Abstract
Purpose
We performed this study to detect preoperative axillary metastases with ultrasound (US)-guided fine needle aspiration biopsy (FNAB), to eliminate the need for time-consuming and costly sentinel lymph node (SLN) scintigraphy and biopsy steps in the treatment of breast cancer patients, and in that of with suspicious US findings, and to evaluate the accuracy of preoperative US-guided FNAB for patients with suspicious lymph node metastases on US.
Methods
Patients with a suspicious breast lump or histopathologically proven breast cancer underwent breast-axillary US. Increase in lymph node size, cortical thickening, non-hilar cortical flow, and hilar changes were evaluated with gray scale-color Doppler US. FNAB was performed if US results were suspicious for malignancy.
Results
Thirty-eight axillary lymph nodes (ALN) underwent FNAB. ALN dissection, SLN scintigraphy, and biopsy steps were bypassed in 23 axillas with positive ALN FNAB (60.5%). The sensitivity of ALN FNAB was 88.46%; specificity and positive predictive value were 100%; and negative predictive value was 66.6% (inadequate cytology included; 76.7%, 100%, 100%, 53.3%, respectively). Asymmetrical cortical thickening, non-hilar cortical flow, and increase in hypoechogenity were only detected in metastatic nodes. Cortical thickening, and lymph node and breast mass size was higher in the metastatic group.
Conclusion
By performing FNAB on suspicious lymph nodes, the routine, high-cost SLN scintigraphy and intraoperative gamma probe steps may be skipped, and axilla dissection can be performed directly. This leads to the elimination of the need for SLN investigation in more than half of the patients. The assessment of ALN metastases with preoperative US-guided FNAB is a cost-effective method with high specificity, that eliminates the need for costly and time-consuming SLN scintigraphy and biopsy steps, and helps in preoperative staging.
Keywords: Axilla, Breast neoplasms, Fine needle aspiration biopsy, Lymph node, Metastasis
INTRODUCTION
Breast cancer is the most common malignant tumor among women, representing 31% of all cancers [1,2]. The presence of axillary involvement in breast cancer determines patient's survival and the staging of the disease, and it plays an important role in local control [1-3]. Until recently, axillary lymph node dissection (ALND) was considered as the reference method for detecting lymph node involvement [4,5]; however, axillary dissection is reported to have a positive result in 30% of palpable tumors and in 10% of non-palpable tumors in patients with clinically negative axillary involvement. The remaining 70% to 90% undergo axillary dissection unnecessarily. Today, the common use of mammography screening has led to the early diagnosis of small size breast tumors. The rate of axillary lymph node (ALN) metastases is very low in patients diagnosed at an early stage [6,7]. The low histopathologic rate of axillary metastases observed in patients with clinically negative axillary involvement has caused a controversy over the performance of axillary dissection, a procedure which involves various complications, in these patients. This led to the development of an alternative method called sentinel lymph node biopsy (SLNB).
The sentinel lymph node (SLN) is the gatekeeper of the lymphatic basin; it is the first node to receive lymphatic drainage of the tumor. In theory, if the SLN does not involve metastases, the other lymph nodes are also negative. Accepting the accuracy of this theory prevents unnecessary axillary dissection. Today, there are two different methods used for SLN determination, including the use of a surgical gamma probe by preoperative lymphoscintigraphy and lymphatic mapping using methylene blue. However, a combined use of these two methods has yielded more successful results.
While SLNB is a commonly accepted method, removing the SLN causes a considerable loss of time during the surgery [8,9]. SLNB is not an entirely perfect procedure, either; in some patients there is no SLN detected that would provide information, or some patients may exhibit involvement of more than 3 SLNs. If axillary metastases can be detected in the preoperative period, the SLNB step can be omitted and ALND can be performed directly. Consequently, efforts in developing new imaging methods for the preoperative staging of lymph nodes in patients with breast cancer are gradually increasing [5,10-15]. Ultrasound (US) is the most commonly investigated method for this purpose [12-15]. Some investigators reported that combined use of US and fine needle aspiration biopsy (FNAB) could provide a highly accurate preoperative lymph node screening [9,14-18]. However, FNAB requires the cooperation of a highly experienced practitioner and an experienced cytologist.
The current trial was designed to evaluate the accuracy of preoperative US-guided FNAB (sensitivity, specificity, positive predictive value [PPV], negative predictive value [NPV]) for patients diagnosed by biopsy with primary breast cancer or for patients with a radiologically detected Breast Imaging Reporting and Data System (BI-RADS) 5 breast mass.
METHODS
The patients gave informed consent and the Medical Trials Local Ethical Committee gave approval to initiate the trial. After axillary examination, the general surgery department referred patients who were diagnosed with primary breast cancer by biopsy or who had a radiologically detected BI-RADS 5 breast mass, to the Hacettepe University Radiology Department Breast Imaging unit for further axillary evaluation and FNAB of suspicious lymph nodes. Bilateral breasts and ALN were evaluated using Siemens Antares US device and a VFX -13.5 Hz probe (Siemens, Karlsruhe, Germany).
The patients with suspicious US findings were included in the study. Findings that were suspicious with respect to metastases included cortical symmetrical and asymmetrical thickening (cortex thickness >3 mm) compared to the lymph nodes on the same or other side, increased size of the lymph nodes, an increase in the sphericity index (short/long diameter ≥0,5), increased cortex hypoechogenicity (cortex more hypoechoic than the subcutaneous fatty tissue), and non-hilar cortical flow. All the patients included in the trial were also suitable candidates for sentinel node biopsy. This study was approved by the institutional review board (approval number: HEK 09/77-207).
FNAB was performed by experts or by a breast imaging fellow using 22 gauge needles in the lymph nodes considered to be suspicious for metastases based on the US results. To achieve local anesthesia 10 mL of 1% xylocaine was used. Guided by US, the needle was inserted parallel to the long axe of the probe toward the cortex of the lymph node. After confirming that the needle tip was in the targeted region, a minimum of three aspirations were performed. Sampling was generally performed from a single lymph node; in the case of high ultrasonographic suspicion, aspiration from two individual lymph nodes was performed. If indicated, a concurrent thick-needle biopsy from the primary mass was performed.
For each passage, effort was made to avoid the vascular hilus. During the procedure, particular care was taken to avoid hemorrhagic aspiration. The aspirated material was spread over the slide, a portion of the slides were placed in alcohol while the remaining portion was left to dry in air. The preparations dried in air were stained using the May-Grünwald Giemsa method; while those fixed in alcohol were stained using the Papanicolau staining method. The materials sent were reported by the cytologists to have one of the following results: inaccurate fixation/artifact, inadequate number of cells, normal or reactive lymph node, suspected malignity, or diagnostic for malignity.
The histopathological results for the US-guided FNAB and ALND were compared. Using US, thickness of the cortex, asymmetrical cortical thickening, increase in the sphericity index, non-hilar cortical flow, and increase in the hypoechogenicity were assessed, and the differences in these properties between the metastatic and non-metastatic lymph nodes were statistically evaluated. Data analysis was performed using SPSS version 17.0 Windows (SPSS Inc., Chicago, USA). For the demographic and descriptive values, the mean±standard deviation, and the maximum and minimum values were calculated. The following tests were used: chi-square tests (Pearson, continuity correction, likelihood ratio, Fisher's exact test, linear-by-linear association, N of valid cases), Mann-Whitney test, test for the difference between two independent groups, and the test for the difference between the two paired groups. The p-values were considered to be statistically significant below 0.05.
The presence of lymph node capsule invasion, the size of the metastatic focus in the lymph node, and the size of the tumor were assessed histopathologically and the correlation of these factors with the cytology results was evaluated.
Patients with positive ALN FNAB underwent directly axillary dissection and the sentinel node dissection step was skipped; all patients with negative ALN FNAB results had SLNB.
RESULTS
FNAB was performed in 38 lymph nodes of a total of 36 females with an age range of 25 to 68 years (mean, 48.52 years). The histopathology of 22 primary breast masses was invasive ductal carcinoma, 8 masses were ductal carcinoma and lobulary mixed invasive carcinoma; 1 was ductal and mucinous mixed invasive carcinoma; 1 was ductal and cribriform mixed invasive carcinoma; 2 were invasive lobulary carcinoma; and 2 were ductal carcinoma in situ. FNAB was performed in two suspicious lymph nodes in the right and left axillae of a patient with a mass in the left breast considered to be suspicious for malignant pathology. Bilateral axillary suspicious lymph nodes were detected in a patient with two masses, in whom the histopathology of the breasts had revealed bilateral lobular carcinoma, and FNAB was performed in both of these nodes. FNAB was performed in the right lymph nodes in 17 patients, in the left lymph nodes in 17 patients, and in bilateral ALN in 2 patients. Of a total of 36 primary breast masses, 16 were located in the right breast and 20 were located in the left breast. Eight masses were of grade 2, and 28 masses were of grade 3.
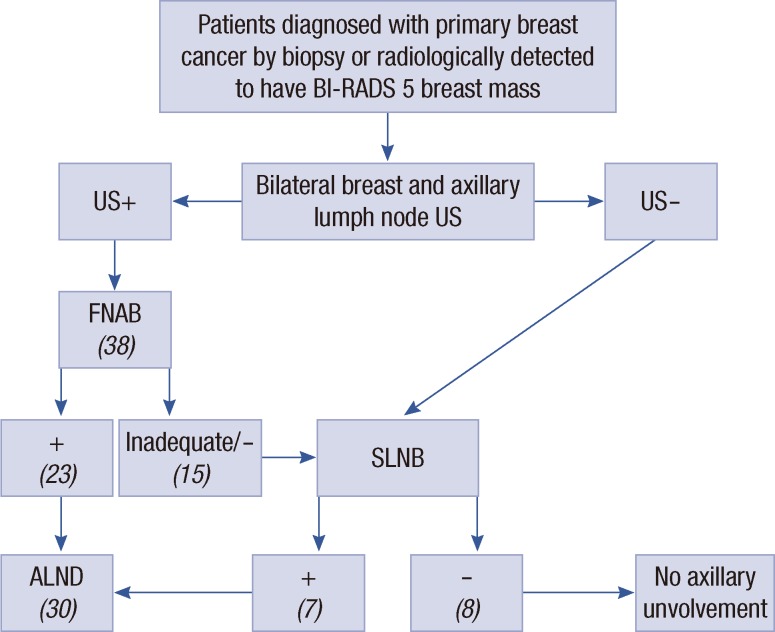
Metastases were detected by FNAB in 23 of the 38 lymph nodes included in the trial. The need for SLN dissection was eliminated in 60.5% of the axillae. Skipping the SLNB step, these patients directly underwent ALND. Aspiration was not adequate in six of the 38 lymph nodes (15.7%). Nine patients did not reveal malignant cells by cytological investigation. SLNB was performed in 15 axillae with a negative or inadequate cytology result, and metastases were detected in seven of these. Thus, metastases were detected by SLNB in three of the nine axillae with a negative cytological result and in four of the six axillae with an inadequate result. There were eight patients in total who had a negative histopathological result (Figure 1).
Figure 1.
Ultrasound (US) evaluation, fine needle aspiration biopsy (FNAB), sentinel lymph node biopsy (SLNB), and axillary lymph node dissection (ALND) algorithm, and number of lymph nodes.
BI-RADS=Breast Imaging Reporting and Data System.
Twenty-three of the 30 patients with lymph node metastases detected by axillary dissection had a positive FNAB result. The sensitivity of US-guided FNAB in assessing the axillary metastases was detected to be 88.46%. Including the cases with inadequate aspiration, the sensitivity decreased to 76.66%. In all the patients with a positive cytology, axillary metastases were detected pathologically. Therefore, the specificity and the PPV of US-guided ALN aspiration in assessing the metastases was 100% (Table 1).
Table 1.
Sensitivity, specificity, positive predictive value, negative predictive value, and false negative rate of FNAB
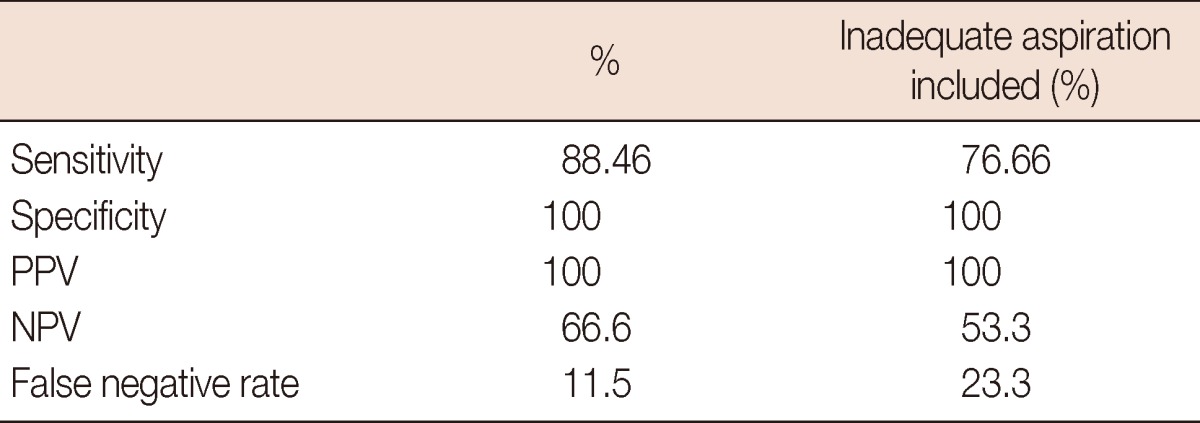
FNAB=fine needle aspiration biopsy; PPV=positive predictive value; NPV=negative predictive value.
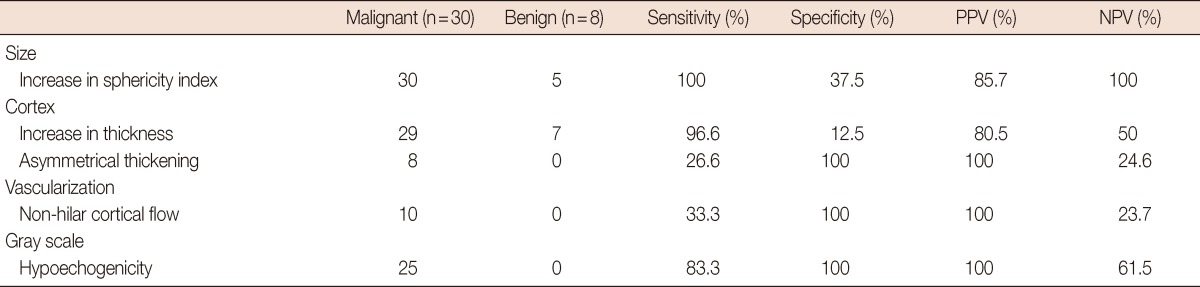
While an increase was detected in the sphericity index of all the lymph nodes in 30 patients with metastases detected on pathology (sphericity index, 0.5-0.91; mean, 0.57), five of the eight lymph nodes without metastases exhibited an increase in the sphericity index (sphericity index, 0.22-0.71; mean, 0.52). An increase in the cortical thickness was detected in 29 of the 30 metastatic lymph nodes and asymmetrical thickening was detected in 8 of the 30 positive nodes. Ten metastatic lymph nodes exhibited non-hilar cortical flow; while increased hypoechogenicity on a gray scale was detected in 25 of the 30 positive nodes; none of the non-metastatic lymph nodes had these two findings (Table 2).
Table 2.
Axillary lymph node evaluation: correlation of ultrasound findings and pathologic results
PPV=positive predictive value; NPV=negative predictive value.
The mean cortical thickness was 8.2 mm (2-32 mm) in metastatic patients and 3.3 mm (2-5 mm) in non-metastatic patients; the lymph node diameter on pathology was 26.4 mm (10-50 mm) in metastatic patients and 17.3 mm (10-30 mm) in non-metastatic patients; the histopathological measurement revealed a mean size of metastatic focus in the lymph node of 21.8 mm (4-50 mm); and the macroscopic mass size was measured to be 55.4 mm (10-160 mm) in metastatic patients and 38.6 mm (10-70 mm) in non-metastatic patients on the pathology specimen.
The difference in cortical thickness and lymph node size between the two groups with a positive and negative pathology was statistically significant. Considering a lymph node cortex thickness ≥4 mm as pathologic, the sensitivity and the specificity of US-guided FNAB in detecting a metastatic lymph node were 86% and 87%, respectively. With the upper limit of cortical thickness given as 3 mm, the sensitivity and specificity were 96.6% and 37.5%, respectively.
Comparing the lymph nodes with capsular invasion detected histopathologically and the nodes with non-hilar cortical flow detected ultrasonographically, the specificity was higher than the sensitivity; however, as indicated above, all the nodes with non-hilar cortical flow detected were metastatic. The sensitivity, specificity, PPV, and NPV of non-hilar cortical flow in detecting capsular invasion were 36.8%, 72.7%, 70%, and 40%, respectively.
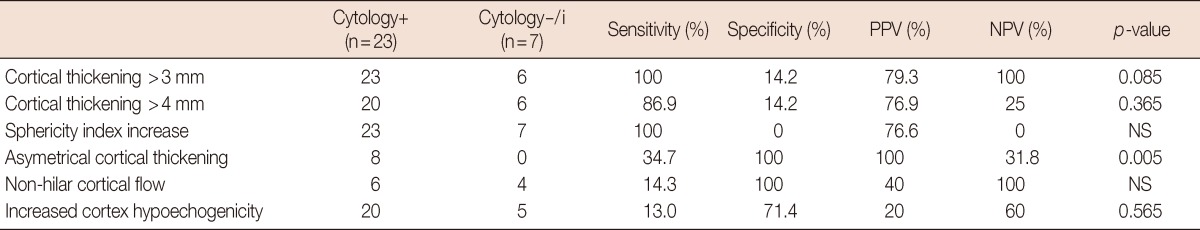
Assessment of the pathologically positive group in two subgroups, those with positive and negative or inadequate cytological result revealed that the group with a positive cytology had a statistically larger lymph node metastatic focus. The cortex thickness was higher in the group with positive cytology. This difference was detected to be at the limit of statistical significance. A comparison of all the parameters evaluated using US revealed no differences in the criteria including the sphericity index, cortical thickening, non-hilar cortical flow, and hypoechogenicity; while asymmetrical cortical thickening was detected significantly more in the cytology-positive group relative to the other groups (Table 3).
Table 3.
Comparison of US findings, between cytology+ metastatic nodes and cytology-/inadequate (i) metastatic nodes
The p-value is 0.005 for asymmetric cortical thickening.
US=ultrasound; i=inadequate; PPV=positive predictive value; NPV=negative predictive value; NS=not specific.
DISCUSSION
The following are used in the routine staging of breast cancer: history, physical examination, breast and lymph node US (for axillary, internal mammary, infraclavicular and supraclavicular lymph nodes), abdominal computed tomography, bone survey, and chest X-ray. The status of the ALN is the most important parameter that affects surgery and medical treatment in patients with breast cancer. Particularly, preoperative detection of axillary metastases is important in determining the mode of ALND.
If no axillary metastases are detected by SLNB in T1 and T2 breast cancers, breast-conservative surgery can be performed. If lymph node metastases or distant metastases are detected in local advanced stage breast cancer, preoperative neoadjuvant chemotherapy is administered [10].
Today, the sentinel node dissection step is skipped, and lymph node dissection is performed on the whole axilla, even in patients known to have a single positive ALN [19,20]. FNAB and cytology of suspicious lymph nodes detected by US can decrease the need for SLNB by 21% to 65%. In our trial, axillary metastases were detected by US-guided FNAB, and the need for SLN dissection was eliminated in 60.5% of the cases. Avoiding unnecessary sentinel node biopsy both shortens the duration of the surgery and decreases the costs of the procedure markedly [19], leading to a reduction in healthcare expenses by nearly 20% [9,18,19,21,22].
The sentinel node dissection performed to detect the status of the gatekeeper (sentinel) lymph node that the primary tumor drains into is quite a complex and time-consuming procedure. The major reason for this is the fact that this procedure requires a multidisciplinary approach. In most cases, radiocolloidal agent is administered to the breast subcutaneous tissue preoperatively and attempts are made to detect the first lymph node into which this agent drains. This procedure prolongs the duration of the operation markedly. In contrast, if the presence of metastases is detected preoperatively in any of the ALNs by fine-needle or thick-needle biopsy, the need for the detection and the dissection of the SLN is eliminated, leading to direct axillary dissection during the operation [23].
Currently, there is no biological tumor marker used to predict ALN metastases. Palpation by hand, US or US-guided FNAB, or thick-needle biopsy procedures are the methods used for this purpose. Physical examination alone is not a sensitive or reliable method for detecting axillary metastases. Metastatic nodes are usually non-palpable and the palpable reactive (non-metastatic) nodes can be misdiagnosed as metastases [10]. The inadequacy of physical examination alone was demonstrated by showing its sensitivity to be between 45.4% and 68% [13]. De Freitas et al. [13] detected the sensitivity, specificity, PPV, and NPV of this method as 68%, 68%, 82%, and 50%, respectively, and reported the total accuracy of the assessment to be 68%. It was found that approximately 15% to 60% of the patients with clinically non-palpable lymph nodes presented with lymph node metastases in the following period [16].
Similar to the results of the physical examination, different rates of accuracy were shown by using US investigation alone. Even based on suspicious imaging results (a lymph node length greater than 10 mm, the absence of a fatty hilus, hypoechoic internal echo, spherical shape, and cortical thickening), the sensitivity of US examination ranged between 35% and 82%; while the specificity was between 73% and 97.9% [16,22,24,25].
De Freitas et al. [13] determined the PPV, the NPV, and the total accuracy value of US as 92%, 49%, and 67%, respectively, when used alone. Evaluating these results collectively, US alone does not seem to have adequate accuracy in detecting the presence of metastases [21].
The use of FNAB to improve the accuracy of preoperative assessment of ALNs has been recommended as a simple, moderately to highly accurate, and minimally invasive method and has been reported to be effective in determining the appropriate approach for the patient [9,15,16,18-22,24]. The results from our trial confirmed that US-guided FNAB was a beneficial method in detecting ALN metastases of breast cancer. The sensitivity and the specificity of US-guided FNAB in assessing the ALN were measured to be 76.6% and 100%, respectively, in our trial. The NPV and the PPV of the procedure were assessed to be 53.3% and 100%, respectively. The aforementioned trials reported a sensitivity of 36% to 86.4%, and a specificity of 95.7% to 100% for assessment of the ALN by US-guided FNAB. The PPV of the procedure was 92% to 100%, the NPV of the procedure was 67% to 70% [9,15,16,18-22,24,25].
The most significant reason for the false negative results detected in these trials was inadequate sampling. By increasing the number of aspirations, the percentage of inadequate samples can be reduced [20]. In our trial, the inadequate sampling rate was 15.7%, similar to the rate reported in the literature. Inadequate aspiration was attributed to the small size of the metastases, the small number of positive lymph nodes, and the deficient imaging of the lymph node during US assessment of the axilla [16,19,20,24]. Dividing the pathologically positive group into two subgroups: one group with positive cytological results, and the second with negative or inadequate cytological results, revealed that the group with positive cytology had a statistically larger lymph node metastatic focus. In addition, the comparison of the cytology-positive metastatic group with the cytology-negative or cytology-inadequate groups revealed that asymmetrical cortical thickening was only detected in the cytology-positive group. Fine-needle aspiration was performed, taking care to target the asymmetrically thickened cortex. Targeting in this manner is observed to increase the success of aspiration, a result similar to that predicted in the literature [26,27]. In our trial, the histopathological measurement of lymph node size was larger in the metastatic group, and this difference was statistically significant. There was no significant difference between the metastatic and non-metastatic groups in tumor grade or size of the mass.
The trials demonstrated that as the tumor size and the size of the lymph node metastases increased, the sensitivity of FNAB and US also increased. It is quite difficult to sample the metastases containing tumor deposits smaller than 5 mm by using FNAB. Such metastases exhibit a cortical thickness smaller than 3 mm in the lymph node and display very small or no desmoplastic reaction in the surrounding tissue. Even if a focus of this size can be assessed as suspicious on US, it would be very difficult to control the needle at an accurate angle in a region of this size [28,29].
In the literature, the most common cause of false positive results was the inaccurate cytological assessment of the cells sampled. Myoepithelial or lymphocyte cell clusters can be cytologically confused with breast epithelial cells. The second most common cause is inappropriate sampling during lymph node dissection. In our trial, there were no false positive results in any of the fine-needle aspiration cases. There are previous trials showing no false positive results, as well as those reporting a false positive result rate of 1.4% to 1.6% [16,17,19,20,25].
We have no clear evidence showing that the FNAB-applied node was the sentinel node or the lymph node detected to be positive on axillary dissection. Abe et al. [27] detected axillary metastases by thick-needle biopsy. In over 15% of cases of positive US-guided core needle biopsy, the positive node was the only one found at subsequent axillary dissection by interference, the sentinel node. This suggested that the node undergoing US-guided thick-needle biopsy was the same node pathologically detected to be metastatic based on dissection. The largest node with the most pathological morphology is selected as the target in FNAB. The possibility that the lymph node closest to the breast is the sentinel node is very high. Even if it has normal morphology, performing FNAB in the lymph node closest to the breast would increase the sensitivity of the procedure [27,30].
US-guided needle biopsies performed in the lymph nodes do not complicate the implementation of the SLNB or ALN biopsy and the histopathological assessment of the sampled node [27].
The increases in the sphericity index, hypoechogenicity, and the cortical thickness on US assessment were statistically significantly higher in the metastatic lymph nodes compared to non-metastatic nodes. These criteria can define the abnormal lymph node together with asymmetrical cortical thickening and color Doppler investigation characteristics. In our trial, we detected malignancy in lymph nodes with a cortical thickness of ≥4 mm at a sensitivity of 86% and specificity of 87%, and of ≥3 mm at a sensitivity of 96% and specificity of 37%. In the literature, the trials with a larger sample size used values ranging between 2.3 mm and 4 mm as the upper limit. In patients with established breast cancer or malignant mass in the breast, FNAB can be performed in lymph nodes with a US cortex thickness >3 mm.
The association between the lymph node capsule invasion and the non-hilar cortical flow was not statistically significant. Assessments specific to the transcortical flow by capsule invasion in trials with a larger sample size could yield more significant results.
Lymph node cytology can also be used in evaluating the chemotherapy response in patients without a surgical indication as well as in preoperative staging [19]. Another advantage of FNAB of suspicious lymph nodes is that it can be performed in hospitals without a nuclear medicine department.
Our sample patient group is not large; a condition caused by the limited number of patients referred to our unit from the general surgery department for ALN assessment and biopsy. Another limitation of our trial is the absence of a cytologist during the FNAB procedure at our unit, leading to inadequate cytology results.
In conclusion, in breast cancer, the morphologic, cortical, and blood flow characteristics of the lymph nodes should be assessed by preoperative axillary gray scale and color Doppler US. In our trial, we detected malignancy in lymph nodes with a cortical thickness of ≥4 mm at a sensitivity of 86% and of ≥3 mm at a sensitivity of 96%. FNAB can be performed in ALNs with a cortical thickness >3 mm in breast cancer patients.
Upon detecting metastases by FNAB in the lymph nodes considered to be suspicious based on preoperative US, the routine, high-cost steps of SLN scintigraphy and intraoperative gamma probe are skipped, and the axillary dissection is performed directly. This can eliminate the need for SLN investigation in more than half of the patients. In this trial, the need for SLNB was eliminated in 60.5% of the patients because US-guided FNAB was performed. FNAB is a sensitive and quite specific method for detecting the presence of metastases in ALNs in patients with breast cancer. The sensitivity, specificity, PPV, and the NPV were 76.6%, 100%, 100%, and 53.3%, respectively. The major reason for the false negative results was the sampling errors associated with very small metastatic foci. Owing to the excellent PPV of this method, ALND can be performed in patients with a positive fine-needle aspiration result. US-guided fine needle aspiration is a highly specific assessment method. Using this method, axillary metastases can be detected at a much lower cost, preoperative staging of the disease can be performed, and the time spent doing SLNB and intraoperative frozen procedure under general anesthesia is eliminated.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Banerjee M, George J, Song EY, Roy A, Hryniuk W. Tree-based model for breast cancer prognostication. J Clin Oncol. 2004;22:2567–2575. doi: 10.1200/JCO.2004.11.141. [DOI] [PubMed] [Google Scholar]
- 2.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9:606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 3.Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer: a multicenter validation study. N Engl J Med. 1998;339:941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds C, Mick R, Donohue JH, Grant CS, Farley DR, Callans LS, et al. Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol. 1999;17:1720–1726. doi: 10.1200/JCO.1999.17.6.1720. [DOI] [PubMed] [Google Scholar]
- 5.Lovrics PJ, Chen V, Coates G, Cornacchi SD, Goldsmith CH, Law C, et al. A prospective evaluation of positron emission tomography scanning, sentinel lymph node biopsy, and standard axillary dissection for axillary staging in patients with early stage breast cancer. Ann Surg Oncol. 2004;11:846–853. doi: 10.1245/ASO.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Swenson KK, Nissen MJ, Ceronsky C, Swenson L, Lee MW, Tuttle TM. Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol. 2002;9:745–753. doi: 10.1007/BF02574496. [DOI] [PubMed] [Google Scholar]
- 7.Mincey BA, Bammer T, Atkinson EJ, Perez EA. Role of axillary node dissection in patients with T1a and T1b breast cancer: Mayo Clinic experience. Arch Surg. 2001;136:779–782. doi: 10.1001/archsurg.136.7.779. [DOI] [PubMed] [Google Scholar]
- 8.McMasters KM, Giuliano AE, Ross MI, Reintgen DS, Hunt KK, Byrd DR, et al. Sentinel-lymph-node biopsy for breast cancer: not yet the standard of care. N Engl J Med. 1998;339:990–995. doi: 10.1056/NEJM199810013391410. [DOI] [PubMed] [Google Scholar]
- 9.de Kanter AY, van Eijck CH, van Geel AN, Kruijt RH, Henzen SC, Paul MA, et al. Multicentre study of ultrasonographically guided axillary node biopsy in patients with breast cancer. Br J Surg. 1999;86:1459–1462. doi: 10.1046/j.1365-2168.1999.01243.x. [DOI] [PubMed] [Google Scholar]
- 10.Murray AD, Staff RT, Redpath TW, Gilbert FJ, Ah-See AK, Brookes JA, et al. Dynamic contrast enhanced MRI of the axilla in women with breast cancer: comparison with pathology of excised nodes. Br J Radiol. 2002;75:220–228. doi: 10.1259/bjr.75.891.750220. [DOI] [PubMed] [Google Scholar]
- 11.Ohta M, Tokuda Y, Saitoh Y, Suzuki Y, Okumura A, Kubota M, et al. Comparative efficacy of positron emission tomography and ultrasonography in preoperative evaluation of axillary lymph node metastases in breast cancer. Breast Cancer. 2000;7:99–103. doi: 10.1007/BF02967197. [DOI] [PubMed] [Google Scholar]
- 12.Yang WT, Ahuja A, Tang A, Suen M, King W, Metreweli C. High resolution sonographic detection of axillary lymph node metastases in breast cancer. J Ultrasound Med. 1996;15:241–246. doi: 10.7863/jum.1996.15.3.241. [DOI] [PubMed] [Google Scholar]
- 13.de Freitas R, Jr, Costa MV, Schneider SV, Nicolau MA, Marussi E. Accuracy of ultrasound and clinical examination in the diagnosis of axillary lymph node metastases in breast cancer. Eur J Surg Oncol. 1991;17:240–244. [PubMed] [Google Scholar]
- 14.Tate JJ, Lewis V, Archer T, Guyer PG, Royle GT, Taylor I. Ultrasound detection of axillary lymph node metastases in breast cancer. Eur J Surg Oncol. 1989;15:139–141. [PubMed] [Google Scholar]
- 15.Deurloo EE, Tanis PJ, Gilhuijs KG, Muller SH, Kröger R, Peterse JL, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer. 2003;39:1068–1073. doi: 10.1016/s0959-8049(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 16.van Rijk MC, Deurloo EE, Nieweg OE, Gilhuijs KG, Peterse JL, Rutgers EJ, et al. Ultrasonography and fine-needle aspiration cytology can spare breast cancer patients unnecessary sentinel lymph node biopsy. Ann Surg Oncol. 2006;13:31–35. doi: 10.1245/ASO.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Bedrosian I, Bedi D, Kuerer HM, Fornage BD, Harker L, Ross MI, et al. Impact of clinicopathological factors on sensitivity of axillary ultrasonography in the detection of axillary nodal metastases in patients with breast cancer. Ann Surg Oncol. 2003;10:1025–1030. doi: 10.1245/aso.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamurthy S, Sneige N, Bedi DG, Edieken BS, Fornage BD, Kuerer HM, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–988. doi: 10.1002/cncr.10786. [DOI] [PubMed] [Google Scholar]
- 19.Kuenen-Boumeester V, Menke-Pluymers M, de Kanter AY, Obdeijn IM, Urich D, Van Der Kwast TH. Ultrasound-guided fine needle aspiration cytology of axillary lymph nodes in breast cancer patients. A preoperative staging procedure. Eur J Cancer. 2003;39:170–174. doi: 10.1016/s0959-8049(02)00501-4. [DOI] [PubMed] [Google Scholar]
- 20.Ciatto S, Brancato B, Risso G, Ambrogetti D, Bulgaresi P, Maddau C, et al. Accuracy of fine needle aspiration cytology (FNAC) of axillary lymph nodes as a triage test in breast cancer staging. Breast Cancer Res Treat. 2007;103:85–91. doi: 10.1007/s10549-006-9355-0. [DOI] [PubMed] [Google Scholar]
- 21.Bonnema J, van Geel AN, van Ooijen B, Mali SP, Tjiam SL, Henzen-Logmans SC, et al. Ultrasound-guided aspiration biopsy for detection of nonpalpable axillary node metastases in breast cancer patients: new diagnostic method. World J Surg. 1997;21:270–274. doi: 10.1007/s002689900227. [DOI] [PubMed] [Google Scholar]
- 22.Davis JT, Brill YM, Simmons S, Sachleben BC, Cibull ML, McGrath P, et al. Ultrasound-guided fine-needle aspiration of clinically negative lymph nodes versus sentinel node mapping in patients at high risk for axillary metastasis. Ann Surg Oncol. 2006;13:1545–1552. doi: 10.1245/s10434-006-9095-8. [DOI] [PubMed] [Google Scholar]
- 23.Filippakis GM, Zografos G. Contraindications of sentinel lymph node biopsy: are there any really? World J Surg Oncol. 2007;5:10. doi: 10.1186/1477-7819-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popli MB, Sahoo M, Mehrotra N, Choudhury M, Kumar A, Pathania OP, et al. Preoperative ultrasound-guided fine-needle aspiration cytology for axillary staging in breast carcinoma. Australas Radiol. 2006;50:122–126. doi: 10.1111/j.1440-1673.2006.01545.x. [DOI] [PubMed] [Google Scholar]
- 25.Sapino A, Cassoni P, Zanon E, Fraire F, Croce S, Coluccia C, et al. Ultrasonographically-guided fine-needle aspiration of axillary lymph nodes: role in breast cancer management. Br J Cancer. 2003;88:702–706. doi: 10.1038/sj.bjc.6600744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedi DG, Krishnamurthy R, Krishnamurthy S, Edeiken BS, Le-Petross H, Fornage BD, et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am J Roentgenol. 2008;191:646–652. doi: 10.2214/AJR.07.2460. [DOI] [PubMed] [Google Scholar]
- 27.Abe H, Schmidt RA, Sennett CA, Shimauchi A, Newstead GM. US-guided core needle biopsy of axillary lymph nodes in patients with breast cancer: why and how to do it. Radiographics. 2007;27(Suppl 1):S91–S99. doi: 10.1148/rg.27si075502. [DOI] [PubMed] [Google Scholar]
- 28.Mainiero MB, Cinelli CM, Koelliker SL, Graves TA, Chung MA. Axillary ultrasound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance. AJR Am J Roentgenol. 2010;195:1261–1267. doi: 10.2214/AJR.10.4414. [DOI] [PubMed] [Google Scholar]
- 29.Hinson JL, McGrath P, Moore A, Davis JT, Brill YM, Samoilova E, et al. The critical role of axillary ultrasound and aspiration biopsy in the management of breast cancer patients with clinically negative axilla. Ann Surg Oncol. 2008;15:250–255. doi: 10.1245/s10434-007-9524-3. [DOI] [PubMed] [Google Scholar]
- 30.Koelliker SL, Chung MA, Mainiero MB, Steinhoff MM, Cady B. Axillary lymph nodes: US-guided fine-needle aspiration for initial staging of breast cancer: correlation with primary tumor size. Radiology. 2008;246:81–89. doi: 10.1148/radiol.2463061463. [DOI] [PubMed] [Google Scholar]