Abstract
Collagen comprises ¼ of the protein in humans and ¾ of the dry weight of human skin. Here, we implement recent discoveries about the structure and stability of the collagen triple helix to design new chemical modalities that anchor to natural collagen. The key components are collagen mimetic peptides (CMPs) that are incapable of self-assembly into homotrimeric triple helices, but are able to anneal spontaneously to natural collagen. We show that such CMPs containing 4-fluoroproline residues, in particular, bind tightly to mammalian collagen in vitro and to a mouse wound ex vivo. These synthetic peptides, coupled to dyes or growth factors, could herald a new era in assessing or treating wounds.
Introduction
Collagen is a helix of three polypeptide strands. Each of these strands consists of ~300 Xaa-Yaa-Gly units, where Xaa is often (2S)-proline (Pro) and Yaa is (2S,4R)-4-hydroxyproline (Hyp). Studies with collagen mimetic peptides (CMPs) show that replacing Pro with Hyp in the Yaa position stabilizes the collagen triple helix.1 Initially, this stability was attributed to water molecules forming bridging hydrogen bonds between the 4-hydroxyl groups and main-chain oxygens.2 We showed, however, that collagen stability was enhanced dramatically by replacing Hyp in the Yaa position with (2S,4R)-4-fluoroproline (Flp; Table 1), which has a side chain that is compromised severely in its ability to form hydrogen bonds.3–5 We concluded that stereoelectronic effects were responsible for the extra stability conferred by Hyp.6–8 Briefly, 4R-configured electronegative substituents enforce a Cγ-exo ring pucker that preorganizes the main-chain dihedral angles of the residue in the Yaa position to be those required in a collagen triple helix.9,10
Table 1.
Thermostability of synthetic collagen triple helices.
In contrast to Hyp in the Yaa position, Pro in the Xaa position of a collagen triple helix adopts a Cγ-endo ring pucker.7,11 Accordingly, we found that replacing Pro in the Xaa position with (2S,4S)-4-fluoroproline (flp), which prefers an endo ring pucker, enhances triple-helical stability.12–14 Even though introducing flp into the Xaa position or Flp into the Yaa position is highly stabilizing, introducing both is highly destabilizing due to steric interactions between proximal fluoro groups within the same cross section of a triple helix.15–18 Nonetheless, heterotrimeric triple helices in which (flp-Flp-Gly)7 and (Pro-Pro-Gly)7 are in a ratio 1:2 or 2:1 are much more stable than the homotrimeric triple helices that assemble from either of these strands alone.16
Natural collagen is not effective in retaining passively absorbed materials. Efforts have been made to deliver and immobilize materials on natural collagen by chemical coupling.19 Such covalent modification requires the use of electrophilic reagents that can have adverse consequences for the structure and attributes of endogenous collagen, as well as damage other biopolymers. Hence, we sought to develop a noncovalent means to anchor a material to natural collagen.
Strand invasion plays a key role in molecular biology. A common example is the invasion of a single DNA or PNA strand into a DNA duplex to form base pairs with one of the parental DNA strands within a displacement loop (or “D-loop”).20,21 Natural collagen contains loops or interruptions in its triple helix,22,23 and these domains are accessible to CMPs.24–26 We sought to take advantage of this phenomenon in wound tissue, which abounds in frayed and broken collagen (Fig. 1). We suspected that fluoroproline-based CMPs might anneal to collagen under physiological conditions, unlike (Pro-Hyp-Gly)n- based peptides, which require a high-temperature pre-treatment to dissociate triple helices into single strands.29,30 Such heating could damage the peptide or a pendant molecule, and is not attractive in a clinical setting. Here, we report on the annealing of CMPs to natural collagen in vitro and ex vivo.
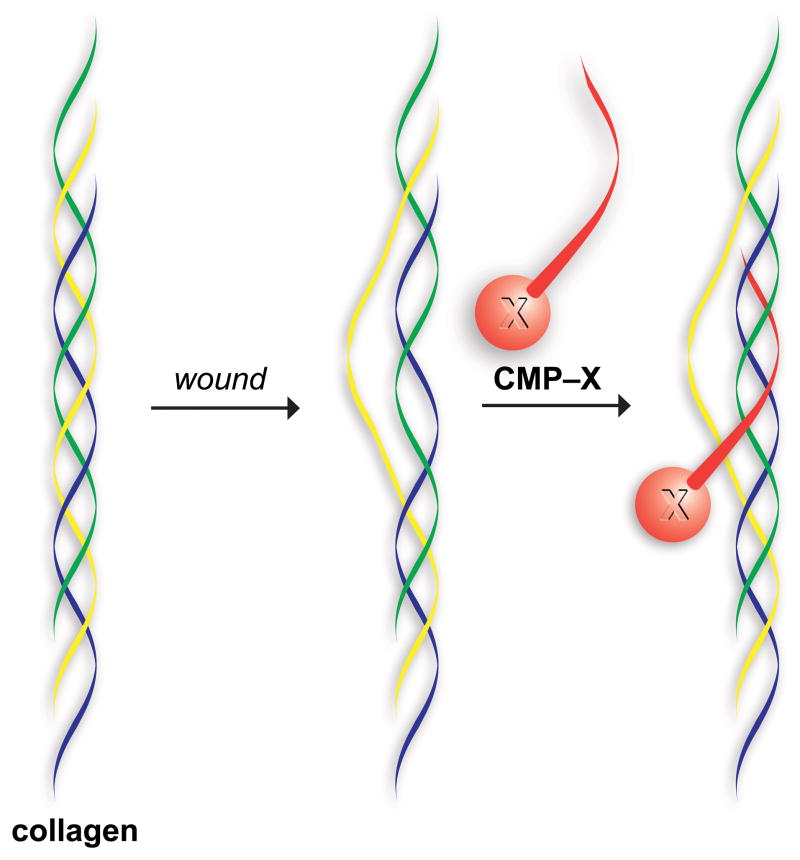
Fig. 1.
Representation of a collagen mimetic peptide (CMP) annealing to damaged collagen to anchor a molecule (X) in a wound bed.
Results and discussion
Design and synthesis of collagen mimetic peptides
CMPs containing 7 Xaa-Yaa-Gly units can be synthesized readily by solid-phase peptide synthesis (SPPS). These peptides can form stable triple helices (Table 1) that resemble those in natural collagen, as is apparent from circular dichroism spectroscopy, analytical ultracentrifugation, X-ray crystallography, fiber diffraction analysis, and electron microscopy.9,31–36 This synthetic strategy facilitates the introduction of nonnatural residues, like flp and Flp,37 into a CMP.
As our preferred CMP, we chose Ac-(flp-Flp-Gly)7-(Gly-Ser)3-LysOH (1). The N-terminal acetyl group precludes any unfavorable Coulombic interactions with natural collagen, and the C-terminal lysine residue provides an amino group for conjugation by N-acylation. The (Gly-Ser)3 unit serves as a flexible, soluble spacer.
As control CMPs, we chose Ac-(Pro-Pro-Gly)7-(Gly-Ser)3-LysOH (2), Ac-(Pro-Pro-Gly)3-(Pro-Pro-Sar)-(Pro-Pro-Gly)3-(Gly-Ser)3-LysOH (3), and Ac-Pro21-(Gly-Ser)3-LysOH (4). We anticipated that CMP 2 should be effective in annealing, though less so than CMP 1 because of its lesser preorganization.6–8 The methyl group of the central sarcosine (Sar) in CMP 3 provides a subtle but strong impediment to triple-helix formation by obviating the interstrand GlyNH···O=CPro hydrogen bond,38 but allows CMP 3 to retain the other physicochemical characteristics of CMP 2. Finally, the linear polyproline strand of CMP 4 is not capable of annealing to natural collagen by triple-helix formation.
CMPs 1–3 were synthesized by a convergent route relying on the condensation of Xaa-Yaa-Gly units. CMP 4 was synthesized the sequential coupling of amino-acid monomers. For annealing experiments, biocompatible dyes were conjugated to the CMPs by O- to N-acyl transfer using an NHS ester of the dyes (Fig. 2).
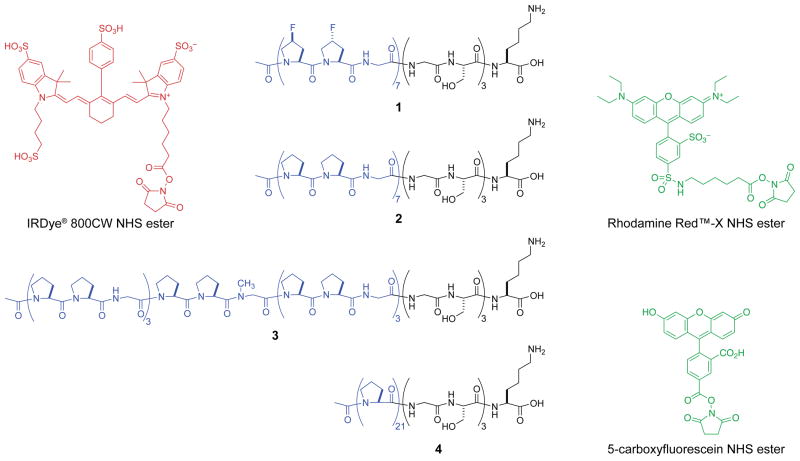
Fig. 2.
CMPs (1–4) and dyes used in this work. Each CMP has a C-terminal (Gly-Ser)3-LysOH segment. CMP–dye conjugates are indicated in the text with a superscript: IRCMP for IRDye® 800CW, RCMP for Rhodamine Red™-X, and FCMP for 5-carboxyfluorescein.
Annealing of collagen-mimetic peptides to collagen in vitro
In initial wound assessment studies, we treated insoluble calf-skin collagen (type I) with fluorescently labeled CMPs 1–4, as well as with the unconjugated fluorophore. We monitored the changes in color and binding by visual inspection over several days. CMPs 1 and 2, which have flp-Flp-Gly and Pro-Pro-Gly units, respectively, annealed to collagen firmly as seen by the persistent color of the insoluble collagen after 12 days (Fig. 3A and 3B). In contrast, collagen treated with CMPs 3 and 4, and free dye lost all apparent color during the first day and retained none after 12 days (Fig. 3C–3E). These initial results validated our strategy (Fig. 1), but did not differentiate between CMPs 1 and 2.
Fig. 3.
Photographs of CMPs annealed to calf-skin type I collagen. Fluorescently labeled CMPs in MeOH were added to collagen, which was washed with PBS, DMSO, and MeOH, and photographed after 12 days. A: RCMP 1; B: RCMP 2; C: RCMP 3; D: RCMP 4; E: Rhodamine Red™-X NHS ester that had been reacted with ethylamine.
Retention of collagen-mimetic peptides on a collagen gel
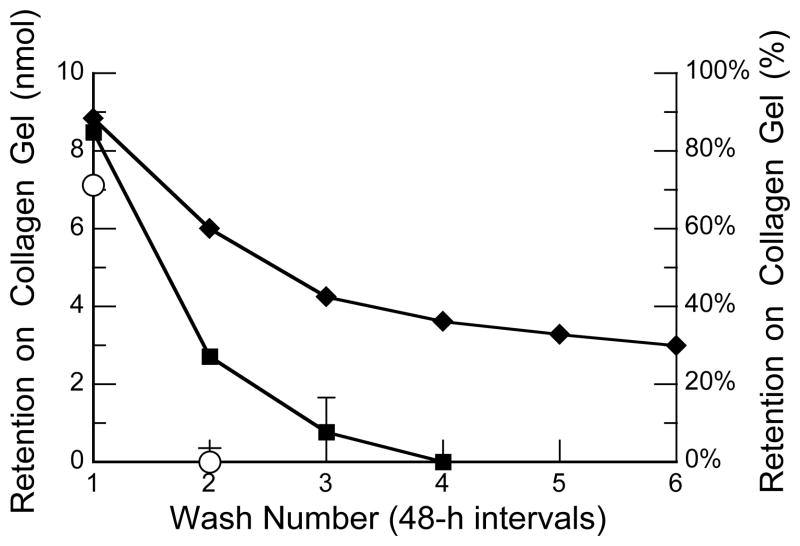
Next, we assessed the time-dependent retention of CMPs 1 and 2 on a gel of rat-tail collagen (type I) under physiological conditions. Over the course of days, CMP 1 exhibited much greater retention than did CMP 2 (Figure 4). Nearly a third of the fluoroproline-containing CMP (1) was retained after two weeks, whereas virtually all of the proline-containing CMP (2) was lost after one week. These data are consistent with the preorganization endowed by the fluoroproline residues.16
Fig. 4.
Plot of the retention of CMPs on a gel of rat-tail type I collagen. Fluorescently labeled CMPs were applied to a gel, which was washed after 90 min (wash number 1) and then at 48-h intervals (wash numbers 2–6). The absorbance of the washes at 494 nm was used to calculate retention on the gel. −: FCMP 1; ■: FCMP 2; □: 5-carboxyfluorescein NHS ester that had been reacted with ethylamine. Values are the mean ± SE from 4 gels.
Annealing of collagen-mimetic peptides to an ex vivo wound
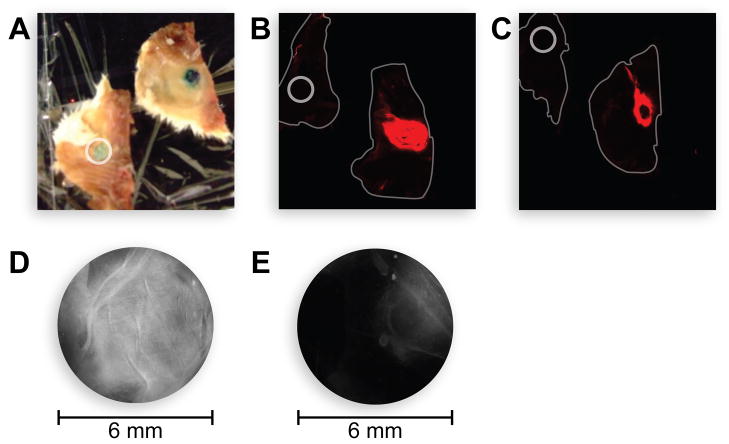
Then, we analyzed the ability of CMPs 1 and 2 to bind to cutaneous wounds on pelts harvested from mice. Identical wounds were created on mice pelts by removing the top layer of the skin, and the consequent wound beds were treated with CMP 1, CMP 2, or the free fluorophore, incubated, and washed. Both CMPs 1 and 2 remained annealed to the wound bed after aggressive washing, whereas the free dye did not (Fig. 5A–C). We repeated the experiment by treating the wound on one side of the pelt with CMP 2 and the other side with CMP 4. As expected, CMP 2 showed much greater binding than did CMP 4 (Fig. 5D and 5E). The fluorescence in these experiments was limited primarily to the wound bed and its edges, where the concentration of damaged collagen is likely to be higher than in the surrounding unbroken skin.
Fig. 5.
Photograph of the annealing of CMPs to mouse collagen ex vivo. Fluorescently labeled CMPs were applied to 6-mm cutaneous wounds on mouse pelts, washed, and imaged. A: IRCMP 1, photograph; B: IRCMP 1, fluorescence image; C: IRCMP 2, fluorescence image; D: RCMP 2, fluorescence image; E: RCMP 4, fluorescence image. In panels A–C, the circles (6 mm) denote wounds treated with IRDye® 800CW NHS ester that had been reacted with ethylamine. In panels B and C, the mouse pelts are outlined.
Toxicity of collagen-mimetic peptides to human cells
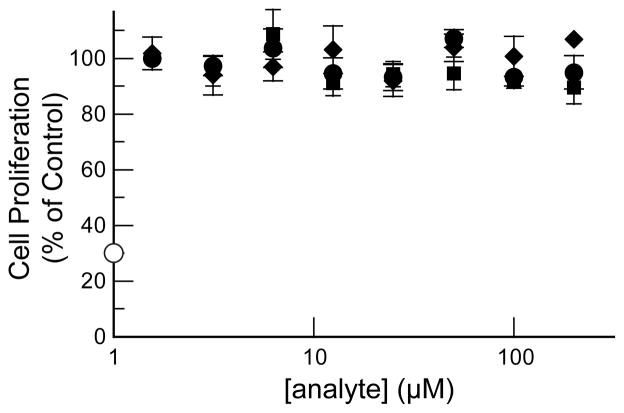
Finally, we performed cytotoxicity assays on CMPs 1 and 2 to determine their suitability for future work in vivo. The CMPs were tested for toxicity towards a relevant model, normal human dermal fibroblast cells. Doxorubicin served as the positive control and (Pro-Hyp-Gly)7 as the negative control. Both peptides proved to be non-toxic to human fibroblast cells (Fig. 6).
Fig. 6.
Cytotoxicity of CMPs. The proliferation of human dermal fibroblast cells was assessed by fluorescence emission using a calcein AM/ethidium homodimer-1 assay after incubation for 72 h with unlabeled CMPs [−, CMP 1; ■, CMP 2; ●, (Pro-Hyp-Gly)7] or doxorubicin (□). Values are the mean ± SE from 4 cultures.
Conclusions
We have demonstrated the efficacy of the strategy depicted in Fig. 1. Both the (flp-Flp-Gly)7-based CMP (1) and the (Pro-Pro-Gly)7-based CMP (2) bind strongly to collagen at room temperature in in vitro and ex vivo. Binding does not require heating the CMP prior to its application. CMP 1 is retained longer on collagen than is CMP 2, providing the option of a long-term attachment of an effector molecule or its sustained release over a shorter time period. Neither peptide is toxic to human fibroblast cells. We anticipate that either CMP 1 or CMP 2 could be used to affix a pendant molecule in a wound bed, obviating the need for repeated application. This methodology avails a myriad of possibilities for the delivery of therapeutic small molecules, peptides, and proteins, and could be especially useful for treating highly traumatized wounds (e.g., in burn patients) or slowly healing wounds (e.g., in diabetic patients).39,40 We foresee a CMP with a pendant dye highlighting areas of maximal tissue damage (which would have many sites for annealing), and a CMP with a pendant growth factor expediting the healing process.
Experimental procedures
General materials and methods
Commercial chemicals were of reagent grade or better, and were used without further purification. Anhydrous THF, DMF, and CH2Cl2 were dispensed from CYCLE-TAINER® solvent delivery systems from J. T. Baker (Phillipsburg, NJ). Other anhydrous solvents, including DMSO, were obtained in septum-sealed bottles. In all reactions involving anhydrous solvents, glassware was either oven- or flame-dried.
Flash chromatography was performed with columns of silica gel 60, 230–400 mesh from Silicycle (Québec City, Canada). Semi-preparative HPLC was performed with a Varian Dynamax C-18 reversed-phase column. Analytical HPLC was performed with a Vydac C-18 reversed-phase column.
IRDye® 800CW NHS ester was from LI-COR (Lincoln, NE). Rhodamine Red™-X NHS ester and 5-carboxyfluorescein NHS ester were from Life Technologies (Grand Island, NY). Insoluble calf-skin collagen from ICN Biomedicals (Irvine, CA) and rat-tail type I collagen (5 mg/mL) from Life Technologies were used for the in vitro annealing and retention studies, respectively. Cryopreserved PrimaPure™ normal human (adult) dermal fibroblasts (NHDF) were from Genlantis (San Diego, CA), and a LIVE/DEAD® Viability/Cytotoxicity Kit for mammalian cells was from Life Technologies.
Mass spectrometry was performed with either a Micromass LCT (electrospray ionization, ESI) mass spectrometer from Waters (Milford, MA) in the Mass Spectrometry Facility in the Department of Chemistry or an Applied Biosystems Voyager DE-Pro (matrix-assisted laser desorption/ionization) mass spectrometer from Life Technologies in the Biophysics Instrumentation Facility at the University of Wisconsin–Madison.
Peptide synthesis
Peptides were synthesized by SPPS using an Applied Biosystems Synergy 432A Peptide Synthesizer from Life Technologies at the University of Wisconsin–Madison Biotechnology Center. The first seven couplings were of a normal duration (30 min), subsequent couplings were extended (120–200 min). Fmoc-deprotection was achieved by treatment with piperidine (20% v/v) in DMF. CMPs 1–4 were synthesized on FmocLys(Boc)-Wang resin (100–200 mesh). CMPs 1–3 were synthesized by segment condensation of their corresponding Fmoc-tripeptides (3 equiv).
For CMP 1, Fmocflp-Flp-GlyOH was synthesized from commercial BocflpOH and BocFlpOH,37 as described previously.16 Briefly, PyBOP-mediated coupling of BocFlpOH to the tosylate salt of glycine benzyl ester yielded a dipeptide, which was converted to its HCl salt, coupled to FmocflpOH, and subjected to hydrogenation to yield the tripeptide. Fmocflp-Flp-GlyOH, FmocGlyOH, and FmocSer(tBu)OH were used in SPPS, resulting in CMP 1.
For CMPs 2 and 3, FmocPro-Pro-GlyOH was synthesized by using N,N′-dicyclohexylcarbodiimide-mediated coupling as reported previously.41 FmocPro-Pro-GlyOH, FmocProOH, FmocSarOH, FmocGlyOH, and FmocSer(tBu)OH were used in SPSS, resulting in CMPs 2 and 3.
CMP 4 was synthesized by the sequential coupling of FmocProOH, FmocGlyOH, and FmocSer(tBu)OH by SPPS.
Peptides were cleaved from the Wang resin by using 95:2.5:2.5 TFA/triisopropylsilane/H2O (total volume: 2 mL), precipitated from t-butylmethylether at 0 °C, and isolated by centrifugation. Peptides were purified by semi-preparative HPLC using the following linear gradients: CMP 1, 5–45% B over 60 min, CMP 2, 10–90% B over 50 min, and CMP 3, 5–85% B over 45 min, where solvent A was H2O containing TFA (0.1% v/v) and solvent B was CH3CN containing TFA (0.1% v/v). All peptides were judged to be >90% pure by analytical HPLC and MALDI–TOF mass spectrometry: m/z [M + H]+ calculated for CMP 1 2631, found 2635; m/z [M + Na]+ calculated for CMP 2 2403, found 2402; m/z [M + Na]+ calculated for CMP 3 2417, found 2416; m/z [M + H]+ calculated for CMP 4 2658, found 2657; m/z [M + H]+ calculated for (Pro-Hyp-Gly)7 1889, found 1889.
CMP–fluorophore conjugate synthesis
The general method optimized for the milligram-scale synthesis of peptide–dye conjugates was as follows. A CMP (1 equiv) was mixed with a fluorescent dye (1.13 equiv) in ~0.3 mL of DMSO containing triethylamine (20 equiv). The resulting solution was allowed to stir at room temperature in the dark for 48 h, and then subjected to purification by reversed-phase HPLC using a linear gradient of 10 mM triethylammonium acetate (pH 7.0) and MeOH. The purified products were characterized by HRMS–ESI or MALDI TOF mass spectrometry. The highly anionic products of conjugation to IRDye® 800CW NHS were stored in glass vials with the ammonium form of a cation-exchange resin. This resin was prepared by stirring Dowex™ 50WX4-50 resin overnight in 1 M NH4OAc. Before use, the resin was washed in 1 M NH4OAc, water, acetone, and hexane, and air-dried.
In vitro annealing
A 660 μM solution (50 μL in 5% v/v DMSO) of RCMPs 1–4 or Rhodamine Red™-X NHS ester (Fig. 2) that had been reacted with ethylamine (2 equiv) was added to calf-skin type I collagen (~10 mg) in a Falcon™ tube. The tubes were incubated in a water bath at 37 °C. After 2 h, each tube was agitated with a vortexing mixer and washed vigorously with phosphate-buffered saline (PBS; 4×), DMSO (4×), and MeOH (4×). These washings were discarded, and the samples were incubated in MeOH at room temperature for 12 days.
Retention on a collagen gel
Rat-tail type I collagen (5 mg/mL in dilute acid), sterile 10× PBS, sterile 1 N NaOH, and sterile H2O were cooled on ice. The amount of each reagent was calculated to make a collagen solution with a final concentration of 3.8 mg/mL in 1× PBS as follows:
The 10× PBS, 1 N NaOH, and H2O were mixed in a sterile tube. The collagen was added slowly to the mixture, which was then mixed thoroughly. This suspension was added to the wells of a 48-well microtiter plate (0.20 mL/plate), which was incubated at 37 °C and 93% humidity in an incubator for 1 h. The resulting gel was rinsed with 1× PBS. Solutions (0.50 mM) of FCMP 1, FCMP 2, and neutralized 5-FAM were prepared in PBS containing DMSO (5% v/v). An aliquot (20 μL) of each solution was added to the wells. The plates were incubated at 37 °C and 93% humidity, and the gel was washed with 4-°C PBS (300 μL). The wells were the re-filled with PBS (500 μL), and the culture plate was incubated at 37 °C with 90% humidity and 5% v/v CO2. The buffer was exchanged with fresh PBS (500 μL) every 48 h. The concentration of FCMP 1, FCMP 2, or neutralized 5-FAM leached during incubation was determined by measuring the absorbance of the removed PBS at 494 nm using a Cary 50Bio spectrophotometer from Varian (Palo Alto, CA).
Ex vivo annealing
Pelts were harvested from euthanized mice and stored at −80 °C. Immediately prior to an annealing experiment, pelts were thawed and shaved with an electric clipper. The treatment area was cleaned in a circular motion with cotton swabs wetted with sterile PBS, and all residual hair was removed. Two identical cutaneous defects were created in each pelt by using a 6-mm biopsy punch, and the top layer of skin was removed by using a forceps and scissors. The wounds were washed with sterile PBS and air-dried. One wound on each pelt was treated with a 50-μM solution (25 μL) of IRCMPs 1 or IRCMP 2, and the other wound was treated with the same amount of the free dye (IRDye® 800CW NHS ester) that had been reacted with ethylamine (2 equiv). The treated wounds were incubated for 1 h at room temperature in a moist environment, and then washed with PBS and DMSO successively for 10 min each. Similar comparisons between CMP 2 and CMP 4 were made by creating identical wounds on mice pelts, and treating one of them with RCMP 2 and the other with RCMP 4. (For the pelts treated with free and conjugated Rhodamine Red™-X dye, it was necessary to rub the wounds with DMSO during the wash due to the highly hydrophobic nature of this dye.) The pelts were then imaged using an Odyssey Imager from LI-COR (for the IRDye® 800CW) and a dissecting fluorescent microscope (for the Rhodamine Red™-X Dye).
Multiplex cytotoxicity assay
Solutions of CMPs 1 and 2, and (Pro-Hyp-Gly)7 (20 mM) were diluted in anhydrous DMSO to a final concentration of 100×. Serial dilutions were made in DMSO in 96-well polypropylene microtiter plates using the Precision XS liquid handler from BioTek (Winooski, VT). Compounds were divided equally into the wells in all 4 quadrants of a 384-well microtiter plate using a Biomek FX liquid handler with 96-channel pipetting head from Beckman Coulter (Brea, CA). Compounds were stored at −20 °C in DMSO until the day of the assay. Freeze-thaw cycles were limited to a maximum of ten per plate.
NHDF cells were maintained as reported previously.42 Cells were harvested by trypsinization using trypsin (0.25% w/v) and EDTA (0.1% w/v), and then counted with a Cellometer Auto T4 cell counter from Nexcelom (Lawrence, MA), before dilution for plating. Cell plating, compound handling, and assay set-up were performed as reported previously,42 except that the cells were plated in 50-μL volumes in 384-well clear-bottom tissue-culture plates from Corning (Lowell, MA). Compounds were added from 384-well stock plates at a 1:100 dilution using a Biomek FX liquid handler. Loaded plates were incubated for 72 h at 37 °C and 5% v/v CO2. Calcein AM (to 10 μM) and ethidium homodimer-1 (to 100 μM) were added (total volume: 30 μL), and the plates were incubated for 30 min at 37 °C. The emission in each well at 530 and 615 nm (calcein AM and ethidium homodimer-1, respectively) was determined by using a Safire-2 microplate reader from Tecan (Männedorf, Switzerland). In addition, CellTiter-Glo reagent (15 μL) from Promega (Madison, WI) was added, and the resulting solution was incubated for 10 min at room temperature with gentle agitation to lyse the cells. The luminescence in each well was determined, and those data were consistent with the data obtained with calcein AM and ethidium homodimer-1.
Supplementary Material
Acknowledgments
We are grateful to N. L. Abbott, M. D. Shoulders, F. W. Kotch, L. J. Martin, and G. A. Ellis for contributive discussions. This work was supported by grants R01 AR044276 and RC2 AR058971 (NIH), and a Wisconsin Institutes for Discovery Seed Grant. MALDI–TOF mass spectrometry was performed at the University of Wisconsin–Madison Biophysics Instrumentation Facility, which was established with Grants BIR-9512577 (NSF) and S10 RR13790 (NIH).
Footnotes
Electronic supplementary information (ESI) available: Analytical HPLC traces and MALDI–TOF mass spectra for CMPs 1–4.
Notes and references
- 1.Inouye K, Sakakibara S, Prockop DJ. Biochim Biophys Acta. 1976;420:133–141. doi: 10.1016/0005-2795(76)90352-4. [DOI] [PubMed] [Google Scholar]
- 2.Bella J, Brodsky B, Berman HM. Structure. 1995;3:893–906. doi: 10.1016/S0969-2126(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 3.Holmgren SK, Taylor KM, Bretscher LE, Raines RT. Nature. 1998;392:666–667. doi: 10.1038/33573. [DOI] [PubMed] [Google Scholar]
- 4.Engel J, Prockop DJ. Matrix Biol. 1998;17:679–680. doi: 10.1016/s0945-053x(98)90119-6. [DOI] [PubMed] [Google Scholar]
- 5.Holmgren SK, Bretscher LE, Taylor KM, Raines RT. Chem Biol. 1999;6:63–70. doi: 10.1016/S1074-5521(99)80003-9. [DOI] [PubMed] [Google Scholar]
- 6.Bretscher LE, Jenkins CL, Taylor KM, DeRider ML, Raines RT. J Am Chem Soc. 2001;123:777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]
- 7.DeRider ML, Wilkens SJ, Waddell MJ, Bretscher LE, Weinhold F, Raines RT, Markley JL. J Am Chem Soc. 2002;124:2497–2505. doi: 10.1021/ja0166904. [DOI] [PubMed] [Google Scholar]
- 8.Kotch FW, Guzei IA, Raines RT. J Am Chem Soc. 2008;130:2952–2953. doi: 10.1021/ja800225k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoulders MD, Raines RT. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoulders MD, Satyshur KA, Forest KT, Raines RT. Proc Natl Acad Sci USA. 2010;106:559–564. doi: 10.1073/pnas.0909592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitagliano L, Berisio R, Mazzarella L, Zagari A. Biopolymers. 2001;58:459–464. doi: 10.1002/1097-0282(20010415)58:5<459::AID-BIP1021>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Renner C, Alefelder S, Bae JH, Budisa N, Huber R, Moroder L. Angew Chem Int Ed. 2001;40:923–925. [PubMed] [Google Scholar]
- 13.Hodges JA, Raines RT. J Am Chem Soc. 2003;125:9262–9263. doi: 10.1021/ja035881z. [DOI] [PubMed] [Google Scholar]
- 14.Doi MN, Uchlyama YS, Nishluchi Y, Nakazawa T, Ohkubo T, Kobayashi Y. J Am Chem Soc. 2003;125:9922–9923. doi: 10.1021/ja035997v. [DOI] [PubMed] [Google Scholar]
- 15.Doi M, Nishi Y, Uchiyama S, Nishiuchi Y, Nishio H, Nakazawa T, Ohkubo T, Kobayashi Y. J Pept Sci. 2005;11:609–616. doi: 10.1002/psc.671. [DOI] [PubMed] [Google Scholar]
- 16.Hodges JA, Raines RT. J Am Chem Soc. 2005;127:15923–15932. doi: 10.1021/ja054674r. [DOI] [PubMed] [Google Scholar]
- 17.Shoulders MD, Guzei IA, Raines RT. Biopolymers. 2008;89:443–454. doi: 10.1002/bip.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoulders MD, Kamer KJ, Raines RT. Bioorg Med Chem Lett. 2009;19:3859–3862. doi: 10.1016/j.bmcl.2009.03.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiller JC, Bonner G, Pan LC, Klibanov AM. Biotechnol Bioeng. 2001;73:246–252. doi: 10.1002/bit.1057. [DOI] [PubMed] [Google Scholar]
- 20.Kasamatsu H, Robberson DL, Vinograd J. Proc Natl Acad Sci USA. 1971;68:2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen PE. Acc Chem Res. 1999;32:624–630. [Google Scholar]
- 22.Paterlini MG, Nemethy G, Scheraga HA. Biopolymers. 1995;35:607–619. doi: 10.1002/bip.360350607. [DOI] [PubMed] [Google Scholar]
- 23.Long CG, Thomas M, Brodsky B. Biopolymers. 1995;35:621–628. doi: 10.1002/bip.360350608. [DOI] [PubMed] [Google Scholar]
- 24.Miles CA, Bailey AJ. Micron. 2001;32:325–332. doi: 10.1016/s0968-4328(00)00034-2. [DOI] [PubMed] [Google Scholar]
- 25.Leikina E, Mertts MV, Kuznetsova N, Leikin S. Proc Natl Acad Sci USA. 2002;99:1314–1318. doi: 10.1073/pnas.032307099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo X, An Y, Yun CS, Yu SM. Angew Chem Int Ed. 2006;45:2267–2270. doi: 10.1002/anie.200504529. [DOI] [PubMed] [Google Scholar]
- 27.Berisio R, Granata V, Vitagliano L, Zagari A. J Am Chem Soc. 2004;126:11402–11403. doi: 10.1021/ja047069h. [DOI] [PubMed] [Google Scholar]
- 28.Nishi Y, Uchiyama S, Doi M, Nishiuchi Y, Nakazawa T, Ohkubo T, Kobayashi Y. Biochemistry. 2005;44:6034–6042. doi: 10.1021/bi047887m. [DOI] [PubMed] [Google Scholar]
- 29.Wang AY, Mo X, Chen CS, Yu SM. J Am Chem Soc. 2005;127:4130–4131. doi: 10.1021/ja0431915. [DOI] [PubMed] [Google Scholar]
- 30.Wang AY, Foss CA, Leong S, Mo X, Pomper MG, Yu MS. Biomacromolecules. 2008;9:1755–1763. doi: 10.1021/bm701378k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenkins CL, Raines RT. Nat Prod Rep. 2002;19:49–59. doi: 10.1039/a903001h. [DOI] [PubMed] [Google Scholar]
- 32.Koide T. Philos Trans R Soc Lond B Biol Sci. 2008;362:1281–1291. doi: 10.1098/rstb.2007.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woolfson DN. Biopolymers. 2010;94:118–127. doi: 10.1002/bip.21345. [DOI] [PubMed] [Google Scholar]
- 34.Fields GB. Org Biomol Chem. 2010;8:1237–1258. doi: 10.1039/b920670a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fallas JA, O’Leary LER, Hartgerink JD. Chem Soc Rev. 2010;39:3510–3527. doi: 10.1039/b919455j. [DOI] [PubMed] [Google Scholar]
- 36.Przybyla DE, Chmielewski J. Biochemistry. 2010;49:4411–4419. doi: 10.1021/bi902129p. [DOI] [PubMed] [Google Scholar]
- 37.Chorghade MS, Mohapatra DK, Sahoo G, Gurjar MK, Mandlecha MV, Bhoite N, Moghe S, Raines RT. J Fluor Chem. 2008;129:781–784. doi: 10.1016/j.jfluchem.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y-S, Chen C-C, Horng J-C. Biopolymers. 2011;96:60–68. doi: 10.1002/bip.21470. [DOI] [PubMed] [Google Scholar]
- 39.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 40.Schultz GS, Wysocki A. Wound Rep Reg. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 41.Jenkins CL, Vasbinder MM, Miller SJ, Raines RT. Org Lett. 2005;7:2619–2622. doi: 10.1021/ol050780m. [DOI] [PubMed] [Google Scholar]
- 42.Langenhan JM, Peters NR, Guzei IA, Hoffmann FM, Thorson JS. Proc Natl Acad Sci USA. 2005;102:12305–12310. doi: 10.1073/pnas.0503270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.