Abstract
Exposure to high levels of glucocorticoids (GCs) during early development results in lasting disturbances in emotional behavior in rodents. Inhibitory GABAergic neurons, classified by their expression of calcium binding proteins (CBPs), also contribute to stress-related behaviors and may be GC sensitive during development. Therefore, in the present study we investigated the effects of prenatal treatment with the glucocorticoid receptor agonist dexamethasone (DEX) on expression of calbindin and calretinin in brain areas critical to emotional regulation (basolateral/lateral amygdala and hippocampal CA1 and CA3 regions). Late gestational treatment with DEX (gestational day 18–22) significantly decreased the density of calretinin immunoreactive cells in the lateral amygdala of adult female offspring with no differences in the basolateral amygdala, hippocampal CA1, or CA3 regions. Moreover, there were no effects of gestational DEX treatment on calretinin expression in males. Calbindin expression in adulthood was unaltered within either amygdala or hippocampal subregion of either sex following prenatal DEX treatment. Together these findings indicate that late gestational DEX treatment causes a targeted reduction of calretinin within the lateral amygdala of females and this may be one mechanism through which developmental glucocorticoid exposure contributes to lasting alterations in emotional behavior.
Keywords: dexamethasone, glucocorticoid, calbindin, calretinin, prenatal, sex difference
Introduction
Circulating glucocorticoid (GC) levels rise prior to birth and are elevated even further under conditions of prenatal stress. Such pathologically elevated levels of GCs are thought to impact the developing brain by altering normal patterns of cell proliferation [22], differentiation [28], migration [8], and death [39]. In addition to endogenous increases in GC levels, exposure to high levels of GCs during development occur following clinical administration of synthetic GCs, such as dexamethasone (DEX), to premature infants or pregnant women at risk for premature delivery. In this case, they promote lung development by stimulating surfactant production [35] and thereby lessen the risk for respiratory distress [36]. However, adverse side effects of DEX have been reported. Infants treated with DEX show dramatic reductions in body weight, detriments in cognitive, motor, and emotional development, and disrupted hypothalamic-pituitary-adrenal (HPA) axis function [2,13,16]. In rodents, perinatal exposure to DEX has repeatedly been shown to have long-term consequences that affect emotional behaviors. These effects include elevated anxiety-related behaviors [23,27], startle responses [14], and depressive-like behaviors [23,27].
The hippocampus and the basolateral amygdalar complex, encompassing the lateral and basolateral amygdalae, play a critical role in the regulation of emotional behaviors including fear, anxiety, and depression [1,10,41]. Within these regions, GABAergic neurons, classified by their expression of calcium binding proteins (CBPs), have been demonstrated to contribute to stress-related behaviors and expression of these cell phenotypes also appears to be GC sensitive during development. Alterations in CBP expression have been linked to anxiety-related behaviors and depression in humans and rodents [18,11,38]. Furthermore, neonatal stress, which involves an elevation in endogenous GC levels, causes region specific increases and decreases in the expression of these cell phenotypes [11,9]. Consistent with the concept that GCs can affect the development of GABA neurons, a recent study in our laboratory indicates that perinatal treatment with DEX induces apoptosis in calbindin and calretinin expressing cells within the amygdala [43].
Given our observation that prenatal DEX increases apoptotic cell death within the amygala and hippocampus [43,44] and CBP-ir neurons may be particularly vulnerable [43], in the present study we determined whether these effects of late gestational GC exposure persist into adulthood by changing the density of calbindin and calretinin immunoreactive neurons in the lateral/basolateral amygdala and CA1 and CA3 hippocampal regions. The results of these studies show that following prenatal DEX treatment, calretinin expression is decreased in the lateral amygdala of adult females, with no effects of DEX treatment found in calbindin or calretinin expression in other areas examined or in males.
Method
Animals
Timed-pregnant Sprague-Dawley dams were obtained from Charles River Laboratories (Wilmington, MA) at 7 days gestation and handled daily for 4 days (GD14-17) to reduce the possibility of investigator-initiated stress responses prior to experimental treatments. Dams were singly housed under a 14/10 L/D cycle (lights on at 0600), with food and water available ad libitum. Pregnant dams were subcutaneously injected with 0.4 mg/kg DEX or vehicle (2% ethanol in safflower oil) once daily at 1000 on gestation days 18–22. This dosage has previously been used in our laboratory and has been shown to produce effects commonly reported following GC treatment including decreased brain and body weight [43,3]. All procedures were approved by the Arizona State University IACUC, under subcontract from the University of Arizona College of Medicine-Phoenix and were in accord with NIH guidelines.
Tissue Processing and Immunohistochemistry
At 60 days of age, male and female offspring were administered a 100 mg/kg intraperitoneal overdose of Nembutal, and intracardially perfused with 100 ml of 0.9% saline followed by 200 ml of phosphate-buffered 4% paraformaldehyde. Brains were extracted and placed in the same fixative at 4°C overnight. Brains were next transferred into a 30% sucrose cryoprotectant solution, where they remained at 4° C until sectioning. For immunohistochemistry, brains were sectioned coronally at 35μm into 4 alternate series using a Leica CM3050S cryostat (Leica, Buffalo Grove, IL). Tissue was placed in cryopreservative at −20° C. Immunohistochemistry was performed on free-floating sections according to our published protocol [43] using primary antisera for (Calbindin D28k (1:20,000, mouse, Millipore, Billerica, MA), or Calretinin (1:5000, mouse, Millipore). Following immunohistochemistry, sections were mounted on gelatin-coated slides, and coverslipped with Permount (Sigma, St. Louis, MO, USA).
Microscopy
Analysis of calbindin-positive cells was conducted on a Zeiss Axioskop microscope equipped with Neurolucida v.7 software (MicroBrightField Inc, Williston, VT). The basolateral/lateral amygdala and CA1/CA3 hippocampal regions were identified using a rat brain atlas [24]. Regions were outlined using Neurolucida software and calbindin and calretinin-ir cells were counted within these areas. Calbindin and calretinin-ir cells were counted bilaterally within 2 consecutive sections near Bregma-2.80mm. The mean of these counts was used as a single value for each brain region for each animal. Calbindin immunoreactivity within the sexually dimorphic nucleus of the preoptic area (SDN-POA) was also assessed as a biological assay of perinatal testosterone levels since calbindin-ir cells form a sub-nucleus within the SDN-POA which is greater in volume and contains more neurons in male compared to female rodents [30,6]. Moreover, this sex difference results from the presence of perinatal androgen secretions in males [30]. One unilateral region from each animal that contained the largest visible calbindin-ir subnucleus of the SDN-POA was chosen for analysis. Calbindin-ir cells were counted within a 500 x 500μm square area surrounding the region.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5.0 (GraphPad, La Jolla, CA). Calbindin- and calretinin-ir cell numbers were compared using 2-way ANOVA with treatment (DEX or vehicle) and sex (male or female) as factors. Significant effects were further analyzed using Bonferonni corrected t-tests. Differences were considered significant when p<0.05 and data are reported as means ± standard error of the mean (SEM).
Results
Amygdala
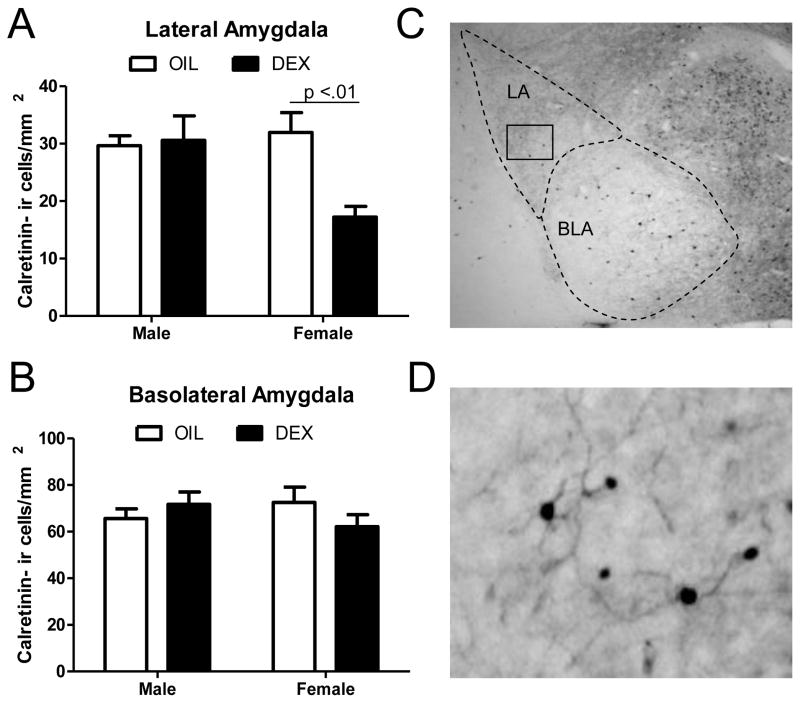
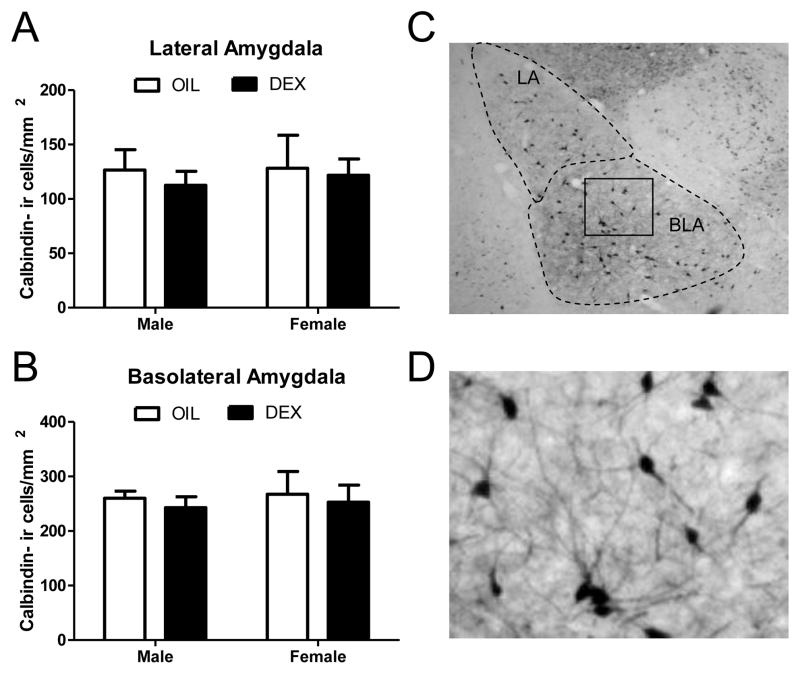
Two way analysis of variance of calretinin-ir cells within the lateral amygdala revealed a significant main effect of treatment (F(1,20) = 5.18, p<.05), but not sex, and a significant sex x treatment interaction (F(1,20) = 6.67, p<.05). Post hoc comparisons indicated that DEX treated females showed a significant decrease in the number of calretinin-ir cells compared to all other groups (p<.01; Figure 1a). No significant main effects or interactions were found in the number of calretinin-ir cells in the basolateral amygdala (Figure 1b). In contrast to calretinin, no significant main effects of treatment, sex, or a sex x treatment interaction were found for calbindin-ir cells in the lateral or basolateral amygdala (Figure 2).
Figure 1. Calretinin immunoreactive cells within the lateral and basolateral amygdala sub-regions following prenatal dexamethasone treatment.
The number of calretinin immunoreactive cells was quantified in the lateral (A) and basolateral (B) amygdala of adult males and females following prenatal treatment with DEX or oil vehicle. (C) Low magnification photomicrograph showing calretinin immunoreactive cells in the amygdala. The lateral and basolateral amygdala are indicated by dashed lines and the box indicates the region that was further magnified in (D). In panels A and B, each bar represents mean +/- SEM of n=6 per group. Numbers above bars indicate levels of significance between groups.
Figure 2. Calbindin immunoreactive cells within the lateral and basolateral amygdala sub-regions following prenatal dexamethasone treatment.
The number of calbindin immunoreactive cells was quantified in the lateral (A) and basolateral (B) amygdala of 60 day old males and females following prenatal treatment with DEX or oil vehicle. No significant differences were found. (C) Low magnification photomicrograph showing calbindin immunoreactive cells in the amygdala. The lateral and basolateral amygdala are indicated by dashed lines and the box indicates the region that was further magnified in panel (D). Each bar represents mean +/- SEM of n=6 per group.
Hippocampus
No significant main effects or interactions were found for the number of calbindin or calretinin-ir cells in the CA1 or CA3 hippocampal regions (Table 1).
Table 1. Numbers of calretinin and calbindin immunoreactive cells within the adult hippocampus and the sexually dimorphic nucleus of the preoptic area following prenatal dexamethasone treatment.
Animals were killed at 60 days old and numbers of immunoreactive cells were counted within an outlined area surrounding the entire CA1 or CA3 subfields of the hippocampus or within a 500 x 500μm square area surrounding surrounding the SDN-POA. Data are expressed as counts per mm².
| Calretinin
|
Calbindin
|
||||
|---|---|---|---|---|---|
| Treatment/Sex | CA1 | CA3 | CA1 | CA3 | SDN-POA |
|
| |||||
| Veh Male | 40.73 ± 3.48 | 30.15 ± 4.87 | 19.88 ± 2.23 | 20.52 ± 2.69 | 145.17 ± 2.89 |
| Dex Male | 50.67 ± 2.24 | 38.14 ± 2.55 | 24.77 ± 2.67 | 27.08 ± 1.43 | 162.33 ± 8.75 |
| Veh Female | 45.24 ± 6.83 | 32.30 ± 4.72 | 22.40 ± 3.52 | 25.51 ± 2.31 | 126.50 ± 8.04* |
| Dex Female | 39.98 ± 8.85 | 30.48 ± 4.15 | 16.58 ± 2.79 | 22.88 ± 2.82 | 122.50 ± 15.31* |
indicates a significant main effect of sex (p<.01) by 2-way ANOVA, with females having fewer calbindin-ir cells than males. N=6 per group.
SDN-POA
Two-way ANOVA of calbindin-ir cells in the SDN-POA revealed a significant main effect of sex (F(1,20) = 8.91, p<.01; Table 1) indicating a greater density in males compared to females. No significant effects of treatment or a sex x treatment interaction were found for calbindin-ir cells in the SDN-POA (Table 1).
Discussion
In this study, we demonstrate that prenatal DEX exposure causes targeted decreases in the number of calretinin-ir cells in the lateral amygdala of adult female rats. This effect was not seen in adult male rats nor was it seen in the basolateral amygdala or CA1 and CA3 hippocampal regions of males or females. Further, prenatal DEX treatment did not affect calbindin cell populations in the amygdala or hippocampus.
The lateral amygdala plays a critical role in regulating emotional behaviors including fear, anxiety, and depressive-like behaviors [1,10,41]. Recent studies show that these behaviors are elevated following prenatal DEX treatment [27,23,14] suggesting that overexposure to GCs during development can permanently affect brain function. The amygdala contains dense populations of cells that express glucocorticoid receptor [20], as early as gestational day 17–18 [37], providing a means through which DEX can exert its effects. However, the specific molecular mechanism/s whereby developmental exposure to GCs alters development of the amygdala is not known.
The amygdala and hippocampus contain a large and heterogenous population of GABAergic neurons that can be sub-classified based on their expression of calcium binding proteins. Calcium binding protein expressing cells, including calbindin and calretinin have distinct cell/synapse morphology and physiology [40,4]. These unique characteristics indicate that subpopulations of CBPs may mediate different inhibitory functions. Hence, a disruption in the expression of one subtype may also cause unique behavioral deficits.
Alterations in the number of GABAergic neurons in the amygdala, including the lateral region, have been shown to correlate with a dysregulation in emotional behaviors in rodents [5]. Therefore, permanent modifications in GABAergic function resulting from prenatal DEX induced decreases in calretinin-ir in the lateral amygdala may contribute to the etiology of these behavioral disturbances. Changes in calretinin-ir cell density in the lateral amygdala may be the consequence of several factors including reduced cell proliferation, altered phenotype differentiation/migration, or increased apoptosis. Each has been shown to be affected by perinatal GC exposure [22,28,8,39,15]. We have hypothesized that one likely mechanism for GC induced alterations in calretinin density is apoptotic cell death. In a recent study, perinatal DEX exposure was shown to preferentially induce apoptosis in amygdala neurons expressing calretinin and calbindin [43].
Interestingly, the observed decrease in lateral amygdala calretinin-ir following in utero DEX treatment occurred exclusively in adult females. Prenatal GC overexposure resulting from either maternal stress or direct administration has been shown to cause a variety of sexually dimorphic modifications in the brain including elevations in apoptotic cell death and permanent cell loss in females [19,33,42]. These sex specific effects of GCs may result from the presence of androgens in males during the perinatal period which spare cells from death in the rodent central nervous system via actions through both androgen and estrogen receptors [21]. Subtle differences in the expression of androgen and estrogen receptors between the lateral and basolateral amygdala (31) may similarly contribute to region specific effects on calretinin immunoreactivity. It is also possible that DEX induced alterations in lateral amygdala calretinin expression occur consequent to sex specific alterations in gonadal hormone receptors. Estrogen receptor beta deficient mice show decreased levels of calretinin within the amygdala (7), therefore a sex specific decrease in this receptor may lead to a decrease in lateral amygdala calretinin. Correlating with sex specific effects of DEX on lateral amygdala calretinin immunoreactivity are reports indicating increased anxiety and depressive-like behaviors in adulthood (23,27). Similarly, prenatal stress has also been reported to lead to female specific increases in these behaviors in adult rats [26,29]. This suggests that a decrease in lateral amygdala calretinin cells may be one factor underlying this behavioral sex difference, although it is not known whether effects of prenatal stress are entirely due to increases in GCs.
Unlike the lateral amygdala, the density of calretinin-ir cells was unaffected in the basolateral amygdala and CA1/CA3 regions of the hippocampus following prenatal DEX treatment, indicating that these regions are less sensitive to the adverse effects of GCs. This difference in sensitivity to DEX on the number of calretinin-ir cells may reflect regional differences in glucocorticoid receptor distribution and/or cellular localization, or reflect tissue specific critical periods for glucocorticoid receptor sensitivity. Cells of the lateral amygdala show a unique distribution of glucocorticoid receptors, in which some receptors are located in non-nuclear membrane translocation sites, including glial processes, dendrites and dendritic spines [12]. Whether this population of glucocorticoid receptors is responsible for the programmed effects of DEX treatment on adult calretinin expression remains to be determined. The amygdala also shows subregion specific alterations in glucocorticoid receptor following prenatal DEX treatment, indicating these discrete regions show different sensitivities to GCs [34]. However, further studies are needed to decipher the specific mechanisms through which DEX differentially affects the lateral and basolateral amygdala.
The density of calbindin expressing cells of adult males and females was unaffected in both the lateral and basolateral amygdala following prenatal DEX treatment. This observation correlates with the results of studies showing that neonatal stress does not affect calbindin immunoreactivity in either the lateral or basolateral amygdala [9] indicating that this phenotype of cells are less sensitive to the effects of GCs. We also did not detect changes in calbindin or calretinin cell numbers in the hippocampus of prenatal DEX treated males and females. By contrast, neonatal stress has been shown to increase calbindin cell number in the hippocampus [9]. However, since GC exposure in our studies was administered prenatally, and Giachino et al [9] administered stress postnatally, the timing of GC overexposure may differentially affect calbindin expression in the hippocampus. It is also possible that stress-induced increases in hippocampal calbindin do not occur exclusively via GC activation of glucocorticoid receptors.
Since prenatal DEX exposure can alter circulating levels of gonadal steroid hormones [17], and gonadal steroids can influence GABA and CBP function [25,32], we addressed the possibility that androgen levels were altered in rodents in the present study and thus may contribute to morphological changes within the lateral amygdala. To this end, we examined the SDN-POA, a forebrain nucleus that is much larger in males than in females and is sensitive to perinatal changes in gonadal steroid hormones [30]. Previous studies have demonstrated that testosterone exposure on postnatal days 0–1 is critical to the formation of sex differences in calbindin-ir in the SDN-POA [30], therefore the calbindin-ir sub-nucleus of the SDN-POA should be altered in prenatal DEX treated animals if there were significant changes in circulating gonadal steroid hormone levels. Our results confirmed the presence of a sex difference in calbindin in rat brains. Since we found no effects of prenatal DEX on SDN-POA calbindin-ir of males or females this indicates that any potential DEX -induced suppression of testosterone in males, or elevation in females, was insufficient to produce morphological effects. Furthermore, the possibility exists that elevated levels of androgens in males during the perinatal period may serve to reduce any effects of DEX, thereby protecting males but not females, from GC induced changes in lateral amygdala morphology.
In summary, these findings indicate that exposure to high levels of GCs during prenatal development can permanently decrease the density of calretinin expressing cells in the lateral amygdala of adult females which subsequently may contribute to disturbances in emotional behaviors reported in prenatal DEX-treated rodents [23,27].
Highlights.
Prenatal dexamethasone decreases calretinin cell density in the female lateral amygdala
Calretinin expression is not altered in the basolateral amygdala and hippocampus
Prenatal dexamethasone did not alter calbindin expression
Acknowledgments
The authors acknowledge Drs. Stuart Tobet and Jill Goldstein for providing helpful input in the design of this study. Support was provided by USPHS Grants NS039951 and MH082679.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamec R, Hebert M, Blundell J. Long lasting effects of predator stress on pCREB expression in brain regions involved in fearful and anxious behavior. Behav Brain Res. 2011;221:118–33. doi: 10.1016/j.bbr.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Barrington KJ. Postnatal steroids and neurodevelopmental outcomes: a problem in the making. Pediatrics. 2001;107:1425–6. doi: 10.1542/peds.107.6.1425. [DOI] [PubMed] [Google Scholar]
- 3.Carbone DL, Zuloaga DG, Hiroi R, Foradori CD, Legare ME, Handa RJ. Prenatal Dexamethasone Exposure Potentiates Diet-Induced Hepatosteatosis and Decreases Plasma IGF-I in a Sex-Specific Fashion. Endocrinology. 2012;153:295–306. doi: 10.1210/en.2011-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham MG, Connor CM, Carlezon WA, Jr, Meloni E. Amygdalar GABAergic-rich neural grafts attenuate anxiety-like behavior in rats. Behav Brain Res. 2009;205:146–53. doi: 10.1016/j.bbr.2009.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev Neurobiol. 2007;67:1371–81. doi: 10.1002/dneu.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan X, Warner M, Gustafsson JA. Estrogen receptor beta expression in the embryonic brain regulates development of calretinin-immunoreactive GABAergic interneurons. Proc Natl Acad Sci U S A. 2006;103:19338–43. doi: 10.1073/pnas.0609663103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukumoto K, Morita T, Mayanagi T, Tanokashira D, Yoshida T, Sakai A, Sobue K. Detrimental effects of glucocorticoids on neuronal migration during brain development. Mol Psychiatry. 2009;14:1119–31. doi: 10.1038/mp.2009.60. [DOI] [PubMed] [Google Scholar]
- 9.Giachino C, Canalia N, Capone F, Fasolo A, Alleva E, Riva MA, Cirulli F, Peretto P. Maternal deprivation and early handling affect density of calcium binding protein-containing neurons in selected brain regions and emotional behavior in periadolescent rats. Neuroscience. 2007;145:568–78. doi: 10.1016/j.neuroscience.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 10.Gos T, Krell D, Bielau H, Steiner J, Mawrin C, Trübner K, Brisch R, Bernstein HG, Jankowski Z, Bogerts B. Demonstration of disturbed activity of the lateral amygdaloid nucleus projection neurons in depressed patients by the AgNOR staining method. J Affect Disord. 2010;126:402–10. doi: 10.1016/j.jad.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Helmeke C, Ovtscharoff W, Jr, Poeggel G, Braun K. Imbalance of immunohistochemically characterized interneuron populations in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience. 2008;152:18–28. doi: 10.1016/j.neuroscience.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–99. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 13.Jones RA. Randomized, controlled trial of dexamethasone in neonatal chronic lung disease: 13- to 17-year follow-up study: I. Neurologic, psychological, and educational outcomes. Pediatrics. 2005;116:370–8. doi: 10.1542/peds.2004-1818. [DOI] [PubMed] [Google Scholar]
- 14.Kjaer SL, Hougaard KS, Tasker RA, MacDonald DS, Rosenberg R, Elfving B, Wegener G. Influence of diurnal phase on startle response in adult rats exposed to dexamethasone in utero. Physiol Behav. 2011;102:444–52. doi: 10.1016/j.physbeh.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity, and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets, and sex selectivity. Neuropsychopharmacology. 2006;31:12–35. doi: 10.1038/sj.npp.1300783. [DOI] [PubMed] [Google Scholar]
- 16.Lajic S, Nordenström A, Hirvikoski T. Long-term outcome of prenatal dexamethasone treatment of 21-hydroxylase deficiency. Endocr Dev. 2011;20:96–105. doi: 10.1159/000321228. [DOI] [PubMed] [Google Scholar]
- 17.Lalau JD, Aubert ML, Carmignac DF, Grégoire I, Dupouy JP. Reduction in testicular function in rats. II. Reduction by dexamethasone in fetal and neonatal rats. Neuroendocrinology. 1990;51:289–93. doi: 10.1159/000125352. [DOI] [PubMed] [Google Scholar]
- 18.Maciag D, Hughes J, O'Dwyer G, Pride Y, Stockmeier CA, Sanacora G, Rajkowska G. Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol Psychiatry. 2010;67:465–70. doi: 10.1016/j.biopsych.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McArthur S, McHale E, Gillies GE. The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex- region- and time-specific manner. Neuropsychopharmacology. 2007;32:1462–76. doi: 10.1038/sj.npp.1301277. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS, Luine VN, Plapinger L, de Kloet ER. Putative estrogen and glucocorticoid receptors in the limbic brain. J Steroid Biochem. 1975;6:971–7. doi: 10.1016/0022-4731(75)90337-4. [DOI] [PubMed] [Google Scholar]
- 21.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–9. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 22.Noorlander CW, Visser GH, Ramakers GM, Nikkels PG, de Graan PN. Prenatal corticosteroid exposure affects hippocampal plasticity and reduces lifespan. Dev Neurobiol. 2008;68:237–46. doi: 10.1002/dneu.20583. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira M, Bessa JM, Mesquita A, Tavares H, Carvalho A, Silva R, Pêgo JM, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. Induction of a hyperanxious state by antenatal dexamethasone: a case for less detrimental natural corticosteroids. Biol Psychiatry. 2006;59:844–52. doi: 10.1016/j.biopsych.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 7. San Diego: Elsevier Academic Press; 2007. [Google Scholar]
- 25.Penatti CA, Porter DM, Jones BL, Henderson LP. Sex-specific effects of chronic anabolic androgenic steroid treatment on GABA(A) receptor expression and function in adolescent mice. Neuroscience. 2005;135:533–43. doi: 10.1016/j.neuroscience.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 26.Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- 27.Roque S, Oliveira TG, Nobrega C, Barreira-Silva P, Nunes-Alves C, Sousa N, Palha JA, Correia-Neves M. Interplay between Depressive-Like Behavior and the Immune System in an Animal Model of Prenatal Dexamethasone Administration. Front Behav Neurosci. 2011;5:4. doi: 10.3389/fnbeh.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rugerio-Vargas C, Ramírez-Escoto M, DelaRosa-Rugerio C, Rivas-Manzano P. Prenatal corticosterone influences the trajectory of neuronal development, delaying or accelerating aspects of the Purkinje cell differentiation. Histol Histopathol. 2007;22:963–9. doi: 10.14670/HH-22.963. [DOI] [PubMed] [Google Scholar]
- 29.Schulz KM, Pearson JN, Neeley EW, Berger R, Leonard S, Adams CE, Stevens KE. Maternal stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiol Behav. 2011;104:340–7. doi: 10.1016/j.physbeh.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sickel MJ, McCarthy MM. Calbindin-D28k immunoreactivity is a marker for a subdivision of the sexually dimorphic nucleus of the preoptic area of the rat: developmental profile and gonadal steroid modulation. J Neuroendocrinol. 2000;12:397–402. doi: 10.1046/j.1365-2826.2000.00474.x. [DOI] [PubMed] [Google Scholar]
- 31.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 32.Stuart EB, Thompson JM, Rhees RW, Lephart ED. Steroid hormone influence on brain calbindin-D(28K) in male prepubertal and ovariectomized rats. Brain Res Dev Brain Res. 2001;129:125–33. doi: 10.1016/s0165-3806(01)00191-2. [DOI] [PubMed] [Google Scholar]
- 33.Tobe I, Ishida Y, Tanaka M, Endoh H, Fujioka T, Nakamura S. Effects of repeated maternal stress on FOS expression in the hypothalamic paraventricular nucleus of fetal rats. Neuroscience. 2005;134:387–95. doi: 10.1016/j.neuroscience.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 34.Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–9. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 35.Whitsett JA, Weaver TE, Clark JC, Sawtell N, Glasser SW, Korfhagen TR, Hull WM. Glucocorticoid enhances surfactant proteolipid Phe and pVal synthesis and RNA in fetal lung. J Biol Chem. 1987;262 :15618–15623. [PubMed] [Google Scholar]
- 36.Yeh TF, McClenan DA, Ajayi OA, Pildes RS. Metabolic rate and energy balance in infants with bronchopulmonary dysplasia. J Pediatr. 1989;114:448–51. doi: 10.1016/s0022-3476(89)80569-4. [DOI] [PubMed] [Google Scholar]
- 37.Yi SJ, Masters JN, Baram TZ. Glucocorticoid receptor mRNA ontogeny in the fetal and postnatal rat forebrain. Mol Cell Neurosci. 1994;5:385–93. doi: 10.1006/mcne.1994.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yilmazer-Hanke DM, Faber-Zuschratter H, Linke R, Schwegler H. Contribution of amygdala neurons containing peptides and calcium-binding proteins to fear-potentiated startle and exploration-related anxiety in inbred Roman high- and low-avoidance rats. Eur J Neurosci. 2002;15:1206–18. doi: 10.1046/j.1460-9568.2002.01945.x. [DOI] [PubMed] [Google Scholar]
- 39.Yu S, Patchev AV, Wu Y, Lu J, Holsboer F, Zhang JZ, Sousa N, Almeida OF. Depletion of the neural precursor cell pool by glucocorticoids. Ann Neurol. 2010;67:21–30. doi: 10.1002/ana.21812. [DOI] [PubMed] [Google Scholar]
- 40.Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex. 2005;15:1178–1186. doi: 10.1093/cercor/bhh218. [DOI] [PubMed] [Google Scholar]
- 41.Zangrossi H, Jr, Viana MB, Graeff FG. Anxiolytic effect of intra-amygdala injection of midazolam and 8-hydroxy-2-(di-n-propylamino)tetralin in the elevated T-maze. Eur J Pharmacol. 1999;369:267–70. doi: 10.1016/s0014-2999(99)00075-8. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Z, Li X, Chen W, Zhao Y, Li H, Qing C, Jia N, Bai Z, Liu J. Prenatal stress causes gender-dependent neuronal loss and oxidative stress in rat hippocampus. J Neurosci Res. 2004;78:837–44. doi: 10.1002/jnr.20338. [DOI] [PubMed] [Google Scholar]
- 43.Zuloaga DG, Carbone DL, Hiroi R, Chong DL, Handa RJ. Dexamethasone induces apoptosis in the developing rat amygdala in an age-, region-, and sex-specific manner. Neuroscience. 2011;199:535–547. doi: 10.1016/j.neuroscience.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuloaga DG, Carbone DL, Quihuis AM, Hiroi R, Chong DL, Handa RJ. Perinatal dexamethasone-induced alterations in apoptosis within the hippocampus and paraventricular nucleus of the hypothalamus are influenced by age and sex. J Neurosci Res. doi: 10.1002/jnr.23026. in press. [DOI] [PubMed] [Google Scholar]