Abstract
Background
Angiotensin-converting enzyme inhibitors improve outcomes in systolic heart failure (SHF). However, doubts linger about their effect in SHF patients with chronic kidney disease (CKD).
Methods
In the Studies of Left Ventricular Dysfunction (SOLVD) Treatment trial, 2569 ambulatory chronic HF patients with left ventricular ejection fraction ≤35% and serum creatinine level ≤2.5 mg/dL were randomized to receive either placebo (n=1284) or enalapril (n=1285). Of the 2502 patients with baseline serum creatinine data, 1036 had CKD (estimated glomerular filtration rate <60 ml/min/1.73 m2).
Results
Overall, during 35 months of median follow-up, all-cause mortality occurred in 40% (502/1252) and 35% (440/1250) of placebo and enalapril patients, respectively (hazard ratio {HR}, 0.84; 95% confidence interval {CI}, 0.74–0.95; p=0.007). All-cause mortality occurred in 45% and 42% of patients with CKD (HR, 0.88; 95% CI, 0.73–1.06; p=0.164), and 36% and 31% of non-CKD patients (HR, 0.82; 95% CI, 0.69–0.98; p=0.028) in the placebo and enalapril groups, respectively (p for interaction=0.615). Enalapril reduced cardiovascular hospitalization in those with CKD (HR, 0.77; 95% CI, 0.66–0.90; p<0.001) and without CKD (HR, 0.80; 95% CI, 0.70–0.91; p<0.001). Among patients in the enalapril group, serum creatinine elevation was significantly higher in those without CKD (0.09 versus 0.04 mg/dL in CKD; p=0.003) during first year of follow-up, but there was no differences in changes in systolic blood pressure (mean drop, 7 mmHg, both) and serum potassium (mean increase, 0.2 mEq/L, both).
Conclusions
Enalapril reduces mortality and hospitalization in SHF patients without significant heterogeneity between those with and without CKD.
Keywords: enalapril, heart failure, chronic kidney disease
1. Introduction
Treatment with angiotensin-converting enzyme inhibitors (ACEIs) has been shown to reduce mortality and hospitalization in patients with systolic heart failure (SHF) or heart failure with reduced ejection fraction (HF-REF) [1–3]. However, these drugs are often underutilized, especially in those with chronic kidney disease (CKD) [4–6]. Although elevation of serum creatinine after initiation of ACEIs is temporary and not harmful to kidney function [7], this has been often cited as a reason for their non-use [7–10]. As most randomized clinical trials (RCT) of ACEIs excluded patients with advanced CKD there is also lack of RCT evidence of their benefit in HF patients with CKD [11]. This is unfortunate as CKD is common among SHF patients and is associated with poor outcomes [12–15]. Further, ACEIs have also been shown to reduce renal failure and prevent death in patients with CKD [16]. Therefore, the purpose of the current study was to evaluate the effect of enalapril on mortality and hospitalization in SHF patients with CKD in the Studies of Left Ventricular Dysfunction (SOLVD)-Treatment trial.
2. Materials and methods
2.1. Source of data and study patients
SOLVD-Treatment was a randomized, double-blind, placebo controlled trial of enalapril, an ACEI, in patients with SHF, the rationale, design, and the results of which have been previously reported [2]. Briefly, 2569 ambulatory chronic HF patients with left ventricular ejection fraction ≤35% who were not currently receiving ACEIs were randomly assigned to receive either placebo (n=1284) or enalapril (n=1285) 2.5 to 20 mg/day. Patients were recruited from 89 hospitals in the United States, Canada, and Belgium between June 1986 and March 1989. Nearly 90% of the patients had New York Heart Association classes II and III symptoms. Patients age >80 years and those with serum creatinine level >2.5 mg/dL were excluded. During an average of 41.4 months of follow-up, 40% and 35% of patients in the placebo and enalapril groups, respectively, died from all causes, which corresponded to a significant 16% risk reduction [2]. The current analysis includes 2502 participants who had data on baseline serum creatinine levels.
2.2. Chronic kidney disease
Overall, 1036 (41% of 2502) patients had CKD defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 using the MDRD (Modification of Diet in Renal Disease) formula [17]. Of these, 538 and 498 patients were randomized to receive placebo or enalapril, respectively. Of the 1466 patients with eGFR ≥60 ml/min/1.73 m2, 714 and 752 were receiving placebo and enalapril, respectively.
2.3. Study outcomes
The primary outcome for the current study was all-cause mortality, which was also the primary end point in the SOLVD-Treatment trial. Secondary outcomes included cause-specific mortality and all-cause and cause-specific hospitalization. Outcomes were ascertained by principal investigator at each center by blinded review of hospital chart and interview of participant relatives.
2.4. Statistical analysis
Baseline characteristics of SOLVD-Treatment participants with CKD receiving placebo and enalapril were compared using Pearson’s chi-square test and Student’s t-test as appropriate. Because MDRD formula underestimates eGFR at higher levels, for between-group comparison of eGFR in those without CKD, we used eGFR estimated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula [18]. Kaplan Meier and Cox proportional hazard analyses were used to estimate the effect of enalapril on outcomes. In SOLVD-Treatment, the effect of enalapril on mortality was most marked during the first 24 months of follow-up, after which there was no mortality difference between patients receiving placebo and enalapril [2]. Therefore, we also examined the effect of enalapril on mortality at various time periods during follow-up. To examine if the effect of enalapril was different in those without CKD, we repeated the above analysis in those without CKD, and formally tested for first-order interaction. We then examined the effect of enalapril on outcomes in SHF patients with CKD stage ≥3B (eGFR <45 ml/min/1.73 m2). We then categorized all patients into those receiving below-target (less than 20 mg daily) and target (20 mg daily or higher) doses of study drugs at Visit 4 and compared all-cause mortality within each dose group, using patients in the placebo group as the reference category. The Visit 4 assessment occurred 2 to 3 weeks after randomization when doses of study drugs were increased to target study dosages and the follow-up began. Finally, we examined the within-group and between-group changes in systolic blood pressure (SBP), serum potassium and serum creatinine during the first 12 months of follow-up. All statistical tests were two-tailed with 95% confidence levels and a p-value <0.05 was considered significant. SPSS for Windows, Version 15 (2006, Chicago: SPSS Inc.) was used for all data analysis.
3. Results
3.1. Patient characteristics
Baseline characteristics of SHF patients with and without CKD in the placebo and enalapril groups are displayed in Table 1. Compared to patients without CKD, those with CKD were generally older, more likely to be women and have ischemic heart disease, and diabetes, and had a higher NYHA functional class, but were similar with respect to other characteristics.
Table 1.
Baseline characteristics of SOLVD-Treatment trial participants with and without chronic kidney disease (CKD) by randomization to enalapril or placebo
| CKD (n=1036)
|
No CKD (n=1466)
|
|||
|---|---|---|---|---|
| Placebo (n=538) | Enalapril (n=498) | Placebo (n=714) | Enalapril (n=752) | |
| Age, years† | 64.5 (±7.6) | 64.1 (±8.3) | 57.7 (±10.0) | 57.6 (±10.4) |
| Female† | 135 (25%) | 119 (24%) | 117 (16%) | 119 (16%) |
| Race† | ||||
| White | 452 (84%) | 414 (83%) | 565 (79%) | 578 (77%) |
| African American | 61 (11%) | 55 (11%) | 119 (17%) | 146 (19%) |
| Other | 25 (5%) | 29 (6%) | 30 (4%) | 28 (4%) |
| Current smoker† | 74 (14%) | 79 (16%) | 189 (27%) | 204 (27%) |
| New York Heart Association class† | ||||
| I | 59 (11%) | 58 (12%) | 111 (16%) | 119 (16%) |
| II | 288 (54%) | 237 (48%) | 398 (56%) | 422 (56%) |
| III | 178 (33%) | 196 (39%) | 189 (27%) | 199 (27%) |
| IV | 13 (2%) | 7 (1%) | 16 (2%) | 12 (2%) |
| Ischemic heart disease† | 394 (73%) | 364 (73%) | 510 (71%) | 516 (69%) |
| Myocardial infarction | 354 (66%) | 336 (68%) | 463 (65%) | 496 (66%) |
| Angina pectoris† | 224 (42%) | 197 (40%) | 267 (37%) | 254 (34%) |
| Hypertension† | 240 (45%) | 248 (50%) | 279 (39%) | 283 (38%) |
| Diabetes mellitus† | 158 (29%) | 150 (30%) | 177 (25%) | 161 (21%) |
| Atrial fibrillation | 45 (8%) | 40 (8%) | 38 (5%) | 74 (10%)* |
| Medications | ||||
| Beta-blockers | 39 (7%) | 34 (7%) | 48 (7%) | 67 (9%) |
| Digitalis† | 350 (65%) | 313 (63%) | 504 (71%) | 512 (68%) |
| Diuretics† | 477 (89%) | 441 (89%) | 589 (83%) | 682 (84%) |
| Potassium-sparing diuretic | 53 (10%) | 47 (9%) | 64 (9%) | 69 (9%) |
| Potassium supplements | 267 (50%) | 275 (55%) | 347 (49%) | 370 (49%) |
| Nitrates† | 246 (46%) | 219 (44%) | 306 (43%) | 279 (37%)* |
| Anti-arrhythmic drugs† | 133 (25%) | 136 (27%) | 128 (18%) | 150 (20%) |
| Calcium-channel blockers† | 184 (34%) | 166 (33%) | 223 (31%) | 200 (27%) |
| Anticoagulants | 84 (16%) | 70 (14%) | 116 (16%) | 128 (17%) |
| Anti-platelet agents | 193 (36%) | 166 (33%) | 238 (33%) | 249 (33%) |
| Weight, kg† | 75 (±8) | 76 (±8) | 78 (±10) | 78 (±9) |
| Heart rate, beats/min† | 78 (±12) | 80 (±13) | 81 (±14) | 80 (±13) |
| Systolic blood pressure, mm Hg† | 126 (±18) | 127 (±19) | 124 (±17) | 124 (±17) |
| Diastolic blood pressure, mm Hg† | 76 (±10) | 77 (±11) | 77 (±10) | 78 (±10) |
| Serum sodium, mmol/liter | 140 (±3) | 140 (±3) | 140 (±3) | 140 (±3) |
| Serum potassium, mmol/liter† | 4.3 (±0.5) | 4.3 (±0.5) | 4.3 (±0.4) | 4.2 (±0.4) |
| Serum creatinine, mg/dl† | 1.49 (±0.27) | 1.50 (±0.27) | 1.06 (±0.18) | 1.06 (±0.18) |
| Estimated glomerular filtration rate, ml/min/1.73m2 | 49 (±8) | 49 (±8) | 78 (±14)‡ | 78 (±14)‡ |
| Cardiothoracic ratio >0.50† | 296 (55%) | 294 (59%) | 401 (56%) | 430 (57%) |
| Ejection fraction, % | 25 (±7) | 25 (±6) | 25 (±7) | 25 (±7) |
P<0.05
P<0.05 between CKD and no-CKD groups
Estimated using the CKD-EPI formula
3.2. Effect of enalapril on all-cause mortality in SHF patients with data on baseline kidney function
Among the 2502 SOLVD-Treatment participants with data on baseline serum creatinine, all-cause mortality occurred in 40% and 35% of patients in the placebo and enalapril groups, respectively (hazard ratio {HR} when enalapril was compared with placebo, 0.84; 95% confidence interval {CI}, 0.74–0.95; p=0.007; Table 2), which is consistent with the findings from the SOLVD-Treatment trial based on 2569 patients [2]. This effect was attenuated, but remained significant, after adjustment for baseline CKD (HR, 0.85; 95% CI, 0.75–0.96; p=0.011) and eGFR (HR, 0.85; 95% CI, 0.75–0.97; p=0.013), and was not significantly different between those with and without CKD (p for interaction, 0.615).
Table 2.
Mortality and hospitalization by randomization in the SOLVD-Treatment trial, in all patients with data on baseline serum creatinine, and those with and without chronic kidney disease (CKD)
| % (events)
|
Absolute risk difference* (%) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Placebo | Enalapril | ||||
| Overall (N=2502) | n=1252 | n=1250 | |||
| All-cause mortality | 40% (502) | 35% (440) | – 5% | 0.84 (0.74–0.95) | 0.007 |
| CKD (n=1036) | n=538 | n=498 | |||
| Mortality | |||||
| All-cause | 45% (242) | 42% (207) | – 3% | 0.88 (0.73–1.06) | 0.164 |
| Cardiovascular | 40% (217) | 36% (177) | – 4% | 0.84 (0.69–1.02) | 0.079 |
| Progressive heart failure | 15% (80) | 15% (75) | 0% | 0.96 (0.70–1.31) | 0.792 |
| Arrhythmia without heart failure | 10% (54) | 9% (43) | – 1% | 0.82 (0.55–1.23) | 0.344 |
| Hospitalization | |||||
| All cause | 76% (408) | 73% (362) | – 3% | 0.83 (0.72–0.96) | 0.012 |
| Cardiovascular | 66% (353) | 59% (293) | – 7% | 0.77 (0.66–0.90) | 0.001 |
| Worsening heart failure | 39% (212) | 27% (134) | – 12% | 0.59 (0.48–0.73) | <0.001 |
| No CKD (n=1466) | n=714 | n=752 | |||
| Mortality | |||||
| All-cause | 36% (260) | 31% (233) | – 5% | 0.82 (0.69–0.98) | 0.028 |
| Cardiovascular | 33% (237) | 28% (211) | – 5% | 0.82 (0.68–0.98) | 0.031 |
| Progressive heart failure | 12% (85) | 10% (78) | – 2% | 0.84 (0.62–1.14) | 0.264 |
| Arrhythmia without heart failure | 9% (62) | 8% (60) | – 1% | 0.89 (0.62–1.27) | 0.522 |
| Hospitalization | |||||
| All cause | 74% (526) | 67% (505) | – 7% | 0.77 (0.69–0.88) | <0.001 |
| Cardiovascular | 62% (439) | 54% (408) | – 8% | 0.80 (0.70–0.91) | <0.001 |
| Worsening heart failure | 35% (246) | 26% (195) | – 9% | 0.68 (0.57–0.83) | <0.001 |
Absolute risk differences were estimated by subtracting event rates in the placebo group from those in the enalapril group
3.3. Effect of enalapril on outcomes in SHF patients with CKD
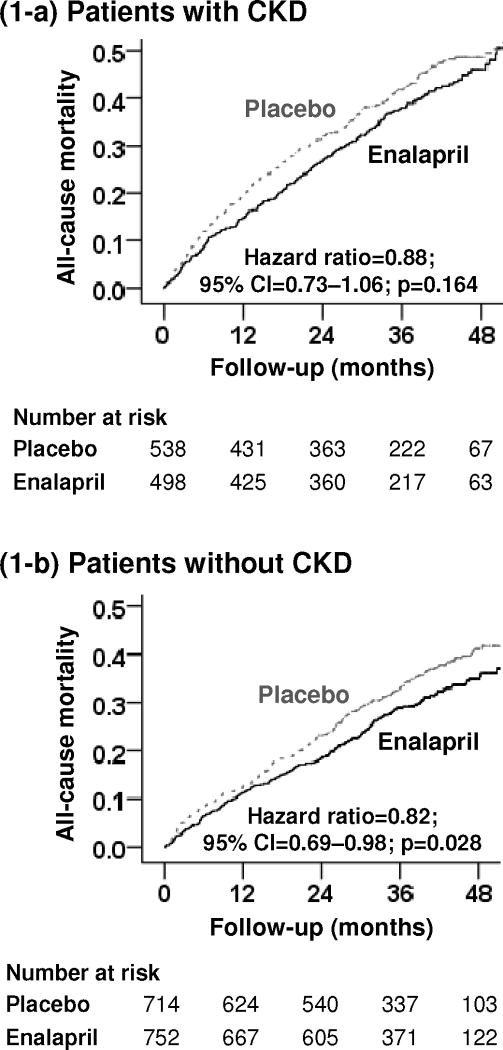
Among the 1036 patients with CKD, all-cause mortality occurred in 45% of those in the placebo group and 42% of those in the enalapril group (HR, 0.88; 95% CI, 0.73–1.06; p=0.164; Figure 1-a and Table 2). Among CKD patients receiving below-target doses, 48% and 40% of those in the placebo and enalapril groups, respectively, died from all causes (HR, 0.78; 95% CI, 0.58–1.02; p=0.066), while respective mortality among those receiving target doses were 42% and 41% (HR, 0.96; 95% CI, 0.73–1.24; p=0.733; Table 3). The effect of enalapril on all-cause mortality at 3, 6, 12, 24, 36 and 48 months of follow-up is displayed in Table 4. The effects of enalapril on cause-specific mortalities are displayed in Table 2. Enalapril significantly reduced hospitalization in SHF patients with CKD (Table 2).
Figure 1.
Kaplan-Meier plots for all-cause mortality in SOLVD-Treatment trial participants (a) with and (b) without chronic kidney disease (CKD); (CI=confidence interval)
Table 3.
Mortality by below-target and target doses of enalapril compared with below-target and target doses of placebo, respectively, in the SOLVD-Treatment trial
| % (events/total)
|
Absolute risk difference* (%) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Placebo | Enalapril | ||||
| CKD (n=987) | n=515 | n=472 | |||
| Below-target dose (n=449) | 48% (114/240) | 40% (84/209) | – 8% | 0.78 (0.58–1.02) | 0.066 |
| Target dose (n=538) | 42% (114/275) | 41% (107/263) | – 1% | 0.96 (0.73–1.24) | 0.733 |
| No CKD (n=1406) | n=688 | n=718 | |||
| Below-target dose (n=542) | 35% (81/235) | 32% (97/307) | – 3% | 0.92 (0.68–1.23) | 0.570 |
| Target dose (n=864) | 37% (166/453) | 29% (120/411) | – 8% | 0.75 (0.59–0.94) | 0.015 |
Absolute risk differences were estimated by subtracting event rates in the placebo group from those in the enalapril group
Table 4.
Effect of enalapril on all-cause mortality at various time periods in patients with and without chronic kidney disease (CKD), in the SOLVD-Treatment trial
| % (events)
|
Absolute risk difference* (%) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Placebo | Enalapril | ||||
| CKD (n=1036) | n=538 | n=498 | |||
| Duration of follow-up | |||||
| 3 months | 6% (32) | 5% (24) | – 1% | 0.80 (0.47–1.37) | 0.419 |
| 6 months | 12% (64) | 9% (43) | – 3% | 0.71 (0.49–1.05) | 0.088 |
| 12 months | 20% (107) | 15% (73) | – 5% | 0.72 (0.53–0.97) | 0.029 |
| 24 months | 32% (171) | 27% (133) | – 5% | 0.80 (0.64–1.01) | 0.057 |
| 36 months | 40% (217) | 36% (181) | – 4% | 0.86 (0.70–1.05) | 0.127 |
| 48 months | 44% (239) | 41% (202) | – 3% | 0.86 (0.72–1.04) | 0.127 |
| At study end | 45% (242) | 42% (207) | – 3% | 0.88 (0.73–1.06) | 0.164 |
| No CKD (n=1466) | n=714 | n=752 | |||
| Duration of follow-up | |||||
| 3 months | 5% (36) | 3% (24) | – 2% | 0.63 (0.37–1.05) | 0.076 |
| 6 months | 9% (61) | 6% (48) | – 3% | 0.74 (0.50–1.07) | 0.110 |
| 12 months | 13% (90) | 11% (85) | – 2% | 0.88 (0.66–1.19) | 0.401 |
| 24 months | 23% (166) | 19% (139) | – 4% | 0.74 (0.62–0.97) | 0.027 |
| 36 months | 32% (226) | 27% (206) | – 5% | 0.84 (0.69–1.01) | 0.064 |
| 48 months | 36% (257) | 31% (229) | – 5% | 0.82 (0.68–0.97) | 0.024 |
| At study end | 36% (260) | 31% (233) | – 5% | 0.82 (0.69–0.98) | 0.028 |
Absolute risk differences were estimated by subtracting event rates in the placebo group from those in the enalapril group
3.4. Effect of enalapril on outcomes in SHF patients with CKD stage ≥3B
Among the 268 patients with CKD stage ≥3B, all-cause mortality occurred in 52% and 44% of patients in the placebo and enalapril groups, respectively (HR, 0.76; 95% CI, 0.54–1.08; p=0.123). Enalapril reduced hospitalizations due to cardiovascular causes (HR, 0.73; 95% CI, 0.54–0.98; p=0.037) and worsening HF (HR, 0.69; 95% CI, 0.46–1.02; p=0.063).
3.5. Effect of enalapril on outcomes in SHF patients without CKD
Among the 1466 patients without CKD, all-cause mortality occurred in 36% of those in the placebo group and 31% of those in the enalapril group (HR, 0.82; 95% CI, 0.69–0.98; p=0.028; Figure 1-b and Table 2). Among patients without CKD receiving below-target doses, 35% and 32% of those in the placebo and enalapril groups, respectively, died from all causes (HR, 0.92; 95% CI, 0.68–1.23; p=0.570), while respective mortality among those receiving target doses were 37% and 29% (HR, 0.75; 95% CI, 0.59–0.94; p=0.015; Table 3). The effect of enalapril on all-cause mortality at various time periods is displayed in Table 4. The effects of enalapril on other outcomes in SHF patients without CKD are displayed in Table 2.
3.6. Changes in systolic blood pressure, serum potassium and serum creatinine during follow-up
During the first year of follow-up, 27 patients had SBP <90 mmHg, 32 and 8 patients had serum potassium ≥5.5 and ≥6 mEq/L respectively, and 99 and 28 patients had serum creatinine ≥2 and ≥2.5 mg/dL respectively. Changes in SBP, serum potassium and creatinine during follow-up are displayed in Table 5. SBP <90 mmHg occurred in 0.2% and 2.1% of placebo and enalapril patients, respectively (p<0.001). Respective rates for those with CKD were 0.0% and 3.4% (p<0.001), and those without CKD were 0.3% and 1.2% (p=0.047; p for heterogeneity=0.061). Among patients in the enalapril group, SBP dropped by 7 mmHg, which was similar in those with and without CKD (p=0.948; Table 5).
Table 5.
Change in systolic blood pressure, serum potassium and creatinine values during first 12 months of follow-up, in patients in the placebo and enalapril groups in the SOLVD-Treatment trial
| Changes during first 12 months Mean (95% CI); paired t-test p value |
Student’s t-test p value | ||
|---|---|---|---|
|
|
|||
| Systolic blood pressure, mm Hg | |||
| CKD (n=983) | Placebo (n=508) | +0.10 (−1.21 to +1.41); 0.880 | <0.001 |
| Enalapril (n=475) | −7.00 (−8.62 to −5.38); <0.001* | ||
| No CKD (n=1411) | Placebo (n=677) | +0.60 (−0.53 to +1.72); 0.297 | <0.001 |
| Enalapril (n=734) | −7.06 (−8.23 to −5.90); <0.001* | ||
| Serum potassium, mEq/L | |||
| CKD (n=970) | Placebo (n=503) | −0.06 (−0.11 to −0.02); 0.008 | <0.001 |
| Enalapril (n=467) | +0.20 (+0.14 to +0.25); <0.001† | ||
| No CKD (n=1384) | Placebo (n=670) | −0.04 (−0.08 to +0.003); 0.069 | <0.001 |
| Enalapril (n=714) | +0.18 (+0.14 to +0.22); <0.001† | ||
| Serum creatinine, mg/dL | |||
| CKD (n=967) | Placebo (n=501) | −0.02 (−0.05 to −0.001); 0.041 | <0.001 |
| Enalapril (n=466) | +0.04 (+0.02 to +0.07); 0.002‡ | ||
| No CKD (n=1383) | Placebo (n=670) | +0.05 (+0.03 to +0.06); <0.001 | <0.001 |
| Enalapril (n=713) | +0.09 (+0.08 to +0.11); <0.001‡ | ||
P=0.948 for differences in patients in the enalapril group with and without CKD
P=0.632 for differences in patients in the enalapril group with and without CKD
P=0.003 for differences in patients in the enalapril group with and without CKD
Serum potassium ≥5.5 mEq/L occurred in 0.9% and 1.8% of patients in the placebo and enalapril groups, respectively (p=0.078). Respective rates for those with CKD were 1.2% and 1.9% (p=0.354), and those without CKD were 0.7% and 1.7% (p=0.115; p for heterogeneity=0.657). Among patients in the enalapril group, serum potassium increased by 0.2 mEq/L, which was similar in those with and without CKD (p=0.632; Table 5). Serum creatinine ≥2.5 mg/dL occurred in 0.5% and 1.9% of placebo and enalapril patients, respectively (p=0.002). Respective rates for those with and without CKD were 1.0% and 4.3% (p=0.001), and 0.1% and 0.3% (p=0.600), respectively (p for heterogeneity=0.507). Among patients in the enalapril group, serum creatinine increased by 0.05 and 0.09 mg/dL in those with and without CKD, respectively (p=0.003; Table 5).
4. Discussion
Findings from the current study demonstrate that while enalapril significantly reduced the risk of all-cause and cardiovascular mortality in SHF patients without CKD, its effect on those with CKD was more modest and lacked significance. Furthermore, SHF patients with CKD seemed to derive benefit during the early years of follow-up and at below-target doses of enalapril. However, there was no significant heterogeneity in the effect of enalapril between SHF patients with and without CKD. Enalapril also significantly reduced all-cause, cardiovascular and HF hospitalizations regardless of CKD. These findings provide important insights into the effect of ACEIs in SHF patients with CKD and suggest that these drugs may play an important role in improving outcomes in SHF patients with CKD, who comprise nearly half of all SHF patients and have poor prognosis, and yet often deprived of these drugs.
As in SHF, CKD is also associated with activation of renin-angiotensin system hormones, suppression of which has been shown to be associated with improved outcomes in patients with CKD [16, 19]. ACEIs have also been shown to improve kidney function in HF patients with rather advanced CKD (mean serum creatinine of 2.3 mg/dL) [8]. Therefore, it is mechanistically plausible that ACEIs would be beneficial for patients with both SHF and CKD. However, considering that treatment effect is generally more pronounced in subsets of patients with more advanced disease and poor outcomes [20], the modest nature of the effect of enalapril on SHF patients with CKD is rather surprising. However, as prognosis worsens with disease progression in HF, the mode of death may also change, which may explain a relatively modest effect of enalapril in SHF patients with CKD. Sudden cardiac death and progressive HF are two common modes of death in HF. With disease progression, progressive HF becomes a more common mode of death. In the main SOLVD-Treatment trial, the effect of enalapril on mortality was mostly driven by reduction in death due to progressive HF as enalapril had little or no effect on sudden cardiac death [2].
If SHF patients with CKD in SOLVD-Treatment were more likely to die from progressive HF and if enalapril was less effective in preventing death due to progressive HF, then the effect of enalapril would be modest in those patients. However, findings from our cause-specific death analysis do not suggest that SHF patients with CKD were more likely to die from progressive cardiac failure which may in part also explain the attenuated effect of enalapril in those with CKD. Yet, enalapril had a rather robust effect on reduction in hospitalization due to worsening HF in those with CKD. Because most HF patients with disease progression would have worsening symptoms with many requiring hospitalizations, these findings suggest that enalapril may have had some effect on symptomatic disease progression. Because in the SOLVD-Treatment trial, mortality reduction associated with enalapril use occurred entirely among patients who had HF hospitalizations during the trial, reduction of HF hospitalization was considered a mechanism by which enalapril reduced mortality [2]. The robust effect of enalapril on hospitalization due to worsening HF regardless of CKD suggests that the underlying mechanism of action of enalapril may be similar in SHF patients with and without CKD.
Prior studies on the effect of ACEIs in SHF patients with CKD based on secondary analysis of existing databases have produced inconclusive results [21–24]. However, to the best of our knowledge this is the first report on the effect of an ACEI on outcomes in SHF patients with CKD based on subgroup analysis of an RCT. These findings are also consistent with those from a propensity-matched study of ACEIs in SHF patients with CKD [25]. Cumulative evidence from those studies and the findings from the current study suggest that as in SHF patients without CKD, ACEIs may reduce mortality and hospitalization in those with CKD. Our findings also suggest that common adverse effects of enalapril such as hypotension, hyperkalemia or elevation of serum creatinine, was low regardless of CKD and was safe.
Our study has several limitations. The exclusion of SHF patients with a creatinine >2.5 mg/dL may limit generalizability of these findings to those with more advanced CKD. However, we observed that enalapril had a similar effect on those with CKD stage ≥3B. We had no data on urine albumin and some patients with early CKD may have been misclassified as having no CKD. Finally, the SOLVD-Treatment trial was conducted in the pre-beta-blocker era of HF care. Whether the modest mortality reduction associated with enalapril in SOLVD-Treatment may be generalizable to contemporary HF patients receiving beta-blockers and other neurohormonal antagonists and devices remains unclear.
In conclusion, enalapril reduces mortality and hospitalization in SHF patients without significant heterogeneity between those with and without CKD. Despite higher mortality in those with CKD, the effect of enalapril seemed somewhat attenuated. However, the effect of enalapril on cardiovascular and HF hospitalization was robust regardless of CKD. Future studies need to examine the effect of ACEIs in more contemporary HF patients with CKD.
Acknowledgments
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [26].
The Studies of Left Ventricular Dysfunction (SOLVD) trial was conducted and supported by the NHLBI in collaboration with the CHS Investigators. This manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the SOLVD or the NHLBI.
Funding and Support: Dr. Bowling is supported by the Birmingham/Atlanta Department of Veterans Affairs Geriatric Research Education and Clinical Center, the John A. Hartford Foundation and the Southeast Center of Excellence in Geriatric Medicine. Dr. Sanders is supported by the NIH through grants (R01 DK046199 and P30 DK079337) and by the Department of Veterans Affairs through a Merit Award. Dr. Allman is supported by the NIH through grant 5UL1 RR025777. Dr. Ahmed is supported by the NIH through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the NHLBI and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
Footnotes
Conflict of Interest/Disclosure Statement: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 2.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 3.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 4.Ghali JK, Giles T, Gonzales M, et al. Patterns of physician use of angiotensin converting enzyme inhibitors in the inpatient treatment of congestive heart failure. J La State Med Soc. 1997;149:474–84. [PubMed] [Google Scholar]
- 5.Jencks SF, Cuerdon T, Burwen DR, et al. Quality of medical care delivered to Medicare beneficiaries: A profile at state and national levels. JAMA. 2000;284:1670–6. doi: 10.1001/jama.284.13.1670. [DOI] [PubMed] [Google Scholar]
- 6.Jencks SF, Huff ED, Cuerdon T. Change in the quality of care delivered to Medicare beneficiaries, 1998–1999 to 2000–2001. JAMA. 2003;289:305–12. doi: 10.1001/jama.289.3.305. [DOI] [PubMed] [Google Scholar]
- 7.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–93. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 8.Dzau VJ, Colucci WS, Williams GH, Curfman G, Meggs L, Hollenberg NK. Sustained effectiveness of converting-enzyme inhibition in patients with severe congestive heart failure. N Engl J Med. 1980;302:1373–9. doi: 10.1056/NEJM198006193022501. [DOI] [PubMed] [Google Scholar]
- 9.Bart BA, Gattis WA, Diem SJ, O’Connor CM. Reasons for underuse of angiotensin-converting enzyme inhibitors in patients with heart failure and left ventricular dysfunction. Am J Cardiol. 1997;79:1118–20. doi: 10.1016/s0002-9149(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed A. Use of angiotensin-converting enzyme inhibitors in patients with heart failure and renal insufficiency: how concerned should we be by the rise in serum creatinine? J Am Geriatr Soc. 2002;50:1297–300. doi: 10.1046/j.1532-5415.2002.50321.x. [DOI] [PubMed] [Google Scholar]
- 11.Himmelfarb J. Chronic kidney disease and the public health: gaps in evidence from interventional trials. JAMA. 2007;297:2630–3. doi: 10.1001/jama.297.23.2630. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin. 2008;4:387–99. doi: 10.1016/j.hfc.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrie CJ, Mark PB, Weir RA. Broken pump or leaky filter? Renal dysfunction in heart failure a contemporary review. Int J Cardiol. 2008;128:154–65. doi: 10.1016/j.ijcard.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Scrutinio D, Passantino A, Lagioia R, Santoro D, Cacciapaglia E. Detection and prognostic impact of renal dysfunction in patients with chronic heart failure and normal serum creatinine. Int J Cardiol. 2011;147:228–33. doi: 10.1016/j.ijcard.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed A, Rich MW, Sanders PW, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–8. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131–40. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005:S57–65. doi: 10.1111/j.1523-1755.2005.09911.x. [DOI] [PubMed] [Google Scholar]
- 20.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–86. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 21.Ezekowitz J, McAlister FA, Humphries KH, et al. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44:1587–92. doi: 10.1016/j.jacc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed A, Centor RM, Weaver MT, Perry GJ. A propensity score analysis of the impact of angiotensin-converting enzyme inhibitors on long-term survival of older adults with heart failure and perceived contraindications. Am Heart J. 2005;149:737–43. doi: 10.1016/j.ahj.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed A, Kiefe CI, Allman RM, Sims RV, DeLong JF. Survival benefits of angiotensin-converting enzyme inhibitors in older heart failure patients with perceived contraindications. J Am Geriatr Soc. 2002;50:1659–66. doi: 10.1046/j.1532-5415.2002.50457.x. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed A, Love TE, Sui X, Rich MW. Effects of angiotensin-converting enzyme inhibitors in systolic heart failure patients with chronic kidney disease: a propensity score analysis. J Card Fail. 2006;12:499–506. doi: 10.1016/j.cardfail.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed A, Fonarow GC, Zhang Y, et al. Renin–angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med. 2011 doi: 10.1016/j.amjmed.2011.10.013. [in press] doi:10.1016/j.amjmed.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010;144:1–2. [Google Scholar]