Abstract
Objectives. We evaluated a community-based, translational lifestyle program to reduce diabetes risk in lower–socioeconomic status (SES) and ethnic minority adults.
Methods. Through an academic–public health department partnership, community-dwelling adults at risk for diabetes were randomly assigned to individualized lifestyle counseling delivered primarily via telephone by health department counselors or a wait-list control group. Primary outcomes (6 and 12 months) were fasting glucose level, triglycerides, high- and low-density lipoprotein cholesterol, weight, waist circumference, and systolic blood pressure. Secondary outcomes included diet, physical activity, and health-related quality of life.
Results. Of the 230 participants, study retention was 92%. The 6-month group differences for weight and triglycerides were significant. The intervention group lost 2 pounds more than did the control group (P = .03) and had decreased triglyceride levels (difference in change, 23 mg/dL; P = .02). At 6 months, the intervention group consumed 7.7 fewer grams per day of fat (P = .05) and more fruits and vegetables (P = .02) than did control participants.
Conclusions. Despite challenges designing effective translational interventions for lower-SES and minority communities, this program modestly improved some diabetes risk factors. Thus, individualized, telephone-based models may be a promising alternative to group-based interventions.
The prevalence of type 2 diabetes continues to rise at an alarming rate in the United States. Approximately 25.6 million adults (11.3% of the US population aged 20 years or older) have diabetes, and another estimated 79 million have prediabetes.1 Greater risk of diabetes is observed for ethnic minority1–5 and lower–socioeconomic status (SES) groups6 compared with White adults of similar ages.
Several clinical trials have tested intensive lifestyle interventions or pharmacological agents in preventing or delaying type 2 diabetes in adults at risk.7–9 These trials showed impressive diabetes risk reductions for lifestyle interventions associated with relatively modest amounts of weight loss and exercise.7–9 Translating this knowledge into lifestyle interventions delivered in real-world settings is thus a major priority.10–12
To reduce observed disparities in risk of diabetes, translational studies need to be community-based and designed for lower-SES and ethnic minority populations. Although many translational lifestyle interventions are available, most were designed for clinical settings;13–21 only a few are offered in community settings.22–26 Of community-based translations, only 3 were designed specifically for lower-SES or minority populations,23–25 and only 1 of these—Project HEED, or Help Educate to Eliminate Diabetes—was evaluated with a randomized controlled trial design.23 HEED was successful in obtaining significant group differences in weight loss at 12 months, but no other significant clinical or behavioral changes were observed.
We conducted a randomized controlled trial of a low-intensity lifestyle intervention for lower-SES, ethnic minority, Spanish- and English-speaking adults. This was a collaborative project between the University of California, San Francisco, and the City of Berkeley Division of Public Health. Public health departments are a good venue for community-based translations to reduce disparities because they serve vulnerable populations most at risk for chronic disease and engage in chronic disease prevention.
METHODS
This 12-month randomized controlled trial compared a lifestyle intervention group with a wait-list control group. After completing the trial, control group participants were offered the lifestyle program. We examined program effectiveness at 6 months (active intervention phase) and 12 months (after maintenance phase) to improve clinical diabetes risk factors, behavioral risk factors, and health-related quality of life.
Study Participants
We focused on community-dwelling adults in 4 distinct low-income neighborhoods in northern California cities: Berkeley, Oakland, and Richmond. Recruitment began with community-based, educational outreach to identify individuals at risk for diabetes.27 Individuals completed a brief self-administered diabetes risk appraisal assessing age, race/ethnicity, history of high blood pressure, abnormal cholesterol level, gestational diabetes, family history of diabetes, regular exercise, and body mass index (BMI; defined as weight in kilograms divided by height in meters squared). The diabetes risk appraisal, adapted from existing diabetes risk tools for use in community settings, used only self-reported variables and a simplified scoring system. Staff scored the diabetes risk appraisal and explained the risk score (0–3 points = low, 4–8 points = moderate, ≥ 9 points = high). Individuals with a moderate or high score (> 4) were educated about their diabetes risk and invited to complete an 8-hour fasting finger-stick test (Accu-chek; Roche Diagnostics, Indianapolis, IN) to determine fasting capillary glucose level.
Individuals who had a capillary blood glucose value between 106 and 160 milligrams per deciliter, who had a moderate to high diabetes risk appraisal score, and who were aged 25 years or older were told about the lifestyle program and study as well as the research process and screened for exclusion criteria. We excluded individuals with diabetes (physician diagnosis, use of insulin or other diabetes medications); diagnosis in past 6 months of myocardial infarction, congestive heart failure, or stroke; heart procedure or heart surgery in past 6 months; implanted defibrillator; hip or knee replacement in past 3 months; insufficient cognitive functioning; and pregnancy. We also excluded individuals not conversant in English or Spanish, with plans to move out of the area within 1 year, and whose spouse or partner had already enrolled. We required physician consent for those with a pacemaker, heart disease, heart rhythm abnormalities, or atrial fibrillation, as well as chest pain or faintness or dizziness in the past 6 months.
Live Well, Be Well Intervention
The lifestyle program28 was designed for lower-SES, minority, and low-literacy adults and adapted from several interventions with established efficacy. It was delivered in Spanish and English and consisted of a 6-month active intervention phase and a 6-month maintenance phase. Trained health department counselors provided education and skills training to modify diet and physical activity through primarily telephone-based counseling (12 calls) with 2 in-person sessions and 5 optional group workshops. In-person and group sessions were held in neighborhood settings. Self-selected and attainable goal-setting and action plans were emphasized to enhance self-efficacy. Motivational interviewing techniques to develop and enhance participants’ motivation were used during the telephone calls. All program materials are available on the Live Well, Be Well Web site (http://iha.ucsf.edu/LiveWellBeWell).
Participation in each program component was tracked. The program consisted of 19 possible “contacts” for a total of 15 possible hours: 1 introductory session, which included a program binder; 1 in-person planning session; 12 telephone counseling calls (10 in active phase, 2 in maintenance phase); and 5 group workshops. Participation was calculated as the total number of contacts received. Minimum compliance was defined as completion of the introductory session, the planning session, and at least 8 telephone calls over the active phase.
We assessed all outcomes at baseline with 6- and 12-month follow-ups. Trained, bilingual research assistants administered questionnaires and performed clinical and anthropometric measurements with standardized protocols. At baseline, we obtained demographic information and a brief medical history. All questionnaires were translated into Spanish.
We selected 7 primary clinical and anthropometric outcomes related to diabetes risk. Laboratory measures included fasting serum glucose, triglyceride, and low- and high-density lipoprotein (LDL and HDL) cholesterol levels measured by enzymatic calorimetric methods (Quest Diagnostics, San Jose, CA). Weight in pounds was measured on a digital scale (Detecto Balance Beam Scale; Cardinal Scale Manufacturing Co, Webb City, MO). We measured waist circumference with a Gullick II (FitnessMart; Country Technology, Inc, Gays Mills, WI) tape spring-tension measure at the site of maximum circumference midway between the lower ribs and the anterior superior iliac spine (mean of 2 measurements). Systolic blood pressure was measured with an Omron (Omron Healthcare, Inc, Lake Forest, IL) automated blood pressure monitor after sitting for 5 minutes (mean of 2 measurements). Participants provided additional consent for serum banking at baseline and 12 months for metabolic biomarker assays. We measured serum fasting insulin level by radioimmunoassay (Millipore, St. Charles, MO) and calculated homeostasis model assessment-insulin resistance (HOMA-IR).29
Secondary behavioral risk factor outcomes targeted diet and physical activity. We assessed dietary intake with the Modified Block Food Frequency Questionnaire available in English and Spanish.30–32 Questionnaires were scored by NutritionQuest (Berkeley, CA). We used 4 measures targeted specifically in the Live Well, Be Well program: (1) total kilocalories per day; (2) total fat per day; (3) total fiber per day; and (4) daily frequency of consumption of fruits, fruit juices, and vegetables. Missing values were assigned to daily energy intake values that did not fall within the range of 500 to 5000 kilocalories per day (9 instances). Physical activity was measured with the CHAMPS Physical Activity Questionnaire.33 Because Live Well, Be Well emphasized increased participation in all physical activities, especially walking, we used 3 measures: (1) hours per week in any physical activity, (2) metabolic equivalent hours per week in any physical activity, and (3) hours per week walking.
We measured 5 secondary health-related quality-of-life outcomes hypothesized to improve with lifestyle changes. Participants rated their overall health from “poor” to “excellent” on a scale from 1 to 5. Three Medical Outcomes Study measures were (internal consistency reliability in parentheses) the Psychological Distress II and Psychological Well-Being II indexes (0.90 and 0.78, respectively)34 and the Sleep Problems Index (0.77).35 We used the Perceived Stress Scale (0.86) to assess stress.36
Sample size estimates assumed equal allocation to experimental groups, correlation between repeated outcomes equaling 0.75, unit-standardized outcomes, 90% retention at 12 months, 80% power, and 2-tailed α equal to .05. Modeled outcomes included difference scores computed by subtracting outcome values assessed at baseline from each corresponding follow-up value. Given the study sample size of 230, the minimum detectable effect corresponded to a group difference in the effect of time equal to 0.28 SDs. This is generally considered a small to small-to-medium effect, suggesting good power.
Eligible participants were stratified by self-reported race/ethnicity (African American, Latino, other) and age categories (25–39, 40–64, ≥ 65 years). We generated stratum-specific sequential identification numbers to randomly allocate individuals to experimental groups in blocks of 4. Study staff was blinded to the linkage between identification numbers and group assignment. At the end of the baseline visit, the participant opened a sealed, opaque envelope preprinted with the sequential identification number to determine the experimental group assignment. Because of the behavioral nature of the intervention, counselors administering the intervention and participants were not blind to group assignment.
Statistical Methods
A randomization check compared experimental groups at baseline via χ2 and t tests. Intention-to-treat linear regressions modeled repeated change scores: outcomes assessed at each follow-up (6 or 12 months) minus the corresponding baseline value. All models included effects of experimental groups, assessment time, and the groups-by-time interaction. Initially, an unstructured residual covariance structure was specified for the model of each outcome, which was subsequently relaxed in 2 steps to consider separate covariance structures for (1) each experimental group and (2) each combination of experimental group and participant sex. The final residual covariance structure was chosen by reference to deviance statistics. The likelihood-based approach to model estimation allowed models to be fit to all available data and invoked the assumption that missing values occurred randomly, conditional on observed values.37
RESULTS
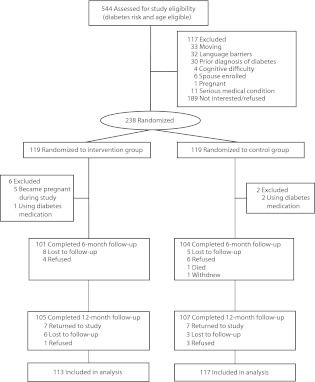
We randomly assigned 238 individuals to an intervention or a control group between July 2006 and July 2008 (Figure 1) with follow-up assessments from December 2006 through August 2009. Of the 119 individuals assigned to the intervention group, 6 were excluded because of use of diabetes medications at enrollment or for interim pregnancy, leaving 113 for analysis. From the control group, 2 were excluded because of use of diabetes medications, leaving 117 for analysis. Twelve-month study retention was 105 (93%) in the intervention group and 107 (91%) in the control group.
FIGURE 1—
Flow of participants from screening to completion of the final follow-up assessment: Live Well, Be Well program, Berkeley, Oakland, and Richmond, California, July 2006–August 2009.
Baseline characteristics indicated a primarily ethnic minority and lower-SES sample, and the average age was older than 55 years, and 73% were women (Table 1). Almost one third were Spanish-speaking, and 77% were from an ethnic minority group. About 20% had no health insurance, about 40% were employed, and approximately 30% reported financial hardship within the prior year. Educational attainment was diverse; about 38% had a high-school diploma or less education. More than half (55%) the sample were obese, and an additional 32% were overweight.
TABLE 1—
Demographic and Socioeconomic Characteristics of Study Participants: Live Well, Be Well Program, Berkeley, Oakland, and Richmond, California, July 2006–August 2009
| Characteristics | Intervention Group (n = 113), % or Mean (SD) | Control Group (n = 117), % or Mean (SD) | P |
| Female | 73 | 74 | .87 |
| Race/ethnicity | |||
| African American | 23 | 23 | .74 |
| Non-Hispanic White | 22 | 23 | |
| Latino | 35 | 39 | |
| Asian | 18 | 13 | |
| Native American/Pacific Islander | 1 | 1 | |
| Multiethnic/mixed | 2 | 1 | |
| Age, y | 58 (16) | 55 (17) | .29 |
| Language of interview | |||
| English | 72 | 66 | .34 |
| Spanish | 28 | 34 | |
| Immigrant to the United States | 47 | 48 | .93 |
| Of immigrants, speak English | |||
| Not at all/poorly/fairly well | 55 | 68 | .16 |
| Well/very well | 45 | 32 | |
| Educational achievement | |||
| < High school | 21 | 25 | .14 |
| High school/GED | 20 | 11 | |
| Some college/tech | 27 | 22 | |
| ≥ Bachelor's degree | 32 | 42 | |
| Health insurance type | |||
| Any private | 63 | 61 | .97 |
| Public | 15 | 17 | |
| None | 22 | 22 | |
| Employed full- or part-time | 35 | 43 | .22 |
| Financial hardship in past ya | 30 | 32 | .8 |
| Family history of diabetes | 53 | 45 | .24 |
| BMI, kg/m2 | 30.1 (5.3) | 29.9 (6.1) | .78 |
| BMI categoriesb | |||
| Normal | 10 | 17 | .2 |
| Overweight | 31 | 32 | |
| Obese | 59 | 50 | |
| Hypertensionc | 50 | 44 | .36 |
| Arthritisd | 35 | 34 | .85 |
Note. BMI = body mass index; GED = general equivalency diploma.
Reponded yes to “In the past 12 months, was there ever a time when you did not have enough money to meet your daily needs?”
BMI categories defined as follows: for non-Asian participants, normal (< 25.0 kg/m2); overweight (25.0 to ≤ 29.9 kg/m2); and obese (≥ 30.0 kg/m2); for Asian participants, normal (< 23.0 kg/m2); overweight (23.0 ≤ BMI to ≤ 24.9 kg/m2); and obese (≥ 25.0 kg/m2).
Hypertension classified if either systolic > 140 mm Hg or diastolic > 90 mm Hg, or if participant reported using any blood pressure medication.
Responded yes to “Has a health professional ever told you that you had arthritis or other joint problems?”
Approximately 72% of the intervention group participants were minimally compliant. Of 19 possible program components, a mean (SD) of 12.5 (4.9) were completed. Of 12 total possible telephone calls, a mean of 8.9 (3.8) were completed. The average number of workshops attended of 5 was 1.7 (1.5). The mean (SD) hours of contact received was 9.0 (3.3) of a possible 15.
Table 2 presents baseline mean scores and 6- and 12-month change scores for the intervention and control groups and the significance of between-group comparisons for the coprimary clinical outcomes. No baseline group differences were seen in these measures.
TABLE 2—
Effect of Intervention and Control Groups on Changes From Baseline in Clinical Outcomes: Live Well, Be Well Program, Berkeley, Oakland, and Richmond, California, July 2006 to August 2009
| Intervention Group
(n = 113) |
Control Group
(n = 117) |
Between-Group Comparison of
Change |
||||||
| Outcomes | Baseline, Mean (SE) | 6-Mo Within-Group Change, Mean (SE) | 12-Mo Within-Group Change, Mean (SE) | Baseline, Mean (SE) | 6-Mo Within-Group Change, Mean (SE) | 12-Mo Within-Group Change, Mean (SE) | 6 Mo, P | 12 Mo, P |
| Weight, lb | 177.85 (3.68) | −2.30*** (0.66) | −1.34 (0.71) | 176.45 (3.68) | −0.44 (0.57) | −0.42 (0.84) | .03 | .4 |
| Waist, cm | 100.56 (1.34) | −0.56 (0.44) | −0.06 (0.44) | 99.30 (1.32) | 0.50 (0.53) | −0.15 (0.48) | .13 | .89 |
| Systolic blood pressure, mm Hg | 126.90 (1.74) | 0.73 (1.39) | 0.34 (1.38) | 127.58 (1.98) | −1.17 (1.32) | 0.27 (1.61) | .32 | .98 |
| Fasting glucose, mg/dL | 93.82 (1.05) | −0.70 (0.87) | −0.88 (1.02) | 93.50 (1.14) | 0.42 (1.04) | −1.39 (0.96) | .41 | .72 |
| Triglycerides, mg/dL | 148.26 (10.71) | −8.76 (7.66) | −1.57 (6.83) | 128.13 (8.56) | 14.39* (6.35) | 4.87 (4.99) | .02 | .45 |
| Low-density lipoprotein, mg/dL | 112.00 (2.95) | −6.62*** (1.84) | −5.78* (2.34) | 114.76 (2.99) | −2.39 (2.07) | −3.61 (2.17) | .13 | .5 |
| High-density lipoprotein, mg/dL | 53.05 (1.56) | 1.76* (0.72) | 3.19*** (0.88) | 54.69 (1.56) | 0.61 (0.71) | 1.69* (0.80) | .26 | .21 |
Note. Each model estimated unstructured residual covariances separately within each combination of experimental groups and respondent sex.
*P < .05; ***P < .001.
Group differences in 6-month change for weight and triglycerides were significant. The intervention group lost 1.9 pounds more than did the control group (P = .03), and a net difference in change in triglyceride levels was found between groups (a difference of about 23 mg/dL favoring the intervention group; P = .02). No significant group differences were observed for other clinical outcomes. However, 2 significant within-group changes were observed in the intervention group: LDL decreased at 6 months (P < .001) and 12 months (P < .05), and HDL increased at 6 months (P < .05) and 12 months (P < .001); HDL also increased in the control group at 12 months (P < .05).
Approximately half of the study participants consented to optional blood banking for the insulin assays. Among those with insulin data, the baseline HOMA-IR among the intervention group was 1.18 ±0.55 and among the control participants was 1.29 ±0.70. The 12-month HOMA-IR in the intervention group was 1.21 ±0.54 and in the control group was 1.34 ±0.63 with no difference between groups.
Table 3 presents baseline mean scores and 6- and 12-month change scores for the intervention and control groups, as well as between-group comparisons, for the secondary behavioral and health-related quality-of-life outcomes. No baseline group differences were found.
TABLE 3—
Effect of Intervention and Control Group on Changes From Baseline in Behavioral and Health-Related Quality-of-Life Outcomes: Live Well, Be Well Program, Berkeley, Oakland, and Richmond, California, July 2006 to August 2009
| Intervention Group
(n = 113) |
Control Group
(n = 117) |
Between-Group Comparison of
Change |
||||||
| Outcomes | Baseline, Mean (SE) | 6-Mo Within-Group Change, Mean (SE) | 12-Mo Within-Group Change, Mean (SE) | Baseline, Mean (SE) | 6-Mo Within-Group Change, Mean (SE) | 12-Mo Within-Group Change, Mean (SE) | 6 Mo, P | 12 Mo, P |
| Total calories, kcal/da | 1870.51 (78.12) | −264.29*** (50.57) | −301.57*** (64.69) | 1915.12 (81.01) | −216.59** (69.23) | −245.88*** (52.68) | .58 | .51 |
| Total fat, g/da | 71.49 (3.58) | −12.95*** (2.41) | −14.35*** (2.89) | 67.93 (3.11) | −5.28 (3.13) | −7.81** (2.39) | .05 | .08 |
| Total dietary fiber, g/db | 17.84 (0.90) | −1.13 (0.70) | −1.97* (0.77) | 19.73 (1.07) | −1.30 (0.82) | −1.79** (0.66) | .88 | .86 |
| Daily frequency fruits and vegetablesc | 3.01 (0.15) | 0.25 (0.16) | 0.12 (0.14) | 3.12 (0.16) | −0.26 (0.15) | −0.30* (0.14) | .02 | .04 |
| Physical activity, h/wka | 7.99 (0.63) | 0.74 (0.62) | 0.68 (0.67) | 7.00 (0.54) | 0.44 (0.61) | 1.07 (0.58) | .73 | .66 |
| Physical activity, metabolic equivalent, h/wka | 25.59 (2.07) | 3.00 (2.20) | 2.24 (2.11) | 23.62 (2.16) | 0.36 (1.97) | 6.37* (2.77) | .37 | .24 |
| Walking, h/wkc | 4.40 (0.38) | 0.37 (0.41) | 0.57 (0.46) | 3.90 (0.36) | 0.31 (0.40) | 0.55 (0.46) | .92 | .97 |
| Self-rated healthb | 3.09 (0.08) | 0.31*** (0.07) | 0.13 (0.07) | 2.92 (0.08) | 0.07 (0.10) | 0.11 (0.09) | .05 | .84 |
| Perceived stressc | 2.26 (0.07) | −0.01 (0.06) | 0.01 (0.06) | 2.37 (0.07) | 0.03 (0.06) | 0.06 (0.06) | .6 | .56 |
| Sleep problemsb | 2.15 (0.07) | 0.06 (0.06) | 0.01 (0.06) | 2.13 (0.07) | 0.07 (0.06) | 0.17** (0.06) | .91 | .05 |
| Psychological well-beingc | 3.74 (0.07) | 0.01 (0.05) | 0.07 (0.06) | 3.75 (0.07) | −0.14* (0.05) | −0.09 (0.06) | .05 | .04 |
| Psychological distressc | 2.09 (0.07) | −0.06 (0.05) | −0.11* (0.05) | 2.21 (0.06) | −0.04 (0.05) | −0.00 (0.05) | .75 | .17 |
Residual covariance unstructured within each combination of experimental groups and respondent sex.
Residual covariance unstructured within each intervention group.
Simple unstructured residual covariance.
*P < .05; **P < .01; ***P < .001.
The intervention group consumed 7.7 fewer grams per day of fat at 6 months (P = .05). Intervention group members reported more frequent consumption of fruits and vegetables than did the control group at 6 months (P = .02) and 12 months (P = .04). No significant group differences were observed in total calories, dietary fiber, or physical activity. However, some within-group changes were significant: within both groups at 6 months, total consumed calories decreased by 264 kilocalories per day for the intervention group (P < .001) and 217 kilocalories per day for the control group (P < .01). Total fiber intake decreased in both groups at 12 months (P < .05 for the intervention group and P < .01 for the control group). Within the control group, metabolic equivalent hours per week in physical activity increased at 12 months (P < .05).
The intervention group had better psychological well-being than did the control group at 6 months (P = .05) and 12 months (P = .04). The intervention group also had greater improvement in self-rated health at 6 months (P = .05) and fewer reports of sleep problems at 12 months (P = .05). There were no group differences in perceived stress or psychological distress, but there was a within-group change: psychological distress was reduced at 12 months in the intervention group (P = .05).
DISCUSSION
In this community-based translational trial of a low-intensity, individually tailored, telephone-based lifestyle intervention in at-risk, lower-SES, ethnic minority adults, we had excellent study retention and program compliance and achieved small improvements in several clinical and behavioral risk factors. The intervention group had significantly more weight loss than did the control group, and the net difference in triglycerides, dietary fat intake, and daily fruit and vegetable consumption favored the intervention group after 6 months of intervention.
The weight loss difference of 1.9 pounds between groups at 6 months was small but statistically significant. Nevertheless, it may be clinically relevant because weight loss was the main predictor of reduced diabetes incidence in the Diabetes Prevention Program: every kilogram of weight loss was associated with a 16% reduction in risk for diabetes.38 Additionally, the intervention group had decreased triglycerides, whereas the control group had increased triglycerides from baseline at both time points; hence we observed a large group difference in change in triglycerides. The magnitude of the group difference in triglyceride change (difference of 23.2 mg/dL at 6 months) was comparable to the difference of 17 milligrams per deciliter in the Finnish Diabetes Prevention Study.8 All mean triglyceride levels, however, were lower than the standard 150 milligrams per deciliter threshold value used to designate elevated triglyceride levels.
The reduction in total fat intake (7.7 g/day less for the intervention group at 6 months) was notable because reduction in fat consumption was a strong predictor of lower diabetes risk in the Diabetes Prevention Program.38 We found changes in consumption of fruits and vegetables of 0.6 servings per day at 6 months. The observed effect sizes of these 2 dietary changes were comparable to those found in a systematic review of other physical activity interventions.39 The improvement in self-rated health was notable because self-rated health consistently predicts future health.40–42
No significant group differences were found in fasting glucose or LDL- or HDL-cholesterol levels, waist circumference, and systolic blood pressure. We have 3 possible explanations: (1) some clinical risk factors were in the normal range at baseline; (2) for some factors, although risk reductions were observed in the intervention group, reductions also were seen in the control group; and (3) the intervention may not have been intensive enough to achieve change.
First, our baseline venous fasting glucose level was in the normal range (mean = 94 mg/dL), which may have precluded observing change in this important risk factor. Other clinical risk factors in near-normal range included systolic blood pressure (mean = 127 mm Hg), LDL (112 mg/dL), and HDL (53 mg/dL). These normal values occurred despite efforts to recruit participants at high risk for diabetes based on elevated fasting capillary blood glucose level and self-reported risk factors. This suggests that community-based studies may have more difficulty recruiting people at greatest risk, in contrast to studies in clinical settings where fasting venous blood test screening can be done. Nevertheless, our sample was at moderate to high risk on other risk factors: about 85% were overweight (more than half were obese), 78% of women and 50% of men had elevated waist circumference, 35% fulfilled metabolic syndrome criteria, and almost 50% had a family history of diabetes.
Second, although the intervention group had significant improvements in LDL and HDL cholesterol and a significant reduction in total caloric intake, similar control group improvements precluded observing between-group differences. This may have occurred because diabetes prevention educational materials were part of outreach and recruitment, and control group participants may have changed behavior to some extent without the program. Indeed, at follow-up, 17% of the control group reported having participated in another lifestyle program at some point during the year. In addition, completing the food frequency questionnaire may have raised awareness about diet and portion sizes. Control group improvements have been observed in other diabetes risk reduction interventions. The DEPLOY (Diabetes Education & Prevention with a Lifestyle Intervention Offered at the YMCA) study found significant weight loss in the control group YMCA setting and similarly had provided diabetes education during recruitment.22 Project HEED found significant control group weight loss; qualitative analyses indicated that control group participants believed they benefited from learning that they were at risk and being given information about diabetes.23 Generally, however, such minimal “interventions” are not effective.12
Third, the Live Well, Be Well program may not have been sufficiently intensive to achieve broad changes. This underscores the substantial challenge to design practical, sustainable programs for underserved populations that obtain risk reductions comparable to those of the Diabetes Prevention Program. One recent community-based translation, Healthy-Living Partnerships to Prevent Diabetes (HELP PD), found significant reductions in fasting glucose level and several other clinical risk factors, but it was a high-intensity program offered to relatively well-educated participants with high levels of risk.26 Designing such programs to be more intensive to achieve greater risk reduction might jeopardize the likelihood of adoption and sustainability by community-based organizations. Live Well, Be Well conformed to contemporary recommendations for translating diabetes prevention lifestyle programs—namely, to use individually tailored goals, self-monitoring, counselors, and other participants to provide support and a problem-solving approach to overcome barriers.11,43 Live Well, Be Well also conformed to criteria for an “individually-adapted health behavior change program,” strongly recommended by the Task Force on Community Preventive Services to increase physical activity,44 and behavior change strategies were similar to those in the diabetes prevention trials.12
By designing the Live Well, Be Well program specifically for lower-SES minority groups that are at higher risk for type 2 diabetes than their counterparts,1–6 this study contributes to the small field of community-based translations aimed at reducing health disparities. Although minority groups are often underrepresented in intervention research,45,46 we obtained a high study retention rate (92%). The other translational intervention designed for underserved groups and tested via a randomized controlled trial design, Project HEED, was a 10-week peer-led group-based program offered in community settings to lower-SES Latino and African American overweight adults with prediabetes.23 Participants were thus at higher risk than were those in Live Well, Be Well. Ninety participants were randomly assigned with 73% study retention. Significant differences in weight loss were reported (at 12 months, the intervention group lost 7.2 pounds compared with 2.4 pounds in the control group; P < .01); however, they found no other clinical or behavioral changes. Their results are notable because the program was less intensive than Live Well, Be Well and was delivered by trained peer educators.
Live Well, Be Well is the only community-based translation that used an individually tailored, primarily telephone-based model rather than a group-based model. The use of telephone counseling and neighborhood settings for in-person sessions made it convenient to participate, possibly allowing people to enroll who otherwise could not have participated. Indeed, completion of telephone calls was substantially higher than workshop attendance; thus, a group-based approach may not have been feasible for this population. Also, in participant interviews after program completion, telephone calls were rated as the most useful program feature. Use of public health department infrastructure and staff for intervention delivery has not been tested previously in diabetes risk reduction studies.
Our approach addressed 4 translation priority areas10 by (1) focusing on vulnerable, understudied groups; (2) having few exclusion criteria, thus being more generalizable; (3) being a partnership between researchers and a public health department, thus reflecting their shared perspectives; and (4) being designed to be sustainable by embedding the program in the public health department's chronic disease prevention program.
Limitations
The study had several limitations. Implementation in 1 city-level public health department setting limited generalizability. However, agencies with larger service areas (e.g., county-level health departments) might find the telephone-based counseling model more attractive than in-person health education workshops. Additionally, because we had difficulty recruiting men, our sample included only 26% men. Use of fasting screening tests limited generalizability to adults available in the morning, similar to other studies.23 Finally, the significant between-group differences in weight and triglyceride levels were small and may have limited clinical benefit.
Conclusions
Our community-based translational study indicated that the Live Well, Be Well intervention was associated with small changes in a few important diabetes risk factors in lower-SES ethnic minority adults, thus providing a promising approach for future translational efforts to reduce disparities. Because so few community-based models for delivering lifestyle interventions are available, our results suggest that individually tailored programs with telephone counseling should be considered along with the more traditional group-based approaches. Testing lifestyle programs that are integrated into a health department's chronic disease prevention infrastructure and delivered by public health department counselors in local community venues provides a novel and sustainable goal for translational research.
Future research could adapt Live Well, Be Well and explore the relative effectiveness of variations in program delivery organization (health department, peer educators), delivery mode (group- vs telephone-based), intensity (number of contacts, duration), and risk-level eligibility (overweight, other diabetes risk factors) in terms of risk reduction and program compliance.
Acknowledgments
Financial support for this project was through a translational research grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R18 DK067896-01A2) and by the Resource Centers for Minority Aging Research program of the National Institute on Aging (grant P30-AG15272).
We acknowledge the substantial contribution of the study staff at the City of Berkeley Division of Public Health (Rainbow Schwartz, Elisa Gallegos, Maria Guerrero, LeConté Dill, Kristen Tehrani, and Kate Clayton) and University of California, San Francisco, staff (Julissa Cabrera, Adriana Delgadillo, Rachel Freyre, Natalie Alvarez, and Deepika Mathur).
Clinical Trials Registration: clinicaltrials.gov identifier NCT00770926.
Human Participant Protection
The research protocol was approved by the University of California, San Francisco, Committee on Human Research, and written consent was obtained from all study participants.
References
- 1.Centers for Disease Control and Prevention National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2011 [Google Scholar]
- 2.Vega WA, Amaro H. Latino outlook: good health, uncertain prognosis. Annu Rev Public Health. 1994;15:39–67 [DOI] [PubMed] [Google Scholar]
- 3.Liao Y, Tucker P, Okoro CA, Giles WH, Mokdad AH, Harris VB. REACH 2010 Surveillance for Health Status in Minority Communities—United States, 2001–2002. MMWR Surveill Summ. 2004;53(6):1–36 [PubMed] [Google Scholar]
- 4.Carter JS, Pugh JA, Monterrosa A. Non-insulin-dependent diabetes mellitus in minorities in the United States. Ann Intern Med. 1996;125(3):221–232 [DOI] [PubMed] [Google Scholar]
- 5.Harris MI, Flegal KM, Cowie CCet al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: the Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21(4):518–524 [DOI] [PubMed] [Google Scholar]
- 6.Robbins JM, Vaccarino V, Zhang H, Kasl SV. Socioeconomic status and diagnosed diabetes incidence. Diabetes Res Clin Pract. 2005;68(3):230–236 [DOI] [PubMed] [Google Scholar]
- 7.The Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuomilehto J, Lindstrom J, Eriksson JGet al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350 [DOI] [PubMed] [Google Scholar]
- 9.Pan XR, Li GW, Hu YHet al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544 [DOI] [PubMed] [Google Scholar]
- 10.Garfield SA, Malozowski S, Chin MHet al. Considerations for diabetes translational research in real-world settings. Diabetes Care. 2003;26(9):2670–2674 [DOI] [PubMed] [Google Scholar]
- 11.Fisher EB, Walker EA, Bostrom A, Fischhoff B, Haire-Joshu D, Johnson SB. Behavioral science research in the prevention of diabetes: status and opportunities. Diabetes Care. 2002;25(3):599–606 [DOI] [PubMed] [Google Scholar]
- 12.Baker MK, Simpson K, Lloyd B, Bauman AE, Singh MA. Behavioral strategies in diabetes prevention programs: a systematic review of randomized controlled trials. Diabetes Res Clin Pract. 2011;91(1):1–12 [DOI] [PubMed] [Google Scholar]
- 13.Almeida FA, Shetterly S, Smith-Ray RL, Estabrooks PA. Reach and effectiveness of a weight loss intervention in patients with prediabetes in Colorado. Prev Chronic Dis. 2010;7(5):A103. [PMC free article] [PubMed] [Google Scholar]
- 14.Pagoto SL, Kantor L, Bodenlos JS, Gitkind M, Ma Y. Translating the diabetes prevention program into a hospital-based weight loss program. Health Psychol. 2008;27(1, suppl):S91–S98 [DOI] [PubMed] [Google Scholar]
- 15.Whittemore R, Melkus G, Wagner J, Dziura J, Northrup V, Grey M. Translating the diabetes prevention program to primary care: a pilot study. Nurs Res. 2009;58(1):2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McTigue KM, Conroy MB, Bigi L, Murphy C, McNeil M. Weight loss through living well: translating an effective lifestyle intervention into clinical practice. Diabetes Educ. 2009;35(2):199–204, 208 [DOI] [PubMed] [Google Scholar]
- 17.Absetz P, Valve R, Oldenburg Bet al. Type 2 diabetes prevention in the “real world”: one-year results of the GOAL Implementation Trial. Diabetes Care. 2007;30(10):2465–2470 [DOI] [PubMed] [Google Scholar]
- 18.McBride PE, Einerson JA, Grant Het al. Putting the Diabetes Prevention Program into practice: a program for weight loss and cardiovascular risk reduction for patients with metabolic syndrome or type 2 diabetes mellitus. J Nutr Health Aging. 2008;12(10):745S–749S [DOI] [PubMed] [Google Scholar]
- 19.Vadheim LM, Brewer KA, Kassner DRet al. Effectiveness of a lifestyle intervention program among persons at high risk for cardiovascular disease and diabetes in a rural community. J Rural Health. 2010;26(3):266–272 [DOI] [PubMed] [Google Scholar]
- 20.Kramer MK, Kriska AM, Venditti EMet al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37(6):505–511 [DOI] [PubMed] [Google Scholar]
- 21.Smith-Ray RL, Almeida FA, Bajaj Jet al. Translating efficacious behavioral principles for diabetes prevention into practice. Health Promot Pract. 2009;10(1):58–66 [DOI] [PubMed] [Google Scholar]
- 22.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community: the DEPLOY pilot study. Am J Prev Med. 2008;35(4):357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parikh P, Simon EP, Fei K, Looker H, Goytia C, Horowitz CR. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. Am J Public Health. 2010;100(suppl 1):S232–S239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boltri JM, Davis-Smith M, Okosun IS, Seale JP, Foster B. Translation of the National Institutes of Health Diabetes Prevention Program in African American churches. J Natl Med Assoc. 2011;103(3):194–202 [DOI] [PubMed] [Google Scholar]
- 25.Merriam PA, Tellez TL, Rosal MCet al. Methodology of a diabetes prevention translational research project utilizing a community-academic partnership for implementation in an underserved Latino community. BMC Med Res Methodol. 2009;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katula JA, Vitolins MZ, Rosenberger ELet al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care. 2011;34(7):1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santoyo-Olsson J, Cabrera J, Freyre Ret al. An innovative multiphased strategy to recruit underserved adults into a randomized trial of a community-based diabetes risk reduction program. Gerontologist. 2011;51(suppl 1):S82–S93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delgadillo AT, Grossman M, Santoyo-Olsson J, Gallegos-Jackson E, Kanaya AM, Stewart AL. Description of an academic community partnership lifestyle program for lower income minority adults at risk for diabetes. Diabetes Educ. 2010;36(4):640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419 [DOI] [PubMed] [Google Scholar]
- 30.Huang MH, Harrison GG, Mohamed MMet al. Assessing the accuracy of a food frequency questionnaire for estimating usual intake of phytoestrogens. Nutr Cancer. 2000;37(2):145–154 [DOI] [PubMed] [Google Scholar]
- 31.Huang MH, Schocken M, Block Get al. Variation in nutrient intakes by ethnicity: results from the Study of Women's Health Across the Nation (SWAN). Menopause. 2002;9(5):309–319 [DOI] [PubMed] [Google Scholar]
- 32.Block G, Mandel R, Gold E. On food frequency questionnaires: the contribution of open-ended questions and questions on ethnic foods. Epidemiology. 2004;15(2):216–221 [DOI] [PubMed] [Google Scholar]
- 33.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcome for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141 [DOI] [PubMed] [Google Scholar]
- 34.Stewart AL, Ware JE, Jr, Sherbourne CD, Wells K. Psychological distress/well-being and cognitive functioning measures. : Stewart AL, Ware JE, Jr, Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992:102–142 [Google Scholar]
- 35.Hays RD, Stewart AL. Sleep measures. : Stewart AL, Ware JE, Jr, Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1992:235–259 [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396 [PubMed] [Google Scholar]
- 37.Little RJA, Rubin DB. Statistical Analysis With Missing Data. 2nd ed. Hoboken, NJ: John Wiley and Sons; 2002 [Google Scholar]
- 38.Hamman RF, Wing RR, Edelstein SLet al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin JS, O'Connor E, Whitlock EP, Beil TL. Behavioral counseling to promote physical activity and a healthful diet to prevent cardiovascular disease in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. −≠+≠;153(11):736–750. [DOI] [PubMed]
- 40.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38(1):21–37 [PubMed] [Google Scholar]
- 41.Miilunpalo S, Vuori I, Oja P, Pasanen M, Urponen H. Self-rated health status as a health measure: the predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. J Clin Epidemiol. 1997;50(5):517–528 [DOI] [PubMed] [Google Scholar]
- 42.Ferraro KF, Kelley-Moore JA. Self-rated health and mortality among black and white adults: examining the dynamic evaluation thesis. J Gerontol B Psychol Sci Soc Sci. 2001;56(4):S195–205 [DOI] [PubMed] [Google Scholar]
- 43.Kriska AM, Delahanty LM, Pettee KK. Lifestyle intervention for the prevention of type 2 diabetes: translation and future recommendations. Curr Diab Rep. 2004;4(2):113–118 [DOI] [PubMed] [Google Scholar]
- 44.Task Force on Community Preventive Services Increasing physical activity: a report on recommendations of the Task Force on Community Preventive Services. MMWR Recomm Rep. 2001;50(RR-18):1–14 [PubMed] [Google Scholar]
- 45.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28 [DOI] [PubMed] [Google Scholar]
- 46.Glasgow RE, Toobert DJ, Hampson SE. Participation in outpatient diabetes education programs: how many patients take part and how representative are they? Diabetes Educ. 1991;17(5):376–380 [DOI] [PubMed] [Google Scholar]