Abstract
Problem
Secretory leukocyte protease inhibitor (SLPI) is an innate immune peptide present on the genitourinary tract mucosa which has antimicrobial activity. In this study, we investigated the interaction of SLPI with Neisseria gonorrhoeae.
Method of study
ELISA and far-western blots were used to analyze binding of SLPI to gonococci. The binding site for SLPI was identified by tryptic digests and mass spectrometry. Antimicrobial activity of SLPI for gonococci was determined using bactericidal assays. SLPI protein levels in cell supernatants were measured by ELISA, and SLPI mRNA levels were assessed by quantitative RT-PCR.
Results
SLPI bound directly to the gonococcal Opa protein and was bactericidal. Epithelial cells from the reproductive tract constitutively expressed SLPI at different levels. Gonococcal infection of cells did not affect SLPI expression.
Conclusion
We conclude that SLPI is bactericidal for gonococci and is expressed by reproductive tract epithelial cells and thus is likely to play a role in the pathogenesis of gonococcal infection.
Keywords: antimicrobial peptide, epithelial cell, gonorrhea, Neisseria gonorrhoeae, reproductive tract
Introduction
Sexually transmitted infections (STI) due to Neisseria gonorrhoeae represent a major world-wide public health problem with an estimated 60 million new cases each year.1 By far, women bear the greatest burden of gonococcal infections, suffering more frequent and more serious complications than men.2 Ascension of gonococci to the upper reproductive tract can lead to pelvic inflammatory disease in approximately 10–20% of infected women who can suffer from the complications of chronic pain, infertility, and are at risk for ectopic pregnancy as a result.3 Control of gonococcal infection is an increasing challenge due to the emergence of multidrug-resistance strains and to the lack of a vaccine.4,5 In addition, infections caused by N. gonorrhoeae increase the likelihood of HIV-1 transmission by increasing both HIV-1 shedding in HIV-1–infected adults and the susceptibility to HIV-1 infection in those who are HIV-1 negative,6 and as such control of gonorrhea needs to be an important component of HIV-1 prevention strategies.7
Infection of the genital mucosa by N. gonorrhoeae involves attachment to and invasion of epithelial cells, which is dependent on the expression of both pili and phase-variable opacity (Opa) proteins.8 The clinical symptoms of gonococcal infections are caused primarily by the intense inflammatory infiltrate of neutrophils responding to cytokines expressed by induction of the innate immune system.9 Previous work from our laboratory and those of other investigators has shown that gonococci can initiate proinflammatory cytokine expression through engagement of innate immune receptors TLR2 and TLR4 resulting in activation of NFκB.10–12 In addition to cytokines, a family of low molecular weight protein antimicrobial mediators of innate immunity including defensins and secretory leukocyte protease inhibitor (SLPI) are found in cervicovaginal secretions of the genital tract in vivo and in secretions from reproductive tract epithelial cells in vitro during gonococcal infection and are thought to influence the outcome of the infection.13–16
SLPI is an 11.7 kDa protein expressed by neutrophils, macrophages, and epithelial cells which is found in various mucosal secretions such as saliva, bronchial mucus, seminal plasma, and cervical mucus of the female genital tract.17 Although SLPI was originally described as a serine proteinase inhibitor able to inhibit host neutrophil proteases, it is now recognized for its pleotropic role in inflammation and immunity through its anti-inflammatory, immune regulatory, and antimicrobial activities. For bacteria, SLPI has been shown to have direct bactericidal activity for Escherichia coli, Staphylococcus aureus, Mycobacterium tuberculosis, and Salmonella typhimurium, although the mechanism is not well understood.18–20 For gonococci, a recent report found that secretions from primary female reproductive tract epithelial cells containing SLPI as well as a number of cytokines and antimicrobial peptides inhibited gonococcal growth, however the specific contribution of SLPI to the bactericidal activity of the secretions was not delineated.13 An association between higher SLPI concentrations in vaginal fluid samples and a reduced rate of perinatal HIV-1 transmission has been reported.21 Conversely, levels of SLPI in vaginal fluid are decreased in women with STI including gonorrhea,14 which may represent an underlying mechanism of increased susceptibility to infection with HIV-1 in individuals with gonorrhea.
In this study, we investigated the interaction of SLPI with gonococci to determine whether SLPI is bactericidal for gonococci and whether gonococcal infection regulates SLPI expression by reproductive tract and intestinal epithelial cells. We demonstrate that SLPI is bactericidal through direct binding to the gonococcal Opa protein and that Opa-negative gonococci are resistant to its bactericidal effect. Moreover, we show that reproductive tract and intestinal epithelial cells constitutively express SLPI and that the regulation of expression is not influenced by either gonococcal adherence to or invasion of cells.
Materials and methods
Bacterial Strains
N. gonorrhoeae strains FA1090, GC56, F62, 1291, and MS11mk LOS variants A (MkA) and C (MkC) and N. meningitidis serogroup A strains 7880 and 7889 and serogroup C strain 89I were used in this study and have been described previously.22–25 Strains were cultured on plates with Difco GC medium base containing IsoVitaleX (Becton, Dickinson, Franklin Lakes, NJ) in the presence of 5% CO2. All strains were selected for expression of Opa protein and piliation by using a dissecting microscope and standard selection criteria.26,27 Opa expression was confirmed by electrophoretic and immunoblotting analyses of whole-cell lysates using Opa MAb B33, which reacts with all Opa protein variants, as described previously.8
Whole Bacteria ELISA
Binding of recombinant human SLPI (R&D Systems, Minneapolis, MN) to whole bacteria was investigated by ELISA assay as described previously.22 In brief, microtiter wells were coated overnight with 100 μl of a suspension of bacteria of absorbance 1.0 at 580 nm. Wells were blocked in DIG buffer (Roche Applied Science, Indianapolis, IN) for 1 h and incubated with 75 μl of 2 μg/ml SLPI for 1 h. For some experiments, increasing concentrations of SLPI were added to the wells to assess dose-response binding. SLPI binding was detected using 2 μg/ml of affinity-purified goat anti-human SLPI (R&D Systems) and alkaline phosphatase conjugated anti-goat IgG Fc (Sigma-Aldrich, St. Louis, MO). The SLPI antibody or the secondary antibody was omitted in control wells. Plates were developed with p-nitrophenyl phosphate substrate (Sigma-Aldrich) and absorbance was measured at 405 nm.
Far-Western Blots
Bacterial outer membrane complex (OMC) was purified from whole bacteria as described previously.28 LOS and proteins were separated by 12% SDS-PAGE under reducing conditions, then blotted to nitrocellulose. Membranes were blocked in DIG buffer (Roche) containing 0.01% Tween-20 for 1 h, and incubated with 0.4 μg/ml SLPI for 1 h. Binding was detected using 0.4 μg/ml affinity-purified goat anti-human SLPI (R&D systems) followed by alkaline phosphatase conjugated anti-goat IgG Fc (Sigma-Aldrich) and Western Blue stabilized alkaline phosphatase substrate (Promega, Madison, WI). For Opa detection, membranes were blocked in DIG buffer and incubated with Opa MAb B33. Opa bands were visualized with alkaline phosphatase conjugated anti-mouse IgG Fc (Sigma-Aldrich) and the Western Blue substrate.
Mass Spectrometric Protein Identification
To confirm the identity of the protein that bound SLPI, the OMC proteins of strain F62 were separated by 12% SDS-PAGE and the gel was stained with Coomassie. Protein in-gel digestion was performed on the protein band in the gel that aligned with the band bound by SLPI in the far-western blots using sequencing grade modified porcine trypsin (Promega) at a final concentration of 12.5 ng/μl according to an established protocol.29 Mixtures of proteolytically generated peptides were analyzed by nanoLC MS/MS utilizing a 2DLC nanoHPLC System (Eksigent, Dublin, CA) interfaced with the Q-Star XL mass spectrometer (AB Sciex, Foster City, CA) equipped with a the nanospray II source (AB Sciex). External calibration was performed in MS/MS mode using fragment ions of Glu-fibrinopeptide as references. An LC Packings Pepmap C18 trap column and a column self-packed with Jupiter Proteo C12 end-capped material were used for desalting and reversed phase peptide separation, respectively. A linear gradient of acetonitrile and formic acid was run at 250 nl/min flow rate. Precursor ion selection employed an automated routine (IDA, Analyst QS 1.1, AB Sciex) that consisted of a series of one survey MS scan (1 s, m/z 400–1700) and two MS/MS scans (2 s, m/z 60–1500). Protein identification was accomplished by using the MASCOT 2.0 search engine (Matrix Science, Boston, MA).
Bactericidal Assay
Opa-positive and Opa-negative variants of gonococcal strains were grown overnight on GC agar containing 1% IsoVitaleX and bacteria were harvested and washed in warm gonococcal buffer as previously described.30 Optical density at 580 nm was used to serially dilute stocks to a concentration of 2 × 105 CFU/ml. Two thousand CFU were incubated in gonococcal buffer for 30 minutes in a 200 μl reaction volume containing 50 μl of increasing tenfold concentrations of recombinant human SLPI (R&D Systems) prepared in gelatin veronal saline buffer with 0.01% bovine serum albumin (Sigma-Aldrich). For some experiments, 50 μl of neutralizing affinity-purified goat anti-human SLPI (R&D Systems) at a concentration of 50 μg/ml was added to the reaction mixture. At time zero and following incubation of the tubes at 37°C for 30 min, 20 μl aliquots of the reactions were plated in duplicate for each concentration of SLPI tested. All plates were incubated overnight and bacterial colonies were counted. Survival was expressed as the percentage of bacteria at time zero that survived to 60 min.
Epithelial Cells
Human endometrial and cervical carcinoma cell lines HEC-1B and ME-180, intestinal epithelial and colon carcinoma cell lines HT-29 and Caco-2, and three HPV16/E6E7 immortalized reproductive tract epithelial cell lines endocervical End 1/E6E7, ectocervical Ect 1/E6E7, and vaginal Vk2/E6E7 were obtained from the American Type Culture Collection (Manassas, VA). HEC-1B cells were cultivated in MEM Eagle’s with Earle’s BSS containing 10% fetal bovine serum, 0.1 mM non-essential amino acids, and 10 μg/ml sodium pyruvate. ME-180 and HT-29 cells were cultivated in McCoy’s 5A containing 10% fetal bovine serum and 0.1 mM non-essential amino acids. Caco-2 cells were cultivated in MEM Eagle’s containing 20% fetal bovine serum and 0.1 mM non-essential amino acids. All reagents were obtained from UCSF Cell Culture Facility unless otherwise noted. The E6E7 cell lines were maintained in keratinocyte serum free medium (Invitrogen, Carlsbad CA) supplemented with 0.05 mg/ml bovine pituitary extract, 0.1 ng/ml recombinant human epidermal growth factor, and 0.44 μg/ml CaCl2. Human primary fallopian tube epithelial cells were obtained from women who had undergone hysterectomies for various benign conditions at Memorial Medical Center in Springfield, IL as previously described.31 Each patient gave consent for tissue to be used in this protocol. Briefly, pieces of fallopian tubes were placed in a sterile petri dish containing an enzyme mixture for 2 h at 37°C as described by Fahey et al.32 Harvested epithelial cells were grown in D-MEM/F-12 media and monolayers took 5–7 days to form.
SLPI ELISA
The constitutive expression of SLPI by reproductive and intestinal tract epithelial cells was measured by ELISA following 8 hours of growth. For the expression of SLPI by cells in response to bacterial infection, 1 × 106 cells were seeded into wells of a six-well cell culture plate and grown overnight prior to infection with a piliated, Opa-positive variant of strain FA1090. Wells were washed 3 times with PBS, fresh media was added, and wells were infected with an MOI of bacteria to cells of 20:1. At specified times, supernatants were collected from the wells and the concentration of SLPI was measured using a SLPI ELISA kit according to the protocol of the manufacturer (R&D Systems).
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted from 1 × 106 cells either infected or uninfected with a piliated, Opa-positive variant of strain FA1090 using the RNeasy mini kit according to instructions of the manufacturer (Qiagen, Germantown, MD). cDNA was synthesized from 1 μg total RNA after DNAse I digestion using QuantiScript reverse transcriptase (Qiagen). qRT-PCR was run on the Applied Biosystems HT 7900 using 50 ng cDNA with QuantiTect human SLPI and GAPDH primers (Qiagen) and LightCycler 480 SYBR Green PCR master mix with ROX passive reference dye (Roche). Samples were analyzed using the ΔΔCt method,33 and expressed as a fold difference compared with the uninfected controls.
Statistical Analysis
Statistical analyses were performed using SigmaStat for Windows version 3.11 (Systat Software, San Jose, CA). Groups of data were analyzed by the Tukey test for multiple pairwise comparisons. Values of p < 0.05 were considered significant for all comparisons.
Results
SLPI Binds to N. gonorrhoeae
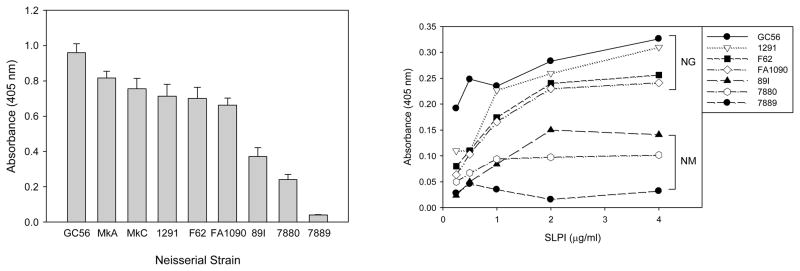
Whole-bacteria ELISA analyses were performed to determine whether gonococci bound SLPI and whether the strains varied in the level of recognition and binding. For comparison with the other pathogenic Neisseria species, we also tested the binding of SLPI to three strains of meningococci. As shown in Figure 1, SLPI bound to all six strains of gonococci, but to variable degrees (p < 0.001). The length of the OS α-chain of the gonococcal LOS did not affect binding, as MkA and MkC bound similar amounts of SLPI despite MkA expressing a truncated lactosyl version of the OS α-chain of MkC.34 Although SLPI also bound to meningococci to variable degrees, all gonococcal strains bound significantly more SLPI than did the encapsulated serogroups A and C meningococcal strains (p < 0.001). For both gonococci and meningococci, the binding of SLPI was dose-dependent and saturable, with gonococci binding higher levels of SLPI than meningococci at all concentrations tested.
Fig. 1.
Binding of SLPI to N. gonorrhoeae and N. meningitidis. Whole-bacteria ELISA analyses were performed to assess the binding of SLPI to N. gonorrhoeae strains GC56, MkA, MkC, 1291, F62, and FA1090, and to N. meningitidis strains 89I, 7880, and 7889. Left panel: SLPI bound to all strains of gonococci and meningococci, but to varying degrees. The gonococcal strains bound significantly more SLPI than did the meningococcal strains (p < 0.001 for all comparisons with gonococcal strains). The bars represent the mean ± s.d of triplicate data points. The results are representative of four independent experiments. Right panel: Dose-response binding of SLPI to gonococci and meningococci. For both species, the binding of SLPI was dose-dependent, and gonococci bound more SLPI at all concentrations tested. The symbols represent the mean of triplicate data points and the results are representative of four independent experiments.
Far-Western Blot Analyses of SLPI Binding
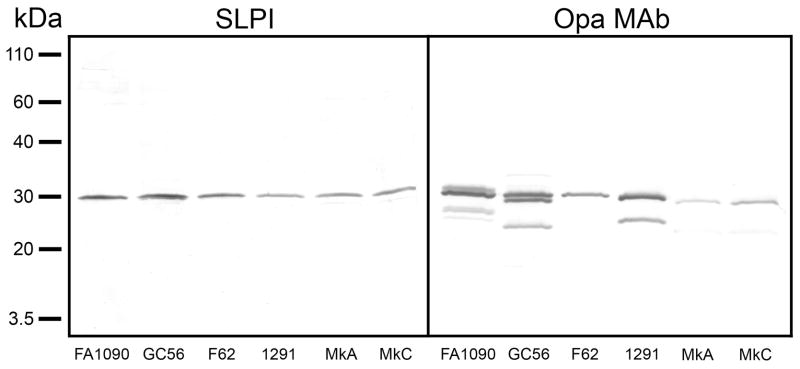
We next investigated the site of binding of SLPI to the gonococcal outer membrane using far-western blots to probe the binding of SLPI to dissociated LOS and protein components of the OMC purified from the six gonococcal strains. As shown in Figure 2, the blots revealed that SLPI bound to a single component of the gonococcal outer membrane with an approximate mass of 30 kDa. As the size of the 30 kDa protein was appropriate for a gonococcal Opa protein, we used Opa MAb B33 to provide evidence for its identity as Opa. The Opa MAb bound to a band of comparable SDS-PAGE migration distance to that bound by SLPI, providing evidence that the Opa protein is the site of SLPI binding. Interestingly, the Opa MAb also revealed that the strains exhibited a repertoire of Opa expression ranging from single variants expressed by strains F62, MkA, and MkC to multiple variants expressed by strains FA1090, GC56, and 1291, however only a single Opa variant of similar electrophoretic mobility from each strain bound SLPI.
Fig. 2.
The site of binding of SLPI to the gonococcal outer membrane was investigated using far-western blots to probe the binding of SLPI to dissociated LOS and protein components of outer membrane complexes purified from the six gonococcal strains. Left panel: Membranes incubated with recombinant human SLPI and probed with anti-human SLPI showed that SLPI bound to a single protein component of the gonococcal outer membrane with an approximate mass of 30 kDa. Right panel: Membranes incubated with Opa MAb B33, which reacts with all Opa protein variants, identified the location of the Opa proteins. The results are representative of four independent experiments.
The identity of Opa was confirmed using in-gel digestion of the candidate protein with modified porcine trypsin to mass fingerprint the peptide fragments. Strain F62 was chosen for the analyses as it expressed only one Opa variant which bound SLPI. Mixtures of proteolytically generated peptides were analyzed by nanoLC MS/MS utilizing a 2DLC nanoHPLC system interfaced with the Q-Star XL mass spectrometer. A probability based Mowse score of 330 with 11 matching peptide queries (p < 0.05) 35 identified the protein as Opa52 based on the Opa nomenclature of Kupsch et al.
Bactericidal Activity of SLPI for Gonococci
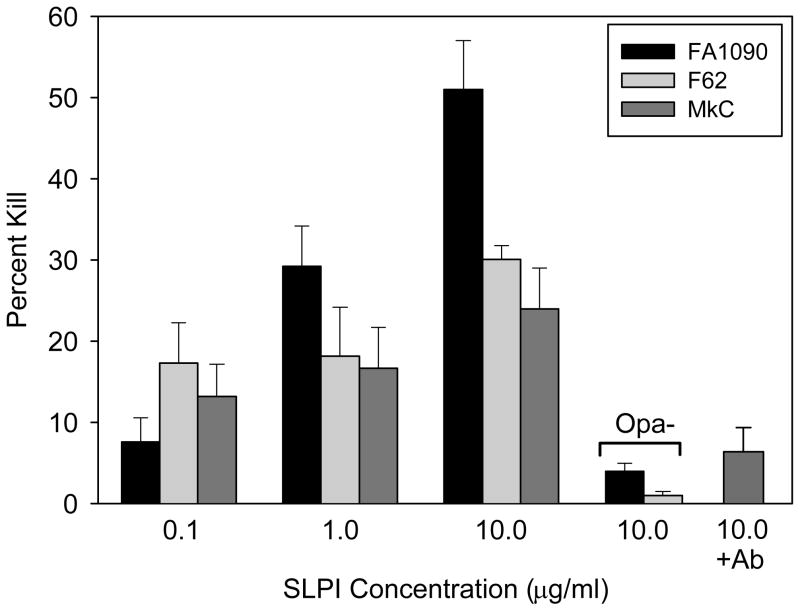
It has been reported that SLPI has antimicrobial activity for the gram-negative bacteria E. coli and S. typhimurium.13,18 We therefore evaluated the bactericidal potential of SLPI for gonococci to assess the biological importance of SLPI binding to the gonococcal outer membrane Opaprotein. As shown in Figure 3, SLPI was bactericidal for the three strains of gonococci tested; FA1090, F62, and MkC. The level of killing varied with strain FA1090 being the most sensitive to killing at the two highest concentrations of SLPI tested. All three strains demonstrated a dose-dependent susceptibility to the bactericidal activity of SLPI at concentrations from 0.1 to 10 μg/ml. Increasing the concentrations of SLPI to 50 μg/ml and 100 μg/ml did not increase the killing of any of the strains (results not shown). To confirm that SLPI was the bactericidal component in the assay, neutralizing anti-SLPI antibody was added to the reaction mixture. In the presence of the SLPI antibody, killing of strain MkC was significantly reduced from 24% to 6% (p = 0.006), which supported the conclusion that SLPI was bactericidal for gonococci. Consistent with the finding that Opa was the target of SLPI binding, Opa-negative variants of FA1090 and F62 were significantly more resistant to SLPI killing. Compared with Opa-positive variants, the killing of Opa-negative variants of FA1090 and F62 was reduced from 51% to 4% and from 30% to 1%, respectively (p < 0.001 for both strains).
Fig. 3.
Bactericidal activity of SLPI for gonococci. Increasing concentrations of SLPI were incubated with Opa-positive colonial variants of gonococci for 30 min and survival was determined. Opa-negative colonial variants of FA1090 and F62 were significantly reduced in their killing by SLPI (p < 0.001 for both strains). In the presence of neutralizing SLPI antibody, killing of strain MkC was significantly reduced (p = 0.006). The bars represent the mean ± s.d of triplicate data points. The results are representative of four independent experiments.
Expression of SLPI by Reproductive and Intestinal Tract Epithelial Cells
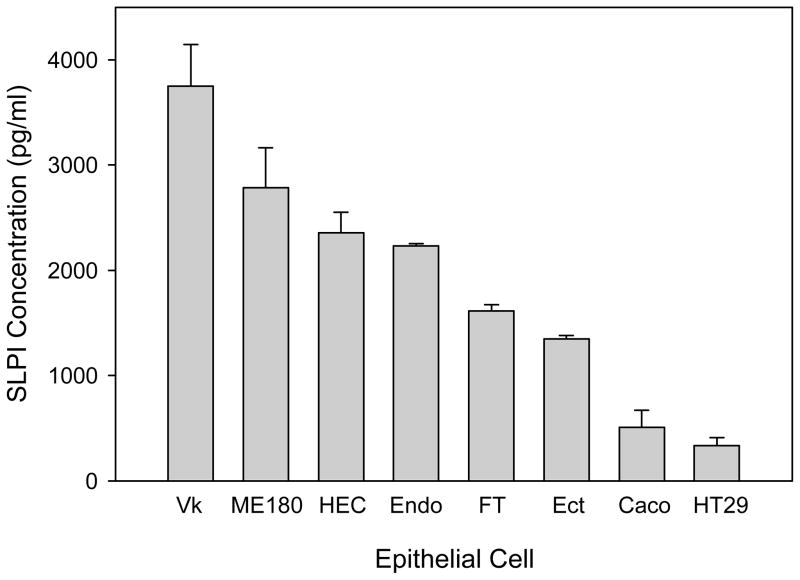
The constitutive expression of SLPI by epithelial cell lines of the female reproductive tract and of the intestinal tract as well as by primary fallopian tube epithelial cells was assessed by ELISA as shown in Figure 4. All cell types constitutively expressed SLPI, however the range of expression was broad. The six reproductive tract epithelial cell types expressed significantly greater amounts of SLPI than the two intestinal tract cells (p < 0.001 for all comparisons). The expression of SLPI by the reproductive tract cells spanned nearly a three-fold range with the vaginal cells expressing the highest levels, perhaps due to the role of vaginal epithelial cells as the first line of innate immune defense against infectious diseases.
Fig. 4.
SLPI is constitutively expressed to differential degrees by mucosal epithelial cells. The expression of SLPI by reproductive tract and intestinal epithelial cells was assessed by ELISA following 8 hrs of cell culture. All cells types constitutively expressed SLPI, with the reproductive tract cells expressing significantly greater amounts than the intestinal cells (p < 0.001 for all comparisons). The bars represent the mean ± s.d of triplicate data points. The results are representative of four independent experiments.
Regulation of SLPI Expression in Response to Gonococcal Cell Adherence and Invasion
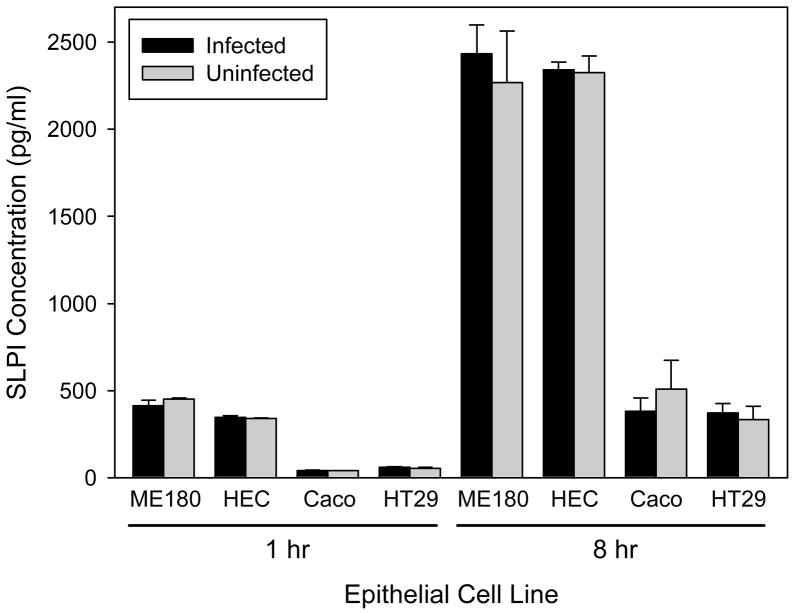
The expression of SLPI by reproductive tract and intestinal epithelial cells in response to gonococcal infection was tested using strain FA1090 (Figure 5). SLPI expression was measured at 1 hour and 8 hours post-infection, which represent known times of adherence to and invasion of HEC-1B cells by this strain, respectively.8 At both time points, all four epithelial cell types infected with gonococci were found to express similar levels of SLPI compared with uninfected cells (p > 0.05 for all comparisons). These results were confirmed by qRT-PCR data which showed no change in the level of SLPI mRNA transcript, consistent with the unchanged SLPI protein levels measured by ELISA (data not shown).
Fig. 5.
Expression of SLPI by reproductive tract and intestinal mucosal epithelial cells infected with a piliated, Opa-positive variant of N. gonorrhoeae strain FA1090. Expression was tested at 1 hour, which is the time of cell adherence by the bacteria, and 8 hours, which is the time of cell invasion. At both time points, epithelial cells exposed to gonococci were found to express similar levels of SLPI compared with unexposed cells (p > 0.05 for all comparisons). The bars represent the mean ± s.d of triplicate data points. The results are representative of four independent experiments.
Discussion
Mucosal epithelial cells secrete a variety of cytokines, chemokines, and antimicrobial peptides to support an innate immune defense barrier against pathogenic organisms.36,37 One of the defense molecules found in mucosal secretions is SLPI, an antimicrobial peptide which has been shown to have direct bactericidal activity against several types of bacteria,18–20,38 however such activity against a bacterial STI pathogen has not been documented to date. In this study, we demonstrate that SLPI is bactericidal for gonococci in a dose-dependent manner through binding to the Opa outer membrane protein. The concentrations of SLPI tested were within the physiological levels of SLPI expressed constitutively in both the female and male urogenital tracts.14,39 Further, the concentrations of SLPI and the degree of bactericidal activity were comparable to that reported for the gram-negative bacteria E. coli and S. typhimurium,18,19 although the bactericidal activity in those studies required a four-fold longer incubation period suggesting that SPLI may be more active against gonococci. Two other innate immune antimicrobial peptides, HE2α and cathelicidin LL-37, which are expressed to greater degrees in the male compared with the female urogenital tract have been reported to exhibit bactericidal activity against gonococci,40,41 although the underlying mechanisms of their antimicrobial activities have not been not identified.
The binding of SLPI to Opa initiated bactericidal killing of gonococci, however the degree of susceptibility to SLPI-mediated killing displayed strain selectivity with FA1090 more susceptible than either F62 or MkC. Although all three strains bound similar amounts of SLPI based on ELISA analyses, FA1090 was the only strain of the three that expressed multiple Opa variants with only a single Opa variant binding SLPI as did all the gonococcal strains. Opa-negative variants of gonococci were resistant to SLPI, suggesting that Opa phenotype phase variation allows gonococci to evade the bactericidal activity of SLPI. Similarly, Opa proteins have been reported to be targets for the C4b and C3b products of complement activation, and as such may play a role in determining bactericidal killing of gonococci through the complement cascade.42
Cationic peptides such as SLPI are thought to kill bacteria by altering the stability of the outer membrane, however the initial binding interaction between SLPI and the bacterial surface leading to microbial killing is not well understood. For mycobacteria, Gomez et al. reported that SLPI binds to several membrane lipids recognized as containing pathogen-associated molecular patterns to facilitate both killing and phagocytosis of mycobacterial strains.20,43 The domain responsible for the bactericidal activity of SLPI has been reported to be located in the cationic N-terminal domain with no apparent contribution by the C-terminal serine proteinase inhibitory domain.18,20 As reported recently by Dewald et al.,44 the significant negative charge of Opa proteins reduces the high propensity of Opa to self-aggregate which facilitates the folding and insertion of the protein into a lipid-containing membrane. As such, the mechanism by which SLPI kills gonococci potentially involves a charge interaction between Opa and the cationic domain of SLPI resulting in the disruption of Opa folding in the membrane leading to overall membrane destabilization and bactericidal activity.
Gonococcal Opa proteins form a family of structurally and functionally related proteins that are encoded by a family of at least 11 independently expressed opa genes.45–47 Antigenic diversity stems from surface-exposed semi- and hypervariable regions within a conserved framework. The expression of Opa is phase variable with the reversible switch between expression and the lack of expression occurring at a rate of approximately 10−3 per generation. Thus a single gonococcus may express from none to several of the Opa proteins in different combinations,48 and this repertoire of Opa expression was represented by the strains in the current study. While the Opa proteins in all strains that bound SLPI had the same apparent molecular weight by SDS-PAGE, we cannot conclude with certainty based on far-western blots that they are the identical Opa variant as several Opa proteins exhibit similar molecular weights.35,49 The data did show however that in those strains which expressed multiple Opa variants, there were variants not bound by SLPI, suggesting that a gonococcal strain expressing only variants not recognized by SLPI would be relatively resistant to bactericidal killing. Of the strains tested, the MS11 variants MkC and MkA have been shown in a previous study to express the Opa52 protein which bound SLPI in strain F62.50 In addition, Bhat et al. reported that the surface-exposed hypervariable region-1 of the Opa52 protein from MS11 shows significant homology with that of the OpaD (or P.IId47,51) protein from FA1090.46 Interestingly, this region has been shown to have a sizeable negative charge and thus may play a role in binding SLPI.51
Opa interacts extensively with human cells, including epithelial cells and phagocytes, through interaction with heparan-sulfate proteoglycans and members of the carcinoembryonic antigen related-cellular adhesion molecule (CEACAM) family, with different Opa variants showing variable specificities for these different ligands.45 Through ligation of CEACAM expressed by cells, Opa influences the innate and adaptive responses to infections by pathogenic Neisseria.52 The Opa52 variant bound by SLPI recognizes the four CEACAM family members, CEA, CEACAM1, CEACAM3, and CEACAM6, which have been shown to bind to Neisserial Opa proteins, and ligation of CEACAM by Opa52 has been reported to inhibit CD4+ T cell activation and proliferation.53 We have previously shown that Opa binds to both mannose-binding lectin of the complement cascade and the cellular receptor TREM-2.22,54 Whether the interactions of these two molecules with Opa interfere with the bactericidal activity of SLPI remains to be investigated.
The expression of both SLPI and Opa is hormonally regulated in the female reproductive tract. For SLPI, maximal expression is in the ovulatory phase and reduced expression in the follicular and luteal phases.55 Interestingly, this relationship is inverse to the reported susceptibility to gonorrhea with regard to menstrual cycle phase. For gonococcal infection, the follicular phase of the menstrual cycle has been reported to have the highest incidence of disease.56 In addition, Sweet et al. found that gonococcal salpingitis infection of the fallopian tube occurred most frequently within seven days of the onset of menses.57 As such, we can speculate that reduced levels of SLPI in the follicular phase may contribute to the increased susceptibility of women to gonorrhea at that point in the menstrual cycle. However, as the female reproductive tract is an immunologically dynamic environment, multiple effector mechanisms certainly play a role in innate immune protection against disease. Opa expression by gonococci is subject to selection in vivo based on the menstrual cycle as the Opa-negative phenotype was found to predominate in cervical isolates from women during the luteal phase,56 and in isolates from fallopian tube salpingitis cases which typically occur near menses.57,58 This finding has been supported recently in a mouse model of gonorrhea in which the stage of the reproductive cycle was reported to play a role in selection of the Opa phenotype.59 Thus, hormones influence both the innate immune protection offered by SLPI as well as the pathogenic potential of the infecting organisms.
SLPI was constitutively expressed to differential degrees by both immortalized and primary mucosal epithelial cells, with the reproductive tract cells expressing significantly greater amounts than the intestinal cells. These results corroborate and expand reports by other investigators on the expression of SLPI by these mucosal cell types.13,19,60 The vaginal cells expressed the highest levels of SLPI, which may be due to their frequent exposure to microbes as the forefront epithelial cell type of the reproductive tract. Similar low levels of constitutive SLPI expression by intestinal epithelial cells have been reported previously,19 suggesting that the importance of SLPI in the innate immune response of the intestine may be diminished when compared with that of the female reproductive tract. SLPI expression by the primary fallopian tube epithelial cells is consistent with the finding of SLPI in biopsies of the fallopian tube epithelium analyzed by immunohistochemistry.61 In women, the constitutive expression of SLPI in the reproductive tract can vary in a similar fashion to that observed for the cell lines. In particular, Cohen et al. found that the cervicovaginal levels of SLPI in healthy, young women in sub-Saharan Africa were significantly lower compared to those from similar women in San Francisco, with no apparent underlying cause.62 Given the bactericidal activity of SLPI for gonococci, this may partly contribute to the higher incidence of gonorrhea in women in Africa than in the U.S.5
The regulation of SLPI expression by epithelial cells in response to microbial infections varies considerably. We found that gonococcal infection of reproductive tract and the intestinal epithelial cells did not influence the expression of SLPI protein or mRNA either at the time of gonococcal adherence at 1 hour or at the time of invasion at 8 hours. Similarly, infection of intestinal epithelial cells by S. typhimurium at 5 hours was reported to result in no change in SLPI expression,19 and infection of cervical epithelial cells by vesicular stomatitis virus induced no change in SLPI.63 Interestingly, infection of an oviductal epithelial cell line by the bacterial STI pathogen Chlamydia trachomatis had no effect on SLPI mRNA expression,61 whereas Wheelhouse et al. reported that C. trachomatis did induce SLPI mRNA in HeLa cervical epithelial cells,64 suggesting differences in the SLPI response of different anatomical locations of the reproductive tract to Chlamydia infection. In contrast, herpes simplex virus and Helicobacter pylori have been reported to down-regulate SLPI expression in epithelial cell lines from the cervix and gastric musosa, respectively.63,65 Conversely, HIV was found to upregulate SLPI expression to a modest degree in cervical epithelial cells,63 and to a significant degree in oral epithelial cells.66 By comparison of SLPI to the induction of other antimicrobial peptides in reproductive tract cells, we previously observed that gonococci upregulated the expression of human α-defensins 5 and 6 in the three vaginal, endocervical, and ectocervical cell lines utilized in this study,16 perhaps indicating differences in the regulation of antimicrobial peptide expression among reproductive tract epithelial cell types.
Levels of SLPI have been reported to be decreased in the cervicovaginal fluids of women with infections of the lower reproductive tract including gonorrhea, chlamydial, trichomoniasis, and bacterial vaginosis in comparison with control subjects.14,67 For gonorrhea, our data on the expression of SLPI by reproductive tract epithelial cells infected with gonococci would have predicted no change in the levels of SLPI in infected women. One possible explanation for this discrepancy is that SLPI is degraded by cysteine proteases such as cathepsin B, which are known to cleave SLPI both in vitro and in vivo.68,69 In inflammatory tissue, concentrations of cathepsins have been shown to be increased significantly.69,70 We recently demonstrated that N. gonorrhoeae induces the enzymatic activity of cathepsin B through the formation of inflammasomes and the induction of IL-1β by LOS in monocytic THP-1 cells.71 Taken together, these observations suggest that the cleavage of SLPI by cathepsin B may account for decreased levels of SLPI in women with gonorrhea, and may represent an underlying mechanism of increased susceptibility to HIV-1 infection in individuals with gonorrhea.6
In summary, our study revealed that SLPI binds directly to gonococci through an interaction with the gonococcal Opa protein and initiates bactericidal killing. In addition, we found that SLPI also bound to encapsulated serogroups A and C meningococci, but to a lesser degree than to gonococci. It is likely that the meningococcal A and C polysaccharide capsules interfer with the binding of SLPI to Opa as demonstrated previously for the binding of the antimicrobial peptide LL-37 to meningococci.72 These data support a new role for SLPI as a innate immune pattern-recognition receptor for N. gonorrhoeae and N. meningitidis. Because of the constitutive expression of SLPI by reproductive tract epithelial cells that are the target of gonococcal invasion, and its affinity for the gonococcal outer membrane Opa protein required for efficient invasion of cells, SLPI is likely to play a role in the pathogenesis of gonococcal infection. Although SLPI binds to gonococci and is bactericidal, gonococci of the Opa-negative phenotype are resistant to its bactericidal effect, and variations in the levels of SLPI expression on the genitourinary tract mucosa in combination with the gonococcal Opa phenotype may influence the clinical course of gonorrhea.
Acknowledgments
This work was supported by the U.S. Department of Veterans Affairs, Office of Biomedical Laboratory Research and Development, and by National Institutes of Health Grant AI063927 (GAJ) from the National Institute of Allergy and Infectious Diseases. Grant administration support was provided by the Northern California Institute for Research and Education. The authors would like to acknowledge Yiming Li for excellent technical assistance and the UCSF Mass Spectrometry Core Facility for the peptide mass mapping which is supported by the Sandler Family Foundation, the Gordon and Betty Moore Foundation, and NIH/NCI Cancer Center Support Grant P30 CA082103. This is paper number 104 from the Center for Immunochemistry.
References
- 1.Tapsall JW. Antibiotic resistance in Neisseria gonorrhoeae. Clin Infect Dis. 2005;41 (Suppl 4):S263–S268. doi: 10.1086/430787. [DOI] [PubMed] [Google Scholar]
- 2.Walker CK, Sweet RL. Gonorrhea infection in women: prevalence, effects, screening, and management. Int J Womens Health. 2011;3:197–206. doi: 10.2147/IJWH.S13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011;9:61–70. doi: 10.1586/eri.10.156. [DOI] [PubMed] [Google Scholar]
- 4.Deguchi T, Nakane K, Yasuda M, Maeda S. Emergence and spread of drug resistant Neisseria gonorrhoeae. J Urol. 2010;184:851–858. doi: 10.1016/j.juro.2010.04.078. [DOI] [PubMed] [Google Scholar]
- 5.Zhu W, Chen CJ, Thomas CE, Anderson JE, Jerse AE, Sparling PF. Vaccines for gonorrhea: can we rise to the challenge? Front Microbiol. 2011;2:124. doi: 10.3389/fmicb.2011.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35:946–959. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 7.Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. 2011;87:183–190. doi: 10.1136/sti.2010.047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiss JM, Lammel CJ, Wang J, Dekker NP, Brooks GF. Neisseria gonorrhoeae coordinately uses Pili and Opa to activate HEC-1-B cell microvilli, which causes engulfment of the gonococci. Infect Immun. 1999;67:3469–3480. doi: 10.1128/iai.67.7.3469-3480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spear GT, Kendrick SR, Chen HY, Thomas TT, Bahk M, Balderas R, Ghosh S, Weinberg A, Landay AL. Multiplex immunoassay of lower genital tract mucosal fluid from women attending an urban STD clinic shows broadly increased IL1β and lactoferrin. PLoS One. 2011;6:e19560. doi: 10.1371/journal.pone.0019560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pridmore AC, Jarvis GA, John CM, Jack DL, Dower SK, Read RC. Activation of toll-like receptor 2 (TLR2) and TLR4/MD2 by Neisseria is independent of capsule and lipooligosaccharide (LOS) sialylation but varies widely among LOS from different strains. Infect Immun. 2003;71:3901–3908. doi: 10.1128/IAI.71.7.3901-3908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-κB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem. 2003;278:46252–46260. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 12.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 13.Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, Frechette GM, Hill ET, Fahey JV. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol. 2011;4:335–342. doi: 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draper DL, Landers DV, Krohn MA, Hillier SL, Wiesenfeld HC, Heine RP. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am J Obstet Gynecol. 2000;183:1243–1248. doi: 10.1067/mob.2000.107383. [DOI] [PubMed] [Google Scholar]
- 15.Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- 16.Klotman ME, Rapista A, Teleshova N, Micsenyi A, Jarvis GA, Lu W, Porter E, Chang TL. Neisseria gonorrhoeae-induced human defensins 5 and 6 increase HIV infectivity: role in enhanced transmission. J Immunol. 2008;180:6176–6185. doi: 10.4049/jimmunol.180.9.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallenave JM. Secretory leukocyte protease inhibitor and elafin/trappin-2: versatile mucosal antimicrobials and regulators of immunity. Am J Respir Cell Mol Biol. 2010;42:635–643. doi: 10.1165/rcmb.2010-0095RT. [DOI] [PubMed] [Google Scholar]
- 18.Hiemstra PS, Maassen RJ, Stolk J, Heinzel-Wieland R, Steffens GJ, Dijkman JH. Antibacterial activity of antileukoprotease. Infect Immun. 1996;64:4520–4524. doi: 10.1128/iai.64.11.4520-4524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Si-Tahar M, Merlin D, Sitaraman S, Madara JL. Constitutive and regulated secretion of secretory leukocyte proteinase inhibitor by human intestinal epithelial cells. Gastroenterology. 2000;118:1061–1071. doi: 10.1016/s0016-5085(00)70359-3. [DOI] [PubMed] [Google Scholar]
- 20.Gomez SA, Arguelles CL, Guerrieri D, Tateosian NL, Amiano NO, Slimovich R, Maffia PC, Abbate E, Musella RM, Garcia VE, Chuluyan HE. Secretory leukocyte protease inhibitor: a secreted pattern recognition receptor for mycobacteria. Am J Respir Crit Care Med. 2009;179:247–253. doi: 10.1164/rccm.200804-615OC. [DOI] [PubMed] [Google Scholar]
- 21.Pillay K, Coutsoudis A, Agadzi-Naqvi AK, Kuhn L, Coovadia HM, Janoff EN. Secretory leukocyte protease inhibitor in vaginal fluids and perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;183:653–656. doi: 10.1086/318535. [DOI] [PubMed] [Google Scholar]
- 22.Quan DN, Cooper MD, Potter JL, Roberts MH, Cheng H, Jarvis GA. TREM-2 binds to lipooligosaccharides of Neisseria gonorrhoeae and is expressed on reproductive tract epithelial cells. Mucosal Immunol. 2008;1:229–238. doi: 10.1038/mi.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarvis GA, Li J, Swanson KV. Invasion of human mucosal epithelial cells by Neisseria gonorrhoeae upregulates expression of intercellular adhesion molecule 1 (ICAM-1) Infect Immun. 1999;67:1149–1156. doi: 10.1128/iai.67.3.1149-1156.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu M, John CM, Jarvis GA. Phosphoryl moieties of lipid A from Neisseria meningitidis and N. gonorrhoeae lipooligosaccharides play an important role in activation of both MyD88- and TRIF-dependent TLR4-MD-2 signaling pathways. J Immunol. 2010;185:6974–6984. doi: 10.4049/jimmunol.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider H, Griffiss JM, Williams GD, Pier GB. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982;128:13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- 26.Kellogg DS, Cohen IR, Norins LC, Schroeter AL, Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968;96:596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey JM. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987;165:1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zollinger WD, Mandrell RE, Griffiss JM, Altieri P, Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979;63:836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiménez C, Huang L, Qiu Y, Burlingame AL. In-gel digestion of proteins for MALDI-MS fingerprint mapping. In: Coligan JE, Dunn BM, Speicher DW, Windfield PT, editors. Current Protocols in Protein Science. John Wiley & Sons; 2006. pp. 16.4.2–16.4.4. [DOI] [PubMed] [Google Scholar]
- 30.Ross SC, Densen P. Opsonophagocytosis of Neisseria gonorrhoeae: interaction of local and disseminated isolates with complement and neutrophils. J Infect Dis. 1985;151:33–41. doi: 10.1093/infdis/151.1.33. [DOI] [PubMed] [Google Scholar]
- 31.Swanson KV, Jarvis GA, Brooks GF, Barham BJ, Cooper MD, Griffiss JM. CEACAM is not necessary for Neisseria gonorrhoeae to adhere to and invade female genital epithelial cells. Cell Microbiol. 2001;3:681–691. doi: 10.1046/j.1462-5822.2001.00147.x. [DOI] [PubMed] [Google Scholar]
- 32.Fahey JV, Humphrey SL, Stern JE, Wira CR. Secretory component production by polarized epithelial cells from the human female reproductive tract. Immunol Invest. 1998;27:167–180. doi: 10.3109/08820139809089454. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Kerwood DE, Schneider H, Yamasaki R. Structural analysis of lipooligosaccharide produced by Neisseria gonorrhoeae, strain MS11mk (variant A): a precursor for a gonococcal lipooligosaccharide associated with virulence. Biochemistry. 1992;31:12760–12768. doi: 10.1021/bi00166a008. [DOI] [PubMed] [Google Scholar]
- 35.Kupsch EM, Knepper B, Kuroki T, Heuer I, Meyer TF. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huttner KM, Bevins CL. Antimicrobial peptides as mediators of epithelial host defense. Pediatr Res. 1999;45:785–794. doi: 10.1203/00006450-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol. 2011;88:185–194. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 39.Moriyama A, Shimoya K, Kawamoto A, Hashimoto K, Ogata I, Kunishige I, Ohashi K, Azuma C, Saji F, Murata Y. Secretory leukocyte protease inhibitor (SLP) concentrations in seminal plasma: SLPI restores sperm motility reduced by elastase. Mol Hum Reprod. 1998;4:946–950. doi: 10.1093/molehr/4.10.946. [DOI] [PubMed] [Google Scholar]
- 40.Liao M, Ruddock PS, Rizvi AS, Hall SH, French FS, Dillon JR. Cationic peptide of the male reproductive tract, HE2α, displays antimicrobial activity against Neisseria gonorrhoeae, Staphylococcus aureus and Enterococcus faecalis. J Antimicrob Chemother. 2005;56:957–961. doi: 10.1093/jac/dki350. [DOI] [PubMed] [Google Scholar]
- 41.Goytia M, Shafer WM. Polyamines can increase resistance of Neisseria gonorrhoeae to mediators of the innate human host defense. Infect Immun. 2010;78:3187–3195. doi: 10.1128/IAI.01301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis LA, Ram S, Prasad A, Gulati S, Getzlaff S, Blom AM, Vogel U, Rice PA. Defining targets for complement components C4b and C3b on the pathogenic neisseriae. Infect Immun. 2008;76:339–350. doi: 10.1128/IAI.00613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Dewald AH, Hodges JC, Columbus L. Physical determinants of β-barrel membrane protein folding in lipid vesicles. Biophys J. 2011;100:2131–2140. doi: 10.1016/j.bpj.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dehio C, Gray-Owen SD, Meyer TF. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 1998;6:489–495. doi: 10.1016/s0966-842x(98)01365-1. [DOI] [PubMed] [Google Scholar]
- 46.Bhat KS, Gibbs CP, Barrera O, Morrison SG, Jahnig F, Stern A, Kupsch EM, Meyer TF, Swanson J. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–1901. doi: 10.1111/j.1365-2958.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 47.Dempsey JA, Litaker W, Madhure A, Snodgrass TL, Cannon JG. Physical map of the chromosome of Neisseria gonorrhoeae FA1090 with locations of genetic markers, including opa and pil genes. J Bacteriol. 1991;173:5476–5486. doi: 10.1128/jb.173.17.5476-5486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Black WJ, Schwalbe RS, Nachamkin I, Cannon JG. Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun. 1984;45:453–457. doi: 10.1128/iai.45.2.453-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barritt DS, Schwalbe RS, Klapper DG, Cannon JG. Antigenic and structural differences among six proteins II expressed by a single strain of Neisseria gonorrhoeae. Infect Immun. 1987;55:2026–2031. doi: 10.1128/iai.55.9.2026-2031.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt KA, Deal CD, Kwan M, Thattassery E, Schneider H. Neisseria gonorrhoeae MS11mkC opacity protein expression in vitro and during human volunteer infectivity studies. Sex Transm Dis. 2000;27:278–283. doi: 10.1097/00007435-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Connell TD, Shaffer D, Cannon JG. Characterization of the repertoire of hypervariable regions in the Protein II (opa) gene family of Neisseria gonorrhoeae. Mol Microbiol. 1990;4:439–449. doi: 10.1111/j.1365-2958.1990.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 52.Sadarangani M, Pollard AJ, Gray-Owen SD. Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria. FEMS Microbiol Rev. 2011;35:498–514. doi: 10.1111/j.1574-6976.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- 53.Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 54.Estabrook MM, Jack DL, Klein NJ, Jarvis GA. Mannose-binding lectin binds to two major outer membrane proteins, opacity protein and porin, of Neisseria meningitidis. J Immunol. 2004;172:3784–3792. doi: 10.4049/jimmunol.172.6.3784. [DOI] [PubMed] [Google Scholar]
- 55.Moriyama A, Shimoya K, Ogata I, Kimura T, Nakamura T, Wada H, Ohashi K, Azuma C, Saji F, Murata Y. Secretory leukocyte protease inhibitor (SLPI) concentrations in cervical mucus of women with normal menstrual cycle. Mol Hum Reprod. 1999;5:656–661. doi: 10.1093/molehr/5.7.656. [DOI] [PubMed] [Google Scholar]
- 56.James JF, Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978;19:332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sweet RL, Blankfort-Doyle M, Robbie MO, Schacter J. The occurrence of chlamydial and gonococcal salpingitis during the menstrual cycle. JAMA. 1986;255:2062–2064. [PubMed] [Google Scholar]
- 58.Draper DL, James JF, Brooks GF, Sweet RL. Comparison of virulence markers of peritoneal and fallopian tube isolates with endocervical Neisseria gonorrhoeae isolates from women with acute salpingitis. Infect Immun. 1980;27:882–888. doi: 10.1128/iai.27.3.882-888.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cole JG, Fulcher NB, Jerse AE. Opacity proteins increase Neisseria gonorrhoeae fitness in the female genital tract due to a factor under ovarian control. Infect Immun. 2010;78:1629–1641. doi: 10.1128/IAI.00996-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fichorova RN, Anderson DJ. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod. 1999;60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- 61.King AE, Wheelhouse N, Cameron S, McDonald SE, Lee KF, Entrican G, Critchley HO, Horne AW. Expression of secretory leukocyte protease inhibitor and elafin in human fallopian tube and in an in-vitro model of Chlamydia trachomatis infection. Hum Reprod. 2009;24:679–686. doi: 10.1093/humrep/den452. [DOI] [PubMed] [Google Scholar]
- 62.Cohen CR, Moscicki AB, Scott ME, Ma Y, Shiboski S, Bukusi E, Daud I, Rebbapragada A, Brown J, Kaul R. Increased levels of immune activation in the genital tract of healthy young women from sub-Saharan Africa. AIDS. 2010;24:2069–2074. doi: 10.1097/QAD.0b013e32833c323b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fakioglu E, Wilson SS, Mesquita PM, Hazrati E, Cheshenko N, Blaho JA, Herold BC. Herpes simplex virus downregulates secretory leukocyte protease inhibitor: a novel immune evasion mechanism. J Virol. 2008;82:9337–9344. doi: 10.1128/JVI.00603-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wheelhouse N, Wattegedera S, Fleming D, Fitch P, Kelly R, Entrican G. Chlamydia trachomatis and Chlamydophila abortus induce the expression of secretory leukocyte protease inhibitor in cells of the human female reproductive tract. Microbiol Immunol. 2008;52:465–468. doi: 10.1111/j.1348-0421.2008.00058.x. [DOI] [PubMed] [Google Scholar]
- 65.Wex T, Treiber G, Venerito M, Leodolter A, Peitz U, Kuester D, Hritz I, Krueger S, Roessner A, Malfertheiner P. Helicobacter pylori-induced downregulation of the secretory leukocyte protease inhibitor (SLPI) in gastric epithelial cell lines and its functional relevance for H. pylori-mediated diseases. Biol Chem. 2006;387:893–901. doi: 10.1515/BC.2006.113. [DOI] [PubMed] [Google Scholar]
- 66.Jana NK, Gray LR, Shugars DC. Human immunodeficiency virus type 1 stimulates the expression and production of secretory leukocyte protease inhibitor (SLPI) in oral epithelial cells: a role for SLPI in innate mucosal immunity. J Virol. 2005;79:6432–6440. doi: 10.1128/JVI.79.10.6432-6440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Novak RM, Donoval BA, Graham PJ, Boksa LA, Spear G, Hershow RC, Chen HY, Landay A. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin Vaccine Immunol. 2007;14:1102–1107. doi: 10.1128/CVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Draper D, Donohoe W, Mortimer L, Heine RP. Cysteine proteases of Trichomonas vaginalis degrade secretory leukocyte protease inhibitor. J Infect Dis. 1998;178:815–819. doi: 10.1086/515366. [DOI] [PubMed] [Google Scholar]
- 69.Taggart CC, Lowe GJ, Greene CM, Mulgrew AT, O’Neill SJ, Levine RL, McElvaney NG. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J Biol Chem. 2001;276:33345–33352. doi: 10.1074/jbc.M103220200. [DOI] [PubMed] [Google Scholar]
- 70.Cox SW, Rodriguez-Gonzalez EM, Booth V, Eley BM. Secretory leukocyte protease inhibitor and its potential interactions with elastase and cathepsin B in gingival crevicular fluid and saliva from patients with chronic periodontitis. J Periodontal Res. 2006;41:477–485. doi: 10.1111/j.1600-0765.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 71.Duncan JA, Gao X, Huang MT, O’Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones A, Geörg M, Maudsdotter L, Jonsson AB. Endotoxin, capsule, and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J Bacteriol. 2009;191:3861–3868. doi: 10.1128/JB.01313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]