Abstract
Gene A protein of bacteriophage phi X174 plays a role as a site-specific endonuclease in the initiation and termination of phi X rolling circle DNA replication. To clarify the sequence requirements of this protein we have studied the cleavage of single-stranded restriction fragments from phi X and G4 viral DNAs using purified gene A protein. The results show that in both viral DNAs cleavage occurs at the origin and at one additional site which shows striking sequence homology with the origin region. During rolling circle replication the single-stranded viral DNA tail is covered with single-stranded DNA binding (SSB) protein. Therefore, we have also studied the effect of SSB on phi X gene A protein cleavage. In these conditions only single-stranded fragments containing the complete or almost complete origin region of 30 bases are cleaved, whereas cleavage at the additional sites of phi X or G4 viral DNAs does not occur. A model for termination of rolling circle replication which is based on these findings is presented. Finally, we present evidence that the second product of gene A, the A* protein, cleaves phi X viral DNA at the additional cleavage site in the presence of SSB, not only in vitro but also in vivo. The functional significance of this cleavage in vivo is discussed.
Full text
PDF







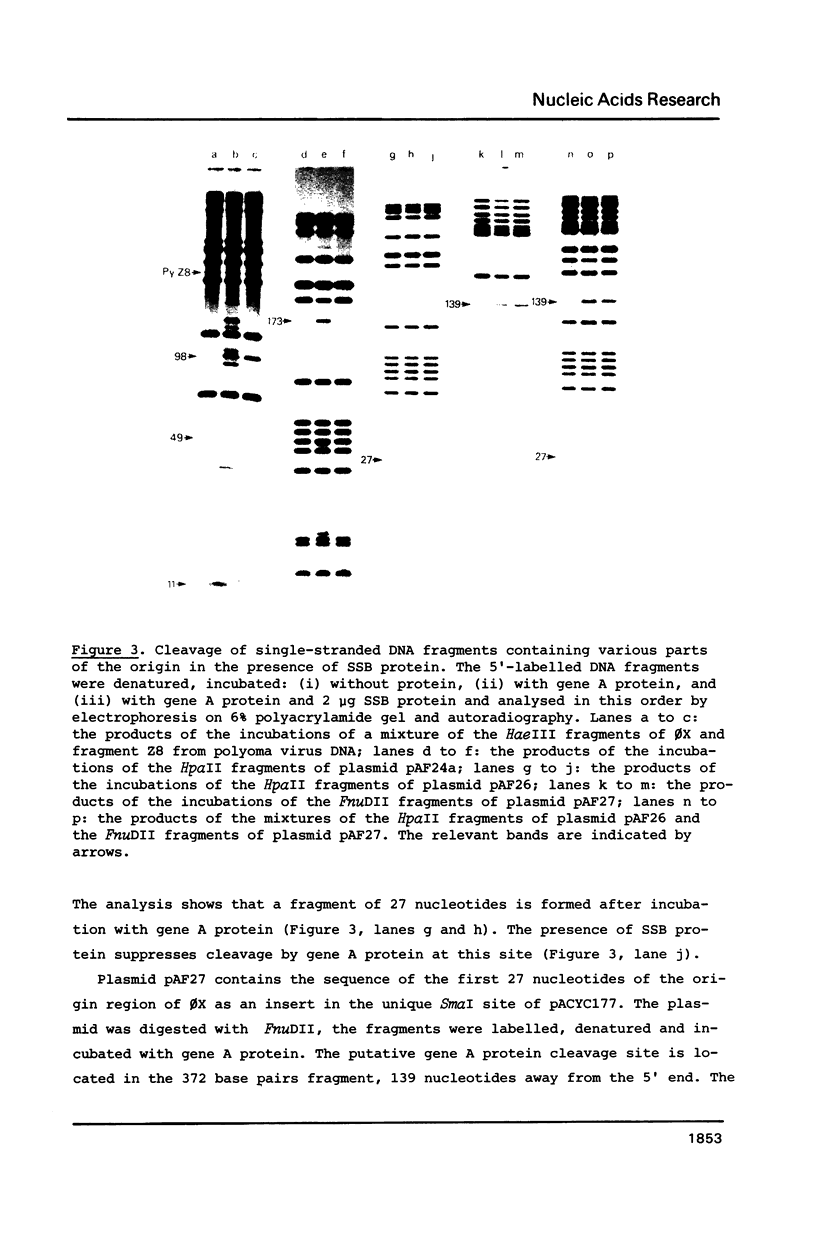







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas P. D., Jansz H. S. Bacteriophage phiX174 DNA synthesis in a replication-deficient host: determination of the origin of phiX DNA replication. J Mol Biol. 1976 Apr 15;102(3):633–656. doi: 10.1016/0022-2836(76)90339-9. [DOI] [PubMed] [Google Scholar]
- Baas P. D., Teertstra W. R., van Mansfeld A. D., Jansz H. S., van der Marel G. A., Veeneman G. H., van Boom J. H. Construction of viable and lethal mutations in the origin of bacteriophage 'phi' X174 using synthetic oligodeoxyribonucleotides. J Mol Biol. 1981 Nov 15;152(4):615–639. doi: 10.1016/0022-2836(81)90120-0. [DOI] [PubMed] [Google Scholar]
- Blakesley R. W., Dodgson J. B., Nes I. F., Wells R. D. Duplex regions in "single-stranded" phiX174 DNA are cleaved by a restriction endonuclease from Haemophilus aegyptius. J Biol Chem. 1977 Oct 25;252(20):7300–7306. [PubMed] [Google Scholar]
- Brown D. R., Roth M. J., Reinberg D., Hurwitz J. Analysis of bacteriophage phi X174 gene A protein-mediated termination and reinitiation of phi X DNA synthesis. I. Characterization of the termination and reinitiation reactions. J Biol Chem. 1984 Aug 25;259(16):10545–10555. [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Colasanti J., Denhardt D. T. Expression of the cloned bacteriophage phi X174 A* gene in Escherichia coli inhibits DNA replication and cell division. J Virol. 1985 Mar;53(3):807–813. doi: 10.1128/jvi.53.3.807-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Ascarelli R. The A* protein of phi X174 is an inhibitor of DNA replication. Nucleic Acids Res. 1981 Apr 24;9(8):1991–2002. doi: 10.1093/nar/9.8.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Kornberg A. Purification and characterization of phiX174 gene A protein. A multifunctional enzyme of duplex DNA replication. J Biol Chem. 1979 Jun 25;254(12):5328–5332. [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kornberg A. An enzyme system for replication of duplex circular DNA: the replicative form of phage phi X174. Proc Natl Acad Sci U S A. 1976 May;73(5):1594–1597. doi: 10.1073/pnas.73.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddes J. C., Barrell B. G., Godson G. N. Nucleotide sequences of the separate origins of synthesis of bacteriophage G4 viral and complementary DNA strands. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1081–1085. doi: 10.1073/pnas.75.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluit A. C., Baas P. D., Van Boom J. H., Veeneman G. H., Jansz H. S. Gene A protein cleavage of recombinant plasmids containing the phi X174 replication origin. Nucleic Acids Res. 1984 Aug 24;12(16):6443–6454. doi: 10.1093/nar/12.16.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Formation of the parental replicative form DNA of bacteriophage phi-X174 and initial events in its replication. J Mol Biol. 1971 Nov 14;61(3):565–586. doi: 10.1016/0022-2836(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Hayashi M. Gene A product of phi X174 is required for site-specific endonucleolytic cleavage during single-stranded DNA synthesis in vivo. J Virol. 1976 Aug;19(2):416–424. doi: 10.1128/jvi.19.2.416-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford W., Model P. Gene X of bacteriophage f1 is required for phage DNA synthesis. Mutagenesis of in-frame overlapping genes. J Mol Biol. 1984 Sep 15;178(2):137–153. doi: 10.1016/0022-2836(84)90136-0. [DOI] [PubMed] [Google Scholar]
- Funk F. D., Snover D. Pleiotropic effects of mutants in gene A of bacteriophage phi chi 174. J Virol. 1976 Apr;18(1):141–150. doi: 10.1128/jvi.18.1.141-150.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Barrell B. G., Staden R., Fiddes J. C. Nucleotide sequence of bacteriophage G4 DNA. Nature. 1978 Nov 16;276(5685):236–247. doi: 10.1038/276236a0. [DOI] [PubMed] [Google Scholar]
- Heidekamp F., Baas P. D., Jansz H. S. Nucleotide sequences at the phi X gene A protein cleavage site in replicative form I DNAs of bacteriophages U3, G14, and alpha 3. J Virol. 1982 Apr;42(1):91–99. doi: 10.1128/jvi.42.1.91-99.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Yudelevich A., Hurwitz J. Isolation and characterization of the protein coded by gene A of bacteriophage phiX174 DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2669–2673. doi: 10.1073/pnas.73.8.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni J. M., Kornberg A. The rho subunit of RNA polymerase holoenzyme confers specificity in priming M13 viral DNA replication. J Biol Chem. 1982 May 25;257(10):5437–5443. [PubMed] [Google Scholar]
- Langeveld S. A., van Arkel G. A., Weisbeek P. J. Improved method for the isolation of the A and A* proteins of bacteriophage phi X174. FEBS Lett. 1980 Jun 2;114(2):269–272. doi: 10.1016/0014-5793(80)81131-8. [DOI] [PubMed] [Google Scholar]
- Langeveld S. A., van Mansfeld A. D., Baas P. D., Jansz H. S., van Arkel G. A., Weisbeek P. J. Nucleotide sequence of the origin of replication in bacteriophage phiX174 RF DNA. Nature. 1978 Feb 2;271(5644):417–420. doi: 10.1038/271417a0. [DOI] [PubMed] [Google Scholar]
- Langeveld S. A., van Mansfeld A. D., van der Ende A., van de Pol J. H., van Arkel G. A., Weisbeek P. J. The nuclease specificity of the bacteriophage phi X174 A* protein. Nucleic Acids Res. 1981 Feb 11;9(3):545–562. doi: 10.1093/nar/9.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E., Hayashi M. Two proteins of gene A of psiX174. Nat New Biol. 1973 Sep 5;245(140):6–8. doi: 10.1038/newbio245006a0. [DOI] [PubMed] [Google Scholar]
- Martin D. F., Godson G. N. Identification of a phiX174 coded protein involved in the shut-off of host DNA replication. Biochem Biophys Res Commun. 1975 Jul 8;65(1):323–330. doi: 10.1016/s0006-291x(75)80096-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Reinberg D., Zipursky S. L., Weisbeek P., Brown D., Hurwitz J. Studies on the phi X174 gene A protein-mediated termination of leading strand DNA synthesis. J Biol Chem. 1983 Jan 10;258(1):529–537. [PubMed] [Google Scholar]
- Roth M. J., Brown D. R., Hurwitz J. Analysis of bacteriophage phi X174 gene A protein-mediated termination and reinitiation of phi X DNA synthesis. II. Structural characterization of the covalent phi X A protein-DNA complex. J Biol Chem. 1984 Aug 25;259(16):10556–10568. [PubMed] [Google Scholar]
- Rowen L., Kornberg A. Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem. 1978 Feb 10;253(3):758–764. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Sanhueza S., Eisenberg S. Bacteriophage phi X174 A protein cleaves single-stranded DNA and binds to it covalently through a tyrosyl-dAMP phosphodiester bond. J Virol. 1985 Feb;53(2):695–697. doi: 10.1128/jvi.53.2.695-697.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Litfin F., Jost E. Stimulation of T7 DNA polymerase by a new phage-coded protein. Mol Gen Genet. 1973 Jul 2;123(3):247–262. doi: 10.1007/BF00271243. [DOI] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Smolar N., Walsh J. E., Griffin B. E. Coding potential and regulatory signals of the polyoma virus genome. Nature. 1980 Jan 31;283(5746):445–453. doi: 10.1038/283445a0. [DOI] [PubMed] [Google Scholar]
- Tessman E. S. Mutants of bacteriophage S13 blocked in infectious DNA synthesis. J Mol Biol. 1966 May;17(1):218–236. doi: 10.1016/s0022-2836(66)80104-3. [DOI] [PubMed] [Google Scholar]
- Van Mansfeld A. D., Baas P. D., Jansz H. S. Gene A protein of bacteriophage phi X174 is a highly specific single-strand nuclease and binds via a tyrosyl residue to DNA after cleavage. Adv Exp Med Biol. 1984;179:221–230. doi: 10.1007/978-1-4684-8730-5_23. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- Weisbeek P., van Mansfeld F., Kuhlemeier C., van Arkel G., Langeveld S. Properties of the A and A proteins of bacteriophage G4. The origin of G4 replicative-form DNA replication. Eur J Biochem. 1981 Mar;114(3):501–507. doi: 10.1111/j.1432-1033.1981.tb05173.x. [DOI] [PubMed] [Google Scholar]
- Zolotukhin A. S., Drygin Iu F., Bogdanov A. A. Izuchenie tipa kovalentnoi sviazi v prirodnom soedinenii belka A* bakteriofaga phi X 174 s nukleotidami. Bioorg Khim. 1984 Aug;10(8):1109–1113. [PubMed] [Google Scholar]
- van Mansfeld A. D., Langeveld S. A., Baas P. D., Jansz H. S., van der Marel G. A., Veeneman G. H., van Boom J. H. Recognition sequence of bacteriophage phi X174 gene A protein--an initiator of DNA replication. Nature. 1980 Dec 11;288(5791):561–566. doi: 10.1038/288561a0. [DOI] [PubMed] [Google Scholar]
- van Mansfeld A. D., Langeveld S. A., Weisbeek P. J., Baas P. D., van Arkel G. A., Jansz H. S. Cleavage site of phiX174 gene-A protein in phiX and G4 RFI DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):331–334. doi: 10.1101/sqb.1979.043.01.039. [DOI] [PubMed] [Google Scholar]
- van Mansfeld A. D., van Teeffelen H. A., Baas P. D., Veeneman G. H., van Boom J. H., Jansz H. S. The bond in the bacteriophage phi X174 gene A protein--DNA complex is a tyrosyl-5'-phosphate ester. FEBS Lett. 1984 Aug 6;173(2):351–356. doi: 10.1016/0014-5793(84)80804-2. [DOI] [PubMed] [Google Scholar]
- van der Ende A., Langeveld S. A., Van Arkel G. A., Weisbeek P. J. The interaction of the A and A* proteins of bacteriophage phi X174 with single-stranded and double-stranded phi X DNA in vitro. Eur J Biochem. 1982 May 17;124(2):245–252. doi: 10.1111/j.1432-1033.1982.tb06584.x. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Keegstra W., Jansz H. S. Complex formation between the adenovirus type 5 DNA-binding protein and single-stranded DNA. Eur J Biochem. 1978 May 16;86(2):389–398. doi: 10.1111/j.1432-1033.1978.tb12321.x. [DOI] [PubMed] [Google Scholar]