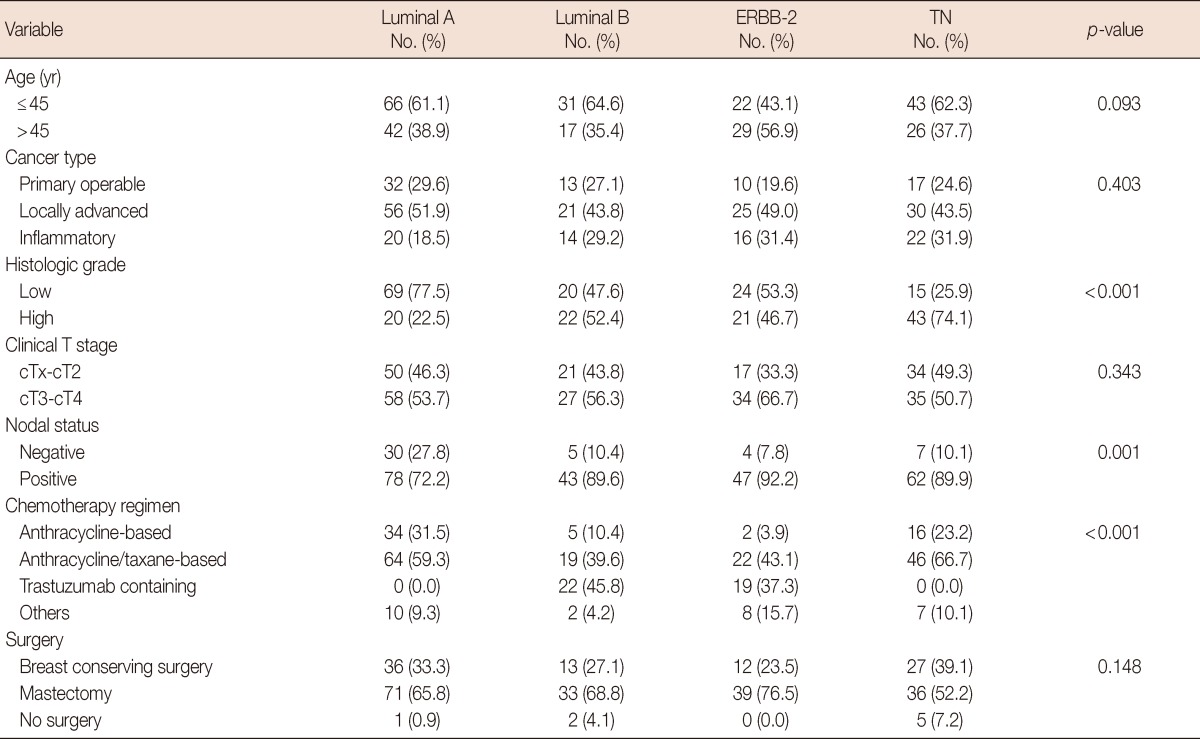
Baseline characteristics of the study population are summarized in Table 1. At the time of diagnosis, 72 patients (26.1%) had primary operable, 132 (47.8%) had locally advanced, and 72 (26.1%) had inflammatory breast cancer. The median age was 44 years (range, 20-78 years). The IHC-based molecular subtypes indicated that 108 patients (39.1%) were luminal A, 48 (17.4%) were luminal B, 51 (18.5%) were ERBB-2, and 69 (25.0%) were triple negative subtype. Breast conserving surgery was performed in 88 patients (31.9%), and most of them (86 patients) had primary operable/locally advanced breast cancer. Surgery was not performed in 8 patients (2.9%) owing to tumor progression or unresponsiveness to chemotherapy. Anthracycline/taxane-based regimen was given to 151 patients (54.7%), anthracycline-based regimen (without taxane) to 57 (20.7%), and trastuzumab-containing regimen to 41 (14.9%; 22 with luminal B and 19 with ERBB-2 subtype). Patients received a median of six cycles (range, 2-12) of NACT. In 63 patients (22.8%), a median of 4 cycles (range, 1-8) of postoperative adjuvant chemotherapy was administered. Table 2 shows the relationship between the clinicopathologic factors and the IHC-based molecular subtypes. There were significant differences in distribution of histologic grade, nodal status, and chemotherapy regimen among the IHC-based molecular subtypes.
Table 1.
Patient characteristics (n=276)
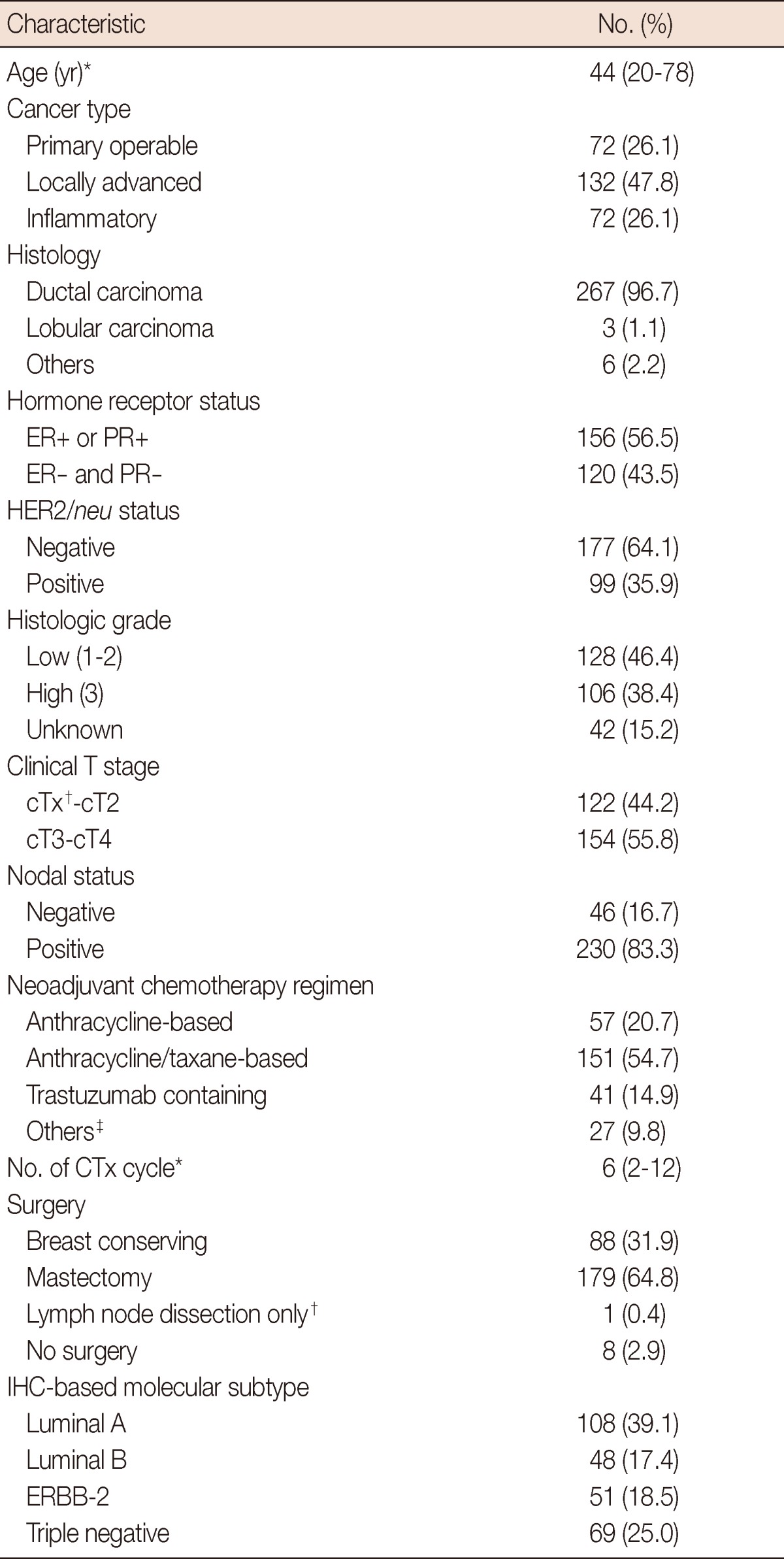
ER=estrogen receptor; PR=progesterone receptor; CTx=chemotherapy; IHC=immnuhistochemistry.
*Median (range); †There was one patient with absence of tumor in primary breast (cTx) and regional lymph node metastasis (cN2). This patient underwent only lymph node dissection; ‡Taxane-based (without anthracycline) and capecitabine-based regimens.
Table 2.
Relationship between clinicopathologic characteristics and IHC-based molecular subtype
IHC=immunohistochemistry; TN=triple negative.
Pathologic response to neoadjuvant chemotherapy
A total of 45 patients (16.3%) achieved pCR in the primary breast and lymph nodes; 16 (5.8%) had residual disease only in the lymph nodes; and 96 (34.8%) had residual disease only in the primary breast. In 119 patients (43.1%), residual tumors were observed in both the primary breast and lymph nodes. The pCR was obtained in 40 patients (19.6%) with primary operable/locally advanced tumors, but obtained in only 5 (6.9%) with inflammatory breast cancer. Among IHC-based molecular subtypes, pCR rates were highest in the ERBB-2 subtypes (29.4%), followed by the triple negative (20.3%), luminal B (16.7%), and luminal A (7.4%) subtypes.
Predictive factors for pCR
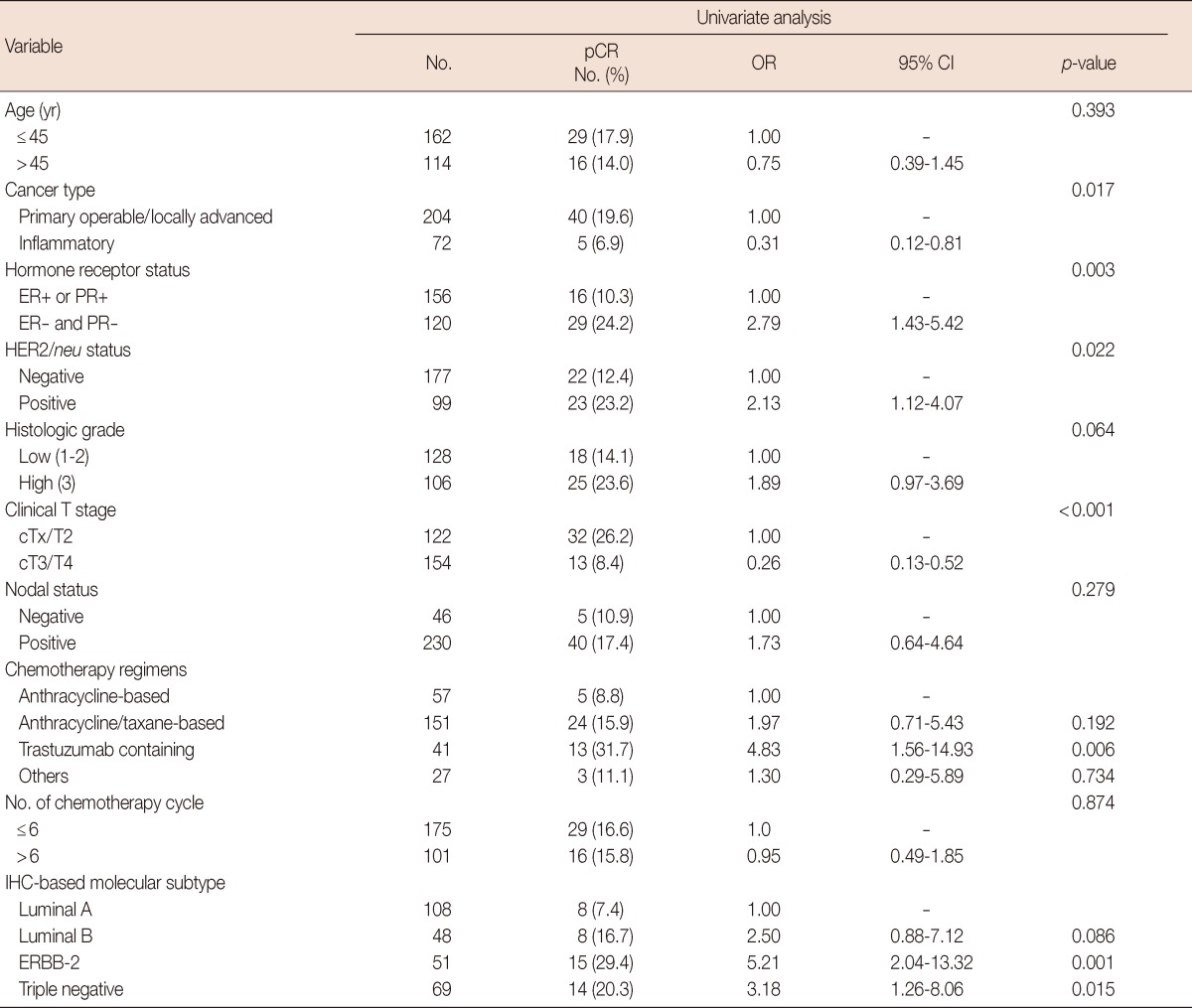
Univariate analyses indicated that cancer type, hormonereceptor status, HER2/neu status, clinical T stage, trastuzumabcontaining regimen, and the ERBB-2 and triple negative subtypes were associated with improved pCR (Table 3).
Table 3.
Univariate analysis for pathologic complete response
pCR=pathologic complete response; OR=odds ratio; CI=confidence interval; ER=estrogen receptor; PR=progesterone receptor; IHC=immnuhistochemistry.
Multivariate analysis was performed with inclusion of cancer type, clinical T stage, nodal status, histologic grade, chemotherapy regimens, and IHC-based molecular subtypes (Table 4). The triple negative subtype was an independent predictive factor for pCR (OR, 3.21; 95% CI, 1.20-8.56; p=0.020), and the ERBB-2 subtype showed a trend for higher pCR rates (OR, 3.03; 95% CI, 0.93-9.89; p=0.067) compared with the luminal A subtype. In addition, clinical T stage (cT3/T4 vs. cT1/T2; OR, 0.21; 95% CI, 0.09-0.46; p<0.001) and trastuzumab-containing regimen (vs. anthracycline-based regimen; OR, 8.23; 95% CI, 1.85-36.71; p=0.006) were significantly associated with pCR. The anthracycline/taxane-based regimen showed a tendency for association with improved pCR (OR, 2.78; 95% CI, 0.93-8.32; p=0.069) compared to the anthracycline-based regimen.
Table 4.
Multivariate analysis for pathologic complete response
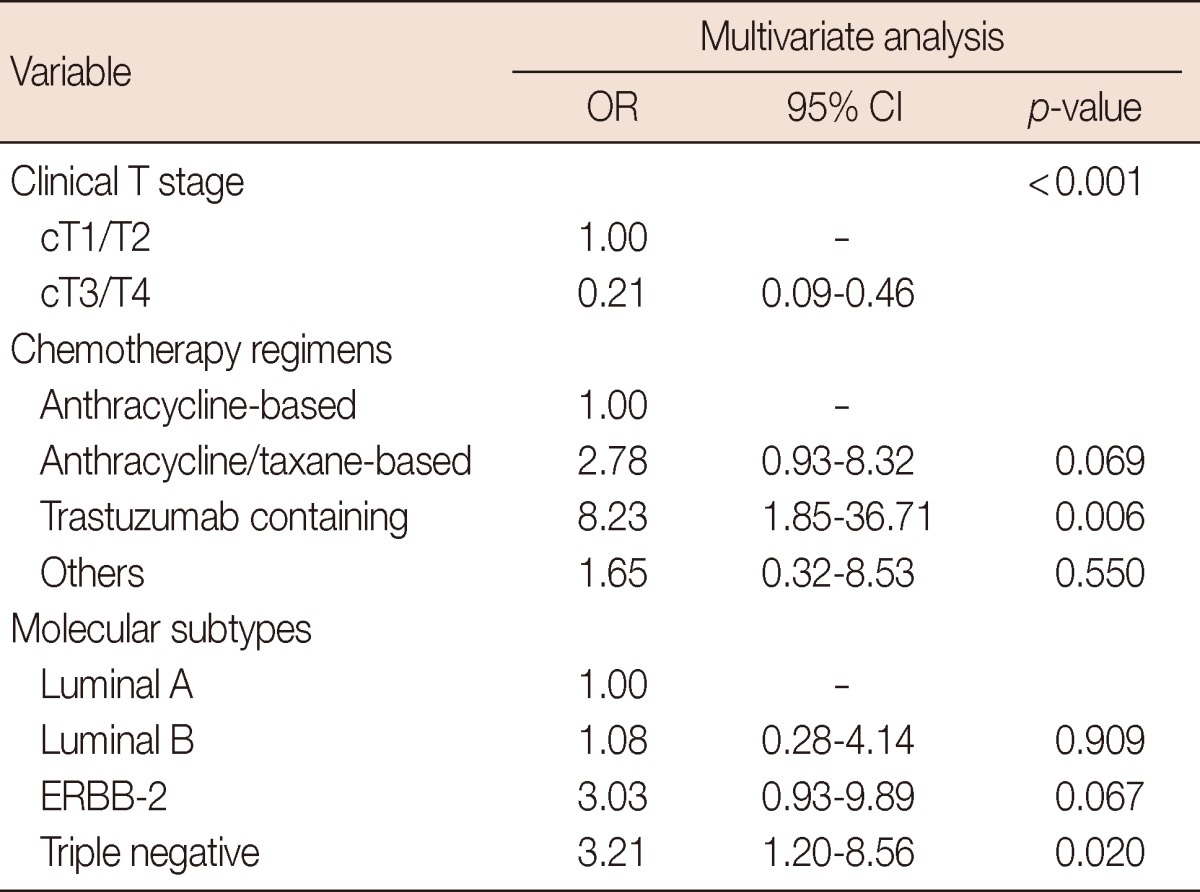
OR=odds ratio; CI=confidence interval.
Trastuzumab as a part of neoadjuvant chemotherapy in HER2/neu-positive breast cancer
In 99 patients with HER2/neu-positive breast cancer, 41 patients (41.4%) were given trastuzumab as a part of NACT. The combination of trastuzumab and paclitaxel was used in all patients, and median six cycles (range, 3-12) were given. Patients who received trastuzumab (31.7%) achieved much higher pCR rates than those treated with conventional chemotherapy regimens (17.2%). Univariate logistic regression analysis showed a tendency for the relationship (p=0.097). However, this was statistically significant (OR, 3.62; 95%CI, 1.19-10.97; p=0.023) after adjustment for confounding factors, such as cancer type, clinical T stage, histologic grade, and hormone receptor status.
Survival outcomes
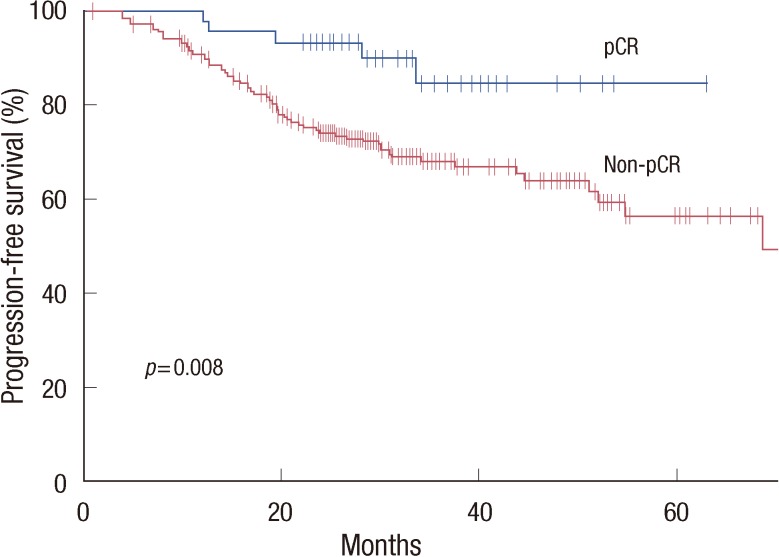
With a median follow-up of 32.3 months (range, 5-110 months), median PFS and OS were not reached in all the patients at the time of the analysis. The pCR was significantly associated with prolonged PFS (hazard ratio [HR], 0.32; 95% CI, 0.13-0.78; p=0.008) (Figure 1). However, we could not find an association with OS (HR, 0.39; 95% CI, 0.12-1.25; p=0.101).
Figure 1.
Progression-free survival according to the status of pathologic complete response (pCR) following neoadjuvant chemotherapy.
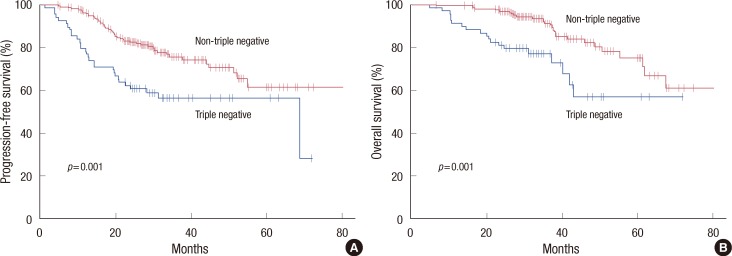
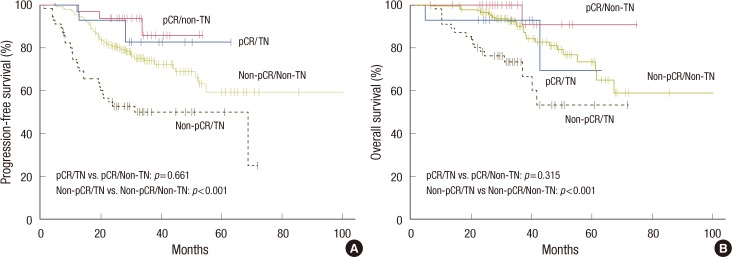
Although patients with the triple negative subtype had significantly higher pCR rates, this subgroup had shorter PFS (HR, 2.20; 95% CI, 1.40-3.46; p=0.001) and OS (HR, 2.81; 95% CI, 1.56-5.08; p=0.001) than those without the triple negative subtype (Figure 2). Patients who had residual disease in the triple negative subgroup showed significantly worse PFS (HR, 2.49; 95% CI, 1.56-3.98; p<0.001) and OS (HR, 3.02; 95% CI, 1.63-5.60; p<0.001) than those in other subgroups. However, among patients who achieved pCR, there was no significant effect of the triple negative status on PFS (p=0.661) or OS (p=0.315) (Figure 3).
Figure 2.
Progression-free survival (A) and overall survival (B) according to the triple negative status.
Figure 3.
Progression-free survival (A) and overall survival (B) according to the pathologic complete response (pCR) and triple negative (TN) statuses.
DISCUSSION
Molecular classification based on gene expression profiling has led to a better understanding of biological phenotypes of breast cancer [15]. This is useful in the prediction of chemosensitivity as well as prognosis [8]. However, technical complexity and high costs of this procedure have limited its clinical application. Thus, combination of immunohistochemical profiles (ER, PR, and HER2/neu) has been investigated as a substitute for the molecular subtypes using gene expression profiles, although these do not exactly correspond [9-12]. In this study, we found that the IHC-based molecular subtypes effectively stratified breast cancer for the likelihood of pCR to NACT. Among the four IHC-based molecular subtypes, the luminal A subgroup showed the least likelihood of pCR to NACT. Patients with the triple negative subtype were more likely to achieve pCR than those with luminal A breast cancer, although this subtype, which largely overlaps the basal-like phenotype [10], is well-known to have aggressive clinical features and poor prognosis [16]. The ERBB-2 subtype showed a tendency for improved pCR. Our results support those of previous reports that used gene expression profiling and immunohistochemical markers, which the basal-like and ERBB-2 phenotypes have better chemosensitivity to NACT than the luminal phenotype [8,10,11]. With regard to the ERBB-2 subtype, however, it is hard to directly compare our results with those of previous studies in which trastuzumab was not used, because 31% of our patients with the ERBB-2 subtype received trastuzumab as a part of NACT.
pCR was observed in 16.3% of our patients and was a good predictor for prolonged PFS, as was shown in previous studies [4,6]. Although we could not find differences in OS according to pCR, it should be considered that the follow-up duration of this study is relatively short and palliative therapy might affect survival after recurrence or progression. Despite its greater sensitivity to NACT, the triple negative subgroup had poor survival outcomes compared to other subgroups. This phenomenon was previously described as the "triple negative paradox" [10,17]. This result was mainly caused by the earlier relapses in patients with this subtype, who failed to achieve pCR. Conversely, triple negative status did not affect the survival of patients with pCR [17]. These findings suggest that the triple negative subtype is a heterogeneous disease, with diverse chemosensitivity, and it could be subdivided in the future.
On the basis of these findings, we believe that tailored therapeutic strategies according to each IHC-based molecular subtype should be considered in patients with breast cancer who are about to receive neoadjuvant therapy. Previous studies have shown that patients with hormone receptor-positive breast cancer are less likely to achieve pCR [6,18]. A recent randomized phase II trial showed that neoadjuvant endocrine therapy is effective in ER-positive tumors, producing similar pCR rates as NACT, and better tolerability than NACT [19]. Therefore, neoadjuvant endocrine therapy may be a promising alternate strategy in luminal A breast cancer [20]. In HER2/neu-positive patients, recent phase III studies have shown that pCR rates were significantly improved with the addition of trastuzumab to conventional chemotherapy [21-23]. We also found that pCR rates in patients with HER2/neu-positive tumors were nearly double for the trastuzumab-treated group, compared with the non-trastuzumab-treated group. The differences between two groups were statistically significant after adjustment for confounding factors. Taking these results together, we believe that trastuzumab should be incorporated in NACT for HER2/neu-positive breast cancer. In patients with the triple negative subtype, because the prognosis of those who failed to achieve pCR is very poor, achievement of the highest possible pCR may be particularly important. Therefore, enhancing the efficacy of NACT with the addition of new drugs, such as platinum or PARP inhibitors, might be required to improve the survival of this subgroup [24].
Our results, which are based on a large number of patients treated at a single medical center, could confirm previous observations regarding predictive factors for pCR and clinical course after NACT. However, our study has several limitations. The data was retrospectively analyzed and the study population was not homogeneous in terms of cancer type and NACT regimens. Although the effects of such variables on the likelihood of pCR were adjusted for in this analysis, the possibility of confounding still exists. In addition, we categorized breast cancer into four subtypes using ER, PR, and HER2/neu status. This may cause misclassification in some patients, compared to original molecular subtypes using the gene expression profiling. However, additional biomarkers including Ki-67 expression, which might be useful for better classification [12], were not available in this study.
In conclusion, we demonstrated that the triple negative and ERBB-2 subtypes of breast cancer are more likely to obtain pCR to NACT, compared to the luminal A subtype. Despite high pCR rates, the triple negative subtype paradoxically showed worse survival outcomes, primarily due to patients who had residual disease following NACT. Individualized strategies in neoadjuvant therapy should be considered in patients with breast cancer, and further studies to define the biological phenotypes are warranted.
Footnotes
This study was presented at the 9th International Conference of the Asian Clinical Oncology Society, August 24-27, 2010, Gifu-city, Gifu, Japan (as oral presentation).
The authors declare that they have no competing interests.
References
- 1.Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, et al. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994;73:362–369. doi: 10.1002/1097-0142(19940115)73:2<362::aid-cncr2820730221>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Mauriac L, Durand M, Avril A, Dilhuydy JM. Effects of primary chemotherapy in conservative treatment of breast cancer patients with operable tumors larger than 3 cm. Results of a randomized trial in a single centre. Ann Oncol. 1991;2:347–354. doi: 10.1093/oxfordjournals.annonc.a057953. [DOI] [PubMed] [Google Scholar]
- 3.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19:4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 4.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 5.Swain SM, Sorace RA, Bagley CS, Danforth DN, Jr, Bader J, Wesley MN, et al. Neoadjuvant chemotherapy in the combined modality approach of locally advanced nonmetastatic breast cancer. Cancer Res. 1987;47:3889–3894. [PubMed] [Google Scholar]
- 6.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 7.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 8.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein NS, Decker D, Severson D, Schell S, Vicini F, Margolis J, et al. Molecular classification system identifies invasive breast carcinoma patients who are most likely and those who are least likely to achieve a complete pathologic response after neoadjuvant chemotherapy. Cancer. 2007;110:1687–1696. doi: 10.1002/cncr.22981. [DOI] [PubMed] [Google Scholar]
- 10.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, et al. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer. 2010;116:1431–1439. doi: 10.1002/cncr.24876. [DOI] [PubMed] [Google Scholar]
- 12.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene FL American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 14.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 15.Desmedt C, Ruíz-García E, André F. Gene expression predictors in breast cancer: current status, limitations and perspectives. Eur J Cancer. 2008;44:2714–2720. doi: 10.1016/j.ejca.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 17.Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 18.Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004;91:2012–2017. doi: 10.1038/sj.bjc.6602235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110:244–254. doi: 10.1002/cncr.22789. [DOI] [PubMed] [Google Scholar]
- 20.Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23:5108–5116. doi: 10.1200/JCO.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 22.Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 23.Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 24.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45(Suppl 1):27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]