Abstract
Over the last three decades, a handful of photochemical mechanisms have been applied to a large number of nanoscale assemblies that encapsulate a payload to afford spatio-temporal and remote control over activity of the encapsulated payload. Many of these systems are designed with an eye towards biomedical applications, as spatio-temporal and remote control of bioactivity would advance research and clinical practice. This review covers five underlying photochemical mechanisms that govern the activity of the majority of photoresponsive nanocarriers: 1. photo driven isomerization and oxidation, 2. surface plasmon absorption and photothermal effects, 3. photo driven hydrophobicity changes, 4. photo driven polymer backbone fragmentation and 5. photo driven de-crosslinking. The ways in which these mechanisms have been incorporated into nanocarriers and how they affect release is detailed, as well as the advantages and disadvantages of each system.
Keywords: photochemical, triggered release, light, near infrared, nanocarriers, nanoparticles
1. Introduction
It is well established that nanocarriers in drug delivery offer many advantages over conventional formulation methods. They can minimize degradation of therapeutic agents upon administration, enhance their in vivo efficiency by delivering higher concentrations of drugs to tumor sites, expose the tumors to active drug for longer periods, and prevent undesirable side effects. In addition, these carriers can protect the therapeutic payload from the harsh in vivo environment. Furthermore, current studies on synergistic therapeutic outcomes of combination therapies are better enabled through the use of drug delivery vehicles.
Organic materials, both natural and synthetic, can be used to tailor nanocarriers to provide specific characteristics, including triggered release on demand. The goal of triggered drug delivery is to control the time and place of release of a therapeutic agent to achieve a higher local concentration, reduce overall injected dose, and reduce systemic toxicity.
Various internal and external triggers, such as pH, specific enzymes, temperature, ultrasound, magnetic field and light are being actively explored. Light is especially attractive, as it can be remotely applied with extremely high spatial and temporal precision. Additionally, a broad range of parameters (wavelength, light intensity, duration of exposure, and beam diameter) can be adjusted to modulate release profiles. Radiation in the UV, visible, and near infrared (NIR) regions can be applied in vivo to induce drug release. Systems responsive to UV and visible irradiation can be used for topical treatments; radiation below 650 nm cannot penetrate deeper than 1 cm into tissue due to high scattering and absorption by hemoglobin, oxy-hemoglobin, and water . NIR light of 650 – 900 nm (water absorbs wavelengths longer than 900 nm) can penetrate up to 10 cm [1] into living tissue and causes minimal tissue damage at the site of application.
This review focuses on light-triggered release from nanosystems. In this size regime one can passively target diseased tissues like tumors by exploiting the enhanced permeation and retention (EPR) effect while at the same time remotely and actively trigger release via light. The structure of this review reflects different mechanisms by which therapeutic agents may be released from nanocarriers upon light exposure. We cover many different nanocarrier types developed to date, including micelles, polymeric nanoparticles, hollow metal nanoparticles, and liposomes as examples of different triggering mechanisms using various photochemical reactions in order to facilitate release of cargo from the nanocarrier. All reactions lead to a change in the nanocarrier assembly either directly or indirectly, which leads to release of the encapsulated bioactive agent. While other reviews have focused on the photo-triggered release of particular nanocarriers (liposomal systems [2-3], micelles [4-6], etc.) separately, we would like to focus on the mechanism of release rather than the nanocarrier. It should be noted that while the choice of nanocarrier can vary based on the application desired, the photochemistry involved could be applied to multiple materials and the challenges with each mechanism need to be addressed. We have also limited the scope of our review to systems for which release of cargo from nanocarriers has been demonstrated.
2. Mechanisms of light-triggered release from nanocarriers
I. Photoisomerization, photocrosslinking, and photosensitization-induced oxidation
Photoisomerization is a process that involves a conformational change about a bond that is restricted in rotation, usually a double bond. In organic molecules with double bonds, this predominantly involves isomerization from a trans orientation to a cis form upon irradiation with light. Azobenzenes, which have -N=N- with phenyl rings on either side, are the most commonly used molecules for this purpose. The planar trans form of azobenzenes is more hydrophobic than the nonplanar cis form, so cis azobenzenes form micelles less easily. UV irradiation-induced conversion of azobenzenes to trans causes disruption of the assemblies. Azobenzenes are attractive because the isomerization is reversible, which is important in applications that require drug delivery on demand.
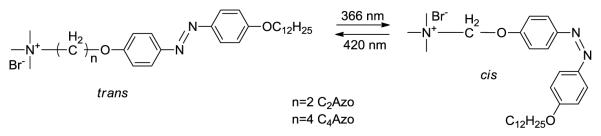
The first report of incorporating azobenzene in a nanocarrier system to effect release was reported by Kano et al. in 1980 [7]. In their work they incorporated an amphiphilic azobenzene (C2Azo and C4Azo, Figure 1) moiety along with dipalmitoylphosphatidylcholine (DPPC) at various molar ratios and were able to modulate the release profiles of liposomes based on the azo moiety of choice, the composition of photo-stationary state, and the degree of incorporation in the liposome. They characterized the photoisomerization process via UV spectroscopy by irradiating the trans azo compound at 366 nm for 10 seconds. The trans compound formed a photo-stationary state with 80% cis isomer which reverts back to trans when irradiated at >420 nm. They also measured the resulting osmotic shrinkage of the vesicles upon incorporation of azo compound (C2Azo & C4Azo) by measuring the optical density of the solutions. The authors encapsulated bromothymol, a blue dye, in the lipid bilayer of liposomes formulated from DPPC and subsequently showed that the permeation of the dye into water increased with greater incorporation of the cis azobenzene moiety (formed by irradiation). In these pioneering studies, percent release and duration of release upon pulsing were not entirely characterized.
Figure 1.
Structure of amphiphatic azobenzene moiety utilized in the study by Kano et al [7].
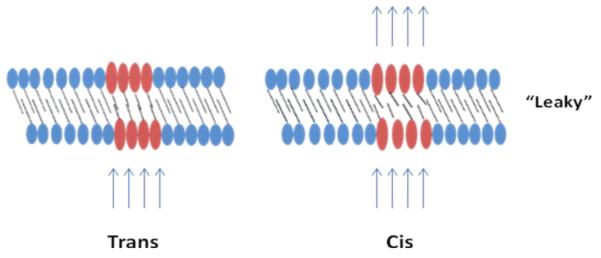
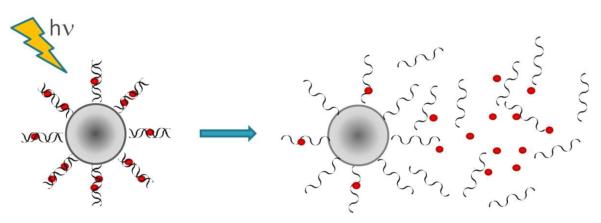
Since this seminal study there have been numerous publications utilizing this concept. Many systems developed since have incorporated azobenzenes in lipid backbones and formulated liposomes that are photo-responsive [8-13]. The photo-responsiveness of the liposomes arises from the fact that in the trans orientation the molecules pack tightly in the bilayer. When irradiated with UV light, they undergo trans-cis isomerization, which leads to distortions in the packing of the bilayer and causes the liposomes to become “leaky,” allowing the encapsulated drugs to be released (Figure 2). Irradiation of azobenzene results in the formation of a photo-stationary state and the composition of this state determines the release rate of the drug. More recently, Smith et al. have used photo-triggerable liposomes to trigger gelation of an alginate solution by releasing calcium chloride upon irradiation with 385 nm light for 1min. Such on demand gelation is important in tissue engineering applications [14].
Figure 2.
Schematic representation of a cross-section of lipid bilayer packed with trans-azobenzenes that photoisomerize upon irradiation into the cis-form and cause distortions in the lipid packing.
The photoisomerization concept has also been successfully utilized in preparation of photo-responsive micelles. These systems take advantage of the change in net dipole moment upon switching from the trans orientation (no net dipole moment) to the cis orientation. This leads to disruption in the hydrophobic-hydrophilic balance of the self-assembled micelles and causes reorganization and subsequent release of encapsulated contents [5].
The first report of utilizing azobenzene to encapsulate a model hydrophobic substance involved an azobenzene-based surfactant, 4-butylazobenzene-4′-(oxyethyl) trimethylammonium bromide (AZTMA). In this preliminary study the authors encapsulated ethylbenzene and showed its release upon irradiation with UV light. They also found the process to be reversible when irradiating with visible light. The authors quantified release by measuring the vapor pressure of ethyl benzene in the headspace, which increased upon irradiation with UV light (260-390nm at 10mW/cm2) for 2 hrs. They found that at an AZTMA concentration of 5mM, the vapor pressure of ethyl benzene increased to values equivalent to those for pure ethyl benzene, suggesting complete release [15-16].
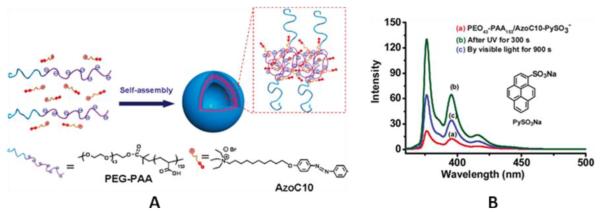
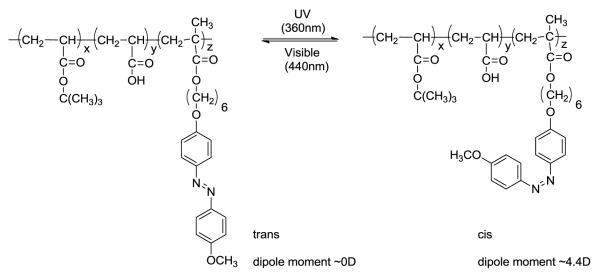
In later work Wang et al. [17] developed a cationic azobenzene-based surfactant to form an ionomer with a doubly hydrophilic block copolymer of polyethylene glycol and poly acrylic acid (Figure 3A) that self assembled into vesicle like aggregates. They showed that when pyrene sulfonic acid-containing aggregates were irradiated ( 360 nm) for 300s, the azo benzene underwent photoisomerization leading to release of the dye, as seen by an increase in fluorescence of the solution. When irradiated by visible light (440 nm) for 900s, they observed partial quenching of the solution, leading them to conclude the dye was re-encapsulated. The authors explain the incomplete re-encapsulation upon visible light irradiation as irreversible release of the dye (Figure 3B).
Figure 3.
A) Schematic representation of the formation of ionomer vesicles from AzoC10 and PEG-PAA. B) fluorescence emission spectra of pyrene sulfonic acid, a) before, b) after UV, and c) after visible irradiation [17].
Recently, Zhao et al. used this concept in preparing the first macromolecular diblock copolymer micelles [18]. While there have been numerous such reports of azobenzene-based block copolymers for photo-responsive micelles, little has been done towards utilizing these diblock copolymer systems for controlled release applications [5-6, 19-21].
Another area where azobenzenes are used is in making nano-impellers for drug delivery applications. Patnaik et al. [22] made azodextran-based nanogels in which 5% and 10 % of linear dextran moieties were functionalized with a hydrophobic derivative of azobenzene (AD-5 and AD-10, Figure 5). This resulted in the self-assembly of the dextran chains to form nanostructures. The self-assembly is driven by stacking of the flat, linear azobenzene groups. When irradiated at 365 nm, these nanogels undergo photoisomerization, which leads to disruption of stacking and release of the contents. The authors showed that release of model dyes and drugs from AD-5 and AD-10 when irradiated with 365nm is directly proportional to irradiation time (0-300min). They also showed that AD-10 encapsulates and releases more drug than AD-5 due to increased azobenzene content.
Figure 5.
Schematic representation of non-covalently crosslinked azo-dextran nanogels, AD [22].
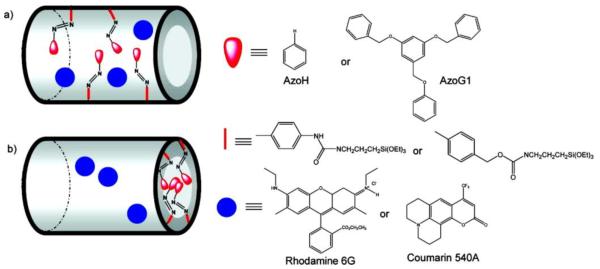
Nano-impellers have also been made to form core-shell mesoporous silica structures. Angelos et al. [23] incorporated azobenzene moieties into mesoporous silica particles that function both as impellers and “gatekeepers” to retain the encapsulated drug and release it on demand (Figure 6). The mechanism of release relies on continuous photoisomerization reactions within the particles, resulting in “wagging” of the polymer strands that form the gates of these structures and release. In order to achieve wagging, the particles were irradiated using 9 mW 457 nm light continuously for 1200s. At this wavelength both cis and trans isomers absorb and photoisomerize with a quantum yield of 0.64 and 0.36, respectively. The released dye was monitored at 540 nm by sampling the solution of particles at one second intervals, which revealed that no dye was released in the absence of irradiation when AzoG1 was used. In comparison, particles formulated with AzoH were leaky even without irradiation. Subsequently, Lu et al. [24] showed that these particles can be used to deliver the anticancer drug camptothecin to cancer cells on demand.
Figure 6.
Photoresponsive materials functionalized with azobenzene derivatives. (a) nano-impellers prepared by the co-condensation ethod derivatized with AzoH; (b) triggered release materials prepared by the post-synthesis modification method derivatized with AzoG1 [23].
The major advantage of the azobenzene systems is reversibilty, which may be used to turn the systems on and off and allow dosed release on demand. In spite of their promise, however, systems that rely predominantly on UV irradiation suffer from a lack of translation in vivo due to low tissue transparency in the UV region.
Photo-crosslinking or photopolymerization as a means of release might seem counterintuitive, because photo-induced crosslinking is generally used in the formation of nanoparticles. However, this photochemical mechanism can also be used for photo-triggered release. Photo-crosslinking is achieved by irradiating a polymerizable double bond directly or in the presence of a radical initiator/sensitizer. Photopolymerization of double bonds incorporated into the hydrophobic domain of a bilayer causes parts of the bilayer to shrink, disrupting the uniform packaging of the molecules and producing pores in the bilayer, which allows release.
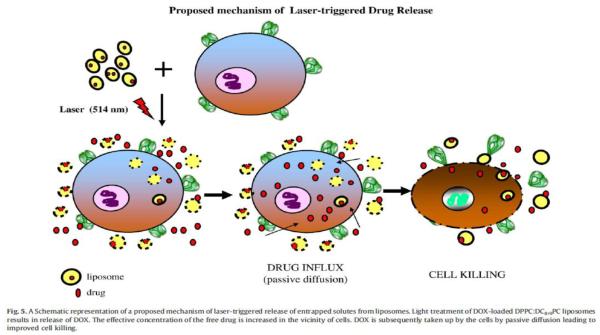
The concept was first realized in liposomes by Regen et al. [25]. Vesicles were formulated with a photo-triggerable (at 254nm) lipid containing two methacrylated phosphatidyl choline derivatives. The resulting vesicles were more stable than non-crosslinked counterparts, which resulted in better circulation. The authors also noted that the leakage rate could be controlled by co-polymerizing the crosslinkable lipids with the homo-polymerizable lipids. Subsequent studies reported systems with modulated rates of release. Some later studies also incorporated sensitizers to absorb at higher wavelengths so that photo-crosslinking could be achieved in the visible region [2-4]. Some recent advances in this area include designing a new class of liposomes containing 1,2 bis-(tricosa-10, 12-dinoyl)-sn-glycero-3-phosphocholine) (DC8,9PC) that have photo-crosslinkable triple bonds [26]. The authors have shown that these liposomes can be used to deliver doxorubicin (DOX). In addition to performing its cell killing functions, doxorubicin also served as a photosensitizer, resulting in photo-crosslinking of the liposomes upon irradiation at 514 nm. The authors showed that when irradiated with 514 nm (166 mW/cm2/min) light for 0-7 min, up to a 22% higher release of DOX occurred compared to the non-irradiated samples. This was the first report of a drug being released photochemically from a liposomal formulation (Figure 7).
Figure 7.
Schematic representation of the proposed mechanism of laser triggered release of entrapped solutes form liposomes. Light treatment of DOX-loaded DPPC:DC8,9PC liposomes results in release of DOX. The effective concentration of the free drug is increased in the vicinity of cells. DOX is subsequently taken up by cells by passive diffusion leading to improved cell killing. [26]
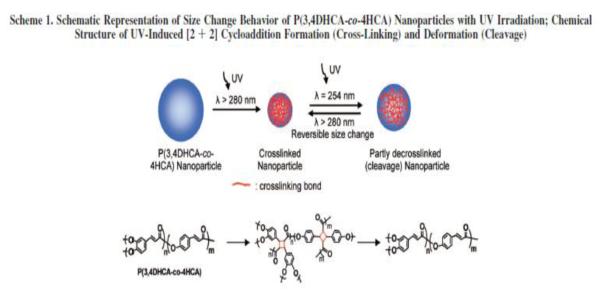
In addition to liposomes, photocrosslinking has also been used as a means for drug delivery in nanocapsules formulated through layer-by-layer deposition of polymers. Park et al. formulated microcapsules by depositing alternating layers of benzophenone-modified poly-(allylamine hydrochloride) and poly-(sodium-4-styrenesulfonate) on polystyrene particles [27-28]. They subsequently dissolved the polystyrene core in organic solvent to obtain hollow capsules. The benzophenone modified poly-(allylamine hydrochloride) moieties are photocrosslinkable and the release rates of encapsulated molecules from the capsules can be controlled by varying the degree of crosslinking of the poly-(allylamine hydrochloride) layer. It is important to emphasize that this work does not use complete photodegradation as a trigger; rather, it uses photocrosslinking as a means to achieve control over release rates. More recent work on this type of polymers incorporates cinnamic acid derivatives in their backbone. The idea in these systems is to utilize the [2+2] cycloaddition reaction of trans cinnamic acids upon photo-irradiation that results in shrinkage of the nanostructures to expel encapsulated contents (Figure 8). [29-30]
Figure 8.
Schematic representation of size change behavior of P(3,4DHCA-co-4HCA) nanoparticles with UV irradiation. Chemical structure of UV-induced [2+2] cycloaddition formation (cross-linking) and deformation (cleavage) [29-30].
Photosensitization-induced oxidation is another photochemical mechanism to impart a change in a nanocarrier through light exposure. Photosensitization-induced oxidation involves generation of a strong oxidizing agent, singlet oxygen, upon illumination of a sensitizer molecule with an appropriate wavelength of light. Singlet oxygen oxidizes plasmogenic lipids and thus causes disruption of biomembranes [31-39]. This mechanism is currently used in photodynamic therapy to disrupt the membrane of cancer cells and induce cell death. The same chemical process can be used to enable photo-controlled release of therapeutic agents from nanocarriers composed of photooxidizable lipids.Lipid photo-oxidation leads to membrane disruption because singlet oxygen formed by irradiation of ZnPc in air leads to the photo-oxidation of the plasmalogen vinyl ether linkage (Fig. 9). [40] The formation of a single-chain surfactant induces a lamellar to hexagonal phase transition, leading to membrane fusion and leakage of the encapsulated content.
Figure 9.
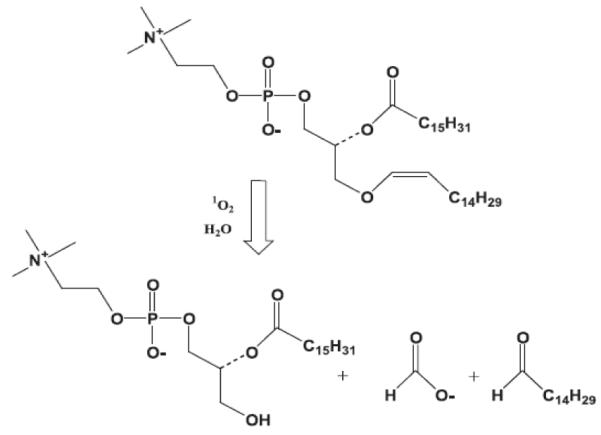
Singlet oxygen mediated photo-oxidation of plasmenylcholine leading to cleavage of vinyl ether linkages [40-41].
The first report of photo-oxidation-controlled release of hydrophilic agents from liposomes was published by Anderson et al. in 1992 [41]. This study demonstrated visible light-triggered release of glucose from liposomes composed of semi-synthetic plasmalogen lipids (1-alk-1′-enyl-2-palmitoyl-sn-glycero-3-phosphocholine (PlasPPC)/ 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) (8:1)) with the photosensitizer zinc phthalocyanine (ZnPc) incorporated within the hydrophobic region of the membrane. Irradiation of air-saturated liposomes with visible light at 37°C for 60 min resulted in release of 62% of encapsulated glucose, twice the amount released in the corresponding dark control experiment.
Several studies utilizing photosensitization to promote endosomal escape of nanoparticles and thus facilitate cargo delivery to the cytosol have appeared in recent years. In this approach light is applied after the particles are endocytosed. Upon irradiation, the photosensitizer encapsulated within the delivery vehicles acts on the lipids that constitute the endosomal membrane, disrupting the lipid bilayer and resulting in the release of particles into cytosol. Berg and coworkers used photosensitizers to mediate endosomal rupture for improved cellular delivery of nucleic acids [42]. Harnessing this mechanism, Kataoka and co-workers reported light-mediated gene delivery to the conjunctival tissue of rats [43].
More recently, Febvay et al. [44] used this approach to achieve cytosolic release of the model cell-impermeable dye Alexa 546 from mesoporous silica nanoparticles internalized by cancer cells. Upon exposure to green light (520 – 570 nm, 500 mW/cm2) the dye acts as a photosensitizer, producing singlet oxygen, which disrupts the endosomal membrane. An increase in membrane permeability was monitored by increase of the fluorescence of Alexa 546 in the cytosol. FITC-labeled dextran (3 kDa) co-internalized with Alexa 546-loaded silica particles was also successfully released into the cytosol upon light exposure for 2 min.
II. Surface plasmon absorption by gold nanoparticles and photothermal effects
NIR (700-950 nm) is preferable to other types of light for triggering release in biological systems because it can pass through blood and tissue to depths of several inches [1]. However, very few organic chromophores absorb in this region, and even fewer are capable of converting the absorbed energy into a chemical or thermal response that can be used to trigger drug release. A few years ago, gold nanostructures (shells [46-48], particles [49-50], rods [51-52], and cages [53-54]) emerged as useful agents for photothermal therapy after they were shown to have strong absorption in the NIR region (four to five times higher than conventional photo-absorbing dyes [55]) and tunable optical resonances. The strong absorption ensures effective laser therapy at relatively low laser energies, rendering this therapy method minimally invasive.
Gold nanoparticles absorb light efficiently in the visible region due to coherent oscillations of metal conduction band electrons in strong resonance with visible frequencies of light. Photoexcitation of metal nanostructures results in the formation of a heated electron gas that cools rapidly within 1 ps by exchanging energy with the nanoparticle lattice. The nanoparticle lattice, in turn, rapidly exchanges energy with the surrounding medium on the timescale of 100 ps, causing localized heating [56]. This fast energy conversion and dissipation can be achieved by using light radiation with a frequency strongly overlapping with the nanoparticle absorption band. The NIR absorption maximum of metal nanostructures can be modulated by changing their size, shape and aggregation [46, 57-59]. This phenomenon has been widely studied as a stand-alone cancer therapy method since the early 2000s [60] and more recently was adopted to trigger the release of entrapped payload from nanocarriers upon exposure to NIR light. Typically, gold nanostructures are incorporated into polymer capsules along with drug molecules. Energy from NIR light generated by a laser absorbed by gold nanostructures and converted into thermal energy. Spontaneous local heating to temperatures well above 600-800°C [57-58] induces significant thermal and mechanical stress within the system and thus causes rupture of the carrier and subsequent payload release.
The first carrier incorporating gold nanoparticles was reported by Radt et al. [61]. Hollow polyelecrolyte microparticles were prepared by layer-by-layer deposition, incorporating 6 nm gold nanoparticles and lysozyme as a model therapeutic between polymer layers. Lysozyme release was observed upon exposure of the microparticle suspension to laser irradiation for 5 min with short pulses (10 ns) at a frequency of 10 Hz at 1064 nm. The amount of protein released upon light exposure was similar to the amount released from a mechanically crushed control sample.
The major challenge in controlled liposomal drug delivery is to create a system that is sufficiently stable in circulation, yet capable of quickly releasing its contents upon stimulus. Wu et al. [58] reported a liposomal delivery system capable of burst release upon absorption of NIR light by hollow gold nanoparticles. In their construct, gold nanoparticles were either encapsulated within dipalmitoylphosphatidylcholine (DPPC) liposomes or tethered to the surface via a PEG linker. 6-carboxyfluorescein was used as a model drug. Leakage from the liposomes was triggered by 130-fs laser pulses at 800 nm, leading to nearly instantaneous release of payload (71% for encapsulated gold nanoparticles and 93% for nanoparticles tethered to the surface) at laser powers exceeding 2.2 W/cm2. At this power setting, only a slight increase of the bulk solution temperature was observed (less then 1°C), while local heating was sufficient to anneal the hollow gold nanoparticles into solid nanoparticles, as evident by transmission electron microscopy (TEM). The observed burst release was ascribed to the formation and collapse of vapor microbubbles upon NIR-induced heating of gold nanoparticles. Thus, exposure of gold nanoparticles to femtosecond NIR laser pulses produces an effect similar to ultrasonication.
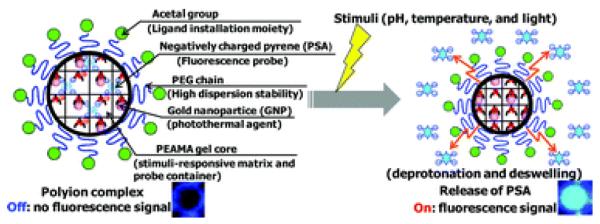
Oishi et al. [62] created multi-stimuli responsive PEGylated nanogels composed of a PEG shell and a cross-linked thermal-responsive poly[2-(N,N-diethylamino)ethyl methacrylate] core with NIR light-absorbing gold nanoparticles immobilized in the core (Figure 10). The PEAMA core acted as a nanoreactor to produce gold nanoparticles from tetrachloroaurate acid (HAuCL4 (III)) without any additional reducing reagents. 1,3,6,8-pyrenetetrasulfonic acid tetrasodium salt (PSA) was encapsulated into polyion complexes as a fluorescent water-soluble probe. While inside the particles, fluorescence of PSA was low due to self-quenching. Release of PSA in PBS solution (pH 7.4) was triggered by irradiation with an argon laser at 514.5 nm. A gradual increase in PSA fluorescence was observed over 8 min of laser irradiation, corresponding to 26% release. Release of the fluorescent probe from nanogels was due to the efficient heat generation by gold nanoparticles, which caused deprotonation and collapse of the temperature-responsive PEAMA core.
Figure 10.
Multi-stimuli responsive PEGylated nanogels composed of a PEG shell and PEAMA core with gold nanoparticles immobilized in the core [62].
Such polyion complex nanoparticles possess great potential as “smart” carriers for delivery of proteins, DNA, and small molecule drugs. Although this particular system cannot be readily translated into in vivo systems because of low tissue transparency at 514 nm, it may find applications in tissue engineering and microscopy.
Another example of NIR light-triggered release from a temperature-responsive nanocarrier was published by Wu et al. [63]. Ag/Au bimetallic nanoparticles were coated with a layer of polystyrene to encapsulate the hydrophobic drug curcumin, followed by an outer layer of nonlinear PEG to improve dispersion, circulation stability, and thermal sensitivity in the physiological range. A 70% release of curcumin was achieved upon irradiation with 1.5 W/cm2 NIR light for 5 min at intervals over 50 hours at 37°C. A similar release profile was observed when the particles were incubated at 41°C for the same time period without irradiation, confirming that the stimulated release is due to thermal sensitivity of the formulated particles triggered by the conversion of NIR energy into thermal energy by the Ag/Au core. Cytotoxicity tests revealed a 4-fold increase in cell killing efficiency of the curcumin-loaded Ag/Au particles compared to free curcumin.
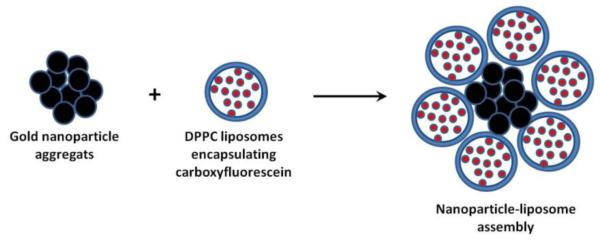
The major concern with gold nanoparticle-mediated light-induced release is stability of the cargo when exposed to the heat generated by the particles upon absorption of NIR energy. Volodkin et al. [59] proposed a solution to this problem by creating gold nanoparticle-liposome assemblies in which the cargo is shielded by a lipid membrane (Figure 11). Large gold nanoparticle aggregates (300 nm) have high cumulative electrostatic charge and attract a large number of liposomes to compensate for their excess charge. The dimensions of the assemblies are controlled by the size and charge of nanoparticles and liposomes as well as their mixing ratio. An additional advantage of this system is higher NIR absorption of aggregated gold nanoparticles compared to single gold nanoparticles.
Figure 11.
Formation of gold nanoparticle-liposome assemblies for NIR light-triggered release of carboxyfluorescein. [58].
Huschka et al. employed the photothermal response of gold nanoshells to NIR irradiation for light-triggered DNA antisense therapy [64-65]. Strands of DNA molecules were covalently attached to the surface of gold nanoshells at the 5′ end via a Au-thiol bond. A complementary non-thiolated DNA sequence was then bound to each strand to form a double helix. Upon illumination with 800 nm light, the double-stranded DNA was dehybridized, releasing the non-thiolated strand with 50% efficiency [65]. The same group later demonstrated that such constructs may be used for release of various guest molecules that can either intercalate between the adjacent base pairs or bind in the major or minor groove of the DNA double helix (Figure 12) [64]. 4′,6-diamidino-2-phenylindole (DAPI) was used to demonstrate light-induced intracellular release. DAPI fluorescence intensity is low in solution, but increases drastically upon binding to DNA. The release of DAPI from the double-stranded DNA inside living H1299 cancer cells upon irradiation with 800 nm for 5 min (1 W/cm2) was evidenced by the decrease in fluorescence of unbound DAPI compared to DAPI inside the DNA helix. One hour after irradiation, delivery of released DAPI to the nucleus was evident; fluorescence intensity recovered upon binding to nuclear DNA. Although no apparent temperature increase was observed in the solution containing the nanoparticles, heat-induced damage to the DNA directly attached to the surface of gold nanoparticles remains a possible limitation.
Figure 12.
Gold nanoparticles with DNA bound to the surface for NIR light-triggered release [63-64].
Delivery systems that utilize gold nanostructures provide an opportunity to take advantage of a combined effect of photothermal ablation and chemotherapy in a single setting. Such combined treatment has been demonstrated to result in higher cytotoxicity compared with chemo- or photothermal treatment alone [27, 66-67]. You et al. investigated the effect of DOX-loaded PEG-coated hollow gold nanoshells (PEG-HAuNS) for anticancer therapy [67]. Exceptionally high loading of DOX into the outer PEG layer of gold nanoshells (1.7 μg DOX/1μg Au) was achieved. Intracellular release of DOX from PEG-HAuNS was observed upon irradiation with four 3 min pulses of 800 nm (2 W/cm2 output power) over 2 hours. DOX-loaded PEG-HAuNS enhanced cell killing compared to free DOX at an equivalent concentration or laser-treated HAuNS alone (86.4% vs. 77% and 40.6%, respectively). The same research group also demonstrated laser-induced release of paclitaxel (PAX) from PLGA microspheres incorporating gold nanoshells and enhanced cytotoxicity compared to non-stimulated PAX-containing microspheres or photothermal treatment alone [27].
III. Photochemical hydrophobicity switch
The formation and stability of micelles and other vesicles relies on the hydrophilic to hydrophobic balance within the amphiphilic molecules constituting the aggregates. When amphiphilic molecules become hydrophilic, the micellar system disintegrates, releasing its cargo. Light-induced conversion of amphiphilic molecules to more hydrophilic forms allows remote control over this process.
A large number of organic molecules that undergo structural rearrangements to generate charged (and therefore more hydrophilic) species upon exposure to light have been developed; such rearrangements are termed photochemical reactions. The majority of organic molecules explored for photo-controlled materials applications respond to UV light. Upon absorption of UV light, these molecules reach an excited state from which they decay non-irradiatively via a chemical transformation. However, some organic chromophores can simultaneously absorb two photons of low-energy NIR light and undergo the same chemical transformation as upon absorption of one photon of high-energy UV light. The phenomenon of two-photon absorption was first theoretically predicted by Maria Goppert-Mayer in the 1930s [68]. The probability of two-photon absorption is generally low and proportional to the square of the intensity of the excitation beam [69]. Therefore, two-photon processes require femtosecond pulsed lasers with high photon density. The first experimental demonstration of two-photon absorption became possible in 1961 soon after the invention of the laser [70]. The efficiency of the two-photon induced chemical transformation is referred to as uncaging action cross-section and is expressed in Goeppert-Mayer units, GM (1 GM = 10−50cm4 s photon−1) [71]. Incorporation of such light-triggering units into delivery vehicles can be employed for controlled photo-triggered release, eliminating the need for inorganic NIR light-absorbing dopants.
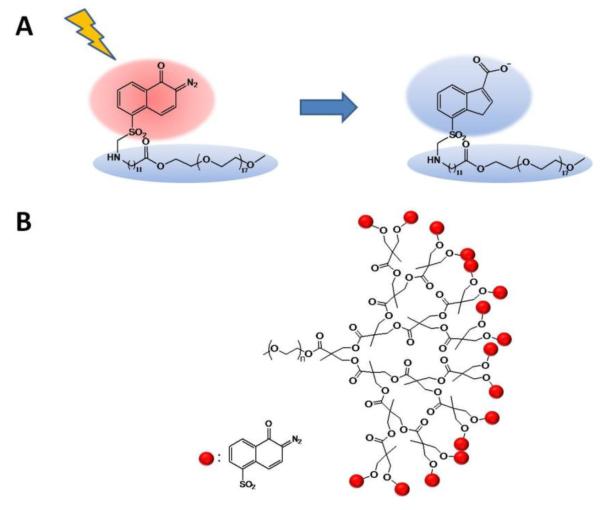
The first micellar system capable of releasing hydrophobic cargo via an NIR light-induced chemical transformation was reported by Goodwin et al. in 2005 [72]. An amphiphilic molecule was constructed by chemically attaching a hydrophobic light-sensitive 2-diazonaphthoquinone (DNQ) to a PEG chain (Figure 13A). This polymer was shown to form micelles above the concentration of 0.15 mg/mL in PBS pH 7.4. Nile Red was encapsulated into the micelles as a reporter dye. Upon irradiation by UV or NIR light (via absorption of one or two photons, respectively), DNQ undergoes a Wolff rearrangement to form a hydrophilic 3-indene carboxylic acid. As a result, the micelles incorporating DNQ dissolve, releasing Nile Red into the aqueous medium, which is evidenced by quenching of the fluorescence of the dye. Over 30 min of irradiation at 795 nm resulted in a 75% decrease in the fluorescence of Nile Red, confirming the dissolution of the micelles. Later, this DNQ-based system was modified by incorporation of dendritic polyester between the PEG and DNQ moieties (Figure 13B), which allowed installation of multiple DNQ molecules per amphiphilic molecule [73]. The new system exhibited lower critical micellar concentration (12 μg/mL) and low cytotoxicity at concentrations as high as 1 mg/mL. However, this stability persisted upon irradiation: irradiation with 795 nm light for 30 min resulted in only a 50% decrease in the fluorescence intensity of encapsulated Nile Red and a decrease in the size of the micelles from 40 to 20 nm.
Figure 13.
A) Diazonaphthoquinone-based molecules undergoing an amphiphilic to hydrophilic switch upon UV and NIR irradiation. [69] B) DNQ-based system modified by incorporation of dendritic polyester to install multiple DNQ moieties [73].
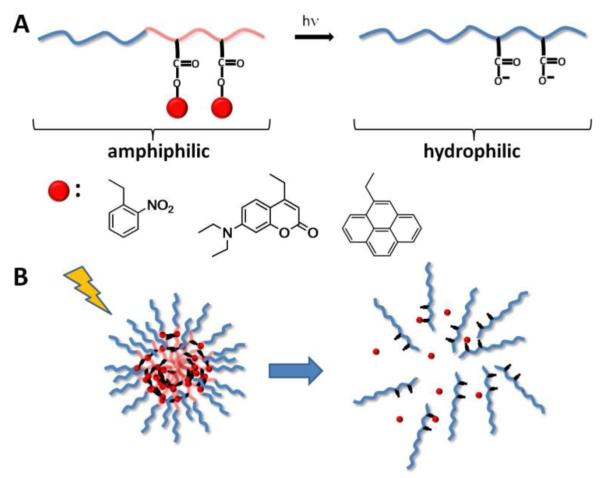
Micelles formed from amphiphilic block copolymers are being actively studied for photo-controlled release. In these constructs, PEG is usually used as the hydrophilic block, while the hydrophobic block is formed by polymethacrylic acid whose carboxyls are masked by various protecting groups that can be removed upon exposure to light (Figure 14A).
Figure 14.
A) Block copolymer with masked carboxyl groups undergoing amphiphilic to hydrophilic switch upon light exposure and examples of light-sensitive protecting groups. (B) Amphiphilic block-copolymer micellar disassembly upon irradiation.
Yue Zhao’s group has been studying light-dissociable block-copolymer micelles. Their first system capable of photo-controlled release of hydrophobic small molecules was based on an amphiphilic block copolymer containing the o-nitrobenzyl protecting group [74]. Micelles encapsulating Nile Red were formed by first dissolving the polymer and the dye in THF and adding water. Photo-controlled release was induced by irradiating the solution with UV light above 365 nm (145 mW/cm). After 420 seconds of irradiation, the fluorescence of Nile Red decreased by 80%, indicating release of the hydrophobic dye into the aqueous environment. Much faster release was observed at higher irradiation powers (75 sec at 500 mW/cm). The same system was also demonstrated to release Nile Red via two-photon uncaging of the carboxylic groups. The two-photon uncaging cross-section of the o-nitrobenzyl group is rather low (0.01 GM [75]); therefore, this process is much slower than the one-photon reaction, requiring irradiation at 700 nm for 210 min to achieve a similar decrease in Nile Red fluorescence.
The [7-(diethylamino)coumarin-4-yl]methyl chromophore (DEACM) has an order of magnitude higher two-photon uncaging cross-section compared to the o-nitrobenzyl group and therefore should be more suitable for use in light-activated drug delivery systems. A block copolymer in which the DEACM caging group masked the carboxylic acid groups was reported later by the same group [76]. However, despite the more NIR-sensitive caging group, the observed release of Nile Red from the micelles was slower than in that for the o-nitrobenzyl-based system (50% decrease in Nile Red fluorescence after 210 min at 500mW/cm).
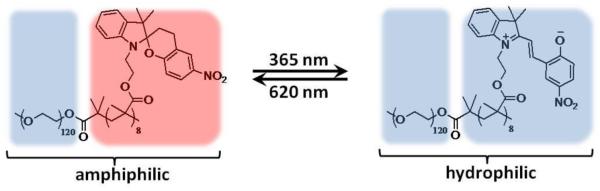
Lee et al. described a similar micellar system for light-triggered release via a hydrophobicity switch [77]. In their system, the hydrophobic block is composed of spiropyran-containing polymethacrylate (Figure 15). Unlike in the previously described systems, the switch between the amphiphilic form and the hydrophilic form of the block copolymer is reversible. Thus, irradiation with UV light converts spiropyran into hydrophilic, zwitterionic merocyanine, while exposure to visible light converts it back to the hydrophobic spiro form. The hydrophobic dye coumarin 102 was encapsulated into block copolymer micelles prepared from spiropyran and its release was induced by irradiating the solution of micelles with 365 nm light for 60 min. Similar to Nile Red, coumarin 102 is insoluble in water, so its fluorescence is quenched when the dye was released into aqueous solution. Complete disruption of the micelles after UV irradiation was observed by AFM. Subsequent irradiation of the solution with 620 nm light for 240 min led to reconstitution of the micelles and partial re-encapsulation of coumarin 102, evidenced by AFM and an increase in the fluorescence intensity of the dye.
Figure 15.
Light-induced hydrophobicity switch of spiropyran-containing block copolymer [74].
Another example of micelles that can be reversibly disrupted was reported by Jiang et al. [78]. Their dual-responsive block copolymer incorporated polyethylene oxide (PEO) as a hydrophilic block and poly(ethoxytriethylene glycol) acrylate-co-poly(o-nitrobenzyl) acrylate (P(TEGA-co-NBA)) as a thermoresponsive hydrophobic block. Above the lower critical solution temperature (LCST) of the P(TEGA-co-NBA) block, the copolymer formed micelles encapsulating Nile Red. Upon continuous UV irradiation for 180 min, the o-nitrobenzyl groups were cleaved and the LCST of the thermoresponsive block increased by 11°C, causing dissociation of the micelles. Further increasing the temperature above the LCST of the new thermoresponsive block reconstituted the micelles and re-encapsulated Nile Red.
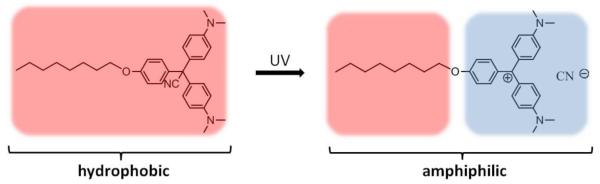
A molecule that switches from fully hydrophobic to amphiphilic was also used for light-triggered release. Malachite green derivative (Figure 16) was incorporated (4.5 mol%) into the membrane of vesicles composed of phosphatidylcholine [79]. Upon UV exposure, this molecule undergoes photoionization and becomes amphiphilic. This results in a decrease of the total free energy of the system, membrane destabilization and eventual solubilization of the membrane components, leading to release of the encapsulated compounds. 80% of the encapsulated dye (8-aminonaphthalene-1,3,6-trisulphonic acid) was released from the vesicles after 15 min of continuous UV irradiation.
Figure 16.
Ionization of Malachite green derivative upon UV light exposure. [76]
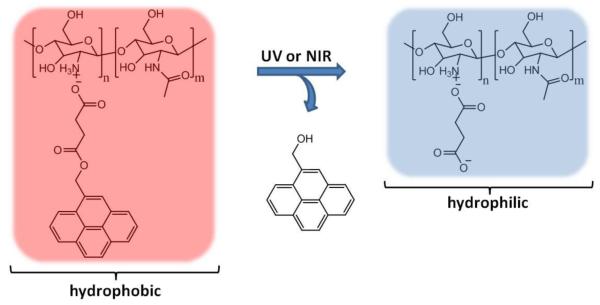
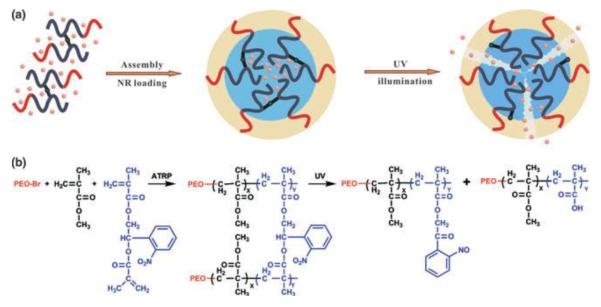
Recently, photosensitive polymeric nanoparticles were prepared by self-assembly of oppositely charged polyelectrolytes [80]. Coulomb interactions between the cationic natural polymer chitosan and an anionic photosensitive pyrene derivative resulted in the formation of hydrophobic polymeric particles in aqueous solution, which were loaded with Nile Red. UV irradiation of the particles for 50 sec (1200 mW cm−2) resulted in an 80% decrease in the fluorescence intensity of Nile Red. However, DLS analysis of the particles after UV exposure showed their shrinking in size but neither complete degradation nor dissolution, which would be expected when the polymer turns hydrophilic (Figure 17). This behavior was attributed to the crosslinking of chitosan by butanoic acid generated after the removal of pyrene group. Some Nile Red release was also observed when the particles were exposed to NIR light (808 nm): a 40% decrease in the fluorescence of Nile Red was achieved after 250 min of exposure, owing to low two-photon absorbance of the pyrene derivative.
Figure 17.
Polyion complex formed between chitosan and pyrene derivative undergoes a hydrophobic to hydrophilic switch upon light exposure [77].
Nanoengineering ion channels to allow optical control is an emerging technology; such channels can be incorporated into liposomes to allow light-triggered release [81]. Channel proteins are excitable pores embedded in cell membranes that, by opening and closing, allow the flow of ions across the membrane. One of these proteins, the mechanosensitive channel of large conductance (MscL) from Escherichia coli, was incorporated into liposomes to function as a remotely controlled valve [82]. In order to make MscL responsive to irradiation, a light-sensitive group was installed at the 22nd amino acid. Removal of the triggering group increases the hydrophilic character of the channel pore and opens the valve, allowing cargo release from the liposomes (Figure 18). Two triggering groups, o-nitrobenzene and spiropyran, have been used to open the valve irreversibly and reversibly, respectively. A 43% release of calcein from the MscL-containing liposomes was observed after exposure to 366 nm light, while only 10% of calcein diffused from non-irradiated liposomes.
Figure 18.
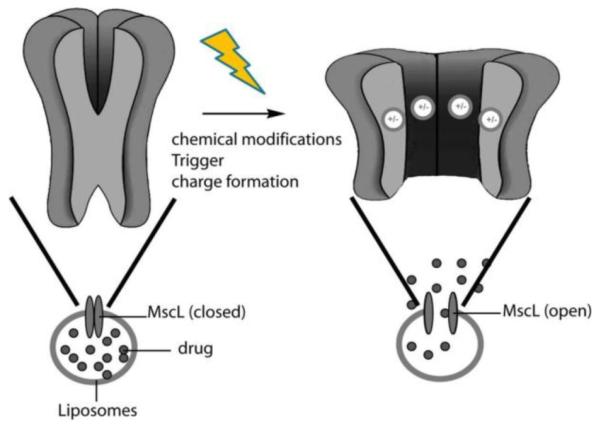
Schematic of triggered liposomal release through an engineered channel protein [78].
Recently, a new way to employ NIR light for triggered release based on lanthanide-doped upconverting nanoparticles (UCNP) has emerged. UCNPs composed of NaYF4 nanocrystals doped with Tm3+ and Yb3+ act as light-harvesting antennae, sequentially absorbing multiple photons of NIR light and converting it into higher energy UV light [83].
The first example of using UCNPs to induce a chemical reaction was demonstrated by the group of N. Branda, when a 2-phenylbenzofurane photoprotecting group masking acetic acid was conjugated to the surface of UCNPs [84]. Exposure of the NPs to 980 nm continuous wave light (4.37 W, 556 W/cm−2) resulted in photocleavage of the 2-phenylbenzofurane group and release of acetic acid. This process occurred via the conversion of NIR light to UV light, since the 2-phenylbenzofurane group is not cleavable by 980 nm irradiation.
More recently, this strategy was further adapted to induce triggered release from a micellar system by the collaborative efforts of N. Branda and Y. Zhao [85]. Micelles composed of an amphiphilic block copolymer containing o-nitrobenzyl photoprotecting groups encapsulating Nile Red (Figure 14) and doped with UCNPs were prepared. Upon exposure to 980 nm light (5 W, 4 hours), Nile Red release was observed, indicating photocleavage of o-nitrobenzyl photo-protecting groups by UV light emitted by the UCNPs and the subsequent hydrophobicity switch and disintegration of the micelles.
The upconversion process happens via sequential absorption of multiple photons and therefore requires 104 – 107 orders of magnitude lower energy densities compared to simultaneous multi-photon absorption processes [86]. However, the potential toxicity and tissue accumulation of UCNPs should be thoroughly investigated before this technique can be developed for in vivo applications.
IV. Polymer backbone photo-degradation
The polymer-based nanocarriers thus far discussed in this review do not degrade into small molecules upon irradiation to effect release. Degradable hydrophobic polymers present an attractive choice as delivery vehicles for therapeutic cargo. Although similar to the hydrophobicity switch mechanism in terms of the chemistry used, the photo-releasing systems described in this section offer the additional advantage of disassembly on both the nanoscale (disintegration of nanocarrier) and the molecular scale (polymer fragmentation). Systems that can degrade into smaller fragments can subsequently be cleared from the body, eliminating any long-term toxicity concerns. Polymers that degrade completely into small molecules via various internal physiological cues like pH, reactive oxygen species, and temperature offer the added advantage of spatio-temporal control. External stimuli, such as ultrasound, light, and magnetic field can also be used to remotely control the release from and final degradation of these delivery vehicles, but have been underutilized. In this regard we have developed the first example of a light degradable polymer that can be formulated into particles for delivery and release of drugs (Figure 19) [87].
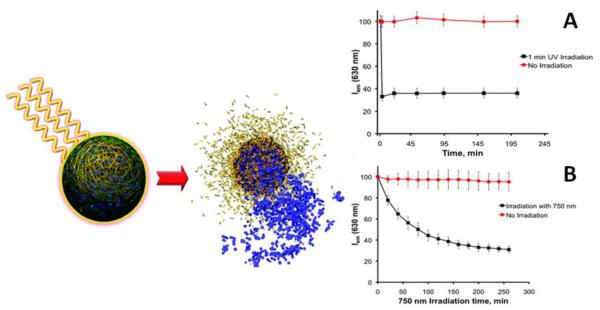
Figure 19.
Schematic representation of degradation of polymeric particles by light and release of cargo. Nile Red release from the polymeric particles upon A) UV irradiation at 300-400 nm B) NIR irradiation at 750nm. [87]
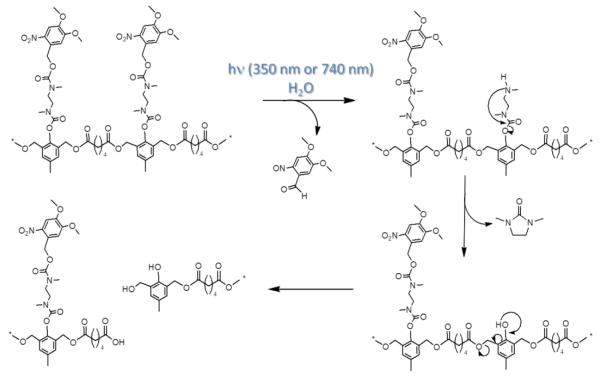
The polymer consisted of a quinone-methide-based backbone with pendant N,N-dimethylethylene diamine groups protected with o-nitrobenzyl phototriggering groups. A cascade of diamine cyclization and quinone-methide rearrangement is activated when the photo-triggering groups are cleaved by one-photon (350 nm) or two-photon (750 nm) photolysis, resulting in the complete degradation of the polymer backbone into small molecules (Figure 20).
Figure 20.
Schematic of quinone-methide-based polymer [83] degradation after cleavage of o-nitrobenzyl triggering group with UV or NIR light.
We have shown subsequently that the polymer can be formulated into nanoparticles via standard and commercially viable emulsion techniques that result in regular particle shapes and sizes. Nile Red was encapsulated as a model drug within these particles to allow measurement of release via fluorescence. While UV irradiation causes an almost immediate 67% decrease in fluorescence intensity upon 60 seconds of irradiation, NIR wavelengths result in more gradual decrease (over 250 min) because of the poor two-photon uncaging cross-section of the o-nitrobenzyl group (0.01 GM). Ongoing studies currently employ much higher efficiency two-photon uncaging groups [88].
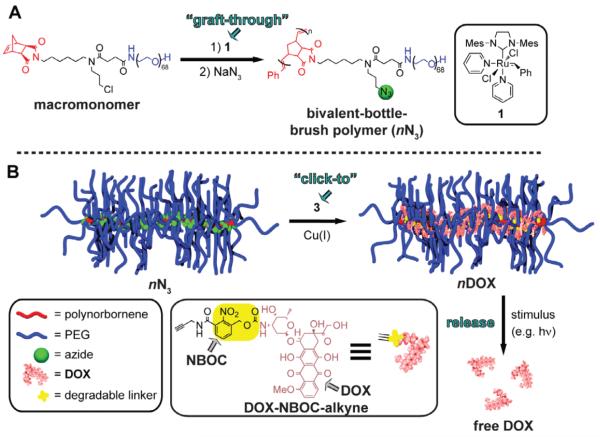
Johnson et al. [89] have adopted a different strategy to release drugs from nanometer sized hydrophobic polymeric systems using light (Figure 21). They have synthesized brush-like nanosystems that have drug covalently bound to the backbone via a photo-degradable o-nitrobenzyl moiety. In their synthesis they utilized a “graft-through” strategy, where a norbornene-containing macromonomer is polymerized by ring-opening metathesis polymerization (ROMP) and DOX is then covalently bound to the backbone using “click” chemistry. The linkage also contains the photo-uncaging o-nitrobenzyl moiety that can be triggered by UV light to release DOX. Irradiation of these 10 nm nanoassemblies using 365 nm light for up to 10 min resulted in a 70% release of the bound DOX.
Figure 21.
Schematic representation of the “graft-through and “click-to” approach. A) Bivalent macromonomer on which “graft-through” ROMP with catalyst 1 was performed followed by in situ chloride-azide exchange. B) The resulting azido-bivalent-brush polymer functionalized with DOX-alkyne by click chemistry allows controlled release of the anticancer agent doxorubicin (DOX) in response to 365 nm UV light [89].
V. Photo de-crosslinking
A particle may be induced to release its payload by breaking up the light-sensitive crosslinks holding it together, making it more porous. The advantage of this approach is that the light-sensitive moieties remain attached to the polymer after light exposure, thus eliminating the issue of potential toxicity of the photocleavage byproducts.
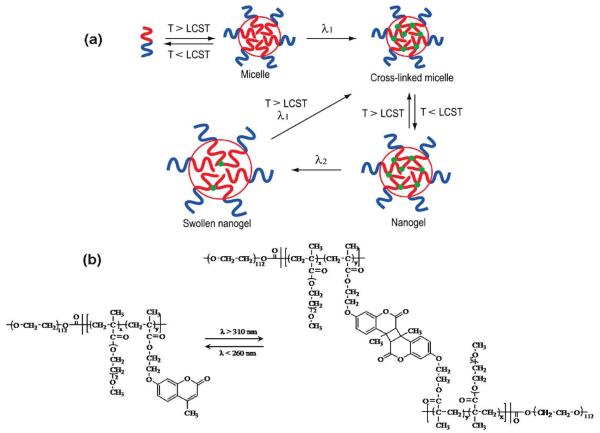
Reversible photo-crosslinked nanoparticles were prepared by the Zhao group [90]. A water-soluble block copolymer containing a block of PEO and a block of poly(2-(2-methoxyethoxy)ethyl methacrylate-co-4-methyl-(7-(methacryloyl)oxyethyloxy)coumarin), denoted as PEO-b-P(MEOMA-co-CMA), was prepared by atom transfer radical polymerization. The polymer solution was heated above its LCST to form block copolymer micelles, which were then photo-crosslinked by irradiation with light at 320 nm (Figure 22) to induce photo-dimerization of the pendant coumarin groups. Cooling the solution below LCST afforded photo-crosslinked nanogel particles. Nanogel swelling by 90% was achieved by irradiating the nanoparticles with light of higher energy (254 nm), inducing photocleavage of the coumarin dimers. Repeated crosslinking could be achieved by bringing the nanogel solution temperature above LCST and irradiating with 320 nm light.
Figure 22.
A) Schematic illustration of the preparation and photo-controlled volume change of nanogel [90]. B) Designed diblock copolymer bearing coumarin side groups for the reversible photo-cross-linking reaction.
The rate of cargo release could be controlled by the reversible photo crosslinking and de-crosslinking of the nanogels. Encapsulated dye, dipyridamole, was released slower from the crosslinked nanogels compared to non-crosslinked micelles, demonstrating the possibility of tuning the release rate by controlling the degree of crosslinking. Further, irradiation of the nanogels with 254 nm light for 3 min reduced the crosslinking density, thus significantly increasing the release rate. The de-crosslinking reaction can also be induced through two-photon absorption of visible light (532 nm). [91]
Crosslinked block copolymers containing an o-nitrobenzyl group in the crosslinkers were used to form micelles that could be disrupted by UV light via cleavage of the crosslinks and generation of hydrophilic carboxylic acid moieties (Figure 23) [92]. Nile Red was released from the micelles upon irradiation with 365 nm for 15 min. Such a strategy may be readily adopted for in vivo applications by replacing the o-nitrobenzyl group with a photosensitive protecting group with a higher two-photon uncaging cross-section.
Figure 23.
A) Photo-controlled release of encapsulated guest molecules upon light irradiation of polymer micelles. B) Synthesis of photocleavable cross-linked block copolymers and their light-triggered dissociation [92].
3. Conclusions
The use of light as an external stimulus is a promising approach to targeted drug delivery that allows precise control over the place, time, and rate of cargo release. Although the first reports of this concept appeared in the 1980s, significant progress has been made in this area over the past decade. A wide range of nanoscale assemblies have employed a handful of photochemical mechanisms to achieve efficient and reproducible release profiles. Most of the systems developed to date respond most efficiently to UV or visible light. However, these systems will most likely be limited to topical applications, where stimulus penetration is not necessary or undesirable. For such systems, the major concern is the damaging effect of high energy irradiation to tissues, especially at shorter wavelengths. Systems that are sensitive to NIR light, such as surface plasmon absorption by gold particles, upconverting nanoparticles, and NIR chromophores, hold more potential in vivo due to the greater depth of penetration of this wavelength of light and the minimal absorption of NIR by endogenous matter. The emerging technology utilizing upconverting nanoparticles is attractive because it does not require expensive high-energy lasers. However, toxicity of these materials and tissue accumulation remain to be thoroughly investigated. In the case of gold nanoparticles used for photothermal effects, thermal stability of the potential cargo must be considered due to local heat generation. Design and synthesis of more efficient NIR light-absorbing organic chromophores will eliminate the need for inorganic dopants.
Expanding the toolbox of photochemical mechanisms that allow release from nanocarriers would increase the likelihood that an efficient system with little biological risk is developed. Furthermore, there is a need to create systems that degrade into small molecules upon irradiation or completion of function. These fully degradable systems would be desirable for a variety of biomedical applications. A recent review focusing in more depth on the drug delivery aspects of light-sensitive nanocarriers was published in this journal [93]; thus, we chose to address the chemical side of this topic in more depth.
As this field continues to grow, there will be a need for more standardized reporting on the photochemical parameters, such as wavelengths, exposure time, laser power per area, and energies per pulse, that govern these processes. Finally, a systematic method for categorizing the change resulting from irradiation (i.e. swelling, de-crosslinking, full degradation, etc.) will have to be implemented so that the literature may be more easily compared. These efforts will allow the community to make more meaningful conclusions when selecting an appropriate system for biomedical applications.
Figure 4.
Scheme showing the generation of a net dipole moment on photoisomerization.
Acknowledgements
The authors thank the NIH Director’s New Innovator Award (1 DP2 OD006499-01) and King Abdulaziz City for Science and Technology for financially supporting this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- [1].Weissleder R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- [2].Shum P, Kim J-M, Thompson DH. Phototriggering of liposomal drug delivery systems. Adv. Drug Del. Rev. 2001;53:273–284. doi: 10.1016/s0169-409x(01)00232-0. [DOI] [PubMed] [Google Scholar]
- [3].Yavlovich A, Smith B, Gupta K, Blumenthal R, Puri A. Light-sensitive lipid-based nanoparticles for drug delivery: design principles and future considerations for biological applications. Mol. Membr. Biol. 27:364–381. doi: 10.3109/09687688.2010.507788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alvarez-Lorenzo C, Bromberg L, Concheiro A. Light-sensitive intelligent drug delivery systems. Photochem. Photobiol. 2009;85:848–860. doi: 10.1111/j.1751-1097.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- [5].Zhao Y. Photocontrollable block copolymer micelles: what can we control? J. Mater. Chem. 2009;19:4887–4895. [Google Scholar]
- [6].Schumers J-M, Fustin C-A, Gohy J-F. Light-Responsive Block Copolymers. Macromol. Rapid Commun. 2010;31:1588–1607. doi: 10.1002/marc.201000108. [DOI] [PubMed] [Google Scholar]
- [7].Kano K, Tanaka Y, Ogawa T, Shimoura M, Okahata Y, Kunitake T. Photoresponsive membranes. Regulation of membrane properties by photoreversible cis-trans isomerization of azobenzenes. Chem. Lett. 1980:421–424. [Google Scholar]
- [8].Bisby RH, Mead C, Mitchell AC, Morgan CG. Fast laser-induced solute release from liposomes sensitized with photochromic lipid: effects of temperature, lipid host, and sensitizer concentration. Biochem.Biophys. Res. Commun. 1999;262:406–410. doi: 10.1006/bbrc.1999.1206. [DOI] [PubMed] [Google Scholar]
- [9].Bisby RH, Mead C, Morgan CG. Photosensitive liposomes as ‘cages’ for laser-triggered solute delivery: the effect of bilayer cholesterol on kinetics of solute release. FEBS Lett. 1999;463:165–168. doi: 10.1016/s0014-5793(99)01612-9. [DOI] [PubMed] [Google Scholar]
- [10].Bisby RH, Mead C, Morgan CG. Wavelength-Programmed Solute Release from Photosensitive Liposomes. Biochem. Biophys. Res. Commun. 2000;276:169–173. doi: 10.1006/bbrc.2000.3456. [DOI] [PubMed] [Google Scholar]
- [11].Bisby RH, Mead C, Morgan CG. Active uptake of drugs into photosensitive liposomes and rapid release on UV photolysis. Photochem. Photobiol. 2000;72:57–61. doi: 10.1562/0031-8655(2000)072<0057:auodip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [12].Morgan CG, Thomas EW, Sandhu SS, Yianni YP, Mitchell AC. Light-induced fusion of liposomes with release of trapped marker dye is sensitised by photochromic phospholipid. Biochim. Biophys. Acta, Biomembr. 1987;903:504–509. doi: 10.1016/0005-2736(87)90057-5. [DOI] [PubMed] [Google Scholar]
- [13].Morgan CG, Yianni YP, Sandhu SS, Mitchell AC. Liposome fusion and lipid exchange on ultraviolet irradiation of liposomes containing a photochromic phospholipid. Photochem. Photobiol. 1995;62:24–29. doi: 10.1111/j.1751-1097.1995.tb05233.x. [DOI] [PubMed] [Google Scholar]
- [14].Alan M. Smith, Jonathan J. Harris, Richard M. Shelton, Perrie Y. 3D culture of bone-derived cells immobilised in alginate following light-triggered gelation. J Control Release. 2007;119:94–101. doi: 10.1016/j.jconrel.2007.01.011. [DOI] [PubMed] [Google Scholar]
- [15].Eastoe J, Vesperinas A. Self-assembly of light-sensitive surfactants. Soft Matter. 2005;1:338–347. doi: 10.1039/b510877m. [DOI] [PubMed] [Google Scholar]
- [16].Orihara Y, Matsumura A, Saito Y, Ogawa N, Saji T, Yamaguchi A, Sakai H, Abe M. Reversible Release Control of an Oily Substance Using Photoresponsive Micelles. Langmuir. 2001;17:6072–6076. [Google Scholar]
- [17].Wang YP, Han P, Xu HP, Wang ZQ, Zhang X, Kabanov AV. Photocontrolled Self-Assembly and Disassembly of Block Ionomer Complex Vesicles: A Facile Approach toward Supramolecular Polymer Nanocontainers. Langmuir. 2010;26:709–715. doi: 10.1021/la9023844. [DOI] [PubMed] [Google Scholar]
- [18].Wang G, Tong X, Zhao Y. Preparation of Azobenzene-Containing Amphiphilic Diblock Copolymers for Light-Responsive Micellar Aggregates. Macromolecules. 2004;37:8911–8917. [Google Scholar]
- [19].Jochum FD, Theato P. Thermo- and light responsive micellation of azobenzene containing block copolymers. Chem. Commun. (Cambridge, U. K.) 2010;46:6717–6719. doi: 10.1039/c0cc01288b. [DOI] [PubMed] [Google Scholar]
- [20].Zhao Y, Tremblay L, Zhao Y. Doubly photoresponsive and water-soluble block copolymers: Synthesis and thermosensitivity. J. Polym. Sci., Part A Polym. Chem. 2010;48:4055–4066. [Google Scholar]
- [21].Han D, Tong X, Zhao Y, Galstian T, Zhao Y. Cyclic Azobenzene-Containing Side-Chain Liquid Crystalline Polymers: Synthesis and Topological Effect on Mesophase Transition, Order, and Photoinduced Birefringence. Macromolecules (Washington, DC, U. S.) 2010;43:3664–3671. [Google Scholar]
- [22].Patnaik S, Ashwani K. Sharma, Garg BS, Gandhi RP, Gupta KC. Photoregulation of drug release in azo-dextran nanogels. Int. J. Pharm. 2007;342:184–193. doi: 10.1016/j.ijpharm.2007.04.038. [DOI] [PubMed] [Google Scholar]
- [23].Angelos S, Choi E, Voegtle F, De Cola L, Zink JI. Photo-Driven Expulsion of Molecules from Mesostructured Silica Nanoparticles. J.Phys. Chem. C. 2007;111:6589–6592. [Google Scholar]
- [24].Lu J, Choi E, Tamanoi F, Zink JI. Light-activated nanoimpeller-controlled drug release in cancer cells. Small. 2008;4:421–426. doi: 10.1002/smll.200700903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Regen SL, Singh A, Oehme G, Singh M. Polymerized phosphatidyl choline vesicles. Stabilized and controllable time-release carriers. Biochem. Biophys. Res. Commun. 1981;101:131–136. doi: 10.1016/s0006-291x(81)80020-4. [DOI] [PubMed] [Google Scholar]
- [26].Yavlovich A, Singh A, Blumenthal R, Puri A. A novel class of photo-triggerable liposomes containing DPPC:DC8,9PC as vehicles for delivery of doxorubicin to cells. Biochim. Biophys. Acta, Biomembr. 2011;1808:117–126. doi: 10.1016/j.bbamem.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Park M-K, Deng S, Rigoberto C. Advincula. pH-sensitive bipolar ion-permselective ultrathin films. J. Am. Chem. Soc. 2004;126:13723–13731. doi: 10.1021/ja0484707. [DOI] [PubMed] [Google Scholar]
- [28].Park M-K, Deng S, Rigoberto C. Advincula. Sustained release control via photo-cross-linking of polyelectrolyte layer-by-layer hollow capsules. Langmuir. 2005;21:5272–5277. doi: 10.1021/la047008x. [DOI] [PubMed] [Google Scholar]
- [29].Shi D, Matsusaki M, Kaneko T, Akashi M. Photo-Cross-Linking and Cleavage Induced Reversible Size Change of Bio-Based Nanoparticles. Macromolecules (Washington, DC, United States) 2008;41:8167–8172. [Google Scholar]
- [30].Shi D, Matsusaki M, Akashi M. Unique size-change behavior of photo-crosslinked cinnamic acid derivative nanoparticles during hydrolytic degradation. Macromolecular Bioscience. 2009;9:248–255. doi: 10.1002/mabi.200800171. [DOI] [PubMed] [Google Scholar]
- [31].Pooler JP. Photomodification of human erythrocytes: external heavy atom effect, selective permeability and properties of the anion permeation pathway. Photochem. Photobiol. 1988;47:369–376. doi: 10.1111/j.1751-1097.1988.tb02739.x. [DOI] [PubMed] [Google Scholar]
- [32].Pooler JP. Photooxidation of cell membranes using eosin derivatives that locate in lipid or protein to study the role of diffusible intermediates. Photochem. Photobiol. 1989;50:55–68. doi: 10.1111/j.1751-1097.1989.tb04129.x. [DOI] [PubMed] [Google Scholar]
- [33].Girotti AW. Photodynamic lipid peroxidation in biological systems. Photochem. Photobiol. 1990;51:497–509. doi: 10.1111/j.1751-1097.1990.tb01744.x. [DOI] [PubMed] [Google Scholar]
- [34].Snipes W, Keller G, Woog J, Vickroy T, Deering R, Keith A. Inactivation of lipid-containing viruses by hydrophobic photosensitizers and near-ultraviolet radiation. Photochem. Photobiol. 1979;29:785–790. doi: 10.1111/j.1751-1097.1979.tb07767.x. [DOI] [PubMed] [Google Scholar]
- [35].Torinuki W, Miura T, Seiji M. Lysosome destruction and lipoperoxide formation due to active oxygen generated from haematoporphyrin and UV irradiation. Br. J. Dermatol. 1980;102:17–27. doi: 10.1111/j.1365-2133.1980.tb05667.x. [DOI] [PubMed] [Google Scholar]
- [36].Dahl TA, Midden WR, Hartman PE. Pure singlet oxygen cytotoxicity for bacteria. Photochem. Photobiol. 1987;46:345–352. doi: 10.1111/j.1751-1097.1987.tb04779.x. [DOI] [PubMed] [Google Scholar]
- [37].Deziel MR, Girotti AW. Photodynamic action of bilirubin on liposomes and erythrocyte membranes. J.Biol. Chem. 1980;255:8192–8198. [PubMed] [Google Scholar]
- [38].Bally MB, Nayar R, Masin D, Hope MJ, Cullis PR, Mayer LD. Liposomes with entrapped doxorubicin exhibit extended blood residence times. Biochimica et Biophysica Acta, Biomembranes. 1990;1023:133–139. doi: 10.1016/0005-2736(90)90018-j. [DOI] [PubMed] [Google Scholar]
- [39].Foote CS, Dobrowolski DC. Singlet oxygen production from photodynamic sensitizers. Oxygen Radicals Chem. Biol., Proc., Int. Conf., 3rd.1984. pp. 465–472. [Google Scholar]
- [40].Thompson DH, Gerasimov OV, Wheeler JJ, Rui YJ, Anderson VC. Triggerable plasmalogen liposomes: Improvement of system efficiency. Biomembranes. 1996;1279:25–34. doi: 10.1016/0005-2736(95)00210-3. [DOI] [PubMed] [Google Scholar]
- [41].Anderson VC, Thompson DH. Triggered release of hydrophilic agents from plasmalogen liposomes using visible light or acid. Biochim. Biophys. Acta, Biomembranes. 1992;1109:33–42. doi: 10.1016/0005-2736(92)90183-m. [DOI] [PubMed] [Google Scholar]
- [42].Hogset A, Prasmickaite L, Selbo PK, Hellum M, Engesaeter BO, Bonsted A, Berg K. Photochemical internalisation in drug and gene delivery. Adv. Drug Del. Rev. 2004;56:95–115. doi: 10.1016/j.addr.2003.08.016. [DOI] [PubMed] [Google Scholar]
- [43].Nishiyama N, Iriyama A, Jang WD, Miyata K, Itaka K, Inoue Y, Takahashi H, Yanagi Y, Tamaki Y, Koyama H, Kataoka K. Light-induced gene transfer from packaged DNA enveloped in a dendrimeric photosensitizer. Nat. Mater. 2005;4:934–941. doi: 10.1038/nmat1524. [DOI] [PubMed] [Google Scholar]
- [44].Febvay S, Marini DM, Belcher AM, Clapham DE. Targeted Cytosolic Delivery of Cell-Impermeable Compounds by Nanoparticle-Mediated, Light-Triggered Endosome Disruption. Nano Lett. 2010;10:2211–2219. doi: 10.1021/nl101157z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tomatsu I, Peng K, Kros A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Del. Rev. 2011;63:1257–1266. doi: 10.1016/j.addr.2011.06.009. [DOI] [PubMed] [Google Scholar]
- [46].Oldenburg SJ, Jackson JB, Westcott SL, Halas NJ. Infrared extinction properties of gold nanoshells. Appl. Phys. Lett. 1999;75:2897–2899. [Google Scholar]
- [47].Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xia YN, Yang PD, Sun YG, Wu YY, Mayers B, Gates B, Yin YD, Kim F, Yan YQ. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003;15:353–389. [Google Scholar]
- [49].Zharov VP, Galitovsky V, Viegas M. Photothermal detection of local thermal effects during selective nanophotothermolysis. Appl. Phys. Lett. 2003;83:4897–4899. [Google Scholar]
- [50].Lee J, Govorov AO, Kotov NA. Nanoparticle assemblies with molecular springs: A nanoscale thermometer. Angew. Chem. Int. Ed. 2005;44:7439–7442. doi: 10.1002/anie.200501264. [DOI] [PubMed] [Google Scholar]
- [51].Huang W, Qian W, El-Sayed MA. Gold nanoparticles propulsion from surface fueled by absorption of femtosecond laser pulse at their surface plasmon resonance. J. Am. Chem. Soc. 2006;128:13330–13331. doi: 10.1021/ja064328p. [DOI] [PubMed] [Google Scholar]
- [52].Takahashi H, Niidome T, Nariai A, Niidome Y, Yamada S. Gold nanorod-sensitized cell death: Microscopic observation of single living cells irradiated by pulsed near-infrared laser light in the presence of gold nanorods. Chem. Lett. 2006;35:500–501. [Google Scholar]
- [53].Chen JY, Wang DL, Xi JF, Au L, Siekkinen A, Warsen A, Li ZY, Zhang H, Xia YN, Li XD. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007;7:1318–1322. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li ZY, Au L, Zhang H, Kimmey MB, Li XD, Xia Y. Gold nanocages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005;5:473–477. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]
- [55].Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B. 2006;110:7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- [56].Link S, El-Sayed MA. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000;19:409–453. [Google Scholar]
- [57].Prevo BG, Esakoff SA, Mikhailovsky A, Zasadzinski JA. Scalable routes to gold nanoshells with tunable sizes and response to near-infrared pulsed-laser irradiation. Small. 2008;4:1183–1195. doi: 10.1002/smll.200701290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wu G, Mikhailovsky A, Khant HA, Fu C, Chiu W, Zasadzinski JA. Remotely Triggered Liposome Release by Near-Infrared Light Absorption via Hollow Gold Nanoshells. J. Am. Chem. Soc. 2008;130:8175–8177. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Volodkin DV, Skirtach AG, Moehwald H. Near-IR remote drug release from assemblies of liposomes and nanoparticles. Angew. Chem., Int. Ed. 2009;48:1807–1809. doi: 10.1002/anie.200805572. [DOI] [PubMed] [Google Scholar]
- [60].Huang XH, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Laser Med. Sci. 2008;23:217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- [61].Radt B, Smith TA, Caruso F. Optically addressable nanostructured capsules. Adv. Mater. (Weinheim, Ger.) 2004;16:2184–2189. [Google Scholar]
- [62].Oishi M, Nakamura T, Jinji Y, Matsuishi K, Nagasaki Y. Multi-stimuli-triggered release of charged dye from smart PEGylated nanogels containing gold nanoparticles to regulate fluorescence signals. J. Mater. Chem. 2009;19:5909–5912. [Google Scholar]
- [63].Wu W, Shen J, Banerjee P, Zhou S. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials. 32:598–609. doi: 10.1016/j.biomaterials.2010.08.112. [DOI] [PubMed] [Google Scholar]
- [64].Huschka R, Neumann O, Barhoumi A, Halas NJ. Visualizing Light-Triggered Release of Molecules Inside Living Cells. Nano Lett. 10:4117–4122. doi: 10.1021/nl102293b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Barhoumi A, Huschka R, Bardhan R, Knight MW, Halas NJ. Light-induced release of DNA from plasmon-resonant nanoparticles: Towards light-controlled gene therapy. Chem. Phys. Lett. 2009;482:171–179. [Google Scholar]
- [66].Park H, Yang J, Lee J, Haam S, Choi IH, Yoo KH. Multifunctional Nanoparticles for Combined Doxorubicin and Photothermal Treatments. ACS Nano. 2009;3:2919–2926. doi: 10.1021/nn900215k. [DOI] [PubMed] [Google Scholar]
- [67].You J, Zhang G, Li C. Exceptionally High Payload of Doxorubicin in Hollow Gold Nanospheres for Near-Infrared Light-Triggered Drug Release. ACS Nano. 4:1033–1041. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Göppert-Mayer M. Über Elementarakte mit zwei Quantensprüngen. Annalen der Physik. 1931;401:273–294. [Google Scholar]
- [69].Marder SR, Lee, Kwang-Sup Photoresponsive Polymers I. Adv. Pol. Sci., Springer. 2008 [Google Scholar]
- [70].Kaiser W, Garrett CGB. Two-Photon Excitation in CaF2: Eu2+ Phys. Rev. Lett. 1961;7:229–231. [Google Scholar]
- [71].Mark JE. Physical properties of polymers handbook. Springer; 2007. [Google Scholar]
- [72].Goodwin AP, Mynar JL, Ma Y, Fleming GR, Frechet JMJ. Synthetic Micelle Sensitive to IR Light via a Two-Photon Process. J. Am. Chem. Soc. 2005;127:9952–9953. doi: 10.1021/ja0523035. [DOI] [PubMed] [Google Scholar]
- [73].Mynar JL, Goodwin AP, Cohen JA, Ma Y, Fleming GR, Frachet JMJ. Two-photon degradable supramolecular assemblies of linear-dendritic copolymers. Chem. Commun. 2007:2081–2082. doi: 10.1039/b701681f. [DOI] [PubMed] [Google Scholar]
- [74].Jiang JQ, Tong X, Morris D, Zhao Y. Toward photocontrolled release using light-dissociable block copolymer micelles. Macromolecules. 2006;39:4633–4640. [Google Scholar]
- [75].Furuta T, Wang SS-H, Dantzker JL, Dore TM, Bybee WJ, Callaway EM, Denk W, Tsien RY. Brominated 7-hydroxycoumarin-4-ylmethyls: Photolabile protecting groups with biologically useful cross-sections for two photon photolysis. Proc. Natl. Acad. Sci. USA. 1999;96:1193–1200. doi: 10.1073/pnas.96.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Babin J, Pelletier M, Lepage M, Allard J-F, Morris D, Zhao Y. A New Two-Photon-Sensitive Block Copolymer Nanocarrier. Angew. Chem. Int. Ed. 2009;121:3379–3382. doi: 10.1002/anie.200900255. [DOI] [PubMed] [Google Scholar]
- [77].Lee H.-i., Wu W, Oh JK, Mueller L, Sherwood G, Peteanu L, Kowalewski T, Matyjaszewski K. Light-Induced Reversible Formation of Polymeric Micelles. Angew. Chem. Int. Ed. 2007;119:2505–2509. doi: 10.1002/anie.200604278. [DOI] [PubMed] [Google Scholar]
- [78].Jiang X, Lavender CA, Woodcock JW, Zhao B. Multiple Micellization and Dissociation Transitions of Thermo- and Light-Sensitive Poly(ethylene oxide)-b-poly(ethoxytri(ethylene glycol) acrylate-co-o-nitrobenzyl acrylate) in Water. Macromolecules. 2008;41:2632–2643. [Google Scholar]
- [79].Uda RM, Hiraishi E, Ohnishi R, Nakahara Y, Kimura K. Morphological Changes in Vesicles and Release of an Encapsulated Compound Triggered by a Photoresponsive Malachite Green Leuconitrile Derivative. Langmuir. 2010;26:5444–5450. doi: 10.1021/la904190c. [DOI] [PubMed] [Google Scholar]
- [80].Cui W, Lu X, Cui K, Wu J, Wei Y, Lu Q. Photosensitive nanoparticles of chitosan complex for controlled release of dye molecules. Nanotechnology. 2011;22:065702. doi: 10.1088/0957-4484/22/6/065702. [DOI] [PubMed] [Google Scholar]
- [81].Gorostiza P, Isacoff EY. Nanoengineering Ion Channels for Optical Control. Physiology. 2008;23:238–247. doi: 10.1152/physiol.00018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kocer A, Walko M, Meijberg W, Feringa BL. A light-actuated nanovalve derived from a channel protein. Science. 2005;309:755–758. doi: 10.1126/science.1114760. [DOI] [PubMed] [Google Scholar]
- [83].Carling CJ, Boyer JC, Branda NR. Remote-Control Photoswitching Using NIR Light. J. Am. Chem. Soc. 2009;131:10838. doi: 10.1021/ja904746s. + [DOI] [PubMed] [Google Scholar]
- [84].Carling CJ, Nourmohammadian F, Boyer JC, Branda NR. Remote-Control Photorelease of Caged Compounds Using Near-Infrared Light and Upconverting Nanoparticles. Angew Chem Int Edit. 2010;49:3782–3785. doi: 10.1002/anie.201000611. [DOI] [PubMed] [Google Scholar]
- [85].Yan B, Boyer J-C, Branda NR, Zhao Y. Near-Infrared Light-Triggered Dissociation of Block Copolymer Micelles Using Upconverting Nanoparticles. J. Am. Chem. Soc. 2011;133:19714–19717. doi: 10.1021/ja209793b. [DOI] [PubMed] [Google Scholar]
- [86].Heer S, Kompe K, Gudel HU, Haase M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 2004;16:2102. + [Google Scholar]
- [87].Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. UV and Near-IR Triggered Release from Polymeric Nanoparticles. J. Am. Chem. Soc. 132:9540–9542. doi: 10.1021/ja102595j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Fomina N, McFearin CL, Sermsakdi M, Morachis JM, Almutairi A. Low Power, Biologically Benign NIR Light Triggers Polymer Disassembly. Macromolecules. 2011;44:8590–8597. doi: 10.1021/ma201850q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Johnson JA, Lu YY, Burts AO, Lim YH, Finn MG, Koberstein JT, Turro NJ, Tirrell DA, Grubbs RH. Core-Clickable PEG-Branch-Azide Bivalent-Bottle-Brush Polymers by ROMP: Grafting-Through and Clicking-To. J. Am. Chem. Soc. 2011;133:559–566. doi: 10.1021/ja108441d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].He J, Tong X, Zhao Y. Photoresponsive Nanogels Based on Photocontrollable Cross-Links. Macromolecules. 2009;42:4845–4852. [Google Scholar]
- [91].Härtner S, Kim H-C, Hampp N. Photodimerized 7-hydroxycoumarin with improved solubility in PMMA: Single-photon and two-photon-induced photocleavage in solution and PMMA films. Journal of Photochem. Photobiol. A. 2007;187:242–246. [Google Scholar]
- [92].Yu L. Photosensitive Cross-linked Block Copolymers with Controllable Release. Photochem. Photobiol. 2011 doi: 10.1111/j.1751-1097.2011.00894.x. [DOI] [PubMed] [Google Scholar]
- [93].Tomatsu I, Peng K, Kros A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Delivery Rev. 2011;63:1257–1266. doi: 10.1016/j.addr.2011.06.009. [DOI] [PubMed] [Google Scholar]