Summary
Multiple myeloma (MM) is characterized by almost exclusive tropism of malignant cells for the bone marrow (BM) milieu. The survival and proliferation of malignant plasma cells have been shown to rely on interactions with nonmalignant stromal cells, in particular mesenchymal stromal cells (MSCs), in the BM microenvironment. However, the BM microenvironment is composed of a diverse array of cell types. This study examined the role of macrophages, an abundant component of BM stroma, as a potential niche component that supports malignant plasma cells. We investigated the proliferation of MM tumour cell lines when cultured alone or together with MSCs, macrophages, or a combination of MSCs and macrophages, using the carboxyfluorescein succinimidyl ester assay. Consistently, we observed increased proliferation of MM cell lines in the presence of either MSCs or macrophages compared to cell line-only control. Furthermore, the combined co-culture of MSCs plus macrophages induced the greatest degree of proliferation of myeloma cells. In addition to increased proliferation, MSCs and macrophages decreased the rate of apoptosis of myeloma cells. Our in vitro studies provide evidence that highlights the role of macrophages as a key component of the BM microenvironment facilitating the growth of malignant plasma cells in MM.
Keywords: mesenchymal cells, macrophages, multiple myeloma, marrow stroma, myeloma
Introduction
The development of haematological malignancies, including multiple myeloma (MM), reflects the accumulation of molecular alterations affecting growth control and/or apoptotic pathways (Hallek, et al 1998, Kastrinakis, et al 2000, Landowski, et al 1997, Plowright, et al 2000). Moreover, it has become clear that the survival and progression of malignances is also dependent on extrinsic events, including interactions with accessory cells that comprise the tumour microenvironment (Bissell and Radisky 2001). Indeed, accumulating evidence supports the hypothesis that the tumour microenvironment or niche ultimately determines the clinical behavior of the disease and has direct impact on overall prognosis (Dalton, et al 2004). There is currently an increasing effort to modulate the survival and proliferation of malignant cells by targeting the stromal components within the tumour niche (Anderson 2007, Galustian and Dalgleish 2009, Kenny, et al 2007, Podar, et al 2009) as a component of multimodality therapeutic strategies.
Mesenchymal stromal cells (MSCs) are a component of the bone marrow (BM) niche with a critical role in the support of normal haematopoietic stem cells and their progeny (Battiwalla and Hematti 2009, Javazon, et al 2004) as well as of malignant cells, including MM plasma cells (Markovina, et al 2010). Because MM is characterized by almost exclusive tropism for BM (Hideshima, et al 2007), the disease serves as a prototypical model to elucidate interactions between malignant cells and their surrounding niche (Dalton 2003). Indeed, recently US Federal Drug Administration-approved MM therapies (i.e., thalidomide and lenalidomide) exert a variety of diverse effects on the tumour microenvironment, including immunomodulatory and anti-angiogenic effects, in addition to direct cytotoxic effects against malignant plasma cells (Chauhan, et al 1996, Damiano, et al 1999, Hideshima, et al 2001a, Pagnucco, et al 2004). Given the complexity of the BM microenvironment, we hypothesized that the maintenance and progression of malignant plasma cells is not restricted to their interactions with MSCs but may also involve other cellular constituents. Macrophages are abundant within the BM stroma (Naito 2008) and recently their role in normal hematopoiesis has begun to be elucidated (Chow, et al 2011, Winkler, et al 2010); however, despite a well-documented role of macrophages as a vital constituent of solid tumour microenvironments (Joyce and Pollard 2009), their role in haematological malignancies is at the earliest stages of investigation (Zheng, et al 2009). Based on our recent work on interactions between BM-derived MSCs and CD14+ derived macrophages (Kim and Hematti 2009), in this study we investigated the effect of interactions between MSCs and macrophages on the growth of MM tumour cell lines.
Methods
Derivation of BM-MSCs
Mononuclear cells (MNCs) were isolated from discarded collection filters after BM donation by normal healthy donors based on an Institutional Review Board (IRB)-approved protocol. MNC layers were separated by density gradient separation technique using Ficoll-Hypaque (GE Lifesciences, Piscataway, NJ, USA), followed by treatment with ACK lysis buffer to reduce contaminating red blood cells (Kim and Hematti 2009). To isolate BM-derived MSCs, MNCs were plated in MSC media (α-minimal essential medium supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acids (NEAA ) and 2 µM L-alanine-L-glutamine). After 24 h, floating cells were discarded and attached cells were allowed to reach near confluency. Cells were then passaged using TrypLE (Invitrogen, Carlsbad, CA, USA) until passage 4, when flow cytometry and differentiation assays were performed to verify MSCs according to established criteria (Dominici, et al 2006). MSCs between passage 4 and 6 were used for experiments.
Isolation and culture of monocytes
We used the AutoMACS Pro Separation System (Miltenyi Biotech, Auburn, CA, USA) to isolate CD14+ cells from the MNC fraction of normal peripheral blood (PB) or BM aspirates from MM patients. Briefly, buffy coat was purchased from Interstate Blood Bank (Memphis, TN, USA) and MM BM aspirates were collected under a University of Wisconsin IRB approved protocol. The mononuclear cell layer was separated by Ficoll Hypaque (GE Lifesciences) density gradient separation. Mononuclear cells were treated with ACK lysis buffer to remove RBC and then incubated with CD14 microbead-conjugated antibody for 15 min at 4°C. CD14 positive cells were then isolated using the positive selection program according to the manufacturer’s protocol. One million CD14+ cells (106) were plated in macrophage culture media (Iscove’s modified Dulbecco’s medium [IMDM] supplemented with 10% human AB serum, 1% NEAA, 2 µM L-alanine-L-glutamine, 1% sodium pyruvate, and 5 µM human recombinant insulin) per each well of a 6-well plate and cultured for 7 days. After incubation, most of the cells were adherent to the plastic surface and stained positive for CD14 and other markers specific for macrophages. When performing co-cultures of MSCs and macrophages with MM cell lines, media for MM cells was used, as the growth of MSCs and macrophages is compatible with this media.
MM Cell line culture
MM cell lines U266 and NCI-H292 were obtained from American Tissue Culture Company (ATCC, Manassas, VA, USA) and were cultured according to the recommended protocol. Briefly, cells were cultured in RPMI 1640 medium with 10% FBS, 1% NEAA, 2 µM L-alanine-L-glutamine and 1% sodium pyruvate; media was changed every two to three days by centrifuging media to collect the cells and adding fresh media.
Cell proliferation assay
MM cell lines U266 or NCI-H292 were stained with 10 uM Carboxyfluorescein succinimidyl ester (CFSE) for 15 min at 37°C then washed twice in phosphate-buffered saline containing 0.5% human serum albumin (HSA). Cells were then added to each condition and were cultured for 3–4 days. As MSCs and macrophages are adherent to the plastic surface, floating cells were collected after washing by pipetting. To rule out the possibility of macrophages phagocytosing CFSE-stained MM cells and becoming positive for CFSE, we use CD14+ staining to negatively select macrophages so that only MM cells were included in the proliferation assay. Data acquisition and analyses were performed with an Accuri C6 Flow Cytometer (Accuri Cytometer, Ann Arbor, MI, USA) and the ModFit Software Program (Verity Software, Topsham, ME, USA). The Proliferative Index, which is the sum of the cells in all generations divided by the calculated number of original parent cells, was used to compare different samples.
Quantitative reverse-transcription polymerase chain reaction assay
RNA was isolated from cells using RNeasy micro kit (Qiagen, Valencia, CA, USA), and the quality of isolated RNA was checked using Nanodrop 1000 (Fisher scientific, Pittsburgh, PA, USA). RNA was converted to cDNA using Quantitect reverse transcription kit (Qiagen). Quantitative polymerase chain reaction was performed using Power SYBR green master mix (Applied Biosystems, Foster City, CA, USA) on StepOne Plus instrument (Applied Biosystems) using standard protocols. Verified primers were purchased from Qiagen. The threshold cycle (Ct) value for each gene was normalized by the average Ct number of three housekeeping genes (RN18S1, GAPDH and ACTB).
Apoptosis Assay
Annexin V staining kit (BD biosciences, San Jose, CA, USA) was used to detect early and late apoptosis using Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide. Apoptosis of the MM cell line U266 was detected in the presence or absence of MSCs or macrophages and was detected using Accuri C6 flow cytometer (Accuri Cytometer) and analyzed with CFlow software (Accuri Cytometer).
Bortezomib preparation
Bortezomib was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was dissolved in sterile fashion in dimethyl sulfoxide (DMSO) to a final concentration of 10 µM. It was then added to tissue culture media at 10 nM concentrations unless otherwise specified. Control cultures included an equivalent % volume DMSO, to exclude the possibility that biological differences were not attributable to the DMSO component.
Statistical analysis
We used the student’s t test to assess the significance of differences observed. The overall α-level for these tests is 0.05. Statistical package PAST (PAlaeontological STatistics) was used for data analysis and visualization.
Results
Derivation of MSCs from BM
We have previously reported the analysis of cell surface markers and differentiation assays of our BM-derived MSCs (Trivedi and Hematti 2008) and shown them to conform to the well-established criteria set forth by International Society for Cellular Therapy (Dominici, et al 2006). Essentially, passage 4–6 BM-MSCs are positive for markers, such as CD29, CD44, CD73, and CD90, and negative for haematopoietic and endothelial markers, such as CD14, CD31, CD34, and CD45; furthermore, they are positive for human leucocyte antigen (HLA)-ABC and negative for HLA-DR molecules (data not shown).
Co-cultured MSCs and macrophages stimulate proliferation of MM cell lines in vitro
Proliferation assays using CFSE staining were performed after three days of coculture. CFSE-stained U266 or NCI-H929 myeloma cell lines were cultured in the following conditions: a) cell line-only control, b) single co-culture MSC alone, c) single co-culture macrophages alone and d) combined co-culture with MSC and macrophages plated at a density of 100,000 cells per well in 6-well plate. The design of the proliferation assay is depicted in Fig 1A. Fig 1B is a representative experiment showing modelling results for proliferation index (PI) calculation. Our results showed increased proliferation of MM cells in the presence of either MSCs or macrophages compared to the cell line-only control (Fig 2). The combined co-culture of MSCs and macrophages induced a notable increase in the proliferation of MM cells. The normalized proliferation indices for NCI-H929 with MM cell line-only control, MSCs, macrophages and MSCs with macrophages were 1.00, 1.83±0.06, 1.83±0.04 and 2.03±0.05, respectively (Fig 2A). For U266 cells, the normalized proliferation indices were 1.00, 1.44±0.02, 1.46±0.04 and 1.98±0.08, respectively (Fig 2B). These results indicate that the highest level of U266 and NCI-H292 MM proliferation occurs in combined co-cultures with MSCs plus macrophages, suggesting that the combination of MSCs and macrophages exerts a synergistic effect on tumour cell growth. Moreover, the addition of macrophages alone appeared to be sufficient in supporting the proliferation of MM cell lines, as was the case with MSCs co-cultured with MM cells. To avoid the potential of macrophage media (IMDM supplemented with 10% human AB serum) to promote proliferation of MM cells, we performed similar experiments using RPMI 1640 medium with 10% FBS and RPMI 1640 medium with 0.5% HSA and obtained comparable results (data not shown).
Figure 1. Schematic diagram of myeloma cell line proliferation assay and analysis.
(A) Experimental outline of co-culture experiments. Macrophages (MQ) are generated by culturing CD14+ monocytes for one week, then mesenchymal stromal cells (MSC) and/or multiple myeloma cell lines are added for 3 days. (B) Sample analysis of proliferation assay. CFSE-stained multiple myeloma cell lines were analysed using flow cytometry.
Figure 2. Proliferation assays of U266 and NCI-H929 MM cell lines.
The mean value was calculated from triplicate experiments and normalized by dividing with the proliferation index obtained from cell line-only control. Combined co-cultures with mesenchymal stromal cells (MSC) and macrophages (MQ)provided a more robust proliferation stimulus than either cell component alone.
Next, we performed proliferation assays of MM cell lines in a macrophage dilution series to ascertain whether there is a dose-response relationship between the number of macrophages and the PI of the MM cell lines. Total PB mononuclear cells (PBMC) were included as a control to account for the possibility that cell crowding might be responsible for the increased proliferation observed previously. Fig 3 summarizes the data from both U266 (Fig 3A) and NCI-H929 (Fig 3B) cell lines. For U266 cell line, the addition of PBMC produced a normalized PI of 0.90±0.15. For different macrophage conditions, the values were 1.16±0.13 (1.25 × 105), 1.28±0.16 (2.5 × 105), 1.36±0.14 (5 × 105) and 1.47±0.10 (1 × 106), respectively. In the case of NCI-H929, the PBMC co-culture condition produced a normalized PI of 1.09±0.13 and macrophage coculture conditions produced values of 1.45±0.15 (1.25 × 105), 1.60±0.16 (2.5 × 105), 1.71±0.18 (5 × 105) and 1.83±0.30 (1 × 106), respectively. As can be seen from the graph, an increased number of macrophages was associated with a higher PI. The presence of PBMC produced a PI that was not statistically different from MM cell line-only control.
Figure 3. Proliferation of myeloma cells in the presence of increasing number of macrophages.
Myeloma cell lines were cocultured in the presence of increasing number of macrophages (MQ). For both U266 (A) and NCI-H929 (B), increasing number of macrophages was associated with increased proliferation index. 1×106 PBMC was added as additional control.
Role of interleukin 6 (IL6) in macrophage support of MM cell proliferation
MSCs secrete IL6 abundantly; moreover, we have previously shown that MSCs instruct co-cultured macrophages to secrete large amounts of IL6 (Kim and Hematti 2009). Therefore, we sought to determine whether the increased proliferation of myeloma cells was due to IL6 secreted from MSCs and/or macrophages. To investigate this possibility, IL6 blocking antibody was added to distinct co-culture conditions at 2.5 µg/ml concentration, and proliferation of U266 cell line was analysed as described above. The proliferation indices for cell line-only control, MSCs, macrophages, and combined MSCs+macrophages cultures were 1.00, 1.63±0.10, 2.18±0.68 and 2.21±0.55, respectively while those treated with IL6 blocking antibody exhibited proliferation indices of 0.81±0.05, 1.06±0.19, 1.42±0.48 and 1.25±0.45, respectively (Fig 4). The difference between control and IL6 blocking was statistically significant across all conditions (p < 0.01). The presence of IL6 antibody reduced proliferation of U266 cells universally, but the most prominent effect was seen when U266 cells were co-cultured with MSCs and macrophages (proliferation ratios between IL6-treated and non-treated U266 cells were as follows: 0.55±0.07 in MSCs plus macrophages combined co-culture, 0.65±0.02 in macrophage-only coculture, 0.65±0.08 in MSC-only coculture and 0.81±0.05 in U266 cell line only-control). The differences between groups were statistically significant except for the comparison between macrophage and MSC co-culture. These findings underscore the potential role of IL6 in the support of MM cell growth by either MSCs or macrophages.
Figure 4. The effect of IL6 neutralizing antibody on proliferation of U266 cells in co-culture with stromal cells.
In the presence of IL6 blocking antibody, the proliferation index of myeloma cells was decreased across all conditions. The changes were more pronounced when myeloma cells were co-cultured in the presence of mesenchymal stromal cells (MSC) and macrophages (MQ).
Effect of bortezomib on growth of U266 cells in presence of MSC and/or macrophages
Next, we investigated the potential of bortezomib-treated stromal cells in supporting MM cell growth. MSCs or macrophages were pre-treated with bortezomib at a final concentration of 1 nM for 24 h and then utilized in co-cultures with U266 myeloma cells (Fig 5A). As macrophages do not attach readily, MSCs were harvested with TrypLE and then added to macrophages in MSCs+macrophages co-culture experiments.
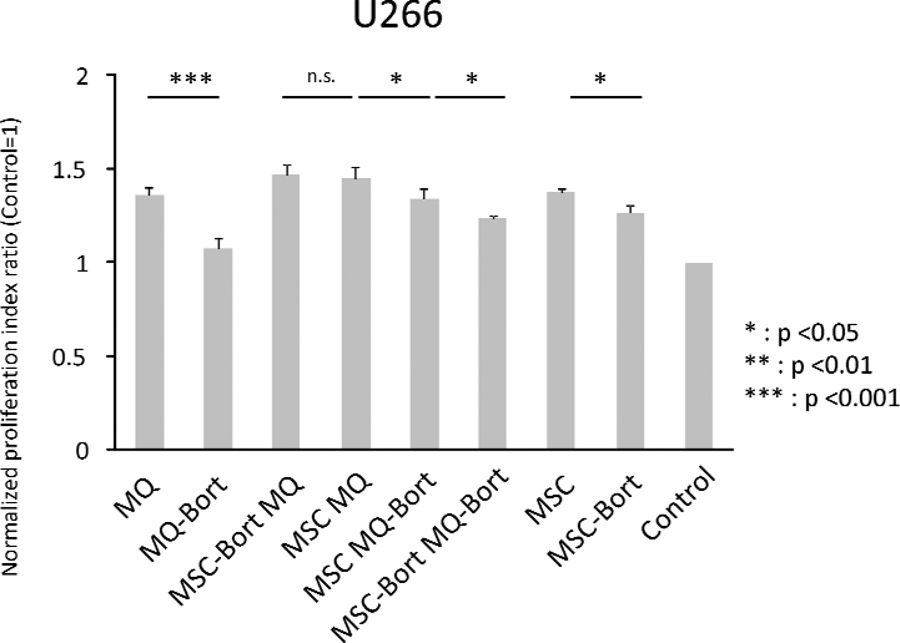
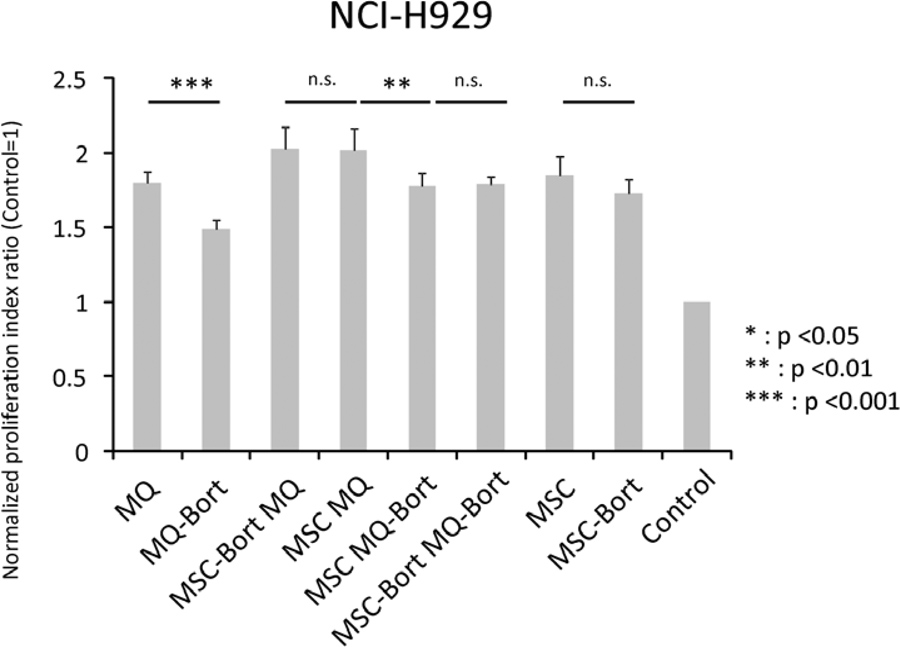
Figure 5. Proliferation of U266 myeloma cells in the presence of bortezomib pre-treated stromal components.
(A) Treatment scheme for generation of bortezomib (Bort) pre-treated macrophages (MQ) and mesenchymal stromal cells (MSC). (B) Proliferation assays of U266 multiple myeloma cell line in the presence of bortezomib pre-treated stromal components. Bortezomib pre-treatment of both macrophages and MSC resulted in decreased proliferation of myeloma cells. Different combinations of pre-treated stromal components are shown. (C) Proliferation assays of NCI-H929 multiple myeloma cell line in the presence of bortezomib pre-treated stromal components. Different combinations of pre-treated stromal components are shown.
When macrophages pre-treated with bortezomib were used in co-culture with U266 cells, the normalized PI of the latter decreased from 1.35±0.04 to 1.07±0.05 (p value, 0.0001). In cocultures with pre-treated MSCs, the PI decreased from 1.37±0.02 to 1.26±0.05 (p value, 0.022). In the case of combined MSCs+macrophages co-cultures with U266 cells, the normalized PI was 1.45±0.06 and the value decreased to 1.34±0.05 (p value, 0.028) when macrophages were pretreated with bortezomib. When MSCs were pre-treated with bortezomib, the PI was 1.47±0.05 (p value, 0.626) and when both MSCs and macrophages were pre-treated with bortezomib, 1.24±0.01 (p value, 0.003) (Fig 5B). In the case of NCI-H929, macrophage co-cultured cells had a PI of 1.80±0.08, which decreased to 1.49±0.06 when the macrophages were pre-treated with bortezomib (p value, 0.0001). In MSC co-cultures, we observed a decrease from 1.85±0.13 to 1.73±0.10 when MSCs were pre-treated with bortezomib (p value, 0.195). In MSC/macrophage co-cultures, we obtained a PI of 2.02±0.14 but when bortezomib pre-treated macrophages were used, it decreased to 1.78±0.08 (p value, 0.004). By contrast, no difference was observed when MSCs were pre-treated with bortezomib (normalized PI of 2.03±0.14, p value 0.953 (Fig 5C)).
Apoptosis of myeloma cell lines in presence of MSCs and/or macrophages
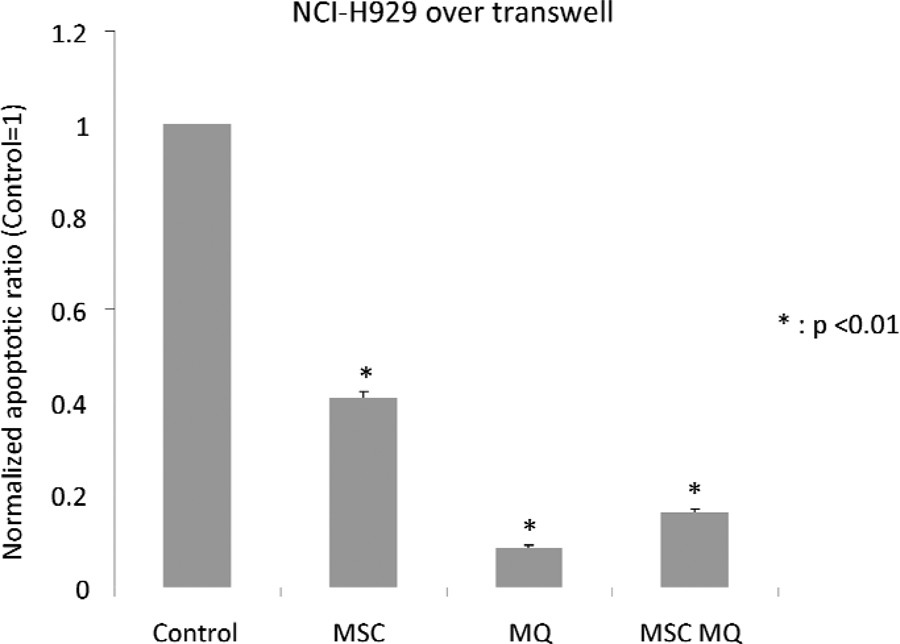
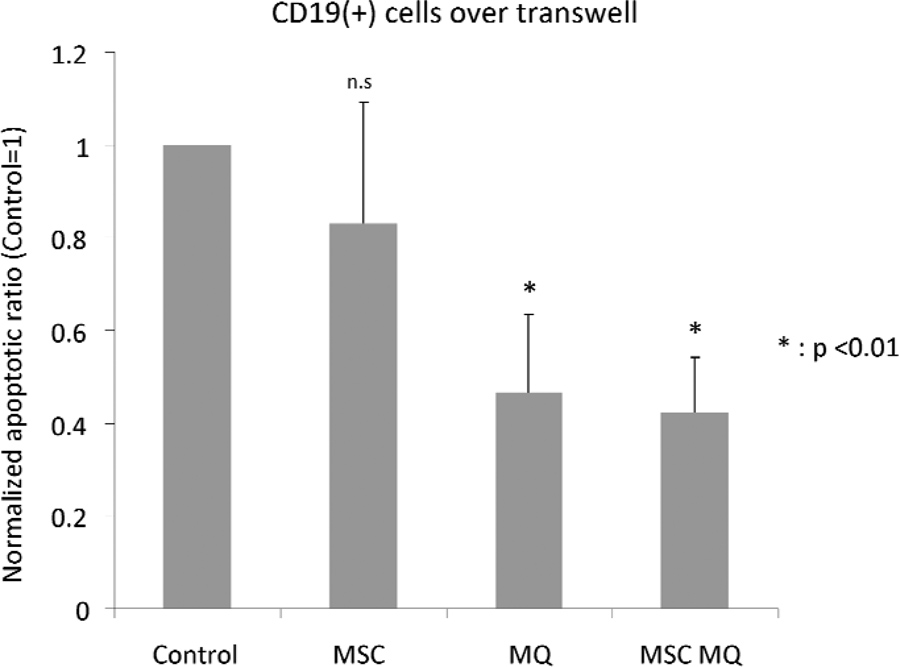
Next, we measured the rate of apoptosis in MM cell lines cultured alone or in co-cultures with MSCs and/or macrophages. We used transwells with pore size of 0.4 um to separate MM cells from MSCs and/or macrophages, as macrophages could phagocytose the apoptotic cells and skew the results. The experimental outline of these co-cultures is depicted in Fig 6A. After three days of co-culture, cells were stained with Annexin V staining kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s protocol. The results showed that both MSCs and macrophages were capable of decreasing apoptosis of myeloma cells compared to cell line-only control (Fig 6B and C). In the case of U266 cells, after normalizing percentage of Annexin V-positive cells to cell line-only control, MSCs co-cultured cells demonstrated an apoptosis ratio of 0.38±0.01, macrophage co-cultured cells demonstrated a ratio of 0.07±0.01 and cells in combined MSCs+macrophages co-cultures had a ratio of 0.14±0.01. For NCI-H929 cells, the values were 0.41±0.01 (MSC co-cultures), 0.09±0.00 (macrophage co-cultures) and 0.16±0.01 (combined MSCs+macrophages co-cultures). Primary CD19+ cells isolated from PB were co-cultured using same experimental design to test if a similar anti-apoptotic effect could be observed by co-culturing with MSC and/or macrophage (Fig 6D). MSC co-cultured primary B cells did not exhibit a statistically significant difference from B cell-alone control (relative apoptotic ratio was 0.83±0.26). In macrophage- and MSCs+macrophages co-cultures, the ratios were 0.47±0.17 and 0.42±0.12 respectively, representing a statistically significant difference from primary B cell alone control.
Figure 6. The effect of mesenchymal stromal cells and/or macrophages on apoptosis of myeloma cells.
(A) Schematic drawing of co-culture for apoptosis testing. (B) U266 cultured on mesenchymal stromal cells (MSCs)+macrophages (MQ), macrophages alone, MSCs alone and cell line only control. (C) NCI-H929 cultured on MSCs+macrophages, macrophages alone, MSCs alone and cell line only control. (D) Primary B cell (CD19 positive cells) apoptosis assay. MSCs, macrophages and MSCs+macrophages all decreased apoptosis of co-cultured multiple myeloma cells. In the case of primary B cells, only macrophages and MSCs+macrophages combination were capable of protecting cells from undergoing apoptosis.
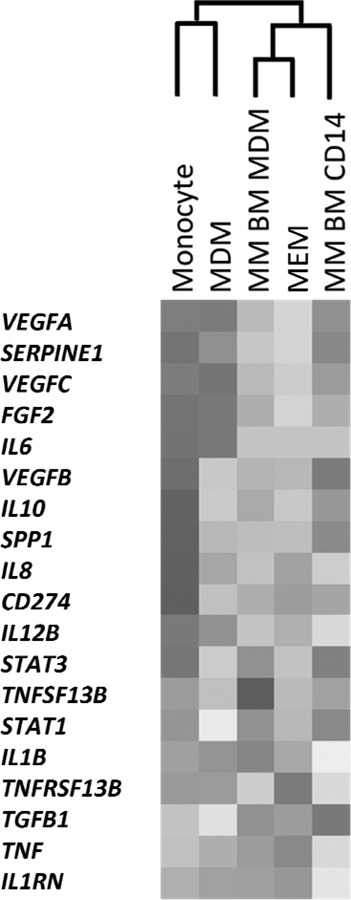
MSC-educated macrophages exhibit similarity with myeloma bone marrow CD14-derived macrophages
A panel of key genes important in MM tumour-stromal interactions was interrogated by reverse-transcription polymerase chain reaction (RT-PCR) in order to compare the phenotype of MSC-educated macrophages (MEM), to that of PB monocyte-derived macrophages (MDM), PB monocytes (monocytes), MM BM CD14+ cells (MM BM CD14) and macrophages derived from myeloma BM CD14+ cells (MM BM MDM). Heat map and associated cluster analysis results are depicted in Fig 7A. Interestingly, MEM showed similar gene expression phenotype with BM monocyte-derived macrophages, while control macrophages derived from the same batch of PB monocytes clustered with PB monocytes. Fig 7B provides a visualization of quantitative PCR data using principal component analysis. In this picture, monocytes were separated from macrophages along the component 2 axis. Among macrophages, control macrophages were distinct from both MM BM CD14-derived macrophages and MSC-educated macrophages by component 1. MSC-educated macrophages showed a distribution pattern similar to MM BM CD14-derived macrophages, again showing similarity between the two groups.
Figure 7. Transcriptomic comparisons of distinct subsets of monocytes and macrophages.
Cluster analysis based on quantitative RT-PCR showed that MSC-educated macrophages (MEM) exhibit similar phenotype to BM CD14+ monocytes isolated from MM bone marrow (MM BM CD14) or MM BM CD14+ cell-derived macrophages (MM BM MDM) (A). SPP1 encodes SPP1 (osteopontin, OPN), CD274 encodes CD274 (Programmed cell death ligand 1, PDL1), TNFSF13B encodes TNFSF13B (B cell activating factor, BAFF) and TNFRSF13B encodes TNFRSF13B (Transmembrane activator and calcium-modulator and cyclophilin ligand interactor, TACI). In contrast, peripheral blood CD14+ monocytes and peripheral blood monocyte-derived macrophages (MDM) generated a separate cluster. Same data visualized using principal component analysis (B). MSC-educated macrophages shared a similar distribution with MM BM CD14-derived macrophages.
Discussion
Much of the current studies of the BM microenvironment in MM concentrate on the interactions between malignant plasma cells and MSCs. While there is sufficient literature to implicate macrophages in the development of solid organ tumours (Hagemann, et al 2009, Mantovani, et al 2009, Sica, et al 2008, Sica, et al 2007, Siveen and Kuttan 2009, Zumsteg and Christofori 2009), the interactions between macrophages and tumour cells in haematological malignancies are not well studied (Byers, et al 2008, Farinha, et al 2005). The contribution of osteoclasts in the pathogenesis of MM has been investigated extensively (Esteve and Roodman 2007, Mitsiades, et al 2007), and there are studies on the role of macrophages as promoters of angiogenesis in MM (Chen, et al 2009, Murdoch, et al 2008, Noonan, et al 2008, Nyberg, et al 2008, Pollard 2008, Ribatti and Vacca 2008, Scavelli, et al 2008, Vacca and Ribatti 2006). However, the role of BM macrophages as a direct-acting component in the MM tumour niche has not yet been investigated in detail, except for a recent study that reported that macrophages protect MM cells from spontaneous and chemotherapy-induced apoptosis (Zheng, et al 2009). IL6 is an important cytokine in the proliferation of normal and malignant plasma cells (Gunn, et al 2006, Hideshima, et al 2004, Karadag, et al 2000, Klein and Bataille 1992, Lauta 2001, Nishimoto and Kishimoto 2006). Both circulating and BM levels of IL6 are reportedly increased in patients with MM as a reflection of disease activity (Hideshima, et al 2004). Although myeloma cells can produce IL6 through an autocrine signalling loop, the predominant source in MM patients has so far been believed to be BM-MSCs (Carter, et al 1990, Gunn, et al 2006). We have previously shown that human BM-derived MSCs change the secretory profile of macrophages to a phenotype characterized by higher expression of IL6 and IL10, and lower expression of IL12 and TNFα (Kim and Hematti 2009). In this study, we propose a novel paradigm in which macrophages are a supplemental source of IL6 and that a combination of high expression of IL6 and IL10 and low expression of IL12 and TNFα could provide a suitable milieu for tumour cell growth. It has been shown that IL10 stimulates the proliferation of MM cells freshly isolated from MM patients in IL6 deprived cultures (Gu, et al 1996). Additionally, both IL12 and TNFα are considered to retain anti-tumour effects (Li, et al 2002, Vilcek 2009, Weiss, et al 2007) and thus a lower expression of these cytokines by macrophages through their interactions with MSCs could provide a favourable milieu for the growth of malignant cells.
Interestingly, we also observed that MSC-educated macrophages have increased levels of VEGFA and VEGFC mRNA expression. It is well known that vascular endothelial growth factors (VEGFs) play a critical role in MM pathology by their effect on vascular endothelial cells, one of the well-known components of the MM niche (Roccaro, et al 2006, Yasui, et al 2006). Traditionally, it has been assumed that MSCs are the major source of VEGFs (Kagiwada, et al 2008, Mayer, et al 2005) but current results suggest the interesting finding that macrophages might be another major contributor of VEGFs, especially when they have been educated by MSCs. Based on an in vivo model of MSC transplantation into rat hind limb ischemia model, we suggested that the source of increased VEGF in the tissues was not transplanted (human) MSCs but recipient (rat) macrophages (Laurila, et al 2009). To further test the hypothesis that combination of MSCs and macrophages can be a useful model for the myeloma BM niche, we also investigated the effect of bortezomib in this model. Bortezomib was first developed in 1998 as a proteasome complex inhibitor (Palombella, et al 1998), reported to be active against MM in 2001 (Hideshima, et al 2001b); with subsequent clinical trials proving its efficacy (Jagannath, et al 2004, Jagannath, et al 2005, Richardson, et al 2003, Richardson, et al 2006). While the major mechanism of action for bortezomib is assumed to be the induction of apoptosis in tumour cells by delaying clearance of apoptotic proteins by proteasome machinery, several other potential mechanisms of action have been suggested (Hideshima, et al 2011). Bortezomib suppresses production of cytokines, such as IL6 and IGF1, and moreover, blocks adhesion of MM cells to MSCs. Furthermore, bortezomib is known to block angiogenic signals, such as VEGFs. Given that increased levels of circulating pro-angiogenic cytokines as well as increased BM vascularization have been reported in MM patients (Vacca, et al 1994), it is very probable that blocking of angiogenic signals could affect the biology of MM. Our data indicate that one potential mechanism of action of bortezomib is through its effects on macrophages. It is also notable that macrophages are a major source of angiogenic factors in tumour microenvironments. Therefore, it is possible that many of the mechanisms currently associated with bortezomib’s clinical effect might actually involve macrophages, in addition to the other targets previously linked to the actions of bortezomib.
The multitude of cellular compartments and the broad constellation of growth factors and cytokines involved in the regulation of tumour niche pose significant therapeutic challenges. Targeting any individual molecular or cell mediator of the MM BM milieu is not sufficient for curative responses due to functional redundancy. Our data shed light on a fundamental, but previously uninvestigated role of macrophage/MSC interactions and suggest that macrophages may play a bigger role than previously thought in the natural history of MM. In addition to yielding new targets for therapeutic approaches, the combination of MSCs and macrophages can provide a more physiologically relevant model to test candidate molecules in vitro (McMillin, et al 2011a, McMillin, et al 2011b, Mitsiades, et al 2011, Negri, et al 2009); and it will be interesting to see the effect of incorporating macrophages into the current drug screening platforms specifically designed to study the effect of stromal microenvironment on the efficacy of drugs (McMillin, et al 2010). Moreover, it should be noted that macrophages can also interact with another major constituent of BM stroma, endothelial cells, through pathways involving serine proteases and angiogenic peptides as well as adhesion molecules. Thus, drugs that target this axis could be also of therapeutic value. Indeed, addition of defibrotide, an agent that could modulate such macrophage-endothelial cell interactions, to other chemotherapeutics resulted in encouraging results both in pre-clinical models (Mitsiades, et al 2009) and in a multicentre phase I/II clinical trial (Palumbo, et al 2010).
Investigating the role of macrophages in the MM tumour niche and their interactions with MSCs presents a novel paradigm in defining the tumour microenvironment for MM, and potentially for other malignancies. Moreover, given that access to human BM macrophages is technically very challenging, we propose MSC-educated macrophages could provide an acceptable substitute for recapitulating the BM microenvironment in vitro as an experimental model.
Acknowledgements
This study was supported by Multiple Myeloma Trillium Research Fund from University of Wisconsin Carbone Cancer Center. Peiman Hematti is recipient of National Heart, Lung and Blood Institute grant K08 HL081076 and. Ryan A Denu is recipient of American Society of Hematology’s Trainee Research Award
Footnotes
Authors’ contributions
Jaehyup Kim, Ryan A Denu, Bridget A Dollar, Leah E Escalante and Justin Kuether performed the research, Jaehyup Kim and Peiman Hematti designed research study, Jaehyup Kim, Fotis Asimakopoulos and Peiman Hematti analysed data. Natalie Callander contributed patient samples and Jaehyup Kim, Natalie Callander, Fotis Asimakopoulos and Peiman Hematti wrote the paper.
Conflicts of interest
The authors have no potential conflicts of interest, including specific financial interests, relationships, or affiliations relevant to the subject of this manuscript.
References
- Anderson KC. Targeted therapy of multiple myeloma based upon tumor-microenvironmental interactions. Exp Hematol. 2007;35:155–162. doi: 10.1016/j.exphem.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11:503–515. doi: 10.1080/14653240903193806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers RJ, Sakhinia E, Joseph P, Glennie C, Hoyland JA, Menasce LP, Radford JA, Illidge T. Clinical quantitation of immune signature in follicular lymphoma by RT-PCR-based gene expression profiling. Blood. 2008;111:4764–4770. doi: 10.1182/blood-2007-10-115915. [DOI] [PubMed] [Google Scholar]
- Carter A, Merchav S, Silvian-Draxler I, Tatarsky I. The role of interleukin-1 and tumour necrosis factor-alpha in human multiple myeloma. Br J Haematol. 1990;74:424–431. doi: 10.1111/j.1365-2141.1990.tb06330.x. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- Chen H, Campbell RA, Chang Y, Li M, Wang CS, Li J, Sanchez E, Share M, Steinberg J, Berenson A, Shalitin D, Zeng Z, Gui D, Perez-Pinera P, Berenson RJ, Said J, Bonavida B, Deuel TF, Berenson JR. Pleiotrophin produced by multiple myeloma induces transdifferentiation of monocytes into vascular endothelial cells: a novel mechanism of tumor-induced vasculogenesis. Blood. 2009;113:1992–2002. doi: 10.1182/blood-2008-02-133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton WS. The tumor microenvironment: focus on myeloma. Cancer Treat Rev. 2003;29(Suppl 1):11–19. doi: 10.1016/s0305-7372(03)00077-x. [DOI] [PubMed] [Google Scholar]
- Dalton WS, Hazlehurst L, Shain K, Landowski T, Alsina M. Targeting the bone marrow microenvironment in hematologic malignancies. Semin Hematol. 2004;41:1–5. doi: 10.1053/j.seminhematol.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Esteve FR, Roodman GD. Pathophysiology of myeloma bone disease. Best Pract Res Clin Haematol. 2007;20:613–624. doi: 10.1016/j.beha.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, Klasa R, Voss N, Connors JM, Gascoyne RD. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- Galustian C, Dalgleish A. Lenalidomide: a novel anticancer drug with multiple modalities. Expert Opin Pharmacother. 2009;10:125–133. doi: 10.1517/14656560802627903. [DOI] [PubMed] [Google Scholar]
- Gu ZJ, Costes V, Lu ZY, Zhang XG, Pitard V, Moreau JF, Bataille R, Wijdenes J, Rossi JF, Klein B. Interleukin-10 is a growth factor for human myeloma cells by induction of an oncostatin M autocrine loop. Blood. 1996;88:3972–3986. [PubMed] [Google Scholar]
- Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113:3139–3146. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallek M, Bergsagel PL, Anderson KC. Multiple myeloma: increasing evidence for a multistep transformation process. Blood. 1998;91:3–21. [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Podar K, Schlossman RL, Richardson P, Anderson KC. Novel therapies targeting the myeloma cell and its bone marrow microenvironment. Semin Oncol. 2001a;28:607–612. doi: 10.1016/s0093-7754(01)90033-8. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001b;61:3071–3076. [PubMed] [Google Scholar]
- Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–618. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Richardson PG, Anderson KC. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther. 2011;10:2034–2042. doi: 10.1158/1535-7163.MCT-11-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, Niesvizky R, Alexanian R, Limentani SA, Alsina M, Adams J, Kauffman M, Esseltine DL, Schenkein DP, Anderson KC. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, McKinley M, Gabayan E, Mazumder A, Schenkein D, Crowley J. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagiwada H, Yashiki T, Ohshima A, Tadokoro M, Nagaya N, Ohgushi H. Human mesenchymal stem cells as a stable source of VEGF-producing cells. J Tissue Eng Regen Med. 2008;2:184–189. doi: 10.1002/term.79. [DOI] [PubMed] [Google Scholar]
- Karadag A, Oyajobi BO, Apperley JF, Russell RG, Croucher PI. Human myeloma cells promote the production of interleukin 6 by primary human osteoblasts. Br J Haematol. 2000;108:383–390. doi: 10.1046/j.1365-2141.2000.01845.x. [DOI] [PubMed] [Google Scholar]
- Kastrinakis NG, Gorgoulis VG, Foukas PG, Dimopoulos MA, Kittas C. Molecular aspects of multiple myeloma. Ann Oncol. 2000;11:1217–1228. doi: 10.1023/a:1008331714186. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468–3474. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B, Bataille R. Cytokine network in human multiple myeloma. Hematol Oncol Clin North Am. 1992;6:273–284. [PubMed] [Google Scholar]
- Landowski TH, Qu N, Buyuksal I, Painter JS, Dalton WS. Mutations in the Fas antigen in patients with multiple myeloma. Blood. 1997;90:4266–4270. [PubMed] [Google Scholar]
- Laurila JP, Laatikainen L, Castellone MD, Trivedi P, Heikkila J, Hinkkanen A, Hematti P, Laukkanen MO. Human embryonic stem cell-derived mesenchymal stromal cell transplantation in a rat hind limb injury model. Cytotherapy. 2009;11:726–737. doi: 10.3109/14653240903067299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauta VM. Interleukin-6 and the network of several cytokines in multiple myeloma: an overview of clinical and experimental data. Cytokine. 2001;16:79–86. doi: 10.1006/cyto.2001.0982. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang X, Xia X. Regression of tumor growth and induction of long-term antitumor memory by interleukin 12 electro-gene therapy. J Natl Cancer Inst. 2002;94:762–768. doi: 10.1093/jnci/94.10.762. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Markovina S, Callander NS, O'Connor SL, Xu G, Shi Y, Leith CP, Kim K, Trivedi P, Kim J, Hematti P, Miyamoto S. Bone marrow stromal cells from multiple myeloma patients uniquely induce bortezomib resistant NF-kappaB activity in myeloma cells. Mol Cancer. 2010;9:176. doi: 10.1186/1476-4598-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer H, Bertram H, Lindenmaier W, Korff T, Weber H, Weich H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005;95:827–839. doi: 10.1002/jcb.20462. [DOI] [PubMed] [Google Scholar]
- McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, Mitsiades N, Schlossman RL, Munshi NC, Kung AL, Griffin JD, Richardson PG, Anderson KC, Mitsiades CS. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin DW, Delmore J, Negri J, Buon L, Jacobs HM, Laubach J, Jakubikova J, Ooi M, Hayden P, Schlossman R, Munshi NC, Lengauer C, Richardson PG, Anderson KC, Mitsiades CS. Molecular and cellular effects of multi-targeted cyclin-dependent kinase inhibition in myeloma: biological and clinical implications. Br J Haematol. 2011a;152:420–432. doi: 10.1111/j.1365-2141.2010.08427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin DW, Delmore J, Negri J, Ooi M, Klippel S, Miduturu CV, Gray NS, Richardson PG, Anderson KC, Kung AL, Mitsiades CS. Microenvironmental influence on pre-clinical activity of polo-like kinase inhibition in multiple myeloma: implications for clinical translation. PLoS One. 2011b;6:e20226. doi: 10.1371/journal.pone.0020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades CS, McMillin DW, Klippel S, Hideshima T, Chauhan D, Richardson PG, Munshi NC, Anderson KC. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am. 2007;21:1007–1034. doi: 10.1016/j.hoc.2007.08.007. vii-viii. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Rouleau C, Echart C, Menon K, Teicher B, Distaso M, Palumbo A, Boccadoro M, Anderson KC, Iacobelli M, Richardson PG. Preclinical studies in support of defibrotide for the treatment of multiple myeloma and other neoplasias. Clin Cancer Res. 2009;15:1210–1221. doi: 10.1158/1078-0432.CCR-08-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades CS, Davies FE, Laubach JP, Joshua D, San Miguel J, Anderson KC, Richardson PG. Future directions of next-generation novel therapies, combination approaches, and the development of personalized medicine in myeloma. J Clin Oncol. 2011;29:1916–1923. doi: 10.1200/JCO.2010.34.0760. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Naito M. Macrophage differentiation and function in health and disease. Pathol Int. 2008;58:143–155. doi: 10.1111/j.1440-1827.2007.02203.x. [DOI] [PubMed] [Google Scholar]
- Negri JM, McMillin DW, Delmore J, Mitsiades N, Hayden P, Klippel S, Hideshima T, Chauhan D, Munshi NC, Buser CA, Pollard J, Richardson PG, Anderson KC, Mitsiades CS. In vitro anti-myeloma activity of the Aurora kinase inhibitor VE-465. Br J Haematol. 2009;147:672–676. doi: 10.1111/j.1365-2141.2009.07891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- Noonan DM, De Lerma Barbaro A, Vannini N, Mortara L, Albini A. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci. 2008;13:6537–6553. doi: 10.2741/3173. [DOI] [PubMed] [Google Scholar]
- Pagnucco G, Cardinale G, Gervasi F. Targeting multiple myeloma cells and their bone marrow microenvironment. Ann N Y Acad Sci. 2004;1028:390–399. doi: 10.1196/annals.1322.047. [DOI] [PubMed] [Google Scholar]
- Palombella VJ, Conner EM, Fuseler JW, Destree A, Davis JM, Laroux FS, Wolf RE, Huang J, Brand S, Elliott PJ, Lazarus D, McCormack T, Parent L, Stein R, Adams J, Grisham MB. Role of the proteasome and NF-kappaB in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci U S A. 1998;95:15671–15676. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A, Larocca A, Genuardi M, Kotwica K, Gay F, Rossi D, Benevolo G, Magarotto V, Cavallo F, Bringhen S, Rus C, Masini L, Iacobelli M, Gaidano G, Mitsiades C, Anderson K, Boccadoro M, Richardson P. Melphalan, prednisone, thalidomide and defibrotide in relapsed/refractory multiple myeloma: results of a multicenter phase I/II trial. Haematologica. 2010;95:1144–1149. doi: 10.3324/haematol.2009.017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright EE, Li Z, Bergsagel PL, Chesi M, Barber DL, Branch DR, Hawley RG, Stewart AK. Ectopic expression of fibroblast growth factor receptor 3 promotes myeloma cell proliferation and prevents apoptosis. Blood. 2000;95:992–998. [PubMed] [Google Scholar]
- Podar K, Tai YT, Hideshima T, Vallet S, Richardson PG, Anderson KC. Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009;14:99–127. doi: 10.1517/14728210802676278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84:623–630. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Vacca A. The role of microenvironment in tumor angiogenesis. Genes Nutr. 2008;3:29–34. doi: 10.1007/s12263-008-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin DH, Rajkumar SV, Srkalovic G, Alsina M, Anderson KC. Extended follow-up of a phase II trial in relapsed, refractory multiple myeloma:: final time-to-event results from the SUMMIT trial. Cancer. 2006;106:1316–1319. doi: 10.1002/cncr.21740. [DOI] [PubMed] [Google Scholar]
- Roccaro AM, Hideshima T, Raje N, Kumar S, Ishitsuka K, Yasui H, Shiraishi N, Ribatti D, Nico B, Vacca A, Dammacco F, Richardson PG, Anderson KC. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res. 2006;66:184–191. doi: 10.1158/0008-5472.CAN-05-1195. [DOI] [PubMed] [Google Scholar]
- Scavelli C, Nico B, Cirulli T, Ria R, Di Pietro G, Mangieri D, Bacigalupo A, Mangialardi G, Coluccia AM, Caravita T, Molica S, Ribatti D, Dammacco F, Vacca A. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene. 2008;27:663–674. doi: 10.1038/sj.onc.1210691. [DOI] [PubMed] [Google Scholar]
- Sica A, Rubino L, Mancino A, Larghi P, Porta C, Rimoldi M, Solinas G, Locati M, Allavena P, Mantovani A. Targeting tumour-associated macrophages. Expert Opin Ther Targets. 2007;11:1219–1229. doi: 10.1517/14728222.11.9.1219. [DOI] [PubMed] [Google Scholar]
- Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97–102. doi: 10.1016/j.imlet.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36:350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca A, Ribatti D. Bone marrow angiogenesis in multiple myeloma. Leukemia. 2006;20:193–199. doi: 10.1038/sj.leu.2404067. [DOI] [PubMed] [Google Scholar]
- Vacca A, Ribatti D, Roncali L, Ranieri G, Serio G, Silvestris F, Dammacco F. Bone marrow angiogenesis and progression in multiple myeloma. Br J Haematol. 1994;87:503–508. doi: 10.1111/j.1365-2141.1994.tb08304.x. [DOI] [PubMed] [Google Scholar]
- Vilcek J. From IFN to TNF: a journey into realms of lore. Nat Immunol. 2009;10:555–557. doi: 10.1038/ni0609-555. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007;7:1705–1721. doi: 10.1517/14712598.7.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Levesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Yasui H, Hideshima T, Richardson PG, Anderson KC. Novel therapeutic strategies targeting growth factor signalling cascades in multiple myeloma. Br J Haematol. 2006;132:385–397. doi: 10.1111/j.1365-2141.2005.05860.x. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumsteg A, Christofori G. Corrupt policemen: inflammatory cells promote tumor angiogenesis. Curr Opin Oncol. 2009;21:60–70. doi: 10.1097/CCO.0b013e32831bed7e. [DOI] [PubMed] [Google Scholar]