Abstract
Study Design
NF-κB activity was pharmacologically and genetically blocked in an accelerated aging mouse model to mitigate age-related disc degenerative changes.
Objective
To study the mediatory role of NF-κB signaling pathway in age-dependent intervertebral disc degeneration.
Summary of Background Data
Aging is a major contributor to intervertebral disc degeneration (IDD), but the molecular mechanism behind this process is poorly understood. NF-κB is a family of transcription factors which play a central role in mediating cellular response to damage, stress, and inflammation. Growing evidence implicates chronic NF-κB activation as a culprit in many aging-related diseases, but its role in aging-related IDD has not been adequately explored. We studied the effects of NF-κB inhibition on IDD using a DNA repair-deficient mouse model of accelerated aging (Ercc1-/Δ mice) previously been reported to exhibit age-related IDD.
Methods
Systemic inhibition of NF-κB activation was achieved either genetically by deletion of one allele of the NF-κB subunit p65 (Ercc1-/Δp65+/- mice) or pharmacologically by chronic intra-peritoneal administration of the Nemo Binding Domain (8K-NBD) peptide to block the formation of the upstream activator of NF-κB, IκB Inducible Kinase (IKK), in Ercc1-/Δ mice. Disc cellularity, total proteoglycan content and proteoglycan synthesis of treated mice and untreated controls were assessed.
Results
Decreased disc matrix proteoglycan content, a hallmark feature of IDD, and elevated disc NF-κB activity were observed in discs of progeroid Ercc1-/Δ mice and naturally aged wild-type compared to young WT mice. Systemic inhibition of NF-κB by the 8K-NBD peptide in Ercc1-/Δ mice increased disc proteoglycan synthesis and ameriolated loss disc cellularity and matrix proteoglycan. These results were confirmed genetically by using the p65 haploinsufficient Ercc1-/Δp65+/- mice.
Conclusion
These findings demonstrate that the IKK/NF-κB signaling pathway is a key mediator of age-dependent IDD and represents a therapeutic target for mitigating disc degenerative diseases associated with aging.
Keywords: NF-kB;aging, proteoglycan, disc degeneration, DNA damage repair, ERCC1-deficient mice
INTRODUCTION
Intervertebral disc degeneration (IDD) is an underlying etiology of many chronic debilitating and pathological disorders, including spinal stenosis, radiculopathy, disc herniation, and low back pain 1-3. Although IDD is complex and multifactorial, aging is clearly the number one risk factor for many IDD-related disorders 4, 5. Aging is closely correlated with a number of degenerative changes in the intervertebral disc (IVD), including cellular senescence, apoptosis, annular fissures, and reduced disc height 6, 7. Loss of matrix proteoglycan (PG), a major structural component essential for disc biomechanical function, is another universal hallmark feature of disc aging 8. Moreover, PG gene expression is decreased while expression of matrix metalloproteinases is increased in aged disc tissue 9, 10. However, the molecular pathway(s) mediating these age-dependent disc degenerative changes are still largely unknown.
NF-κB is a family of transcription factors which play a central role in mediating cellular response to damage, stress, and inflammation 11. Mammalian NF-κB family consists of five subunits, but the most common and abundant form is the p50/p65 heterodimer 12. Under basal condition, NF-κB is localized primarily in the cytoplasm in an inactive state as it is being sequestered by the IκB proteins. NF-κB becomes activated in response to many different types of stress, including inflammatory, oxidative, genotoxic, mechanical and chemical stress. The canonical NF-κB activation pathway involves activation of IκB kinase (IKK), a heterotrimer consisting of two catalytic subunits, IKKα and IKKß, and a regulatory subunit termed IKKγ or NEMO (NF-κB Essential Modulator) 12. Activated IKK then phosphorylates IκB, leading to its ubiquitination and subsequent proteosomal degradation 11. IkB degradation allows NF-κB to translocate into the nucleus where it binds to its cognate DNA site to induce transcription of NF-κB -targeted genes 13 that regulate cell survival and growth, and production of inflammatory cytokines.
An expanding body of literature link chronic activation of NF-κB to tissue aging and many age-related degenerative diseases, including the musculoskeletal disorders such as, muscular dystrophy 14, osteoarthritis 15, osteoporosis 16. NF-κB is chronically up-regulated in various tissues of aged rodents 17-19. Increased NF-κB activity is also observed in cells derived from elderly persons and patients with Hutchinson-Gilford progeria syndrome, a disease of dramatically accelerated aging. Moreover, a recent modeling study identified NF-κB as the transcription factor most associated with mammalian aging and demonstrated that expression of a subset of NF-κB effectors is increased with aging 20.
The role of NF-κB signaling in age-related IDD is far less clear, although some existing evidence suggest its involvement the process of age-associated IDD. Elevated levels of several NF-κB-targeted genes, the pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and IL-8, in aged and/or painful degenerated IVDs have been reported 21-23. Increased level of oxidative stress, a key activator of NF-κB activity, has been reported in aged and degenerative disc tissue 24. Moreover, immunohistochemical studies also showed positive correlation between the level of disc NF-κB activity and IVD degeneration grade and patient age 24. Intra-discal injection of ‘naked’ NF-κB decoy oligonucleotide proved effective in partially restoring IVD height in a rabbit annular stab disc degeneration model, indicating that activation of NF-κB is involved in disc structural changes. 25.
Based on these observations, we hypothesize that activation of NF-κB signaling pathway plays a central role in mediating age-related degenerative changes in the intervertebral disc. We tested this hypothesis by blocking NF-κB activity pharmacologically and genetically using an in vivo rodent model of accelerated aging due to DNA repair deficiency (Ercc1-/Δ mice) previously reported to exhibit accelerated disc aging symptoms 5.
MATERIALS AND METHODS
Mice breeding and isolation of intervertebral discs
Experiments involving mice were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and in accordance with the NIH guidelines for humane care of animals. Wild-type and Ercc1-/Δ mice of a mixed genetic background (FVB/n:C57Bl/6J) were bred and genotyped by PCR as previously described 26. Knockin mice of C57Bl/6J genetic background expressing eGFP under the control of an NF-κB regulatory element (NF-κBeGFP) were provided by Christian Jobin, UNC Chapel Hill 27. p65+/- C57Bl/6J mice were provided by Denis Guttridge, Ohio State University, and bred with Ercc1+/- C57Bl/6J mice to generate Ercc1+/-p65+/- C57Bl/6 mice. These were then bred with Ercc1+/Δ FVB/n mice to generate Ercc1-/Δp65+/- mice and littermate controls (Tilstra et al. submitted) 28. Mice were euthanized at the established time points and the spines were isolated and dissected with the aid of a 5x magnifier. Entire intervertebral discs (IVDs) were removed en bloc from the surrounding vertebral bodies through an incision along the endplates using a surgical no. 11 blade. To harvest NP tissue, an axial cut was made on the disc side of the endplate to expose the disc center, followed by gentle aspiration of the NP tissue using a sterile P-10 pipette tip under a dissecting microscope (20-40 x magnification, Nikon SMZ645).
8K-NBD treatment of Animals
8K-NBD (KKKKKKKKGGTALDWSWLQTE) peptide was synthesized by the peptide synthesis facility at the University of Pittsburgh, Pittsburgh, PA. Ercc1-/Δ mice were intraperitoneally treated with 8K-NBD 10 mg/kg three times per week. Treatment began at 5 weeks of age before the animals were symptomatic and continued until 18-20 weeks of age (Tilstra et al. submitted) 28. Disc tissues were isolated for analyses.
Immunofluorescence
Wild-type NF-kBeGFP mice were sacrificed at 5-6 months and 25-30 months of age. Tissues were placed in 10% formalin for 2 hours, and then transferred to 30% sucrose in phosphate buffered saline (PBS) overnight at 4°C. The tissues were then frozen in 2-methylbutane and embedded in optimal cutting temperature (OCT) at -20°C. Five micron sections were cut using a cryostat. Tissues were stained using HOESCT dye (Sigma) and mounted using gelvatol, as previously described 29. Samples were allowed to incubate overnight at 4°C and were analyzed for cells expressing eGFP by fluorescence microscopy (Nikon Eclipse Ts100).
Histological Staining
Isolated spines were decalcified and embedded in paraffin (Tissue Tek processor and Leica embedder). Seven micrometer sections were stained with either hamatoxylin and eosin (H&E) or safranin O and fast green dyes (Fisher Scientific, Pittsburgh, PA) by standard procedure and photographed under 40-200x magnification (Nikon Eclipse Ts100).
1,9-Dimethylmethylene Blue (DMMB) Colorimetric Assay for Sulfated Glycosaminoglycans (GAGs)
NP isolated from six lumbar IVDs of each mouse was pooled and digested using papain at 60°C for two hours. GAG content was measured in duplicate by the DMMB procedure 30 using chondroitin-6-sulfate (Sigma C-8529) as a standard. The DNA concentration of each sample was measured using the PicoGreen assay (Molecular Probes) and used to normalize the GAG values. Average values from six reaction samples (two duplicates x three mice per group) were calculated and reported ± standard error.
Quantitation of proteoglycan synthesis
Disc organ cultures of isolated functional spine units (FSU), each consisting of vertebra, disc, vertebra, were established as previously described 31. Four thoracic FSUs were cultured in complete growth medium (F-12/D-MEM containing 10% FCS, 1% PS, and 25 μg/ml L-ascorbic acid) for two days to equilibrate after the trauma of surgical dissection, followed by 12 hour labeling incubation with 35S-sulfate (20 μCi/ml). Proteoglycan synthesis was measured by 35S-sulfate incorporation as described previously 32. The rate of proteoglycan synthesis was calculated as the fmoles of sulfate incorporated per μg DNA. Average values from six reaction samples (two duplicates x three mice per group) were calculated and reported ± standard error.
Quantitation of matrix gene expression
Total RNA was purified from whole discs using the RNeasy Plus Universal Kit (Qiagen). The RNA was analyzed in duplicate reactions by real-time RT-PCR (iCycler IQ4, Bio-Rad) to determine mRNA levels of these selected NF-κB gene targets.
| Gene | Forward (5’→3’) | Reverse (5’→3’) |
|---|---|---|
| GAPDH | GAGGCCGGTGCTGAGTAT | GCGGAGATGATGACCCTTTTGG |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| IL-6 | GACTTCCATCCAGTTGCCTTC | ATTTCCACGATTTCCCAGAG |
| MMP-1β | TCTTTATGGTCCAGGCGATGAA | CCTCTTCTATGAGGCGGGGAT |
| MMP-3 | GGTACAGAGCTGTGGGAAGTC | GATGAGCACACAACCACACAC |
| iNOS | ATGACACTCTTCACCACAAGG | CAATGGCATGAGGCAGGAG |
The cycle threshold (Ct) values were obtained and normalized to the housekeeping gene GAPDH. The ΔΔCt method 33 was used to calculate the relative mRNA levels of each target gene between Ercc1-/Δ mice and wild-type littermates. Average values from six measurements (duplicate × 3 mice per group) are shown ± one standard error.
Statistical analysis
Values represent the average of 6 trials ± standard error (SE), with 95% confidence intervals calculated to determine statistical significance. The confidence intervals were calculated based on the t-distribution because of the small sample size.
RESULTS
NF-κB is activated in intervertebral discs of natural and accelerated aging mice
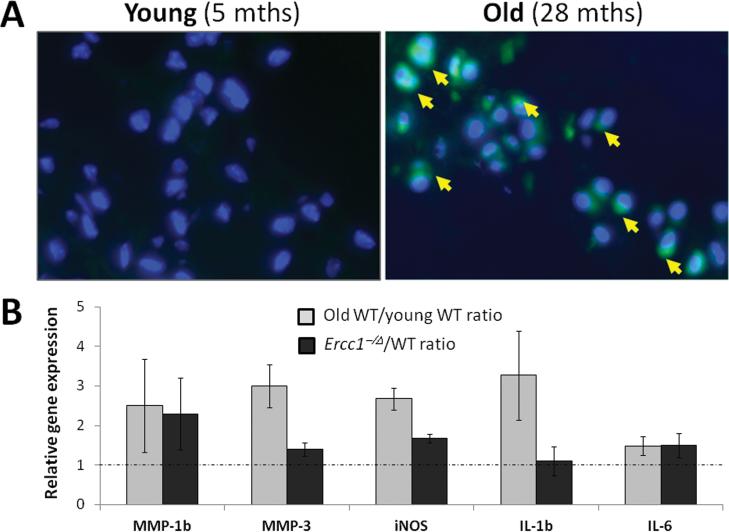
Previous analysis of human discs reported a positive correlation between NF-κB activation in disc tissue and aging 24. To verify this finding in mice, we used knockin mice expressing eGFP under the control of an NF-κB regulatory element (NF-κBeGFP) (Clauson et al, in preparation). In these mice, green fluorescence from eGFP expression indicates activation of the NF-κB pathway. Through fluorescent microscopy, eGFP was detected in the nucleus pulposus of old (>2 yrs), but not young (5-6 months) wild-type (WT) NF-κBeGFP mice (Fig. 1A), indicating increased NF-κB activity disc tissues of aged mice. To further confirm this result, we analyzed disc expression of selected genes known to be induced by activated NF-κB, including the interleukins (IL-1β, IL-6), the matrix metalloproteinases (MMP-1β, MMP-3), and inducible nitric oxide synthase (iNOS). The level of mRNA expression of these NF-κB gene targets was higher in discs of old WT mice compared to young WT mice (Fig. 1B). Similarly, expression of these NF-κB responsive genes also was generally greater in discs of accelerated aging progeroid Ercc1-/Δ mice (5-6 mths) compared to those in their WT littermates (Fig. 1B). Together, these data provide evidence of increased NF-κB activity in discs with aging.
Figure 1. Increased NF-κB activation in disc tissue of natural and accelerated aging mice.
A, Disc sections from NF-κBeGFP young (5 months, left image) and old (28 months, right image) WT mice were imaged using fluorescent microscopy to detect eGFP expression (green) and nuclei were counter-stained with Hoechst (blue). Representative images of the nucleus pulposus region are shown. Arrows, eGFP-positive cells. B, disc mRNA levels of selected NF-κB target genes as determined by qRT-PCR. Gray, the ratios of disc mRNA level of old (23-28 months) to young (5-6 months) wild-type mice. Black, the ratios of disc mRNA level of progeroid Ercc1-/Δ mice (5-6 months) to their WT littermates.
Genetic reduction of NF-κB mitigates loss of disc matrix proteoglycan in progeroid Ercc1-/Δ mice
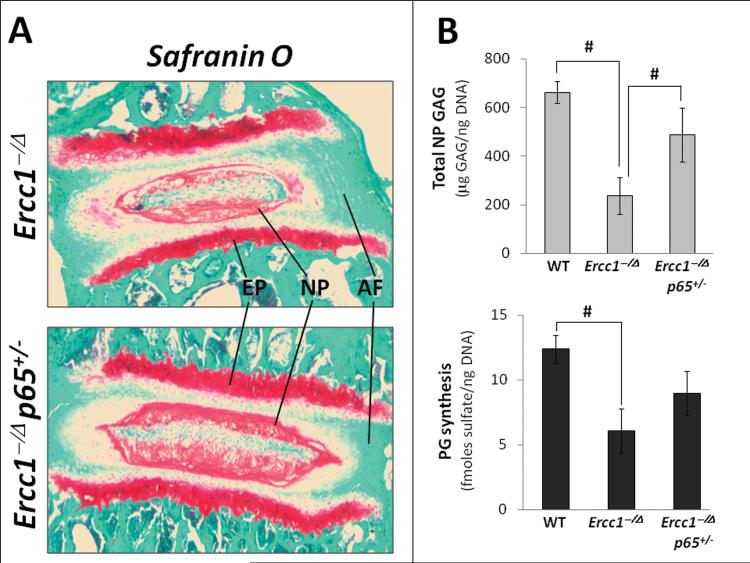
Accelerated aging Ercc1-/Δ mice exhibit distinct age-related histopathologic changes in their discs, including reduced safranin O staining for disc proteoglycans (PG) 5. To test whether these degenerative changes are mediated by NF-κB, we measured disc PG content using the Ercc1-/Δ mouse strain containing a genetic deletion of one allele of the NF-κB subunit p65 (Ercc1-/Δp65+/- mice). Compared to Ercc1-/Δ mice, Ercc1-/Δp65+/- mice exhibited higher levels of disc PG content as assessed qualitatively by histological safranin O staining (Fig. 2A) and quantitatively by DMMB assay for total disc GAG content (Fig. 2B). In addition, net loss of disc PGs in Ercc1-/Δ mice could be caused by decreased PG synthesis. We determined PG synthesis by measuring the level of 35S incorporated into the discs of mice ex vivo. Disc PG synthesis from Ercc1-/Δp65+/- mice (9.1 ± 1.7 fmoles sulfate/ng DNA) was 30% higher than that from Ercc1-/Δ mice (6.1 ± 1.7 fmoles sulfate/ng DNA), but was still about 30% lower than that from WT mice (12.4 ± 1.1 fmoles sulfate/ng DNA) (Fig. 2B). Thus, genetic reduction of NF-κB mitigated PG loss in progeroid Ercc1-/Δ mice in part through enhancing PG matrix synthesis.
Figure 2. Genetic depletion of the p65 NF-κB subunit mitigates age-associated disc proteoglycan loss and histopathologic changes.
A, Safranin O/fast green histologic staining of disc sections. Endplate (EP), nucleus pulposus (NP), and annulus fibrosus (AF) are indicated. Red, safranin O staining of proteoglycan. B, DMMB assay for total GAG of NP tissue (top graph) and proteoglycan synthesis as measured by 35S incorporation by disc organotypic culture (bottom graph). # denotes p < 0.05.
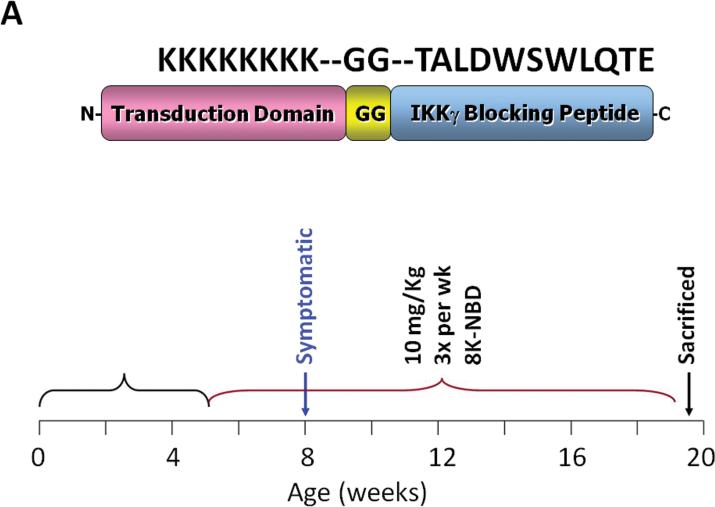
8K-NBD inhibition of IKK/NF-κB attenuates disc histopathological changes and PG loss associated with aging in progeroid Ercc1-/Δ mice
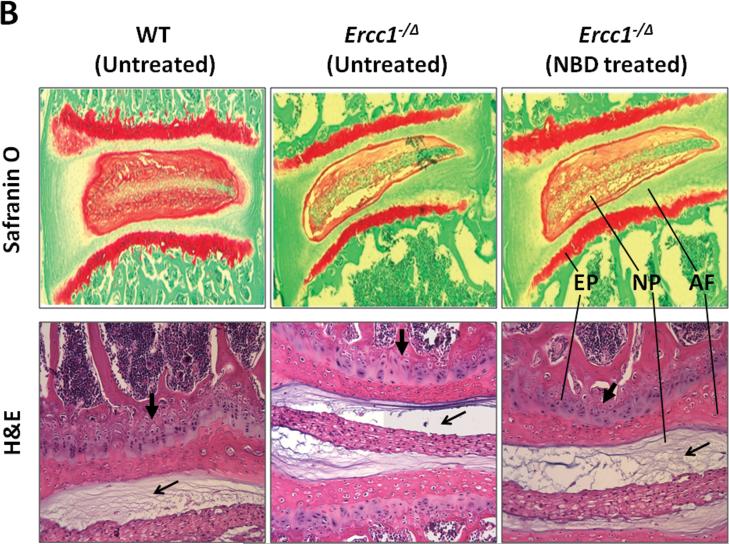
To independently confirm the beneficial effects of reducing NF-κB activity by genetic depletion, we treated Ercc1-/Δ mice using the 8K-NBD peptide that reduces NF-κB activation by inhibiting the formation of the IKK protein complex (Fig. 3). The 8K-NBD peptide, consisting of an 8 lysine protein transduction domain 34 fused to the 11 amino acid 8K-NBD peptide, was shown previously to have therapeutic efficacies in animal models of inflammatory bowel disease, muscular dystrophy, and arthritis 14, 35, 36. Ercc1-/Δ mice at 5 weeks of age, were either untreated or treated with 10 mg/kg of 8K-NBD three times per week intraperitoneally until the age of 18-20 weeks (Fig. 4A). This dose and treatment regiment was selected because it was previously shown to be therapeutic in mouse models of muscular dystrophy14 and inflammatory bowel disease 36. Compared to untreated control, 8K-NBD-treated mice showed noticeable improvement in nucleus pulposus (NP) matrix proteoglycan content by safranin O histological staining (Fig. 4B). 8K-NBD treatment also resulted in increased cellularity in the endplate and denser matrix network within the NP as assessed by H&E staining (Fig. 4B).
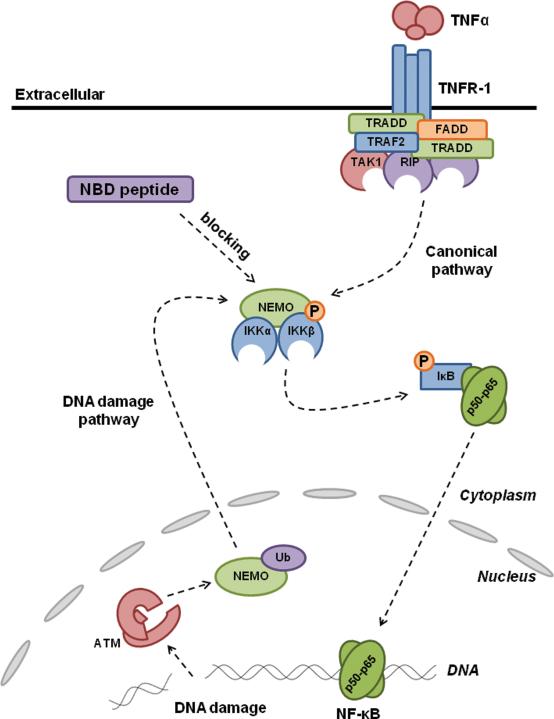
Figure 3. Inhibition of NF-κB activation by the NBD peptide.
NF-κB activation: Pro-inflammatory stress (canonical pathway) or stress from unrepaired DNA damage (non-canonical pathway) induces the formation of the IKK complex which leads to the phosphorylation of IκB and its subsequent degradation. NF-κB is released and translocated into the nucleus to activate transcription of its target genes. NBD inhibition of NF-κB: NBD binds to the NEMO protein (also known as IKKγ) and prevents it from associating with the α and β subunits. This prevents the formation of the IKK complex and hence NF-κB activation.
Figure 4. Pharmacologic suppression of IKK/NF-κB activation ameliorates age-associated disc proteoglycan loss and histopathologic changes.
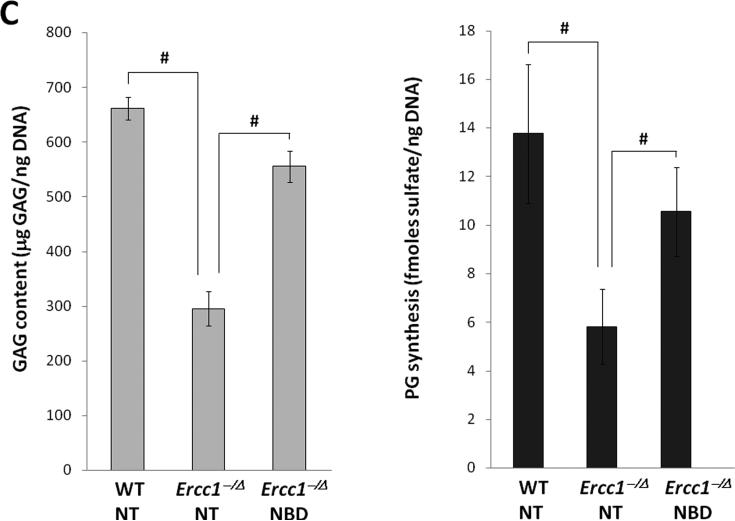
A, 8K-NBD peptide structure and amino acid sequence (top) and 8K-NBD treatment regimen (bottom). Ercc1-/Δ mice were treated with 10 mg 8K-NBD per kg body weight three times per week intraperitoneally starting from 5 wks of age until 20 wks of age. B, Safranin O and H&E histological staining of disc sections of 8K-NBD-treated Ercc1-/Δ mice and untreated Ercc1-/Δ mice and WT control. Endplate (EP), nucleus pulposus (NP), and annulus fibrosus (AF) are indicated. Red, safranin O staining of proteoglycan. EP cellularity (thick arrow) and NP matrix density (thin arrow) are indicated. C, DMMB assay for total GAG of NP tissue (left graph) and proteoglycan synthesis as measured by 35S incorporation by disc organotypic culture (right graph). NT, not treated. # denotes p < 0.05.
To confirm the therapeutic effects of 8K-NBD, we also measured disc matrix PG content using the quantitative DMMB assay for total GAG. Total GAG of NP tissue of 8K-NBD treated Ercc1-/Δ mice (555 ± 21 μg GAG/ng DNA) was greater than that of untreated Ercc1-/Δ controls (295 ± 31 μg GAG/ng DNA) but was still lower than that of WT mice (662 ± 29 μg GAG/ng DNA) (Fig. 4C, left graph). Disc PG synthesis from 8K-NBD treated Ercc1-/Δ mice (11 ± 2 fmoles sulfate/ng DNA) also showed a substantial increase over that of the untreated Ercc1-/Δ mice (6 ± 2 fmoles sulfate/ng DNA) (Fig. 4C, right graph). Thus consistent with the effects observed using the NF-κB genetic depletion approach, 8K-NBD inhibition of NF-κB also appeared to mitigate PG loss in progeroid Ercc1-/Δ mice in part through enhancing PG matrix synthesis.
DISCUSSION
Increasing evidence implicates chronic activation of NF-κB as a major mediator of many age-associated degenerative diseases. In this report, we used a mouse strain harboring a NF-κBeGFP reporter to demonstrate that there is a significant increase in the percent of cells in the disc with activated NF-κB in aged mice. Moreover, in addition to demonstrating that NF-κB is upregulated in the disc with aging, we demonstrate that activation of NF-κB plays an important role in age-associated disc degeneration. Systemic inhibition of NF-κB activation by chronic administration of the 8K-NBD peptide in Ercc1-/Δ mice mitigated age-related IDD, including loss of disc matrix proteoglycan and cellularity. This result was confirmed genetically by crossing Ercc1-/Δ mice into a p65 haploinsufficient background to reduce NF-κB, thus demonstrating that the therapeutic effect of 8K-NBD on disc health is a result of direct inhibition of NF-κB pathway and not other nonspecific targets. These results extend our previous study demonstrating that genetic depletion of one allele of the p65 subunit of NF-κB or pharmacologic inhibition of NF-κB attenuated numerous age-related pathologies in Ercc1-/Δ mice including impaired gait, epidermal atrophy, sarcopenia, anemia, liver and kidney dysfunction, incontinence and trembling 28.
Previous studies reported evidence of increased NF-κB activation in aged and degenerated human disc 24. Elevated levels of NF-κB target genes, including the pro-inflammatory cytokines TNF-α, IL-1β, IL-6 a n d I L-8, have been documented in human degenerative discs 21-23, 37, 38. Immunomorphological analysis revealed a higher level of carboxymethyl-lysine (CML), a biomarker of oxidized protein, in degenerated intervertebral discs from aged patients compared to young normal discs 24. Age-associated oxidative damage in disc is also evident by the accumulation of advanced glycation end products (AGEs), produced by nonenzymatic glucosylation and oxidation of proteins and lipids 39, 40. These findings suggest that cells in aged discs are exposed to oxidative stress, a known activator of the NF-κB pathway.
Our study also indicates enhanced disc NF-κB activity in mice with age. This is consistent with the idea that NF-κB is activated in response to cellular stress and damage, including DNA damage, which stochastically accumulate with age 11, 41. In the case of Ercc1-/Δ mice, which age rapidly due to a DNA repair deficiency, accumulation of DNA damage is likely the source that triggers NF-κB activation in disc tissue. Indeed, in a separate study we have demonstrated through immuno and biochemical analyses that NF-κB is activated in a variety of tissue types in the ERCC1-deficient mouse model of accelerated aging, including liver, kidney and muscle 28, as well as in tissues of naturally aged mice including liver, muscle, gastrointestinal tract, and bone marrow (Clauson et al, in preparation). Together, these observations strongly suggest that during the aging process the NF-κB pathway plays a vital role in mediating the effects of various stressors on the changes in cellular transcription program in disc tissue leading to the development of age-related IDD.
Aging causes many degenerative changes to intervertebral discs, particularly loss of disc proteoglycan matrix which is highly detrimental as this is typically accompanied by a concomitant decrease in disc hydration and reduced ability of the tissue to resist compression 42, 43. Our study shows that by decreasing NF-κB activity in Ercc1-/Δ mice, PG synthesis was improved and PG loss was alleviated. Ercc1-/Δ mice harbor a high level of senescent cells in the disc 5. Senescent chondrocytes also have been reported to have compromised capacity to synthesize new matrix 44. Thus the decline in disc PG synthesis in Ercc1-/Δ mice could be due to increased cellular senescence. Since cell senescence is a well-demonstrated consequence of cellular response to DNA damage 41, 45, our results suggest that the accumulation of DNA damage in the DNA repair-deficient Ercc1-/Δ mice promotes cellular senescence in their intervertebral discs through the mediatory action of NF-κB.
It should be noted that neither our pharmacologic (8K-NBD treatment) nor genetic (p65+/-) intervention completely revert the age-related disc degenerative changes in Ercc1-/Δ mice to the normal disc phenotype observed in their control littermates. This is not unexpected because disc aging process is complex and likely involves other molecular pathways besides the NF-κB signaling pathways. Moreover, it is not possible to attribute how much of the observed therapeutic effects in our study are the direct effects of NF-κB inhibition in disc tissue. Alternatively, it is also possible that the therapeutic effects on discs are also due to the systemic response to NF-κB inhibition which, as we have shown in a separate study, delays the onset of many other age-related pathologies in ERCC1-deficient mice and improves the overall health of these animals 28.
In summary, our findings suggest that DNA damage promotes age-dependent intervertebral disc degeneration and aging and that NF-κB activation mediates both natural and accelerated disc aging and age-related disc diseases. This study also suggests that the IKK/NF-κB signaling pathway is a potential therapeutic target for treating age-related disc degenerative disorders.
KEY POINTS.
■ NF-κB activity is elevated in aged and degenerated disc tissue of naturally aging mice and accelerated aging Ercc1-/Δ mice.
■ Reduction of NF-κB activity in accelerated aging Ercc1-/Δ mice either by genetic depletion of an allele of the NF-κB subunit p65 or by pharmacologic inhibition using the Nemo Binding Domain (8K-NBD) peptide to block the IκB Inducible Kinase mitigates loss of disc matrix proteoglycan.
■ NF-κB-mediated signal transduction pathway plays an important role in age-related IDD and represents as a potential therapeutic target for treating age-dependent disc pathologies.
Acknowledgements
The authors thank Douglas Bentley, Lauren Taylor, William Witt, and SoRa Choe for assistance with histological and gene expression analyses. We thank Dr. Denis Guttridge for providing the PCR primers to assess expression of NF-κB responsive genes and for the p65+/- mice.
The device(s)/drug(s) that is/are the subject of the manuscript is/are not intended for human use. This work was supported in part by the Albert B. Ferguson, Jr. M.D. Orthopaedic Fund of the Pittsburgh Foundation, Italian Society of Spine Surgery Grant, and NIH grants. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Livshits G, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung KM. The relationship between disc degeneration, low back pain, and human pain genetics. Spine J. 10:958–60. doi: 10.1016/j.spinee.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Spine: Low Back and Neck Pain. The Burden of Musculoskeletal Diseases in the United States. National Center for Health Statistics: National Health and Nutrition Examination Survey Data, 1998-2004. 2011 [Google Scholar]
- 4.Miller JA, Schmatz C, Schultz AB. Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988;13:173–8. [PubMed] [Google Scholar]
- 5.Vo N, et al. Accelerated aging of intervertebral discs in a mouse model of progeria. J Orthop Res. 28:1600–7. doi: 10.1002/jor.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am 88 Suppl. 2006;2:10–4. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 7.Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247–61. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 2004;29:2691–9. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 9.Cs-Szabo G, et al. Changes in mRNA and protein levels of proteoglycans of the anulus fibrosus and nucleus pulposus during intervertebral disc degeneration. Spine. 2002;27:2212–9. doi: 10.1097/00007632-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 10.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–20. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–80. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 13.Furia B, et al. Enhancement of nuclear factor-kappa B acetylation by coactivator p300 and HIV-1 Tat proteins. J Biol Chem. 2002;277:4973–80. doi: 10.1074/jbc.M107848200. [DOI] [PubMed] [Google Scholar]
- 14.Acharyya S, et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berenbaum F. Signaling transduction: target in osteoarthritis. Curr Opin Rheumatol. 2004;16:616–22. doi: 10.1097/01.bor.0000133663.37352.4a. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, et al. Antioxidant alpha-lipoic acid inhibits osteoclast differentiation by reducing nuclear factor-kappaB DNA binding and prevents in vivo bone resorption induced by receptor activator of nuclear factor-kappaB ligand and tumor necrosis factor-alpha. Free Radic Biol Med. 2006;40:1483–93. doi: 10.1016/j.freeradbiomed.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 17.Helenius M, Hanninen M, Lehtinen SK, Salminen A. Aging-induced up-regulation of nuclear binding activities of oxidative stress responsive NF-kB transcription factor in mouse cardiac muscle. J Mol Cell Cardiol. 1996;28:487–98. doi: 10.1006/jmcc.1996.0045. [DOI] [PubMed] [Google Scholar]
- 18.Xiao ZQ, Majumdar AP. Induction of transcriptional activity of AP-1 and NF-kappaB in the gastric mucosa during aging. Am J Physiol Gastrointest Liver Physiol. 2000;278:G855–65. doi: 10.1152/ajpgi.2000.278.6.G855. [DOI] [PubMed] [Google Scholar]
- 19.Korhonen P, Helenius M, Salminen A. Age-related changes in the regulation of transcription factor NF-kappa B in rat brain. Neurosci Lett. 1997;225:61–4. doi: 10.1016/s0304-3940(97)00190-0. [DOI] [PubMed] [Google Scholar]
- 20.Adler A, Sinha S, Kawahara TLA, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-kB activity. Genes Dev. 2007;21 doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachmeier BE, et al. Analysis of tissue distribution of TNF-alpha, TNF-alpha-receptors, and the activating TNF-alpha-converting enzyme suggests activation of the TNF-alpha system in the aging intervertebral disc. Ann N Y Acad Sci. 2007;1096:44–54. doi: 10.1196/annals.1397.069. [DOI] [PubMed] [Google Scholar]
- 22.Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008;47:809–14. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- 23.Burke JG, et al. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 24.Nerlich AG, et al. Immunomorphological analysis of RAGE receptor expression and NF-kappaB activation in tissue samples from normal and degenerated intervertebral discs of various ages. Ann N Y Acad Sci. 2007;1096:239–48. doi: 10.1196/annals.1397.090. [DOI] [PubMed] [Google Scholar]
- 25.Akeda K, et al. ISSLS. 2005.
- 26.Ahmad A, et al. ERCC1-XPF endonuclease facilitates DNA double-strand break repair. Mol Cell Biol. 2008;28:5082–92. doi: 10.1128/MCB.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karrasch T, Kim JS, Muhlbauer M, Magness ST, Jobin C. Gnotobiotic IL-10-/-;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol. 2007;178:6522–32. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- 28.Tilstra JS, et al. Inhibition of IKK/NF-κB delays the onset of age-related degenerative diseases in a mouse model of accelerated aging. Science Translational Medicine. 2011 In Review. [Google Scholar]
- 29.Mitala CM, et al. Impact of microdialysis probes on vasculature and dopamine in the rat striatum: a combined fluorescence and voltammetric study. J Neurosci Methods. 2008;174:177–85. doi: 10.1016/j.jneumeth.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, et al. Bupivacaine decreases cell viability and matrix protein synthesis in an intervertebral disc organ model system. Spine J. 11:139–46. doi: 10.1016/j.spinee.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbertson L, et al. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. Spine J. 2008;8:449–56. doi: 10.1016/j.spinee.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Sobajima S, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine. 2005;30:15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 34.Tilstra J, et al. Protein transduction: identification, characterization and optimization. Biochem Soc Trans. 2007;35:811–5. doi: 10.1042/BST0350811. [DOI] [PubMed] [Google Scholar]
- 35.Dai S, Hirayama T, Abbas S, Abu-Amer Y. The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks osteoclastogenesis and bone erosion in inflammatory arthritis. J Biol Chem. 2004;279:37219–22. doi: 10.1074/jbc.C400258200. [DOI] [PubMed] [Google Scholar]
- 36.Dave SH, et al. Amelioration of chronic murine colitis by peptide-mediated transduction of the IkappaB kinase inhibitor NEMO binding domain peptide. J Immunol. 2007;179:7852–9. doi: 10.4049/jimmunol.179.11.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–45. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulrich JA, Liebenberg EC, Thuillier DU, Lotz JC. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976) 2007;32:2812–9. doi: 10.1097/BRS.0b013e31815b9850. [DOI] [PubMed] [Google Scholar]
- 39.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(Pt 1):345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sivan SS, et al. Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem J. 2006;399:29–35. doi: 10.1042/BJ20060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niedernhofer LJ, Robbins PD. Signaling mechanisms involved in the response to genotoxic stress and regulating lifespan. Int J Biochem Cell Biol. 2008;40:176–80. doi: 10.1016/j.biocel.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cell Mater. 2006;12:92–101. doi: 10.22203/ecm.v012a11. [DOI] [PubMed] [Google Scholar]
- 43.Singh K, Masuda K, Thonar EJ, An HS, Cs-Szabo G. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine (Phila Pa 1976) 2009;34:10–6. doi: 10.1097/BRS.0b013e31818e5ddd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin JA, Buckwalter JA. Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa Orthop J. 2001;21:1–7. [PMC free article] [PubMed] [Google Scholar]
- 45.Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7:979–87. doi: 10.1038/nrd2656. [DOI] [PubMed] [Google Scholar]