Abstract
The Eph family of receptor tyrosine kinases and their ligands the ephrins play an essential role in the targeting of retinal ganglion cell axons to topographically correct locations in the optic tectum during visual system development. The African claw-toed frog Xenopus laevis is a popular animal model for the study of retinotectal development because of its amenability to live imaging and electrophysiology. Its visual system undergoes protracted growth continuing beyond metamorphosis, yet little is known about ephrin and Eph expression patterns beyond stage 39 when retinal axons first arrive in the tectum. We used alkaline phosphatase fusion proteins of EphA3, ephrin-A5, EphB2, and ephrin-B1 as affinity probes to reveal the expression patterns of ephrin-As, EphAs, ephrin-Bs and EphBs, respectively. Analysis of brains from stage 39 to adult frog revealed that ephrins and Eph receptors are expressed throughout development. As observed in other species, staining for ephrin-As displayed a high caudal to low rostral expression pattern across the tectum, roughly complementary to the expression of EphAs. In contrast with the current model, EphBs were found to be expressed in the tectum in a high dorsal to low ventral gradient in young animals. In animals with induced binocular tectal innervation, ocular dominance bands of alternating input from the two eyes formed in the tectum; however, ephrin-A and EphA expression patterns were unmodulated and similar to those in normal frogs, confirming that the segregation of axons into eye-specific stripes is not the consequence of a respecification of molecular guidance cues in the tectum.
Keywords: visual system, axon guidance, topographic mapping, optic tectum, ocular dominance
INTRODUCTION
The Eph family of receptor tyrosine kinases and their ligands the ephrins play an important role in the targeting of retinal ganglion cell (RGC) axons within the optic tectum during visual system development (Feldheim and O'Leary, 2010; McLaughlin and O'Leary, 2005). The complementary gradients of Ephs and ephrins that exist in both the eye and the tectum help guide the incoming axons to arborize at appropriate tectal locations, ensuring that the axes of the retina are represented in the tectum in a well-organized and topographic fashion (O'Leary and McLaughlin, 2005).
Eph receptors and ephrin ligands are subdivided into A and B classes based both on structural homology and binding affinity: EphAs preferentially bind ephrin-As, which are GPI-anchored, while EphBs bind more strongly to transmembrane ephrin-Bs. Ephs and ephrins will bind several different receptors/ligands within their class with different affinities, although lower affinity cross-class binding has been reported in limited cases (Flanagan and Vanderhaeghen, 1998; Gale et al., 1996; Himanen et al., 2004; Pasquale, 2004).
The interaction of Ephs and ephrins requires cell-to-cell contact due to their cell surface expression (Flanagan and Vanderhaeghen, 1998). Following binding, Eph/ephrin activation influences cell and/or axon motility and migration through regulation of cellular adhesion and cytoskeletal dynamics. Activation of Rho family small GTPases, integrin-mediated signalling, the Ras-MAP-kinase pathway, or interactions with SH2-domain-containing adaptor proteins can occur in both the Eph-expressing cell (forward signalling) and the ephrin-expressing cell (reverse signalling), resulting in attraction or repulsion depending on the circumstance (Egea and Klein, 2007; Murai and Pasquale, 2003). In the retinotectal system, Ephs and ephrins expressed on the same cell can also interact in cis, which results in their inactivation, and this type of interaction has been proposed to heighten the steepness of the gradient of receptor or ligand available for binding (Carvalho et al., 2006; Hornberger et al., 1999; Yin et al., 2004).
The mapping of the rostrocaudal axis of the tectum is guided by EphAs and ephrin-As: EphAs expressed in a high temporal, low nasal gradient on RGCs interact with ephrin-As expressed in a high caudal to low rostral gradient in the tectum, activating forward signalling and resulting in temporal RGC repulsion (McLaughlin and O'Leary, 2005). RGCs in mice initially grow past their termination zones (TZs) in the superior colliculus (SC), and ephrin-As expressed in the SC both inhibit RGCs from arborizing too caudally, and encourage topographic branching along the axon shaft following overshoot (Yates et al., 2001). Tracing the retinocollicular projection in mice lacking ephrin-As reveals the presence of TZs at normal as well as ectopic locations, a phenotype which becomes more severe when additional ephrin-A homologues are knocked out (Feldheim et al., 2000; Frisen et al., 1998). In frogs, ephrin-As guide RGCs directly to their TZs, where an arbor forms through back-branching behind the growth cone (Harris et al., 1987). Additional factors contributing to the formation of an accurately patterned rostrocaudal tectal axis have been identified, including Engrailed (Wizenmann et al., 2009), Repulsive Guidance Molecule (Monnier et al., 2002), and the activation by tectal EphAs of retinal ephrin-As signalling through p75NTR (Lim et al., 2008b; Rashid et al., 2005;).
It has been proposed that the dorsoventral axis of the tectal map is specified by gradients of Ephs and ephrins of the B class, but the mechanism of action may differ in mice and amphibians (Hindges et al., 2002; Mann et al., 2002). In frogs, RGCs express ephrin-Bs in a high dorsal to low ventral gradient in the eye (Mann et al., 2002; Scalia et al., 2009). It has been reported that EphB1 is expressed in Xenopus tectum in a high ventral to low dorsal gradient, which has been suggested to attract dorsal retinal axons into ventral tectum through activation of reverse signalling (Mann et al., 2002). EphB2-and EphB3-deficient mice exhibit ectopic laterally-located TZs, indicating that dorsoventral patterning in mouse appears to be mediated by forward rather than reverse signalling, with ephrin-B acting bifunctionally as both an attractive and repellent cue depending on the levels of EphBs on the axons (Hindges et al., 2002; McLaughlin et al 2003b). More recent evidence has put forward several other candidates for dorsoventral guidance: Wnt3 expressed in optic tectum may repel Ryk-expressing RGCs from the midline (Schmitt et al., 2006) and Sema3D may encourage RGCs to terminate dorsally in zebrafish tectum (Liu et al., 2004).
The formation of an accurate topographic map relies not only on guidance by molecular cues, but also patterned activity. Patterned waves of spontaneous activity in mammalian retina contribute to the formation of discrete TZs of retinocollicular axons, as revealed in ß2 nicotinic acetylcholine receptor knockout mice, in which RGCs exhibit abnormal spontaneous activity lacking the normal structure of waves (McLaughlin et al., 2003a; Stafford et al., 2009). The contribution of activity-dependent mechanisms to map formation can also be studied in a frog in which two eyes have been surgically induced to innervate one normally monocular tectal lobe (“binocular innervation”), a condition which induces the formation of ectopic ocular dominance bands (Law and Constantine-Paton, 1980; Reh and Constantine-Paton, 1985; Ruthazer et al., 2003; Straznicky and Glastonbury, 1979; Straznicky et al., 1980). Blockade of activity or N-methyl-D-aspartate glutamate receptors (NMDARs) inhibits eye-specific band formation and causes desegregation of existing bands, indicating that activity is an instructive cue both in the formation and maintenance of the amphibian visual map (Cline et al., 1987; Reh and Constantine-Paton, 1985; Ruthazer et al., 2003). It has been assumed that binocular innervation does not alter the level of retinal activity or the expression of molecular guidance cues; thus ODC segregation is instructed by the low correlation of activity patterns of one eye compared to the other (Cline et al., 1987; Ruthazer and Cline, 2004). Somewhat surprisingly, however, the patterns of expression of the molecular guidance cues in this classic model of activity-dependent map instruction have never been assessed.
Xenopus laevis is a popular model for studying retinotectal development because of its amenability to live imaging and electrophysiology. Using alkaline phosphatase fusion proteins and immunohistochemistry, we have characterized the expression patterns of Ephs and ephrins in the visual system of Xenopus laevis throughout development. Additionally, to investigate whether the maintenance of eye-specific segregation in binocularly-innervated frogs relies on the respecification of molecular guidance cues, we compared the expression pattern of ephrin-As and EphAs to the arrangement of ocular dominance bands in binocularly-innervated animals.
METHODS
Animals
Tadpoles were produced from adult Xenopus laevis frogs primed with human chorionic gonadotrophin (Sigma-Aldrich, Oakville, ON) and raised in Modified Barth's Solution with Hepes (MBSH; 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4×7H20, 0.33 mM Ca(NO3)2×4H20, 0.41 mM CaCl2, 10 mM HEPES, pH 7.4). Tadpoles (from stage 45 to stage 66) were fed once daily on a finely ground mixture of distilled water and two parts Frog Brittle (Nasco, Fort Atkinson, WI), one part Nutrafin goldfish pellets (Hagen, Montreal, CA) and one part NU-Way trout ration (Unifeed, Edmonton, CA). Stage 66 froglets were maintained in dechlorinated water and fed Frog Brittle (Nasco, Fort Atkinson, WI) once every two days. All animals were raised from embryos except for the fully-grown adults, which were obtained from Nasco (Fort Atkinson, WI) and some stage 59 and 66 animals, which were purchased from Boreal Northwest (St. Catharines, ON). Staging was according to Nieuwkoop and Faber (1967). All procedures were approved by the Animal Care Committee of the Montreal Neurological Institute of McGill University under the guidelines of the Canadian Council on Animal Care.
Whole-mount staining
Fusion proteins of human placental heat-stable alkaline phosphatase (Flanagan et al., 2000) linked to the extracellular region of chick EphB2, mouse ephrin-A5, mouse EphA3, or human ephrin-B1 were used to assay the presence of ephrinBs, EphAs, ephrinAs, and EphBs respectively, as previously described (Bach et al., 2003; Scalia and Feldheim, 2005, Scalia et al., 2009). The pia mater was removed from brains stage 59 and older prior to incubation with the fusion protein. Whole-mount brains were stained at several important developmental timepoints: stages 45 and 48 (tadpole), 59 (during metamorphosis), 66 (early postmetamorphic frog), and late postmetamorphic frog. To reveal patterns of expression over a wide sensitivity range, several different concentrations of ephrin-B1-AP were tested on brains from frogs at stage 45, 48, 59, and 66.
Labelling of retinal projection
To label the retinal innervation of the tectum (Figure 1), animals at stage 45 and 48 were euthanized by immersion in 0.2% MS-222 followed by incubation overnight in 4% paraformaldehyde (in 0.1 M phosphate buffer; Polysciences Inc., Warrington, PA). The left eye of each tadpole was injected with 0.2% DiI (in ethanol; Invitrogen, Burlington, ON), and the animals were incubated at room temperature to allow for transport (4 days for stage 45, 7 days for stage 48). Images were collected on a custom-built two-photon microscope at 710nm with an Olympus 20× (UIS2 UPlanFL N 0.5 NA) air objective. Autofluorescence from the surrounding tissue allowed for visualization of the anatomy of the tectum, Images were denoised in ImageJ (NIH, Bethesda, MD) using the Remove Outliers function (threshold=50), and linearly corrected for contrast. The stage 48 image is a z-projection (Fig. Civ), but because of the small size of the tectum at stage 45, a single optical section was used (Fig. Biv).
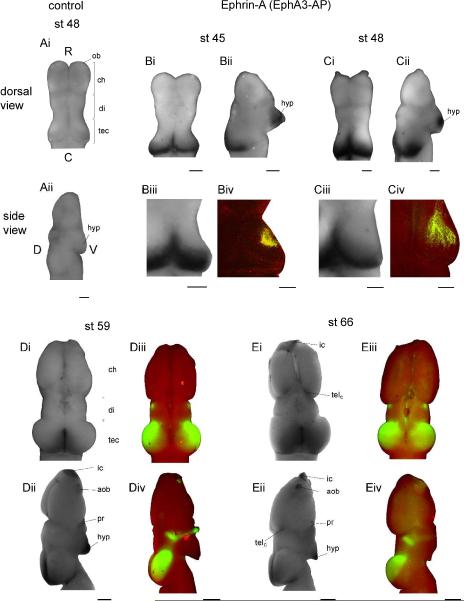
Figure 1. Ephrin-A expression.
(A) Dorsal (Ai) and side (Aii) views of a stage 48 whole-mount Xenopus brain reacted with the negative control AP-only probe. The tissue is a pale uniform pink and has no discernible staining pattern. Rostral (R), caudal (C), dorsal (D), and ventral (V) axes are labelled, and this orientation is consistent in all other whole-mount images. (B–C) Patterns of ephrin-A expression (EphA3-AP binding) in representative stage 45 (B) and 48 (C) brains (dorsal: Bi, Ci, side: Bii, Cii). Additional examples of ephrin-A tectal expression patterns (Biii, Ciii) are shown next to examples of the bulk DiI-labeled retinal projection (Biv, Civ) in animals of the same ages (RGCs, green; autofluorescence, red). Two-photon optical section at stage 45 (Biv) and z-projection at stage 48 (Civ) highlight the relative location of retinorecipient tectal neuropil. (D–E) Ephrin-A expression and retinal innervation pattern in stage 59 (D) and 66 (E) brains; dorsal views are above (Di, Diii, Ei, Eiii), and side views are below (Dii, Div, Eii, Eiv). Ephrin-As are intensely expressed at stage 45 and 48 in the hypothalamus and in a very clear high caudal to low rostral gradient in the tectum. The gradient of tectal ephrin-A expression shifts slightly in late developmental stages to a low rostrolateral to high caudomedial gradient. Throughout development, retinorecipient tectal neuropil is located rostrolateral to the caudal pole of the tectum and to the peak of ephrin-A expression, but within the zone of graded ephrin-A expression. Paired dorsal and side view images are of the same brains for A–E except Dii, for which a more representative side view image was chosen. Scale bars: A–C=100 μm, D–E=500 μm.
The retinal innervation pattern in the brain of stage 59 and 66 animals (Fig. 1) was labelled using fluorescently-tagged wheat germ agglutinin conjugated to Alexa Fluor 488 (2% in PBS; Invitrogen, Burlington, ON) and imaged on an epifluorescence microscope. The outline of the brain was captured using the autofluorescence signal.
To acquire an image of RGC axons in the living tadpole tectum (Figure 4), the eye was electroporated with eGFP (Haas et al., 2002), and 48 hours later the ventricle was injected with a counterstain (5 mM BODIPY TR Methyl Ester in DMSO; Invitrogen, Burlington, ON). Images were collected on a two-photon microscope as above, at 910nm.
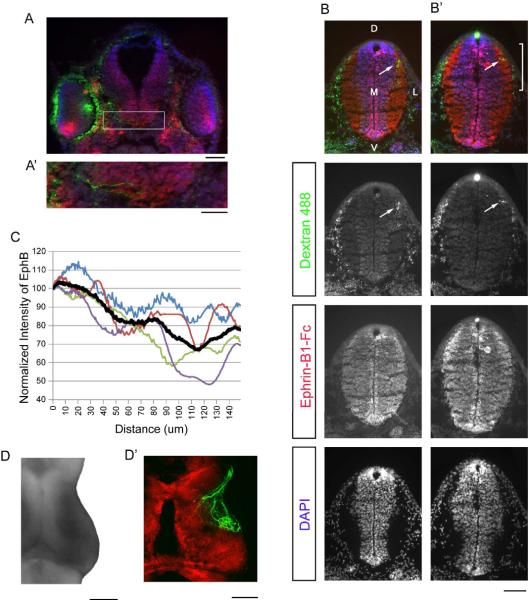
Figure 4. Dorsoventral gradient of EphB expression in young tadpoles.
(A,A') A coronal section of a stage 40/41 tadpole rostral to the tectum shows DAPI (blue), Alexa Fluor 488 dextran iontophoresed into the eye (green), and a high ventral to low dorsal gradient of EphBs in the eyes (red, ephrin-B1-Fc). The white box indicates the region magnified in A'. (A') Several labelled retinal axons (green) can be seen traversing the optic chiasm. (B) A coronal section of the brain of a stage 40–41 animal, showing Alexa Fluor 488 dextran (retinal axons, green), ephrin-B1-Fc (EphBs, red), and DAPI (cell bodies, blue). Each channel is represented in a separate panel below the composite image, and the terminations of RGC axons in the neuropil are indicated with white arrows. Dorsal (D), ventral (V), medial (M), and lateral (L) axes are labelled. (B') One section caudal to B (B') is the most caudal section containing retinal axons. A distinct high dorsal to low ventral gradient of EphBs can be seen across the retinorecipient neuropil (indicated by the white bracket). (C) A graph indicating the EphB reactivity (normalized to the dorsal-most value) across the dorsal-most 150 μm of the retinorecipient neuropil as defined by the presence of RGC axon terminals. The black line is the average intensity, and the red line indicates the intensity of the image shown in B'. (D,D') The high caudomedial to low rostrolateral gradient of EphBs across the dorsal surface of the tectum (D) outlines the region of tectal innervation of retinal axons (D'), as seen in two different stage 48 brains. Scale bars: A=100 μm, A'=50 μm, B,B',D,D'=100 μm.
Staining of sections
The left eye of stage 34–38 tadpoles was iontophoresed with a 5% (in water) 10KD dextran conjugated to Alexa Fluor 488 (Invitrogen, Burlington, ON) using 70V pulses at a frequency of 200 Hz with 1ms duration. Positive current was passed through a glass micropipette filled with the dextran solution, and a silver wire placed below the animal served as the cathode (Ruthazer et al., 2005). The following day, at stage 40/41, the animals were anaesthetized in 0.2% MS-222 (Sigma-Aldrich, Oakville, ON) in MBSH, and incubated in 20% fish gelatin with 15% sucrose (Norland Products, Cranbury, NJ) for 20 minutes. The tadpoles were then immersed in Tissue-Tek O.C.T. Compound (Sakura Finetek, Torrance, CA) and flash frozen without fixation using 2-methylbutane (Sigma-Aldrich, Oakville, ON) chilled with dry ice. Coronal sections were cut at 30 μm, mounted onto gelatin-coated slides (LabScientific, Livingston, NJ) and left overnight to dry. A mouse ephrin-B1-Fc chimera (1:100, R & D Systems, Minneapolis, MN) and a goat anti-human Alexa Fluor 555 secondary antibody (1:250, Invitrogen, Burlington, ON) were used to visualize EphBs in a standard immunohistochemistry protocol. To amplify the Alexa Fluor 488 dextran signal, rabbit anti-Alexa Fluor 488 primary (1:250) and goat anti-rabbit Alexa Fluor 488 (1:500) secondary antibodies were also applied (Invitrogen, Burlington, ON). Cell nuclei were stained with DAPI. Sections were only analysed if a sequence of sections was intact showing retinal axons coursing along the optic tract and arborizing in the optic tectum. The most caudal section containing retinal axons was used for measurement of the EphB gradient.
Binocularly-innervated frogs
To induce binocular innervation, stage 44 Xenopus laevis tadpoles were first anaesthetized using 0.02% MS-222, then the left tectal lobe was cut away from the brain with a 30 G 1/2 syringe tip (Becton Dickinson, Franklin Lakes, NJ) and aspirated out using a glass micropipette. Once the animals grew to be stage 66, the eyes were injected with WGA 488 or 594. The animals were maintained for two days to allow for anterograde transport. Following sacrifice, the brains which exhibited ocular dominance bands were imaged with an Olympus 20× (UIS2 UPlanFL N 0.5NA) air objective on a custom-built two-photon microscope at 880nm, and then processed as whole-mounts for visualization of ephrin-As or EphAs as described above. It was decided that a respecification of Eph or ephrin expression patterns following dual innervation would be most easily detectable in the case of EphA or ephrin-A because they are normally expressed in a gradient that is offset from the rostocaudally-oriented ocular dominance bands, whereas EphB is expressed uniformly in metamorphic and post-metamorphic tecta, Following ephrin staining, the WGA labelling was imaged again on an epifluorescence microscope, and the image was linearly adjusted for contrast. Montages were made from the two-photon images using Adobe Photoshop, and were linearly and uniformly adjusted for contrast.
Image Analysis
Images of whole-mount tissue were linearly corrected for contrast in Adobe Photoshop. For coronal sections, the images were first processed with a Gaussian Blur (10 pixel radius) to smooth sudden drops in intensity due to gaps in the tissue. Using ImageJ software (NIH, Bethesda, MD), a line was drawn freehand along the surface of the neuropil (150 μm long, 38 μm wide), starting dorsally at the border between neuropil and cell body layer and extending laterally and ventrally along the surface. The intensity of EphB signal was measured along this line and normalized to the EphB staining value at the dorsal border between the neuropil and cell body layer.
RESULTS
Ephrin-A Expression
Staining of stage 48 Xenopus brain tissue with a negative control AP tag vector (containing no Eph or ephrin sequence) results in tissue with a uniform, faint pink color and no regions of strong staining (Figs. 1Ai, Aii, n=10). In stage 45 and 48 tadpoles, ephrin-A staining is strongest in the hypothalamus, tectal midline, and across the dorsal surface of the optic tectum in a low rostral to high caudal gradient (Figs. 1Bi, Bii, Ci, Cii, stage 45, n=8; stage 48, n=8). From stage 59 onward, strong expression of ephrin-As persists along the midline of the tectum, and the angle of the gradient of expression rotates slightly to become low rostrolateral to high caudomedial (Figs. 1Di, Dii, Ei, Eii, stage 59, n=4; stage 66, n=3). The older brains also show ephrin-A expression in the hypothalamus, preoptic area, implantation cone of the olfactory bulb, accessory olfactory bulb, and a caudal region of the telecephalon.
Throughout development the tectal neuropil in which the retinal axons arborize is located rostrolateral to the peak of ephrin-A expression but within its high caudal to low rostral gradient (Fig. 1Biii, Biv, Ciii, Civ, Diii, Div, Eiii, Eiv). Caudal- and medial-most tectum remains uninnervated in the Xenopus brain from stage 45–66.
EphA Expression
In early development, EphA is expressed in the cerebral hemispheres, in several regions of the diencephalon, and faintly in the anterior tectum (Figs. 2A,A', n=7). Starting at stage 59, EphAs become much more strongly expressed in a tectal gradient that is high rostrolateral to low caudomedial across the dorsal surface (Figs. 2B,B', n=6). EphAs can also be seen expressed in the optic tract, striatum, pretectum, precommisural area, habenular nuclei, habenular commissure, and cerebral hemispheres. As demonstrated in a composite image of brains from stage 66 froglets, the expression pattern of EphAs (left) is largely complementary to that of ephrin-As (right) in the optic tectum in these older animals (Fig. 2C, EphA, n=5; ephrin-A, n=7).
Figure 2. EphA expression.
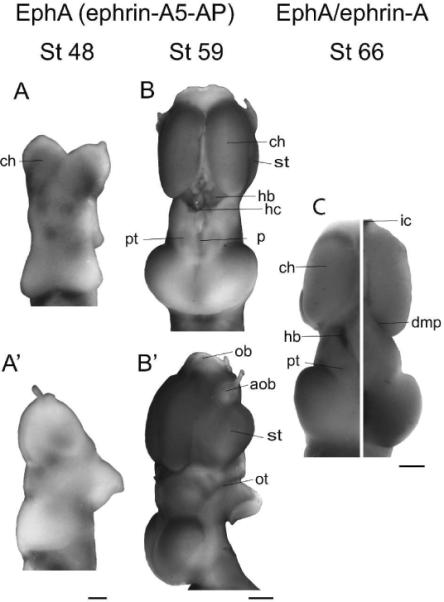
(A,B) Dorsal views of stage 48 (A) and stage 59 (B) brains showing EphA expression. (A',B') Side views of the same brains. EphAs are highly expressed throughout development in the cerebral hemispheres. In younger stages, EphAs are expressed in several regions of the diencephalon and faintly in rostral tectum. From stage 59 onward, a strong high rostrolateral to low caudomedial gradient is seen in the tectum. Staining is also present in older stages in the pretectum, striatum, habenular commissure, habenular nuclei, precommissural area, and the optic tract, although is notably absent in the olfactory regions. (C) Composite image showing EphA expression (left) and ephrin-A expression (right) in the stage 66 brain. The complementarity of the gradients of expression of EphAs and ephrin-As in the tectal lobes is striking. Scale bars: A,A'=100 μm, B–C=500 μm.
EphB Expression
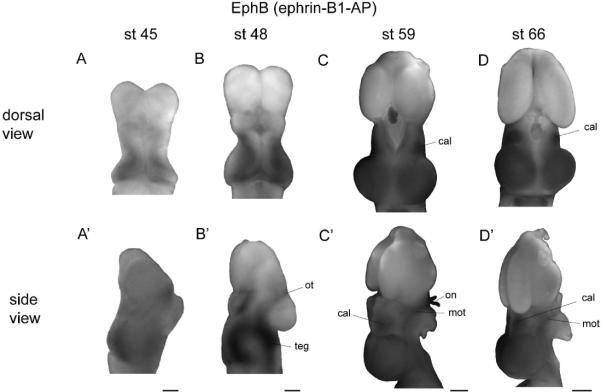
In stage 45 and 48 brains reacted with undiluted ephrin-B1-AP reagent, staining for EphB was present in the optic tract, and appeared to be uniformly strong across the tectal lobes, with decreased staining at the midline (data not shown). Because of the intensity of staining across the tectum, we suspected that an underlying gradient may have been saturated by our staining conditions. We therefore reacted stage 45 and 48 brains with ephrin-B1-AP at half concentration, which revealed that EphB is expressed in a high caudomedial to low rostrolateral gradient in the tectum (Figs. 3A–B', Suppl. Fig. 1, stage 45, n=7; stage 48, n=6). The orientation of this pattern shows slight variability, with some animals displaying a high caudomedial to low rostrolateral gradient, and others showing a gradient oriented high medially (dorsally) to low laterally (ventrally) (Fig. 3B). This gradient was similar to that found in Rana pipiens (Suppl. Fig. 2), and the same range of orientations of the EphB expression gradient was observed in early larval Rana pipens with further dilutions of the fusion protein (Scalia et al., 2009). However, in marked variance with an earlier report in Xenopus (Mann et al., 2002), in no case did we observe a high lateral (ventral) to low medial (dorsal) gradient. At stage 59 and 66, the tectal lobes are intensely and uniformly stained, with decreased staining at the tectal midline and the caudal edge of the tectal lobes (Figs. 3C–D', stage 59, n=5; stage 66, n=2). EphB expression can also be seen in the central anterolateral nucleus and outer margins of the optic tract. Application of ephrin-B1-AP at half-concentration to stage 59 (n=6) and 66 (n=4) brains revealed no underlying gradient of expression at these later stages.
Figure 3. EphB expression.
(A–D) Dorsal views of stage 45 (A), 48 (B), 59 (C), and 66 (D) brains stained for EphB expression. (A'–D') Side views of brains at the same age. Dorsal and side views are of the same brains, except at stage 45, where a more representative side view was chosen. At both stage 45 and 48, EphBs are expressed in a high caudomedial to low rostrolateral gradient in the tectum, and at stage 48 also in the tegmentum and optic tract. (C–D') At st 59 and 66, EphBs are expressed uniformly across the surface of the tectum, as well as in the central anterolateral nucleus and the outer margins of the optic tract. Scale bars: A–B'=100 μm, C–D'=500 μm.
To investigate whether the high caudomedial to low rostrolateral expression of EphBs seen in whole-mount preparations of young Xenopus brain was present at the initial stages of retinal axon ingrowth, we examined stage 40/41 tadpoles. Because the expression pattern of EphBs within the retinorecipient region of tectal neuropil is most relevant to its putative role in dorsoventral guidance of RGC afferents, we identified the precise location of the axon terminals in the tectum by labelling RGC axons from the contralateral eye. In brief, Alexa Fluor 488 dextran was iontophoresed into the retina to label RGCs; then the unfixed tadpoles were flash frozen, cryosectioned at 30 μm thickness in the coronal plane and labelled immunohistochemically for EphBs using an ephrinB1-Fc chimera and anti-human Fc secondary antibody conjugated to Alexa Fluor 555.
A coronal section of the tadpole head anterior to the tectum shows high ventral to low dorsal EphB staining in the eye (Fig. 4A), confirming previous reports of a retinal gradient of EphBs in frogs (Mann et al., 2002; Scalia et al., 2009). In the brain, tectal tissue labels very strongly for EphBs, with particularly high staining in the tissue surrounding the ventricle. In a magnified region of the same section, several dextran-labeled RGC axons can be seen crossing the optic chiasm (Fig 4A').
Sequential coronal sections from rostral to caudal revealed a number of Alexa Fluor 488 dextran-labelled RGC axons (green) leaving the eye, crossing the chiasm, and progressing superficially towards the dorsal surface, to finally terminate in the most dorsal part of the tectal neuropil. In the most caudal of the sequence of sections containing RGC axons, EphBs are clearly expressed in a high dorsal to low ventral gradient over the dorsal-most 150 μm of tectal neuropil where RGC axons (white arrows) terminate (Fig. 4B', n=4). This gradient was observed in the most caudal section that contained RGC axons, and in several cases in the section immediately rostral to it (Fig. 4B). In the four animals in which EphBs were expressed in a graded pattern, the gradients of EphB intensity have approximately the same slope (Fig 4C). In an additional two animals, no gradient could be measured as EphBs appeared to be expressed uniformly across the dorsoventral axis in retinorecipient tectum. The zone of highest EphB expression (Fig. 4D) appears to fall at the medial or caudomedial border of the retinorecipient area of tectum, illustrated in a stage 48 tadpole with GFP-labelled RGC axons counterstained with BODIPY (Fig. 4D').
Ephrin-B Expression
Ephrin-B staining intensely labels the cerebral hemispheres and olfactory bulb in animals at stage 48 (Fig 5A,A', n=8). In stage 59 brains, ephrin-Bs are expressed in the cerebral hemispheres, olfactory bulb, accessory olfactory bulbs, hypothalamus, and at the tectal midline (Fig 5B,B', n=4). Staining of the tectal lobes is not evident at any stage. A composite image showing expression of EphBs (left) and ephrin-Bs (right) at stage 66 highlights the complementarity of the two patterns; very high EphB staining in the tectum and diencephalon contrasts with high ephrin-B staining only in the cerebral hemispheres and olfactory regions (Fig. 5C, ephrin-B, n=3; EphB, n=2).
Figure 5. Ephrin-B expression.
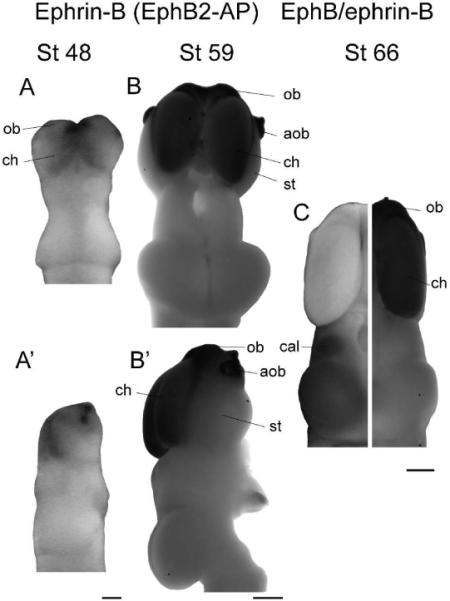
(A,B) Dorsal views of stage 48 (A) and 59 (B) brains stained for ephrin-B expression. (A',B') Side views of the same brains. Ephrin-Bs are expressed very strongly in the cerebral hemispheres and olfactory regions at all stages. In older animals specifically, strong expression can be seen in the olfactory bulb, accessory olfactory bulb, cerebral hemispheres, and at the midline of the optic tectum, although staining is undetectable in the striatum and the tectal lobes. (C) Composite image showing EphB (left) and ephrin-B (right) expression in the stage 66 brain. The overall expression patterns of EphBs and ephrin-Bs are complementary; the telencephalon stains for ephrin-Bs, while the diencephalon and optic tectum show staining only for EphBs. Scale bars: A,A'=100 μm, B-C=500 μm.
Eph and Ephrin Expression Patterns in Adult Xenopus Brain
The graded expression patterns of EphAs and ephrin-As in the tectum are very striking throughout development, consistent with protracted visual system development in this species (Straznicky and Hiscock, 1984). However, staining for EphAs and ephrin-As in the tecta of late postmetamorphic frogs several years old no longer revealed graded expression. In contrast to early postmetamorphic (stage 66) brains (Fig 6A, left, n=7), ephrin-A staining is very faint if not absent in the late postmetamorphic tectum (Fig 6A, right, n=1) and no clear gradient can be detected. Staining for ephrin-As persists in the hypothalamus and implantation cones of the olfactory bulb, however, indicating that the lack of tectal staining for ephrin-As is not due to an inability to detect ephrin-As in more mature tissue (data not shown).
Figure 6. Expression of Ephs and ephrins in adult Xenopus.
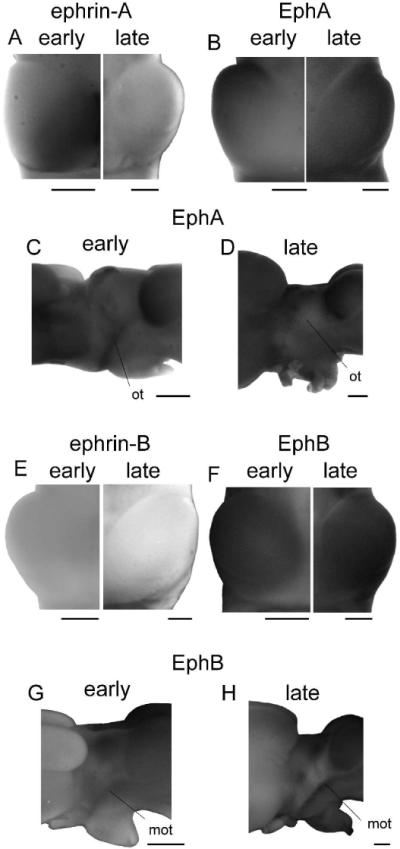
(A,B) A composite image of tectal lobes from early (stage 66, left) and late (right) postmetamorphic animals stained for ephrin-A (A) and EphA (B) expression. The very striking gradients present in early postmetamorphic animals are faint if not absent in the late postmetamorphic brains stained either for EphAs or ephrin-As. (C,D) Side view of EphA expression in an early (stage 66; C) and late postmetamorphic brain (D). EphAs appear to be expressed in the optic tract at stage 66, but do not persist in the late postmetamorphic animal. (E,F) Tectal lobes from early (stage 66, left) and late (right) postmetamorphic animals stained for ephrin-B (E) and EphB (F) expression. The very low expression of ephrin-Bs and very high expression of EphBs appear to continue unchanged into adulthood. (G, H) The side view of an early (stage 66; G) and late (H) postmetamorphic brain stained for expression of EphBs. EphBs are strongly expressed in the marginal zone of the optic tract in both froglet and fully mature animal, but ephrin-Bs were not detected in the optic tract at any time point. Scale bars = 500 μm.
Similarly, the high rostrolateral to low caudomedial gradient of EphAs seen in early postmetamorphic brains (Fig 6B, left, n=5) is subtle if not absent in late postmetamorphic tissue, where EphAs are highly and uniformly expressed across the tectum (Fig 6B, right, n=1). The expression of EphAs in the optic tract at stage 59 (Fig. 2B') and 66 (Fig. 6C) is also no longer present in the late postmetamorphic frog (Fig. 6D). The complementarity of the gradients of EphAs and ephrin-As throughout development persists in the postmetamorphic adult tectum, where EphA expression is high and uniform, and ephrin-A expression is uniformly subtle.
In contrast, EphBs and ephrin-Bs show no change between early and late post-metamorphic adults. Ephrin-B staining is not detected in the tectal lobes in early (Fig. 6E, left, n=3) or late postmetamorphic animals (Fig. 6E, right, n=1). However, persistent staining in the cerebral hemispheres, olfactory bulbs, and accessory olfactory bulbs serves as a positive control. EphBs are persistently and uniformly expressed in the tectum in early (Fig. 6F, left, n=2) and late postmetamorphic (Fig. 6F, right, n=1) brains, and are also present in the marginal zones of the optic tract at both stages (Fig 6G,H). At no point in development was ephrin-B staining detected in the optic tract.
EphA and Ephrin-A Expression Pattern in Binocularly Innervated Xenopus Tectum
Although the retinotectal projection in Xenopus is normally entirely crossed, early developmental ablation of one of the two tectal lobes results in a significant ectopic projection from the ipsilateral eye (Ruthazer et al., 2003; Straznicky and Glastonbury, 1979). The afferents from the two eyes, now projecting to the same tectal lobe, segregate into eye-specific ocular dominance bands. The dependence of ocular dominance band formation and maintenance on patterned neural activity is well established, and activity patterns and guidance cues appear to work in concert to drive the eye-specific segregation of RGC axons (Cline, 2003; Constantine-Paton et al., 1990; Ruthazer and Cline, 2004). However, it remains possible that altered activity patterns in a binocularly-innervated animal could respecify tectal expression of one or more molecular guidance cues in a manner that might guide axons to segregate into ocular dominance bands (Hanson and Landmesser 2004; Nicol et al 2007). Alternatively, the axons of one eye may be repelled by high local levels of ephrin-As expressed on a group of axons from the other eye, strengthening band formation (Gallarda et al., 2008). In either of these scenarios, we might expect to see ephrins or Ephs expressed in a banded pattern aligned with or offset to the bands of innervation. Because the tectal gradients of EphAs and ephrin-As are oriented rostrolaterally/caudomedially in stage 66 animals, a banded EphA or ephrin-A pattern could potentially contribute to the formation or maintenance of innervation bands that are typically aligned along the rostrocaudal axis (Cline et al., 1987; Law and Constantine-Paton, 1980; Straznicky and Glastonbury, 1979). We therefore examined the pattern of ephrin-A and EphA expression in the binocularly innervated optic tectum.
Following the stage 44 removal of one tectal lobe and subsequent confirmation of binocular innervation, the patterns of expression of ephrin-As (n=4) and EphAs (n=1) at stage 66 showed no evidence of adopting a banded pattern reflecting the pattern of eye-specific innervation, but rather maintained a pattern similar to that in normal frogs. Injection of wheat germ agglutinin-Alexa Fluor 488 conjugate (WGA 488) in the left eye and WGA 594 in the right eye of a binocularly innervated stage 66 animal indeed labelled stripes in the tectum that were innervated alternately by one or the other eye (Fig. 7A–B'). The stripes were aligned mostly longitudinally along the rostrocaudal axis, with a width of approximately 100 μm, consistent with previous descriptions of ocular dominance bands (Cline et al., 1987; Law and Constantine-Paton, 1980; Straznicky and Glastonbury, 1979). Most regions of strong innervation by one eye were only very weakly innervated by the other eye. Following staining for ephrin-As, WGA 488 and 594 labelling was still weakly visible in the wholemounts (Fig. 7C, C'). Ephrin-As in the same brain were expressed in a gradient that was strong caudomedial to weak rostrolateral, without evidence of banding (Fig. 7D, D'). This pattern is similar to that in normal stage 66 animals. One feature consistently observed in brains with only one tectal lobe was a point of very intense ephrin-A staining at the caudomedial aspect of the binocularly-innervated tectum. This point may correspond to the very high staining we see at the midline of normal stage 66 animals stained for ephrin-As.
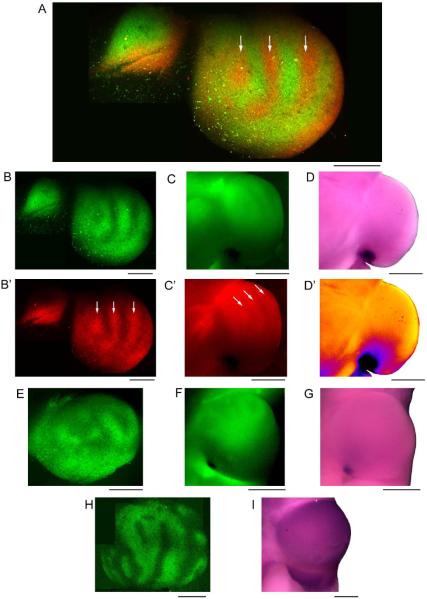
Figure 7. Ephrin-A and EphA expression patterns are unbanded in binocularly-innervated tecta.
(A) A two-photon montage of an early postmetamorphic (stage 66) frog tectum following left tectal lobe removal at a larval stage shows a binocular banded innervation pattern 48 hrs after left eye wheat germ agglutinin Alexa 488 (WGA 488) injection and right eye WGA 594 injection. The arrows in A indicate bands of high intensity (strong innervation) from the right eye. The smaller structure to the left is the remains of the left tectal lobe. (B,B') The green channel (B) and red channel (B') of the two-photon image seen in A. The white arrows refer to the same bands of WGA 594 maxima. (C,C') The WGA labelling persists faintly following the ephrin staining protocol, as seen in an epifluorescence image of the entire tectal lobe (green channel: C, red channel: C'). (D) The expression of ephrin-As in the same tectum is strongest caudomedially and decreases in a gradient rostrolaterally, similar to the expression of ephrin-As in normal adults. This pattern does not align with the pattern of bands seen in the WGA labelling. (D') A thermal map of the image shown in D. The images in B were collected at an angle to best visualize the banding, whereas the angle for corresponding images in C and D was optimized for the ephrin-A gradient. (E) A two-photon montage of another optic tectum following left tectal lobe removal and right eye injection with WGA 488 shows patches of innervation as well as unlabeled patches presumably innervated by the contralateral eye. (F,G) The bands of WGA 488 labelling persist after the ephrin staining protocol (F) but do not align with the pattern of ephrin-A expression (G). (H) A two-photon montage of the innervation pattern in a tectum following left tectal lobe removal and right eye injection with WGA 488. (I) The EphA expression pattern in the same tectum is strong, uniform, and unbanded. Scale bars: A–B'=300 μm, C–D'=500 μm, E=300 μm, F,G=500 μm, H=400 μm, I=500 μm.
A two-photon montage image of the innervation pattern of another animal following injection in the right eye with WGA 488 shows bright patches of innervation and dark patches presumably innervated by the other eye (Fig. 7E). After the ephrin staining protocol, a smooth, high caudomedial to low rostrolateral gradient of ephrin-A staining was observed (Fig. 7G) that bore no correspondence to the WGA488-labeled ocular dominance bands (Fig. 7F).
Ocular dominance bands seem to be most evident in rostral tectum where ephrin-A levels are low, therefore we also examined the pattern of EphA staining. The right eye of a binocularly innervated animal was injected with WGA 488 and its tectal innervation pattern was imaged on the two-photon microscope (Fig. 7H). The animal illustrated had undergone metamorphosis approximately one year prior, so the borders of the stripes of innervation were very well defined, and the widths of the stripes were between 100–250 μm. EphA expression in the remaining tectal lobe of this animal was strong and uniform across the tectal surface (Fig. 7I). Because of the age of the animal, the EphA staining pattern more closely resembles that of an adult (Fig. 6B, right) than that of an immature froglet (Fig. 6B, left). However, there is no indication that the pattern of ocular dominance bands is reflected in the EphA expression pattern.
DISCUSSION
This study provides a description of the expression patterns of Ephs and ephrins throughout the life of a classic model for the study of retinotectal development, Xenopus laevis. The tectal gradient of ephrin-A expression (low rostral to high caudal), and the complementary tectal gradient of EphAs (high rostral to low caudal) are robust until the end of metamorphosis, but become more subtle in late postmetamorphic animals, after the rate of cell addition in the retinotectal system declines. EphBs are expressed in a high caudomedial (dorsal) to low rostrolateral (ventral) gradient in retinorecipient tectum early in development, and are expressed uniformly across the tectal surface from metamorphosis on. Ephrin-Bs are expressed at the tectal midline and in the telencephalon. We also report normal graded ephrin-A and EphA expression patterns in binocularly-innervated animals exhibiting ocular dominance bands, indicating that the presence of ocular dominance bands is unlikely to be due to remapping of guidance cues.
The tectal gradient of ephrin-A protein responsible for rostrocaudal patterning that is seen throughout development is more subtle, if not absent, in late postmetamorphic animals. Likewise, in late postmetamorphic animals the optic tract did not have detectable levels of EphAs, which during development are the retinally-expressed receptors through which ephrin-As activate forward signalling and repulsion of RGC axons. The persistence of these guidance molecules throughout metamorphosis, but not in fully mature animals, corresponds well with the timeline of retinotectal development; new RGCs continue to send projections to the tectum until several months after metamorphosis at which point retinal ganglion cell proliferation declines to negligible levels (Grant and Keating, 1986; Straznicky and Gaze, 1971; Straznicky and Hiscock, 1984). In addition, the isthmotectal projection through which tectal cells receive indirect binocular input starts to form around stage 53 and only remains plastic until about 4 months post-metamorphosis (Udin and Grant, 1999). Although our technique does not allow for quantification of absolute expression levels, it appears that the gradients of expression of ephrin-As and EphAs that are necessary for development of the Xenopus visual system may become more subtle when they are no longer necessary.
In contrast, the high dorsal to low ventral gradient of EphBs over the tectal neuropil becomes high uniform staining during early metamorphosis, when retinotectal development is ongoing. Ephrin-Bs were not detected in the tectal lobes at any timepoint we examined. The lack of change in the expression pattern of EphBs or ephrin-Bs between development and adulthood suggests that EphBs and ephrin-Bs may have roles in the tectum that are independent of the timeline of retinal axon ingrowth.
Overall, the expression patterns of Ephs and ephrins that we report are similar to those described in larger Ranid frogs (Bach et al., 2003; Scalia et al., 2009; Scalia and Feldheim, 2005; Yagita et al., 2005). The expression of ephrin-As in Xenopus and Rana is quite similar, although there is staining in some diencephalic nuclei in Rana (e.g., the lateral geniculate nucleus) that we did not detect in Xenopus. We also see strong staining for ephrin-As in a caudal region of the telencephalon in older stages which is not observed in Rana. The patterns of EphA and ephrin-B staining are also generally comparable across frog species. The rotation of the EphA and ephrin-A gradients from rostral/caudal to rostrolateral/caudomedial with age is common between Xenopus and Rana. Furthermore, in both Xenopus and Rana, EphBs are expressed tectally in a high caudomedial to low rostrolateral gradient in young animals that becomes high uniform staining in adults (Scalia et al., 2009). In contrast with the overall similarities of ephrin expression patterns in different species of frog, in Rana the gradients of EphAs and ephrin-As appear to persist at low levels into adulthood, which is not the case in Xenopus (Bach et al., 2003).
Additionally, ephrin-B has been reported to be expressed at the optic chiasm of Xenopus frogs starting at metamorphosis as well as in embryonic mice, acting to repel EphB-expressing axons from entering the contralateral optic tract, and resulting in an ipsilateral projection (Nakagawa et al., 2000; Williams et al., 2003). Elevated ephrin-B staining was not evident at the ventral surface of the optic chiasm in our whole-mount stage 59 or 66 preparations, which is likely due the fact that the ephrin-B2-expressing midline radial glia are located directly dorsal to the optic chiasm, closer to the ventricle, and therefore less readily revealed in whole-mount preparations than in tissue sections.
We were surprised to find that the conspicuous expression pattern of EphB protein that we observed in the optic tectum was not consistent with that predicted by the prevailing model for dorsoventral RGC axon guidance by ephrin-B/EphB attractive signaling. The dorsoventral gradient of EphB protein that we report in young animals had the opposite orientation to the graded expression of EphB1 mRNA described by Mann et al. (2002) in stage 39 Xenopus larvae. The contrasting results could possibly be explained by differential localization or regulation of EphB message and protein. Additionally, our ephrin-B1-Fc probe is expected to bind to several EphB isoforms in the tissue, whereas the published in situ assay (Mann et al., 2002) was specific for EphB1 (although EphB1 is the preferred binding partner for ephrin-B1) (Flanagan and Vanderhaeghen, 1998). One other report also claimed high ventral to low dorsal expression of EphB protein in Xenopus (Lim et al., 2010). This study also examined coronal sections, but did not identify the zone of RGC axon targeting in the tectal neuropil, and therefore may have mistaken the tegmentum, (a structure immediately ventral to the tectum which stains intensely for EphBs; Fig. 3B', Suppl. Fig. 1) for ventral tectal neuropil.
Furthermore, we also found that the early gradient of EphBs became uniform staining across the tecta of late stage tadpoles. Both the high dorsal to low ventral gradient of EphBs that we observed and the uniform EphB staining in older animals are inconsistent with dorsoventral RGC axon guidance through an attractive interaction mediated by EphB/ephrin-B reverse signalling.
In the present study, staining of whole-mount brains with EphB2-AP revealed only midline staining for ephrin-Bs in the tectum, and did not detect ephrin-Bs in the optic tract. In agreement with our data, Lim and coworkers reported uniform, low ephrin-B staining in the stage 45 tectum using EphB2-Fc probes (Lim et al., 2010), and several papers have reported a lack of staining for ephrin-B in the amphibian optic tract (Scalia and Feldheim 2005; Scalia et al., 2009) despite detection of a high dorsal to low ventral gradient of ephrin-Bs in the retina (Scalia et al., 2009). In contrast, Mann et al. (2002) reported staining for ephrin-Bs on neurites from explants of dorsal but not ventral retina using affinity probes. It is possible that ephrin-B is not expressed on RGC axons but rather on amacrine cells or ganglion cell dendrites in the retinal inner plexiform layer (Braisted et al., 1997). Alternatively, this apparent discrepancy in expression patterns may be explained by the phenomenon of “masking”, in which the Eph receptor-reporter fusion proteins are unable to displace existing Eph/ephrin binding already present in the tissue, and therefore underreport absolute protein levels (Sobieszczuk and Wilkinson, 1999). It should be noted that the binding of fusion proteins would consequently be expected to report the amounts of protein available to interact with newly ingrowing axons, which is presumably the parameter most relevant for topographic axon guidance. It is therefore possible that ephrin-Bs are indeed expressed on RGC axons, but at far lower levels than EphBs in the tectum. It may be possible to resolve this issue through the use of immunohistochemistry, as specific antibodies against other epitopes on Xenopus Ephs and ephrins become available, or by performing in situ hybridization of tectal cells.
The presence of a gradient of available EphB protein in retinorecipient tectum in the opposite orientation to that predicted by in situ hybridization calls for a reconsideration of the prevailing model of dorsoventral mapping in Xenopus, in which ephrin-B-expressing axons from dorsal retina (Mann et al., 2002) are directed to the hypothetical high levels of EphBs in ventral tectum. EphB/ephrin-B forward signalling would be an alternative candidate mechanism, given its implication in topographically-directed axonal branching in murine retinocollicular development (Hindges et al., 2002; McLaughlin et al., 2003b). However, fusion protein staining reveals very low levels of ephrin-Bs on the surface of Xenopus tectum, and certainly no mediolateral gradient. These results suggest that the primary function of EphB/ephrin-B interactions in the tectum may not be dorsoventral mapping.
EphBs may perform a role independent of axon guidance in the tectum. One possibility is suggested by the close relationship of the peak of EphB expression in the early larva to the zone of transition between proliferation and differentiation during tectal cellular development; an EphB isoform expressed on postmitotic cells could help guide them out of the proliferative zone surrounding the ventricle to their destination below the pial surface (Scalia et al., 2009). Our whole-mount experiments and others report ephrin-B expression only at the tectal midline (Scalia et al., 2009; Scalia and Feldheim, 2005), but it is possible that ephrin-Bs are expressed deeper within the tectum on glial fibres, at levels high near the ventricle and low at the tectal surface.
Another possible role for the tectal EphB expression patterns may be in retinotectal synapse formation and functioning, give the well-established role for EphBs in synaptogenesis (Dalva et al., 2000; Kayser et al., 2006; Kayser et al., 2008). The neuropil region in early stage tadpoles appears to roughly coincide with the region of graded EphB expression that we see in early Xenopus development (Fig. 4D,D'). In addition, application of EphB2-Fc to the Xenopus optic tectum has been reported to enhance the formation and efficacy of presynaptic sites in RGC axon terminals, probably through reverse signaling (Lim et al., 2008a). Tectal cell proliferation is complete at stage 58 (Straznicky and Gaze, 1972), around the same time the tectal EphB gradient adopts a more homogeneous expression pattern. It is possible that the gradient of EphB expression may reflect both synaptogenesis and synapse maintenance roles, and the pattern of expression normalizes after the peak of synaptogenesis is complete.
If EphB/ephrin-B signalling does not specify dorsoventral mapping in the retinotectal projection of frogs, how is it achieved? Alternative candidates have recently been put forward for regulation of dorsoventral retinotectal mapping. Wnt3 is expressed in a high medial to low lateral gradient in chick tectum and mouse colliculus, and appears to have a bifunctional mapping role: high concentrations of Wnt3 repel retinal axons away from the zebrafish tectal midline through the high ventral to low dorsal retinal gradient of Ryk receptor, whereas low concentrations of Wnt3 attract dorsal RGC axons to the tectum through a lower-affinity interaction with Frizzleds (Schmitt et al., 2006). It has also been suggested that a high ventral to low dorsal gradient of semaphorin-3D may play a role in dorsoventral mapping by repelling axons from ventral tectum, thereby ensuring that they correctly innervate dorsal tectum (Liu et al., 2004). Furthermore, some degree of pretarget sorting of RGC axons in the dorsoventral, but not nasotemporal, axis appears to take place in the optic tract (Plas et al., 2005).
The second aim of this paper was to investigate whether a respecification of the normal pattern of ephrins could contribute to the formation or maintenance of ocular dominance bands in the experimentally-induced binocularly innervated tectum. To the contrary, we found that the main guidance molecules involved in patterning the rostrocaudal axis, EphAs and ephrin-As, were expressed in a relatively normal, unmodulated gradient in early postmetamorphic brains from Xenopus with binocularly innervated tecta.
Binocular innervation of the tectum has been proposed to lead to a situation in which the graded expression of guidance molecules should favor the contiguous and overlapping retinotopic mapping of afferents from both eyes, while patterns of neural activity should promote the organized aggregation of inputs from the same eye to the exclusion of axons from the other eye (Ruthazer and Cline, 2004). Coincident spiking of neighboring RGCs in one eye is detected by NMDA receptors on the postsynaptic tectal cells onto which they converge (Cline et al., 1987). These synaptic contacts are stabilized (Rajan et al., 1999), thereby encouraging coactive RGCs to remain neighbors, and branches projecting to inappropriate (opposite-eye) locations are selectively withdrawn (Ruthazer et al., 2003). In a tectal lobe innervated by axons from both eyes, the formation of ocular dominance columns appears to be a compromise between guidance through molecular cues and eye-specific correlated activity patterns, each of which favors a different innervation outcome (Cline et al., 1987; Ruthazer and Cline, 2004).
The pattern of ephrin-A expression was assayed at stage 66 to ensure the animals had well-established ocular dominance bands. However, by looking specifically at older stages we may have missed an early developmental period during which the pattern of guidance cues was altered by binocular innervation. This seems unlikely, as the maintenance of ocular dominance bands even in postmetamorphic froglets requires ongoing retinal activity and tectal NMDAR activation (Cline et al., 1987; Reh and Constantine-Paton, 1985). Thus our data support the notion that the formation and maintenance of ocular dominance bands reflect a instructive mechanism by which neural activity and correlated firing with postsynaptic cells directly shapes axonal growth and branching, though the possibility remains that other unexamined guidance molecules could have been respecified by binocular innervation,
This study provides insight into how Eph and ephrin expression patterns change over the life of Xenopus laevis. Our findings are consistent with models in which the graded expression of ephrin-As and EphAs specifies mapping of the nasotemporal axis of the retina onto the rostrocaudal axis of the tectum. However, the specific mechanisms by which ephrin-B/EphB signaling contributes to dorsoventral retinotopic mapping in Xenopus require reconsideration. Additionally, the presence of relatively normal expression patterns of Ephs and ephrins in binocularly-innervated animals renews the question of what cues are responsible for the retrograde signalling of correlations detected by the postsynaptic tectal neuron back to its presynaptic RGC axonal partners.
Supplementary Material
A side view color image of a stage 48 Xenopus brain stained with ephrin-B1-AP at half-concentration. From the side, the region of maximum EphB expression appears as a crescent shape with a graded decrease in staining in the rostrolateral direction. Scale bar=100 μm.
A side view color image of a TK-I Rana pipiens brain (the equivalent of stage 46 in Xenopus) stained with ephrin-B1-AP at a 1:10 dilution. EphB is expressed in a crescent shape with high caudomedial and low rostrolateral expression. Scale bar=250um.
Acknowledgments
Many thanks to John Flanagan for the initial gift of fusion proteins and expression vectors. The authors would also like to acknowledge the contributions of Jena Yamada, Anne Schohl, Jisheng Liu, and Kirill Satanovsky to these experiments. This study was supported by CIHR grant MOP-77567 to ESR and NIH grant R01-EY014689 to DAF. VH holds a doctoral Canada Graduate Scholarship from NSERC. ESR is a tier 2 Canada Research Chair.
Table of Abbreviations
- ot
optic tract
- tec
tectum
- aob
accessory olfactory bulb
- ob
olfactory bulb
- ch
cerebral hemisphere
- cal
central anterolateral nucleus
- ic
implantation cones of the olfactory bulb
- hb
habenular nuclei
- hc
habenular commissure
- tel
telencephalon
- di
diencephalon
- p
precommissural area
- po
preoptic area
- hyp
hypothalamus
- st
striatum
- mot
marginal zone of the optic tract
- on
optic nerve
- teg
tegmentum
- telc
caudal region of the telencephalon
References
- Bach H, Feldheim DA, Flanagan JG, Scalia F. Persistence of graded EphA/Ephrin-A expression in the adult frog visual system. J Comp Neurol. 2003;467(4):549–65. doi: 10.1002/cne.10941. [DOI] [PubMed] [Google Scholar]
- Braisted JE, McLaughlin T, Wang HU, Friedman GC, Anderson DJ, O'Leary DD. Graded and lamina-specific distributions of ligands of EphB receptor tyrosine kinases in the developing retinotectal system. Dev Biol. 1997;191(1):14–28. doi: 10.1006/dbio.1997.8706. [DOI] [PubMed] [Google Scholar]
- Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9(3):322–30. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- Cline H. Sperry and Hebb: oil and vinegar? Trends Neurosci. 2003;26(12):655–61. doi: 10.1016/j.tins.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Cline HT, Debski EA, Constantine-Paton M. N-methyl-D-aspartate receptor antagonist desegregates eye-specific stripes. Proc Natl Acad Sci U S A. 1987;84(12):4342–5. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–54. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, Greenberg ME. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103(6):945–56. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Glennemeier KA, Boorse GC. Endocrinology of Complex Life Cycles: Amphibians. In: Pfaff DW, Arnold AP, Etgen AM, Rubin RT, Fahrbach SE, editors. Hormones, Brain, and Behaviour. Elsevier; 2002. pp. 469–513. [Google Scholar]
- Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17(5):230–8. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Kim YI, Bergemann AD, Frisen J, Barbacid M, Flanagan JG. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron. 2000;25(3):563–74. doi: 10.1016/s0896-6273(00)81060-0. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, O'Leary DD. Visual map development: bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harb Perspect Biol. 2010;2(11):a001768. doi: 10.1101/cshperspect.a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JG, Cheng HJ, Feldheim DA, Hattori M, Lu Q, Vanderhaeghen P. Alkaline phosphatase fusions of ligands or receptors as in situ probes for staining of cells, tissues, and embryos. Methods Enzymol. 2000;327:19–35. doi: 10.1016/s0076-6879(00)27264-9. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–45. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Frisen J, Yates PA, McLaughlin T, Friedman GC, O'Leary DD, Barbacid M. Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron. 1998;20(2):235–43. doi: 10.1016/s0896-6273(00)80452-3. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17(1):9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Gallarda BW, Bonanomi D, Müller D, Brown A, Alaynick WA, Andrews SE, Lemke G, Pfaff SL, Marquardt T. Segregation of axial motor and sensory pathways via heterotypic trans-axonal signaling. Science. 2008;320(5873):233–6. doi: 10.1126/science.1153758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Keating MJ. Ocular migration and the metamorphic and postmetamorphic maturation of the retinotectal system in Xenopus laevis: an autoradiographic and morphometric study. J Embryol Exp Morph. 1986;92:43–69. [PubMed] [Google Scholar]
- Haas K, Jensen K, Sin WC, Foa L, Cline HT. Targeted electoporation in Xenopus tadpoles in vivo-from single cells to the entire brain. Differentiation. 2002;70(4–5):148–54. doi: 10.1046/j.1432-0436.2002.700404.x. [DOI] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43(5):687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Harris WA, Holt CE, Bonhoeffer F. Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos: a time-lapse video study of single fibres in vivo. Development. 1987;101(1):123–33. doi: 10.1242/dev.101.1.123. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7(5):501–9. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O'Leary DD. EphB forward signaling controls directional branch extension and arborization required for dorsalventral retinotopic mapping. Neuron. 2002;35(3):475–87. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Hornberger MR, Dutting D, Ciossek T, Yamada T, Handwerker C, Lang S, Weth F, Huf J, Wessel R, Logan C, Tanaka H, Drescher U. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron. 1999;22(4):731–42. doi: 10.1016/s0896-6273(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26(47):12152–64. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser MS, Nolt MJ, Dalva MB. EphB receptors couple dendritic filopodia motility to synapse formation. Neuron. 2008;59(1):56–69. doi: 10.1016/j.neuron.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MI, Constantine-Paton M. Right and left eye bands in frogs with unilateral tectal ablations. Proc Natl Acad Sci U S A. 1980;77(4):2314–8. doi: 10.1073/pnas.77.4.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Cho SJ, Sumbre G, Poo MM. Region-specific contribution of ephrin-B and Wnt signalling to receptive field plasticity in developing optic tectum. Neuron. 2010;65(6):899–911. doi: 10.1016/j.neuron.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Lim BK, Matsuda N, Poo MM. Ephrin-B reverse signaling promotes structural and functional synaptic maturation in vivo. Nat Neurosci. 2008a;11(2):160–9. doi: 10.1038/nn2033. [DOI] [PubMed] [Google Scholar]
- Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O'Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008b;59(5):746–58. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Berndt J, Su F, Tawarayama H, Shoji W, Kuwada JY, Halloran MC. Semaphorin3D guides retinal axons along the dorsoventral axis of the tectum. J Neurosci. 2004;24(2):310–8. doi: 10.1523/JNEUROSCI.4287-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann F, Ray S, Harris W, Holt C. Topographic mapping in dorsoventral axis of the Xenopus retinotectal system depends on signaling through ephrin-B ligands. Neuron. 2002;35(3):461–73. doi: 10.1016/s0896-6273(02)00786-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, O'Leary DD. Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol. 2003a;13(1):57–69. doi: 10.1016/s0959-4388(03)00014-x. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, Yates P, O'Leary D. Bifunctional action of ephrin-B1 as a repellent and attractant to control bidirectional branch extension in dorsal-ventral retinotopic mapping. Development. 2003b;130:2407–2418. doi: 10.1242/dev.00467. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, O'Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–55. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Macchi P, Deitinghoff L, Andersen JS, Mann M, Flad M, Hornberger MR, Stahl B, Bonhoeffer F, Mueller BK. RGM is a repulsive guidance molecule for retinal axons. Nature. 2002;419(6905):392–5. doi: 10.1038/nature01041. [DOI] [PubMed] [Google Scholar]
- Murai KK, Pasquale EB. 'Eph'ective signaling: forward, reverse and crosstalk. J Cell Sci. 2003;116(Pt 14):2823–32. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Brennan C, Johnson KG, Shewan D, Harris WA, Holt CE. Ephrin-B regulates the ipsilateral routing of retinal axons at the optic chiasm. Neuron. 2000;25(3):599–610. doi: 10.1016/s0896-6273(00)81063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X, Voyatzis S, Muzerelle A, Narboux-Nême N, Südhof TC, Miles R, Gaspar P. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat Neurosci. 2007;10(3):340–7. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, McLaughlin T. Mechanisms of retinotopic map development: Ephs, ephrins, and spontaneous correlated retinal activity. Prog Brain Res. 2005;147:43–65. doi: 10.1016/S0079-6123(04)47005-8. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin promiscuity is now crystal clear. Nat Neurosci. 2004;7(5):417–8. doi: 10.1038/nn0504-417. [DOI] [PubMed] [Google Scholar]
- Plas DT, Lopez JE, Crair MC. Pretarget sorting of retinocollicular axons in the mouse. J Comp Neurol. 2005;491(4):305–19. doi: 10.1002/cne.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan I, Witte S, Cline HT. NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J Neurobiol. 1999;38(3):357–68. doi: 10.1002/(sici)1097-4695(19990215)38:3<357::aid-neu5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rashid T, Upton AL, Blentic A, Ciossek T, Knoll B, Thompson ID, Drescher U. Opposing gradients of ephrin-As and EphA7 in the superior colliculus are essential for topographic mapping in the mammalian visual system. Neuron. 2005;47(1):57–69. doi: 10.1016/j.neuron.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Reh TA, Constantine-Paton M. Eye-specific segregation requires neural activity in three-eyed Rana pipiens. J Neurosci. 1985;5(5):1132–43. doi: 10.1523/JNEUROSCI.05-05-01132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301(5629):66–70. doi: 10.1126/science.1082545. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Cline HT. Insights into activity-dependent map formation from the retinotectal system: a middle-of-the-brain perspective. J Neurobiol. 2004;59(1):134–46. doi: 10.1002/neu.10344. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Haas K, Javaherian J, Jensen K, Sin WC, Cline HT. In: In Vivo Time-lapse Imaging of Neuronal Development in Imaging in Neuroscience and Development: A Laboratory Manual. Yuste R, Konnerth A, editors. Cold Spring Harbor Press; Cold Spring Harbor, NY: 2005. [Google Scholar]
- Scalia F, Currie JR, Feldheim DA. Eph/ephrin gradients in the retinotectal system of Rana pipiens: developmental and adult expression patterns. J Comp Neurol. 2009;514(1):30–48. doi: 10.1002/cne.21968. [DOI] [PubMed] [Google Scholar]
- Scalia F, Feldheim DA. Eph/ephrin A- and B-family expression patterns in the leopard frog (Rana utricularia) Brain Res Dev Brain Res. 2005;158(1–2):102–6. doi: 10.1016/j.devbrainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439(7072):31–7. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Sobieszczuk DF, Wilkinson DG. Masking of Eph receptors and ephrins. Curr Biol. 1999;9(13):R469–70. doi: 10.1016/s0960-9822(99)80296-6. [DOI] [PubMed] [Google Scholar]
- Stafford BK, Sher A, Litke AM, Feldheim Spatial-temporal patterns of retinal waves underlying activity-dependent refinement of retinofugal projections. Neuron. 2009;64(2):200–12. doi: 10.1016/j.neuron.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straznicky C, Glastonbury J. Anomalous ipsilateral optic fibre projection in Xenopus induced by larval tectal ablation. J Embryol Exp Morphol. 1979;50:111–22. [PubMed] [Google Scholar]
- Straznicky C, Tay D, Hiscock J. Segregation of optic fibre projections into eye-specific bands in dually innervated tecta in Xenopus. Neurosci Lett. 1980;19(2):131–6. doi: 10.1016/0304-3940(80)90183-4. [DOI] [PubMed] [Google Scholar]
- Straznicky K, Gaze RM. The growth of the retina in Xenopus laevis: an autoradiographic study. J Embryol Exp Morphol. 1971;26(1):67–79. [PubMed] [Google Scholar]
- Straznicky K, Gaze RM. The development of the tectum in Xenopus laevis: an autoradiographic study. J Embryol Exp Morphol. 1972;28(1):87–115. [PubMed] [Google Scholar]
- Straznicky K, Hiscock J. Post-metamorphic retinal growth in Xenopus. Anat Embryol (Berlin) 1984;169(1):103–9. doi: 10.1007/BF00300592. [DOI] [PubMed] [Google Scholar]
- Udin SB, Grant S. Plasticity in the tectum of Xenopus laevis – binocular maps. Prog Neurobiol. 1999;59(2):81–106. doi: 10.1016/s0301-0082(98)00096-3. [DOI] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39(6):919–35. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Wizenmann A, Brunet I, Lam JS, Sonnier L, Beurdeley M, Zarbalis K, Weisenhorn-Vogt D, Weinl C, Dwivedy A, Joliot A, Wurst W, Holt C, Prochiantz A. Extracellular Engrailed participates in the topographic guidance of retinal axons in vivo. Neuron. 2009;64(3):355–66. doi: 10.1016/j.neuron.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita Y, Barjis I, Hecht M, Bach H, Feldheim DA, Scalia F. Partial nucleotide sequences and expression patterns of frog (Rana pipiens) ephrin-A2 and ephrin-A5 mRNA. Brain Res Dev Brain Res. 2005;159(1):72–7. doi: 10.1016/j.devbrainres.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Yates PA, Roskies AL, McLaughlin T, O'Leary DD. Topographic-specific axon branching controlled by ephrin-As is the critical event in retinotectal map development. J Neurosci. 2001;21(21):8548–63. doi: 10.1523/JNEUROSCI.21-21-08548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Yamashita Y, Noda H, Okafuji T, Go MJ, Tanaka H. EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci Res. 2004;48(3):285–96. doi: 10.1016/j.neures.2003.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A side view color image of a stage 48 Xenopus brain stained with ephrin-B1-AP at half-concentration. From the side, the region of maximum EphB expression appears as a crescent shape with a graded decrease in staining in the rostrolateral direction. Scale bar=100 μm.
A side view color image of a TK-I Rana pipiens brain (the equivalent of stage 46 in Xenopus) stained with ephrin-B1-AP at a 1:10 dilution. EphB is expressed in a crescent shape with high caudomedial and low rostrolateral expression. Scale bar=250um.