Summary
Converging lines of evidence from varied scientific disciplines suggest that cutaneous melanomas comprise biologically distinct subtypes that arise through multiple causal pathways. Understanding the respective relationships of each subtype with etiologic factors such as UV radiation and constitutional factors is the first necessary step toward developing refined prevention strategies for the specific forms of melanoma. Furthermore, classifying this disease precisely into biologically distinct subtypes is the key to developing mechanism- based treatments, as highlighted by recent discoveries. In this review, we outline the historical developments that underpin our understanding of melanoma heterogeneity, and we do this from the perspectives of clinical presentation, histopathology, epidemiology, molecular genetics, and developmental biology. We integrate the evidence from these separate trajectories to catalog the emerging major categories of melanomas and conclude with important unanswered questions relating to the development of melanoma and its cells of origin.
Keywords: Melanoma, melanocytes, risk factors, gene mutation, pathology
Introduction
The original description of melanoma as a disease entity is attributed to René Laennec, who published an article on ‘The Melanoses’ in 1812 (Laennec, 1812). Even at that time, Laennec already distinguished two forms of the disease, a firm form and a softening form. However, he was likely referring to progression stages, rather than distinct subforms of the disease. Since then, it has become clear that melanocytic neoplasms, whether benign or malignant, display a kaleidoscope of phenotypic variation and, with certainty, are comprised of biologically distinct subtypes. The precise number of diseases that are encompassed within the realm of melanocytic neoplasia is currently unclear, and criteria precisely defining them are in evolution. Multiple distinct categories of melanocytic neoplasms are distinguished in the current WHO classification of skin tumors (LeBoit et al. 2006). The benign neoplasms, melanocytic nevi, can present in large multiplicity or solitarily, can arise before or after birth, are small to giant, involve the epidermis or the dermis, and come in a range of cytologies. Similarly, melanomas express significant clinical and histopathological variation, often depending on the anatomical site on which they arise.
Despite these facts, attempts to classify melanocytic neoplasia into biologically uniform subsets have had little impact on the clinical management, in particular that of metastatic melanoma. For example, current staging protocols do not distinguish between different categories of cutaneous melanomas. In part, this is attributable to the fact that independent of the nature of the primary, melanoma undergoes a uniformly grim clinical course once metastases have developed. Furthermore, the cytotoxic and immunological therapies available to date do not target specific functional or regulatory pathways in cells, and most do not show much therapeutic effectiveness at all and none that consistently varies among the type of the primary from which the metastatic disease originated. Finally, many of the classification criteria that have been proposed show considerable overlap, making it difficult to categorize cases reproducibly based on histopathological criteria alone (Weyers et al., 1999). As a consequence, only a small minority of investigative studies have emphasized potential differences between disease subgroups. In recent years, emerging molecular data have provided strong genetic support for the notion of biologically distinct disease subsets. It has become apparent that the neglect of the heterogeneous nature of melanocytic neoplasia is obstructing progress in developing rationally based approaches to melanoma treatment and prevention, because critical effects and associations will be missed if biologically distinct disease groups are not separated.
At the outset, however, we acknowledge that the concept of melanomas arising through multiple different causal pathways has been understood implicitly for decades. Melanomas arising from internal tissues and melanomas arising on special areas of the glabrous (non-hair-bearing) skin such as plantar and palmar surfaces and the nail apparatus (hereafter referred to as acral melanomas) occur at low but predictable frequencies in all populations independent of ethnicity and climatic conditions. As such, they have long been suspected of having causes that differ from those arising on other body sites (Fleming et al., 1975; Hinds, 1979; Lewis, 1967; Stevens et al., 1990). However, even for cutaneous melanomas, i.e., melanomas arising from the non-glabrous skin, etiologic diversity is not a new concept. More than 40 yr ago, Lee and Merrill stated:
Since the beginning of this century, it has been recognised that malignant melanomas are heterogeneous, and that different clinical appearances are also associated with different distributions by site and age and have different outcomes. (Lee and Merrill, 1970)
Given the phenotypic diversity of melanocytic neoplasia, the wide variations in age and anatomical site of primary manifestation, and the different distribution of genetic alterations in specific subsets, the question also arises whether or not cells within the melanocytic lineage are comprised of different subtypes of melanocytes. If so, then it needs to be determined whether these subtypes and/or their separate differentiation stages have specific susceptibilities to environmental and genetic insults, which in turn may give rise to different types of melanocytic neoplasms.
What follows is an account of how the current theories about the causal pathways to melanoma and melanoma subtypes were developed. Here, we use the term ‘causal pathway’ in its broadest sense to describe the sequence of events or conditions or characteristics that together transform a melanocyte into a neoplasm. The term is inclusive and incorporates the mutagenic factors that presumably initiate the process, as well as the myriad host factors (be they susceptibility, protective, reparative molecular, or immunological factors) that also play a role in governing progression of melanocytic neoplasia. Equally, we use subtype as a taxonomic term in which categories are placed into hierarchical order, based on their biological and pathogenetic relationships. We contend that melanomas may be grouped according to common components of their mechanistic alterations and pathogenesis and further that such groupings are important to pave the way for developing strategies to clinically manage and prevent melanomas, specified by subtype. We have attempted to capture the key contributions to knowledge and placed them in the context of what was understood at the time of their discovery. Our account recognizes the different perspectives of the disciplines that have sought to understand the origins of melanoma and is therefore presented firstly in terms of clinical and histologic observations, before turning to epidemiological investigations and discoveries of molecular factors and aspects of melanocyte development. In the closing section, we provide an overview of the categories of melanocytic neoplasms, as they currently emerge considering and synthesizing information from the above areas of research. We acknowledge that this is an incomplete and subjective selection of information from a huge trove on published data on these diverse topics. We apologize in advance for having omitted important studies.
Clinical and histopathological aspects
There are distinct patterns of clinical and histopathological appearance that led to the distinction of ‘histogenetic’ types of melanoma put forward in the first classification system proposed by Wallace Clark and colleagues (McGovern et al., 1973). The classification employed histopathology, anatomical site, and degree of sun damage to distinguish four types of melanoma. A prominent feature for classification was the distribution of melanocytes within the early phase of melanoma growth, in which the tumor expands on the surface of the skin, the radial growth phase (RGP). Three different types were distinguished based on their intraepidermal growth pattern, and for the third type, its anatomical site of origin—superficial spreading melanoma (SSM), lentigo maligna melanoma (LMM), and acral lentiginous melanoma (ALM). A fourth type, nodular melanoma (NM), was proposed as a separate category because it presented without a significant radial growth phase. In SSM, a phase of superficial, radial growth clinically leads to a well-circumscribed polycyclic patch with variegating shades of brown, gray, and black, in which subsequently a nodule develops (vertical growth phase, VGP). Histopathologically, the telltale sign of SSM is the presence of enlarged melanocytes, often arranged as small aggregates or nests, that display marked upward scatter within the epidermis, a pattern referred to as ‘pagetoid’, because it resembles the intraepidermal spread of breast cancer in the epidermis of the nipple (Paget’s disease). By contrast, LMM and ALM display a lentiginous growth pattern, in which the melanocytes are arranged as solitary units along the basilar epidermis, without notable pagetoid growth. The lentiginous growth pattern typically fades continuously into the adjacent non-lesional skin, making it difficult or impossible to determine the boundary between melanoma in situ and normal skin. LMM and ALM also show a propensity to involve appendageal structures (such as hair follicles in LMM and sweat glands in ALM). By contrast, NM, by definition, does not have a significant RGP, suggesting an accelerated transition to the VGP.
Additional categories of melanomas that have distinctive clinical and/or histopathological features include desmoplastic melanoma, nevoid melanoma, melanoma arising from a blue nevus, melanoma arising from giant congenital nevus, and melanoma in childhood. In addition, melanomas can originate from extracutaneous sites such as the mucosal epithelium, primarily that of the anogenital region, oropharynx, and paranasal sinuses as well as the conjunctiva. Finally, melanomas can originate from melanocytes of the uvea, encompassing intraocular structures such as the iris, ciliary body, and choroid.
Epidemiological investigations into melanoma etiology
Latitude gradient
Melanoma has long been known to occur at highest incidence in fair-skinned populations, casting strong suspicion on sunlight as the likely environmental carcinogen (McGovern, 1952). The relationship between sunlight and melanoma was first demonstrated systematically by Herbert Lancaster (Lancaster, 1956) in an elegant epidemiological analysis in which he compared melanoma mortality between populations of European extraction residing at latitudes at varying degrees from the equator. He reported almost threefold higher melanoma mortality among the predominantly Anglo-Celtic populations residing in the tropical and subtropical states of Australia compared with those living in more temperate states. Markedly higher melanoma mortality was similarly observed in the lower latitudes of the North Island of New Zealand than the higher latitudes of the South Island. Further, melanoma mortality among white people residing in the lower latitudes of South Africa and the United States was systematically higher than white people residing at higher latitudes in Canada, the British Isles, and continental Europe. From these data, Lancaster hypothesized that ambient sunlight was the principal environmental determinant of the occurrence of melanoma within a population. Such patterns have been reported repeatedly from populations around the globe (Bulliard et al., 1994; Crombie, 1979b; Magnus, 1973; Swerdlow, 1979; Teppo et al., 1978) and, together with the observation that melanoma is overwhelmingly a disease of white people (Crombie, 1979a), still provide the most persuasive evidence that sunlight is the primary cause of cutaneous melanoma.
Anatomic distribution
Testing the hypothesis that sunlight was the principal cause of melanoma, it was expected that sites habitually exposed to the sun would have the highest incidence. Moreover, it was suspected that the anatomical sites experiencing the highest rates of melanoma might differ across populations, yet it was quickly shown that the rank order of body sites experiencing progressively higher incidence of melanoma was similar across fair-skinned populations, regardless of the overall incidence of melanoma in the populations under study (Lee and Merrill, 1970). Thus, in almost all reports, melanomas were shown to be most numerous overall on the back and limbs (particularly, the lower limb in women), followed by the head and neck, and then the anterior trunk (Bulliard, 2000; Green et al., 1993). A convincing argument was made however that comparisons of melanoma incidence by anatomic site that do not account for surface area may lead to erroneous inferences, because the ‘malignant potential’ for each target cell at a given site was really the measure of interest. On an area-adjusted basis, studies reported consistently that melanoma was most common on habitually sun-exposed sites (such as the ears in men and face and neck in both sexes), followed by intermittently exposed sites (back, shoulders, and limbs; Bulliard, 2000; Bulliard et al., 1997; Elwood and Gallagher, 1998; Franceschi et al., 1996; Green et al., 1993). Melanomas were noted to be particularly rare on sites habitually covered by clothing such as the buttocks, but also, paradoxically, on the dorsum of the hand. As mentioned previously, certain types of melanomas were observed to arise in areas that are well protected from UV radiation (such as the non-hair-bearing skin of the palms and soles and areas under the nails), and still others were recorded at sites that have no UV exposure at all (such as those on mucosal membranes). Remarkably, these two forms of melanoma, acral and mucosal, arise with similar absolute incidences in most populations (Elwood and Gallagher, 1998), underscoring the inference that these melanomas arise independently of sun exposure and genetic factors associated with constitutional skin pigmentation.
Insights from comparisons of occupational groups
To explore further the associations between sunlight exposure and melanoma risk, epidemiologists compared melanoma incidence across occupations (indoor versus outdoor work) as a surrogate measure of historical sun exposure. Somewhat unexpectedly, early investigations found that indoor workers experienced higher rates of cutaneous melanoma than outdoor workers (Lee and Strickland, 1980). However, more detailed analyses showed that outdoor workers experienced significantly higher rates of melanoma on habitually sun-exposed sites (face, head, and neck), whereas office workers were found to have a significant excess of melanomas on habitually covered body sites (Beral and Robinson, 1981; Vagero et al., 1986). These findings provided the first epidemiological evidence that melanomas arising on different body sites may be associated with sunlight in different ways.
Melanoma incidence by age and anatomical site
In 1998, Elwood and Gallagher performed a novel analysis in which they compared the incidence of melanoma across strata of age group and body site, being careful to adjust for the relative surface area of each site (Elwood and Gallagher, 1998). For their analyses, body sites were categorized as being ‘minimally exposed’ to the sun (viz. hip, thigh, buttock, abdomen and sole of foot in both sexes, plus scalp and chest in women), ‘maximally exposed’ (face, ear, dorsum of hand), or ‘intermittently exposed’ (all other sites). Among the young (<35 yr), overall melanoma incidence was low, but those tumors that did occur were most common on intermittently exposed sites and were exceptionally rare at maximally exposed sites. In early middle-age (35–49 yr), the area-adjusted incidence of melanomas was more than threefold higher on intermittently than maximally exposed sites. At older ages, the distributions were reversed so that above age 65 yr, the incidence of melanomas on maximally exposed sites was twice that of intermittently exposed sites, and more than 12 times higher than that of minimally exposed sites. Similar observations were made subsequently using registry data from New Zealand (Bulliard, 2000), USA (Lachiewicz et al., 2008), and Australia and Scotland (Whiteman et al., 2007). In all populations, it appeared that melanomas arising at younger ages occurred predominantly on the trunk and limbs, while at older ages, melanomas became more common on habitually sun-exposed sites such as the head and neck.
Analytical epidemiology: directly comparing those with and without melanoma
The preceding descriptive epidemiological studies made use of routinely collected data from large populations, often with only a limited number of variables (e.g., age, sex, site of melanoma). In contrast, analytical epidemiological studies are purposefully designed to collect pre-specified characteristics from targeted participants to enable comparisons between those with (‘cases’) and without (‘controls’) the disease of interest. Analytical studies have consistently shown that a suite of phenotypic factors are associated with increased risks of melanoma, including a large number of melanocytic nevi on the skin (Green et al., 1985; Holly et al., 1987; Holman and Armstrong, 1984b), a family history of melanoma (Bliss et al., 1995; Olsen et al., 2010a), fair skin that burns and does not tan (Bliss et al., 1995; Olsen et al., 2010b), and a propensity to freckling (Bliss et al., 1995; Olsen et al., 2010b). Of these, the highest risks of melanoma are conferred by having large numbers of nevi, and this fact, coupled with the observation that upwards of 30% of melanomas have histological evidence of pre-existing nevus remnants, suggests that nevi are both risk markers and precursors for melanoma although the absolute rate of progression is exceedingly small (Tsao et al., 2003). More recently, a number of constitutional genotypes associated with significantly increased risks of cutaneous melanoma have been identified through candidate approaches (MC1R; Bastiaens et al., 2001; Palmer et al., 2000) and genome-wide scans (TYR, MTAP; Bishop et al., 2009). Genome-wide studies have also identified some of the same genes as being associated with nevi (MTAP; Falchi et al., 2009) and pigmentation traits (MC1R, TYR; Sulem et al., 2007), confirming the epidemiological inference that these constitutional factors are likely heritable contributors to melanoma risk (Gudbjartsson et al., 2008). The role of heritability, particularly for nevi, is explored further below.
Melanoma heterogeneity by anatomic site
Rather than assume that all cutaneous melanomas arise in association with the same risk factors, a number of investigators have explored whether risk factors for melanoma differ according to the anatomic site of the primary tumor. While these earlier studies were not specifically designed or statistically powered to detect differences in melanoma risk by site, they found that people who develop melanomas on the trunk have nevus counts consistently higher than people whose melanomas arise on the head and neck (Chen et al., 1996; Kruger et al., 1992; Rieger et al., 1995; Weinstock et al., 1989). Two Australian studies corroborated and extended these findings by observing that the extent and direction of the associations with solar keratoses (markers of cumulative solar skin damage) and nevi are governed by the anatomical site of melanoma (Bataille et al., 1998; Whiteman et al., 1998). Thus, whereas the risks of truncal melanomas are associated with large numbers of nevi, they are inversely associated with numbers of solar keratoses. In contrast, melanomas arising on the head and neck are associated with large numbers of solar keratoses, but only weakly with numbers of nevi. Whether these site-specific associations reflect differences in sun exposure, melanocyte susceptibility, or constitutional factors is not clear from these observational studies, but they hint at the likely complexity underlying the development of cutaneous melanoma.
Melanoma heterogeneity by histopathological characteristics
The concept of etiologic heterogeneity of cutaneous melanoma was pursued in other ways, for example by performing analyses separately for each of the commonly recognized subtypes of melanoma (Holman and Armstrong, 1984a). Melanomas of the lentigo maligna subtype were found to be associated with high levels of accumulated sun exposure (as predicted from clinical experience), but no consistent differences were found across the other histological subtypes. By the mid-1980s, Ackerman and David had proposed a unifying concept for melanoma and asserted that distinct histological subtypes of melanoma did not exist (Ackerman, 1980). Nonetheless, they left the door ajar on the possibility of etiologic heterogeneity by speculating…
It would not be surprising, however, if one day it were shown that the causes and pathogenesis of malignant melanomas at different anatomic sites and those that arise de novo, rather than in association with melanocytic nevi, are very different. (Ackerman and David, 1986)
Pursuing a similar line of reasoning, Green (Green, 1992) proposed that the anatomic distribution of melanomas might be explained by melanocytes at different body sites having different propensities to undergo malignant change. Specifically, it was proposed that nevi on the head and neck are less likely to progress to melanoma than nevi on the trunk and would therefore give rise to fewer nevus-associated melanomas. In support of this theory, Green found that nevus-associated melanomas occur far more commonly on the trunk than on the head and neck. Studies in other populations confirmed the non-uniform distribution of nevus-associated melanomas across the skin surface (Kruger et al., 1992; Skender-Kalnenas et al., 1995). Further, it was found that patients with nevus-associated melanomas are significantly more likely to have high nevus counts, whereas de novo melanomas (i.e., those without evidence of a pre-existing nevus) are more likely to arise in older patients, on the head and neck, and be associated with solar elastosis (Carli et al., 1999; Purdue et al., 2005). As discussed below, recent molecular genetic studies strongly support the concept that melanomas arising on the central body parts of younger individuals with numerous melanocytic nevi are biologically distinct from melanomas arising on the cumulatively sun-damaged skin of older individuals and that the nevi and melanomas of the former pathway are driven by the same genetic alterations (BRAF mutations).
Numbers of nevi are determined by genes and sunlight
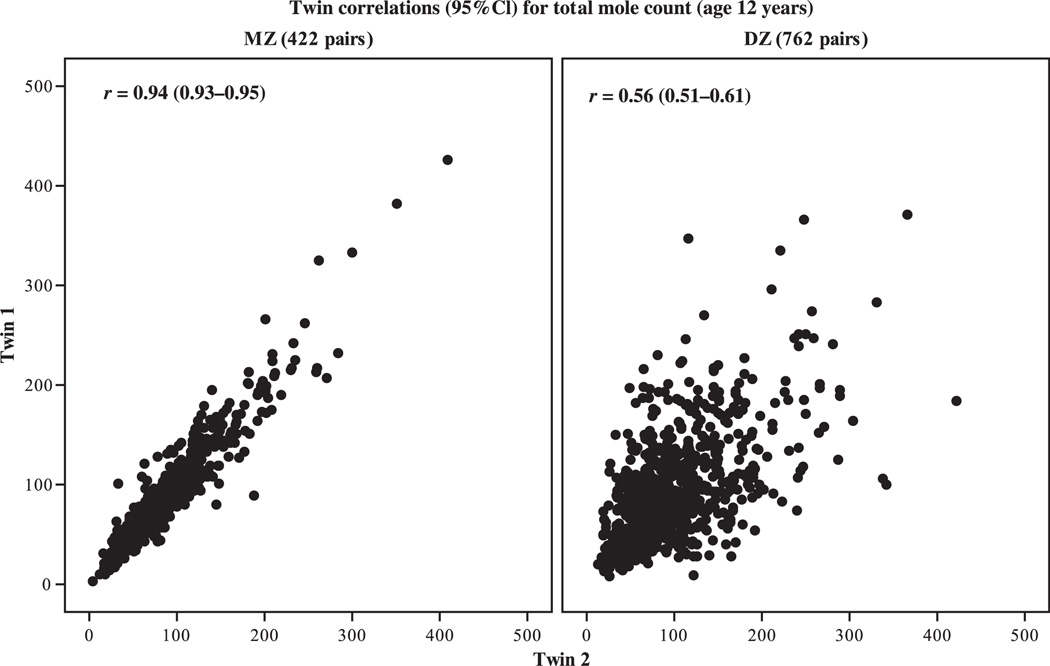
Given the strong epidemiological associations of nevi with cutaneous melanoma and the inference that at least a proportion of melanomas appear to arise directly from nevi, substantial efforts were made to identify those factors that drive the development of nevi in humans. Epidemiological studies quickly established that high levels of sun exposure predicted higher numbers of nevi in early childhood (English and Armstrong, 1994; Fritschi et al., 1994; Harrison et al., 2000; Kelly et al., 1994; Whiteman et al., 2005). Subsequent studies have since provided convincing evidence that the number of nevi on the skin is under strong genetic control by comparing nevus counts among twins. Whereas monozygotic (MZ, or identical) twin pairs share all of their genes and have extremely highly correlated nevus counts (twin1 versus twin2, r = 0.94), dizygotic (DZ, or fraternal) twin pairs share on average only half of their genes, and their nevus counts are considerably less correlated (r = 0.60, Figure 1; Zhu et al., 1999).
Figure 1.
Correlations of nevus counts for 422 identical (MZ) and 762 fraternal (DZ) twin pairs in Queensland, Australia (unpublished figure: Dr. Gu Zhu and Prof Nick Martin, Queensland Institute of Medical Research).
The nevus data in Figure 1 are informative beyond heritability. The range of nevus counts in that sample of 12-yr-old Queensland twins was very large (1 to 350+ nevi; Zhu et al., 1999). A parallel study using identical methodology but conducted in the United Kingdom [an environment of markedly lower solar irradiance (Diffey and Gies, 1998)] yielded almost identical correlations for MZ (r = 0.94) and DZ (r = 0.61) twins but reported substantially lower nevus counts (Wachsmuth et al., 2005). These paired studies of nevus heritability conducted in environments of markedly different insolation show that within fair-skinned populations, there exist some people with a high propensity to develop melanocytic nevi, while others have a low propensity, regardless of the ambient solar environment in which they are raised. Thus, genes appear to determine a person’s potential for nevus development, and the final expression of this potential is determined to some degree by that person’s exposure to sunlight. Efforts to locate the genes that influence nevus number are intensifying, with a number of candidates identified through genome-wide approaches (Falchi et al., 2009). Moreover, there is evidence emerging that at least one of these loci (thought to be MTAP) may also be associated with melanoma risk (Bishop et al., 2009).
Epidemiological summary
The accumulated data from epidemiological studies make a compelling case that different subtypes of cutaneous melanomas may be defined through different combinations of causal factors operating at different times in the evolution of the cancer. Those causal factors for which the evidence for heterogeneous effects is strongest include the pattern and dose of sun exposure and the age and phenotype of the host. Factors for which the evidence is rapidly emerging include the anatomic location of the pigment cell (potentially related to the tissue microenvironment of the developing pigment cells, as described below) and the constitutional genotype of the host. The following section extends the concept of melanoma heterogeneity by exploring whether the pathways to melanoma might be reflected in disruptions to molecules that control critical functions within the pigment cell.
Molecular aspects
The marked differences in the role of UV radiation and clinical phenotype as causal factors for melanoma according to body site distribution suggest differences in underlying genetic aspects, constitutional and somatic, of the disease. Over the last decade, significant progress has been made in uncovering critical somatic alterations, discoveries that are beginning to revolutionize the treatment of melanoma. It has also become apparent that specific genetic alterations are associated with particular clinical and histopathological features of the disease, suggesting that they will be helpful in refining existing disease classification schemes.
RAS and melanoma
The first melanoma oncogene to be identified was RAS (Albino et al., 1984, 1989), with NRAS being the most frequently affected RAS family member. The frequency of NRAS mutations is approximately 15% in most melanoma types, while HRAS or KRAS are infrequently mutated. This distribution differs from the overall RAS mutation frequency in cancer, in which KRAS is by far (85%) the most commonly mutated RAS allele (Downward 2003). van Elsas et al. (1996) found that one of 68 melanomas analyzed had an HRAS mutation and none had any KRAS mutation, and Curtin et al. (2005) found one HRAS mutation and no KRAS mutations in 126 primary melanomas of different types. Whether specific types of melanoma have a higher frequency of NRAS mutations is not entirely resolved. Earlier studies by van‘t Veer et al. (1989) and Ball et al. (1994) found a higher frequency of NRAS mutations in melanomas on sun-exposed sites (Ball et al., 1994; van‘t Veer et al., 1989). In a subsequent larger study of 175 primary tumor samples, 63 metastases, and 32 cell lines, van Elsas et al. (1996) also found the highest incidence of mutant NRAS in tumors arising on body sites such as the face or head (22%), compared with the limbs (15%) or the trunk (11%). By contrast, other studies did not find a significant association of NRAS mutations with anatomical site of origin or degree of sun exposure of the primary as assessed by the degree of solar elastosis in the adjacent skin (Broekaert et al., 2010; Curtin et al., 2005; Viros et al., 2008; Wagner et al., 1995).
Similarly, some studies reported NRAS mutations associated with certain histopathological subtypes, while others found no such associations (Lee et al., 2010). van Elsas et al. (1996) reported a higher mutation frequency in NM (28%) and LMM (23%), compared with SSM (14%), and Saldanha et al. (2006) found six of eleven (55%) NMs had NRAS mutations. By contrast, several larger studies did not see higher frequencies of NRAS mutations in NM or LMM than in SSMs (Broekaert et al., 2010; Curtin et al., 2005; Viros et al., 2008). In part, these discrepancies may be attributable to the lack of certainty with which the diagnosis of NM can be made. The original definition stipulates that any RGP flanking the VGP should not exceed more than three rete ridges into the flanking epidermis. This assessment is often impractical as the entire specimen may not be available for histologic inspection and only a small fraction of the circumference is typically examined to assess the degree of circumscription. Finally, NRAS mutations were initially reported to be associated with tumor thickness and level of invasion, but such associations have not been consistently observed throughout studies (Ball et al., 1994; Demunter et al., 2001; Edlundh-Rose et al., 2006; van Elsas et al., 1996; Omholt et al., 2002; Poynter et al., 2006).
BRAF and melanoma
Contrasting with the findings for NRAS, BRAF mutations, originally discovered in melanoma cell lines (Davies et al., 2002), have been reproducibly associated with specific clinical and histopathological characteristics of melanoma. Multiple studies have shown that patients with BRAF-mutated melanomas are significantly younger than patients with melanomas without BRAF mutations (Edlundh-Rose et al., 2006; Goel et al., 2006; Liu et al., 2007; Maldonado et al., 2003; Thomas et al., 2007). Furthermore, BRAF-mutant melanomas are more commonly of the SSM subtype (Broekaert et al., 2010; Curtin et al., 2005; Lang and MacKie, 2005; Liu et al., 2007; Maldonado et al., 2003; Saldanha et al., 2006; Sasaki et al., 2004; Thomas et al., 2007; Viros et al., 2008) and arise more commonly on the trunk (Broekaert et al., 2010; Curtin et al., 2005; Edlundh-Rose et al., 2006; Lang and MacKie, 2005; Liu et al., 2007; Maldonado et al., 2003; Thomas et al., 2007; Viros et al., 2008) and on the intermittently rather than chronically sun-exposed skin (Broekaert et al., 2010; Curtin et al., 2005; Davison et al., 2005; Deichmann et al., 2006; Lang and MacKie, 2005; Liu et al., 2007; Maldonado et al., 2003; Poynter et al., 2006; Thomas et al., 2007; Viros et al., 2008). Several studies have also shown that BRAF-mutant melanomas are more pigmented than melanomas without BRAF mutations by histopathological (Viros et al., 2008; Broekaert et al., 2010) and clinical inspection (Liu et al., 2007).
BRAF mutations are also commonly found in acquired melanocytic nevi (Pollock et al., 2002), and as these nevi tend to arise in the first two decades of life, it is likely that BRAF-mutant melanomas and nevi may be part of the same spectrum of melanocytic neoplasia. In support of this notion, several studies have found that BRAF-mutant melanomas are more likely to be associated with the presence of multiple melanocytic nevi on the patients’ skin than melanomas without BRAF mutations (Maldonado et al., 2003; Poynter et al., 2006; Thomas et al., 2007). This suggests that BRAF mutation is an early event that by itself is insufficient to cause melanoma. Further genetic alterations have to be acquired by the BRAF-mutant melanocytes within a nevus to result in melanoma formation, a process that takes additional time and could explain the difference in latency between the appearance of nevi in the first two decades and melanomas, typically in the third decade and later. The observation that the BRAF mutation frequency decreases in older individuals indicates a window of vulnerability to develop these mutations early in life (Curtin et al., 2005), consistent with epidemiological findings discussed above.
A detailed morphologic analysis of primary melanomas revealed that a combination of phenotypic features of the RGP of a primary tumor had a higher predictive value for the presence of a BRAF mutation than the histologic subtype of melanoma or any of the other features listed above (Viros et al., 2008). These features were the presence of upward scatter of intraepidermal melanocytes, predominance of melanocytes arranged in nests rather than single cells, and the degree of melanization of the constituent melanocytes. The associations between clinical and histopathological features were confirmed in a separate cohort in an independent multiobserver study (Broekaert et al., 2010). In both studies, BRAF-mutant melanomas were also found to be more likely to metastasize to regional lymph nodes than melanomas without BRAF mutations.
In aggregate, the reproducible association with younger age, clinical and histopathological features, and pattern of metastasis strongly suggest that melanomas with BRAF mutation are part of a biological subtype of melanoma. It is likely that this subtype is not fully defined by the BRAF mutation status, even if all activating mutations were considered. (Most studies have analyzed only exon 15 or codon 600.) The oncogenic alterations equivalent to BRAF or NRAS are not known in a substantial proportion of melanomas, and it is to be expected that there are other mutations or combinations thereof that are functionally equivalent to BRAF mutation and therefore result in (or are associated with) similar phenotypic alterations. This was suggested in a recent study in which melanomas that were predicted to be BRAF mutant based on the three features listed above, but that did not have BRAF or NRAS mutations, and were also more similar in other features associated with BRAF mutations (Broekaert et al., 2010). It remains to be demonstrated whether these ‘BRAF-like’ melanomas have other genetic or biological similarities to melanomas with BRAF mutations.
KIT and melanoma
Mutations in KIT are found in melanomas arising on glabrous skin or the nail apparatus, the mucosa, or skin with cumulative sun-induced damage (CSD melanomas) and are relatively absent in melanomas on skin without chronic sun-induced damage (Beadling et al., 2008; Curtin et al., 2006). In the melanoma types in which KIT mutations are found, BRAF mutations are relatively uncommon, and therefore, the two mutation spectra represent somewhat of a mirror image of each other. Acral and mucosal melanomas and melanomas on chronically sun-damaged skin have several features in common. Their incidence rises with age—as opposed to melanomas with BRAF mutation which peak around 50 yr of age—and they frequently show a lentiginous growth pattern, i.e., a growth pattern in which intraepidermal melanocytes are arranged as solitary units rather than nests within the basal layer or the epidermis. The melanomas in these categories tend to have in situ components that tend to be poorly circumscribed, with melanocytes gradually decreasing in number toward the periphery of the lesion, making it difficult or impossible for pathologists to evaluate excision margins. For acral melanomas, a pronounced field effect has been demonstrated in which melanocytes that are genetically abnormal, but histopathologically bland, can extend more than a centimeter into the seemingly normal skin adjacent to the melanoma. These attributes are in contrast to those found in melanomas with BRAF mutations, which tend to have a sharply demarcated intraepidermal component (Viros et al., 2008). The poor circumscription is likely to be due to an increased lateral mobility of the neoplastic melanocytes, which can be functionally linked to KIT pathway activation (Alexeev and Yoon, 2006). The SCFKIT signaling pathway is essential during melanocyte migration from the neural crest to the skin via the dorsolateral pathway, and reactivation of this pathway may therefore induce a histopathological growth pattern that reflects increased cell motility. The propensity for lateral migration in these melanoma types is also a plausible explanation for their notoriety to persist locally, i.e., recur at the excision site after an apparently complete excision. As opposed to the melanomas with BRAF mutation, acral, mucosal, and CSD melanomas tend not to arise in association with melanocytic nevi. Instead, they are characterized by a period of prolonged intraepidermal growth, which can last many years before an invasive component develops.
Despite the similarities between acral, mucosal, and CSD melanomas outlined above, several clear differences have been observed. Acral and mucosal melanomas both have a distinctive type of genomic instability that results in numerous focused gene amplifications and deletions scattered throughout the genome (Bastian et al., 2000, 2003; Curtin et al., 2005; van Dijk et al., 2003). Fluorescence in situ hybridization studies in acral melanoma indicate that these gene amplifications arise very early during progression, as they can already be identified in the in situ portions and the subtle extension of those in situ portions that escapes histopathological detection (field cells; Bastian et al., 2000; North et al., 2008). The mechanism underlying this marked instability is currently not understood. Comparisons of the aberration patterns between different tumor deposits from the same patient have yielded relatively stable patterns of aberrations, suggesting a transient period of genomic instability early in the development of these melanomas (Bastian, unpublished). This could be related to breakage–fusion–bridge cycles during telomere crisis or the recently described phenomenon of chromothripsis (Stephens et al., 2011). In both scenarios, chromosomes break repeatedly, forming complex rearrangements. In the case of telomere crisis, the breakages are thought to arise over several generations of cell divisions or, in the case of chromothripsis, as a single dramatic event shattering regions of a chromosome that are subsequently being patched together by the DNA repair machinery. Amplification of the genomic region harboring the catalytic subunit of telomerase has been observed in acral melanoma (Bastian et al., 2000; Curtin et al., 2005) and has been shown to coincide with the development of the vertical growth phase in some cases (North et al., 2008). While amplifications can also be found in a subset of melanomas arising outside of acral or mucosal sites, they typically develop late during progression, do not arise in multiplicity across the genome, and are not detectable in the early progression phase of these melanomas. Despite the relative paucity of BRAF mutations and the presence of KIT mutations that are shared between acral, mucosal, and CSD melanomas, the latter category is set apart by the absence of the numerous high-level amplifications that are found consistently in acral and mucosal melanomas. Furthermore, the genomic regions affected by amplification or other copy number changes differ between the two types, suggesting that acral and mucosal melanomas are biologically distinct. For example, amplifications in acral melanomas most frequently involved chromosome 11q13, centering on the cyclin D1 locus, as well as hTERT on chromosome 5p. By contrast, cyclin D1 is infrequently amplified in mucosal melanomas, where amplifications frequently involve the CDK4 and MDM2 locus on chromosome 12q (Curtin et al., 2005).
G-protein mutations in melanoma
Recently, mutations in G-proteins of the Gαq family of GTPases have been described in certain subsets of melanocytic neoplasia. G-proteins operate as a heterotrimeric complex of alpha, beta, and gamma subunits downstream of G-protein-coupled receptors, where they act as a molecular switch, relaying extracellular signals transduced by the receptor to intracytoplasmic effector proteins. A role for the two closely related Gαq family members Gq and G11 (encoded by the genes GNAQ and GNA11, respectively) in melanocyte biology was suggested because hypermorphic mutations in both genes were found to result in skin darkening in a mutagenesis screen in mice (Van Raamsdonk et al., 2004). A subsequent study in benign and malignant melanocytic neoplasms identified recurrent mutations of GNAQ in blue nevi and uveal melanomas (Van Raamsdonk et al., 2009). The mutations were different from the mutations initially found in the germline of mice, in that they exclusively affected codon 209 and by consequence effectively crippled the GTPase activity of GNAQ, leading to a GTP-bound, constitutively activated state. Subsequent studies have confirmed that the mutations are likely to occur early in the progression of melanocytic neoplasia, as suggested by their presence in benign nevi and the fact that they are not associated with outcome (Bauer et al. 2009; Onken et al. 2008). GNAQ mutations have also been identified in melanocytomas of the central nervous system, benign melanocytic neoplasms closely resembling blue nevi (Küsters-Vandevelde et al., 2009). A more recent study has identified recurrent mutations of GNA11 in the same spectrum of melanocytic neoplasms in which GNAQ mutations are observed, albeit with different mutation frequencies (Van Raamsdonk et al., 2010). While GNAQ mutations are common (approximately 80%) in blue nevi and less common in malignant tumors, GNA11 mutations are most common in uveal melanoma metastases, with a substantially lower mutation frequency in blue nevi (<10%), suggesting that it may have more powerful oncogenic effects than GNAQ. Strikingly, the mutations in GNAQ and GNA11 are restricted to blue nevi and uveal melanomas, with virtually no mutations in other benign or malignant melanocytic neoplasms harboring these mutations (Van Raamsdonk et al., 2009, 2010). To date, additional searches for GNAQ and GNA11 mutations have yet to yield other human neoplasms with recurrent mutations of these two Gαq family members (Eom et al., 2009; Lamba et al., 2009).
Clues to melanoma diversity from melanocyte development
The striking epidemiological, clinical, histopathological, and genetic differences observed for melanomas raise the possibility that they are related to properties inherent to the precursor cells from which melanomas derive, the melanocyte lineage. Differences in the susceptibility of melanocytes to becoming cancerous might be related to differences in developmental origins of melanocytes, different states of embryonic development, or differences in environments through which melanocytes migrate and differentiate. What follows are a series of issues that are the focus of current research and which may yield fundamental insights as to how melanomas arise.
Melanocytes derive from different developmental pathways
The most visually apparent location of melanin pigment-producing cells is in the integument, resulting in profound hair and skin coloration variations. However, pigment cells are found throughout the body including, but not limited to, the eye (uvea and retinal pigment epithelium), inner ear (stria vascularis), central nervous system (leptomeninges, substantia nigra), heart, and muscle. Some of these pigment-producing cells are derived from the central nervous system, including the retinal pigment epithelium and the substantia nigra. The majority of the other pigment cells are melanocytes having a common embryonic origin, the neural crest (Rawles, 1947). As outlined below, even our definition of some of the cells broadly classified as melanocytes may have to be revised as studies have demonstrated histologic and functional differences between melanocytes found throughout the organism.
The neural crest from which melanocytes derive is a transient population of migratory and multipotent cells that also gives rise to Schwann cells and neurons of the peripheral, enteric, and cranial nervous system, bone, cartilage, and adrenal and mesenchymal derivatives. Melanocytes have traditionally been described as arising later than other derivatives and migrating along a pathway on the most exterior surface of the fetus through the presumptive dermal skin with subsequent entry into the epidermal layer. It has also been proposed that melanocytes can populate the skin via migration along peripheral nerve projections that terminate within the skin (Cramer, 2009). Recent developmental studies using model organisms and lineage tracing have been able to trace melanocytes arising from migration of a multipotent precursor cell along nerve projections. These cells are retained in a ‘stem cell’-like state until competitive signaling at the nerve termini promotes these cells to differentiate into melanocytes (Adameyko and Lallemend, 2010; Adameyko et al., 2009). In mice, the choice to become Schwann cell versus melanocyte is governed by multiple factors including inhibitory and promoting pathways. For example, Neuregulin–ERBB3 signaling, while not needed for initial melanoblast development, is involved in inhibiting precursors cells to differentiate into melanocytes (Adameyko et al., 2009; Buac et al., 2009) and in the regulation of skin pigmentation (Choi et al., 2010) and is altered in melanoma (Buac et al., 2009). As melanocytes migrate and differentiate, they interact with multiple cell types before settling in their final locations. For example, melanocyte precursors migrating along a dorsolateral pathway first interact with mesenchymal cells; subsequently, they translocate to the epidermis where they interact with epidermal cells, including keratinocytes. The differentiation of a melanocyte from the neural crest is also likely to involve intermediates with the potential to differentiate into multiple cell types. While these intermediates have not clearly been identified in vivo, neural crest cells of several different lineages can be induced toward a melanocyte lineage in vitro following appropriate stimuli in the tissue environment (Dupin and Le Douarin, 2003; Le Douarin et al., 2004). The developmental potential of the neural crest also varies along the axis. While the neural crest of all regions can give rise to melanocytes, cephalic regions also give rise to neuronal, glial, and mesodermal cells. Neural crest cells in the trunk region have more restricted destinies, not being able to give rise to mesodermal tissues. The most caudal neural crest cells have the most restrictive lineages, also losing capacity to give rise to neurons (Catala et al., 2000). Given that melanocyte precursors develop from differing rostral–caudal portions of the neural tube and encounter different tissue environments along their developmental pathways, it is also likely that epigenetic modifications occur, which may still present as signatures within the melanocytes. Taken together, these differences in melanocyte development may also partially explain the variation of melanocyte populations to genetic insults.
Melanocytes are influenced by anatomic locations
Work by several groups has demonstrated that while the density of epidermal melanocytes for a given anatomic region is symmetrical within one individual, there is extensive variation both between individuals and interestingly across body regions within an individual (Gilchrest et al., 1979; Mitchell, 1963; Quevedo et al., 1965, 1969; Staricco and Pinkus, 1957; Szabo, 1954, 1967; Whiteman et al., 1999). Anatomical regions with the highest density of melanocytes are the genitalia (higher density in the men) and the head and neck area, followed by regions with intermediate density such as the back and limbs, while chest and abdomen have the lower densities. While some of these differences in melanocyte density may be secondary to environmental exposures such as UV exposure, it is likely that there are also inherent differences in these regions owing either to cellular interactions or to differential growth of the body. The head, for example, is proportionally large at birth and therefore grows in area less so than other body parts. In contrast, the limbs and trunk must expand. One might imagine that regional differences in the rates of expansion of body surfaces would translate into different rates of melanocyte proliferation as they strive for confluence. Both the abundance of melanocytes at a given location and the relative rates of proliferation of these cells during the course of growth may influence the frequency of melanoma occurrence and the types of pathways involve.
In the skin, melanocytes do not act in isolation but rather have extensive interactions with keratinocytes, Langerhans cells, and fibroblasts. Interactions include not only the transfer of melanin to keratinocytes, but extensive cytokine, hormonal, and growth factor signaling. It has been shown that co-cultures of fibroblasts with melanocytes can influence proliferative and pigmentary functions (Choi et al., 2010; Yamaguchi et al., 2007, 2008). For example, the less pigmented palmoplantar skin is proposed to result from an increased expression by fibroblasts of DKK1. DKK1 is an inhibitor of canonical Wnt signaling, which in turn modulates the growth and differentiation of melanocytes. Can similar interactions explain other regional differences? Comparison of gene expression profiles of human fibroblast populations from different anatomical sites has demonstrated that expression patterns vary according to location across the body surface (Rinn et al., 2006). In fact, patterns of gene expression of skin fibroblasts can be used to distinguish between cells derived from three broad locations: the rostral–caudal axis, the proximal–distal axis, and the dermal–non-dermal axis. Therefore, it is possible that differences in susceptibility to melanoma may be indirectly due to regional differences in supporting cells such as the fibroblasts or keratinocytes to maintain the melanocytes in a quiescent state.
Melanocytes exhibit differing morphologies
Within the integument, melanocytes can be observed at numerous locations: the basal layer of the dermal–epidermal junction, within hair follicles and interfollicular spaces of the epidermis, in sebaceous glands, and within the dermis. One might assume that all differentiated pigment producing cells were identical; however, several studies have demonstrated that melanocytes observed in each separate location have distinctive morphological and functional properties. Even within an anatomical region, variation in morphology has been noted. Szabo (1954) described two morphologically distinct types of melanocytes within the epidermis of the human cheek. One class of melanocytes exhibited a large cell body and multiple branches of dendrites, whereas a second class of melanocytes demonstrated a much smaller cell body. In addition to these histologically distinguishable melanocytes, less apparent, non-pigmented cells capable of forming melanocytes are also present. For example, melanocyte stem cells located within the bulge region of the hair follicle are needed to replenish follicular melanocytes after each hair cycle (Nishimura et al., 2002). Stem cells with multi-lineage potential can be derived from skin and induced to differentiate into melanocytes (Sviderskaya et al., 2009), and cells isolated with melanoblast markers can be induced along multiple lineages including melanocytes. Evidence is also mounting for a stem cell residing in the dermis and/or peripheral nerve projections that can give rise to the epidermal melanocytes (Cramer, 2009; Li et al., 2010).
Melanocytes in differint anatomical locations can also exhibit differences in gene expression (Medic et al., 2010). In cranial regions of developing embryos, melanoblasts near the neural tube express KIT and subsequently express MITF when migrating. In the trunk, both KIT and MITF are expressed in melanoblasts observed near the neural tube (Opdecamp et al., 1997; Wilson et al., 2004). Furthermore, differences are found between melanocytes within different microanatomic regions of the skin: dermal, epidermal, follicular, and stem cell melanocytes exhibit differing cadherin expression during development and postnatally (Jouneau et al., 2000; Nishimura et al., 1999), and GSTA4 is specifically expressed in stria vascularis melanocytes but not other melanocyte subtypes (Uehara et al., 2009).
The differences observed between melanocytes from different sites through gene expression and morphologic studies are also supported by functional studies. Gain-of-function experiments using transgenic mice showed that overexpression of endothelin 3 and HGF results in increased dermal melanocytes, while KITL overexpression results in increased numbers of interfollicular melanocytes (Aoki et al., 2009; Kunisada et al., 1998). Misexpression of the metabotropic glutamate receptor GRM1 in melanocyte lineage also results in an increased dermal melanocyte expansion, pigmentation, and nevus formation (Pollock et al., 2003). Interestingly, both ENDRB, the receptor for endothelin 3, and GRM1 are G-protein-coupled receptors that signal through Ga subunits of the Gq family of which two members, GNAQ and GNA11, are frequently mutated in intradermal proliferations and uveal melanoma. These comparisons suggest that epidermal and dermal melanocytes require or respond to different functional growth factors. In support of this idea, a modest reduction in KIT signaling affects melanocytes in uvea, cochlea, and Harderian gland much less than melanocytes in the skin (Aoki et al., 2009). Using in vivo and in vitro analyses with inhibitors for KIT, EDNRB, and MET signaling, it has been proposed that cutaneous epidermal melanocytes are more dependent on KIT signaling, whereas cutaneous dermal and non-cutaneous melanocytes are more dependent on EDNRB and MET signaling (Aoki et al., 2005, 2009, 2011).
Melanocyte subtypes have also been proposed by analysis of cells early in development. Boissy et al. (1990) used an interesting chicken mutant, called recessive albino, to assess the relationship of uveal and feather follicular melanocytes. This mutant has pigmented uveal melanocytes but albino feather melanocytes. Culturing neural crest cells isolated from the recessive white mutant chick embryos results in both albino (presumed epidermal) and pigmented (presumed uveal) melanocytes, supporting an intrinsic difference between the two populations that is not overcome by culture conditions. Separating these two populations by FACS analysis demonstrated that these two populations retained their fate. These two cell types were found to be derived throughout the rostral–caudal axis, yet in vivo, they were found only in the uvea. Boissy proposes that they represent evolutionary remnants, originally used by amphibians and reptiles as dermal derivatives for colorations, but which are no longer needed in haired species. This could be due to selective environments for epidermal and dermal melanocyte growth factors.
Activation of genetic pathways affects subsets of melanocyte derivatives
As described previously, genetic analyses have found that mutations in specific pathways are more prevalent in some melanoma subtypes than others. For example, mutations in uveal melanoma, blue nevi and central nervous system harbor mutations in GNAQ and GNA11, but typically lack mutations in the BRAF, NRAS, or KIT pathways. Is it that the GTPase pathway genes are particularly prone to mutate in uveal, dermal, and CNS melanoma precursors, or rather that certain pathways are constitutively activated only in certain sublineages of melanocytes? Functional studies of melanocyte development and analysis of mutations in animal models support the latter conjecture.
In animal models, many mutations exist that affect melanocyte development and function. If specific pathway mutations act only within some subsets of melanocytes (as proposed above), then one would expect to see distinct patterns of melanocyte defects and melanoma. In fact, this seems to be the case. From genetic screens for darker skin and hair in mice, mutations have been identified that result in elevations of melanocyte numbers in dermal versus epidermal compartments (Fitch et al., 2003; Van Raamsdonk et al., 2004). For example, mutations of the GTPases, Gαq, and Gα11, which act downstream of EDNRB, cause dermal hyper-pigmentation owing to expansion of dermal melanocytes. This is consistent with the observation that overexpression of the EDNRB ligand, ET3, causes an increase in the number of dermal melanocytes (Garcia et al., 2008). In contrast, overexpression of KITL either through transgene expression (Kunisada et al., 1998) or by mutations in ribosomal proteins RPS19 and RPS20 (McGowan et al., 2008) leads to increases in the number of epidermal melanocytes. Notch signaling has been shown to be necessary for maintenance of epidermal melanocytes, while dermal and uveal melanocytes are not affected by loss of Notch (Schouwey et al., 2007). These findings clearly indicate that subsets of melanocytes respond to activation of distinct pathways, supporting the hypothesis of different sublineages of melanocytes. This does not appear to be the case for all genetic mutations however, as overexpression of mutant BRAFV600E in mouse melanocytes affects both dermal and epidermal melanocytes (Dankort et al., 2009; Dhomen et al., 2010).
The emerging subtypes of melanoma: a synthesis
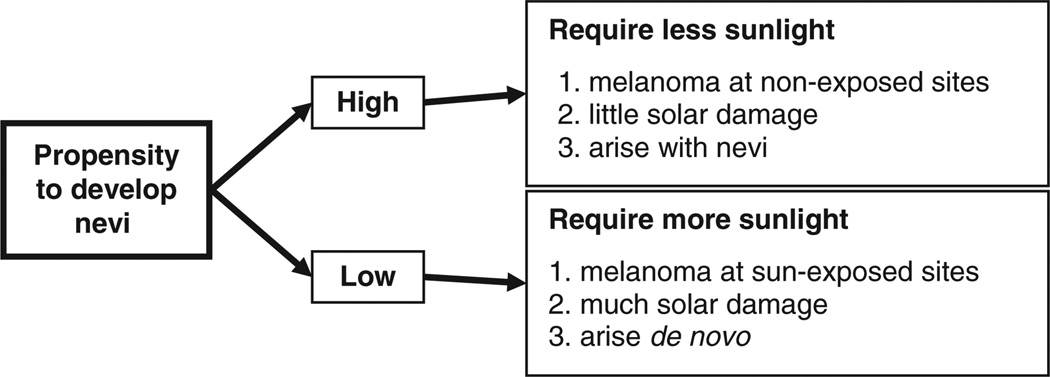
This historical account has shown that by the late 1990s, the accumulating data rendered a ‘single common pathway’ model to explain the development and behavior of all cutaneous melanomas untenable. In a first attempt to explain the complex epidemiological features of melanoma, a simple two-pathway model was proposed, which incorporated accepted risk factors (sunlight, nevi, pigmentation) with the newer concept of melanocytic proliferative potential (Whiteman et al., 1998) (Figure 2). The model posited that among people with high nevus counts (presumed to have melanocytes with inherently high proliferative potential), sun exposure is required to initiate melanoma development, after which other host factors drive progression to cancer. In contrast, among those with low nevus counts, it was postulated that chronic exposure to sunlight is necessary to drive melanocytes to develop into melanomas. The hypothesis predicted that, all other things being equal, melanomas in the former group would arise on sites with large populations of ‘unstable’ or ‘proliferative’ melanocytes, whereas melanomas in the latter group would arise most often on habitually sun-exposed anatomical sites.
Figure 2.
Divergent pathway hypothesis for cutaneous melanoma. The divergent pathway hypothesis proposed that one determinant of melanoma development is the propensity of the host to develop nevi. For people with a tendency to develop large numbers of nevi, melanomas are more likely to be initiated after only modest amounts of sun exposure, after which host factors appear to largely drive further progression of the tumor. For people with little tendency to develop nevi, repeated exposure to the sun appears necessary for melanoma development. These ‘divergent pathways’ are likely to explain, at least in part, the diverse anatomic distributions and risk factor associations observed for cutaneous melanomas.
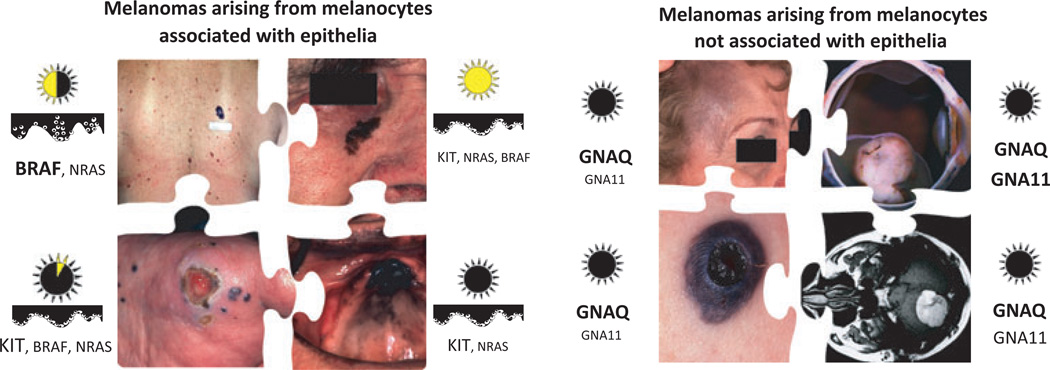
A number of studies have since provided support for this hypothesis with the general observation that patients with melanomas on the trunk are significantly younger and more likely to have high nevus counts than patients with melanomas on the head or neck but are significantly less likely to have evidence of high levels of cumulative sun exposure (Newton-Bishop et al., 2010; Olsen et al., 2009; Whiteman et al., 2003, 2006). The notion of at least two different types of melanoma on the sun-exposed skin was independently confirmed by the molecular studies associating BRAF and KIT mutations with distinct clinical and histopathological features. While the precise delineating criteria of the melanoma subsets have yet to be formulated, the following subtypes emerge (Figure 3):
Figure 3.
The emerging subtypes of melanoma: a synthesis. The emerging subtypes of melanoma: Melanomas can be subdivided into epithelia-associated (left panel) and non-epithelia associated types (right panel). The left panel shows from the upper left clockwise non-CSD, CSD, acral, and mucosal melanoma, and the right panel shows nevus of Ota, uveal melanoma, melanoma arising within a blue nevus, and melanocytoma. The sun icons indicate the relative cumulative UV exposure typical for the respective scenarios. The cartoons underneath illustrate the intraepidermal growth pattern, and the font size for the genes reflects their relative frequency for each type.
Melanomas arising from epithelial melanocytes
CSD melanomas
Chronic sun damage (CSD) melanomas represent the second most common type of melanoma in peoples of European descent and start to arise after the fifth decade of life, with an incidence progressively increasing with older age. They primarily affect the chronically sun-exposed skin of the head and neck, face, and distal and dorsal extremities. The surrounding skin typically shows marked solar elastosis. They typically do not arise in association with a precursor melanocytic nevus or in patients with numerous melanocytic nevi. Instead, afflicted patients often have other cutaneous neoplasms associated with chronic sun exposure such as actinic keratoses, solar lentigines, or other types of non-melanoma skin cancers. Together, these findings suggest that CSD melanomas require high cumulative doses of UV radiation to develop.
Microscopically, these melanomas show a radial growth phase comprised of melanocytes arranged as solitary units with poor lateral circumscription. Molecularly, CSD melanomas have a lower prevalence of mutant BRAF than non-CSD melanomas, with 30–40% showing mutations in KIT or NRAS, and a considerable proportion likely to have mutations in as yet undiscovered oncogenes. Similar to the other melanoma categories that also have activation of the KIT pathway (acral and mucosal types), CSD melanomas tend to be poorly circumscribed, with areas of melanoma in situ gradually fading into the non-lesional skin. It is conceivable that the lentiginous growth pattern prevailing in these melanoma types reflects the effects of activated KIT signaling on melanocyte migration.
Non-CSD melanomas
This subtype represents the most common type of melanoma in people of European descent. The incidence of non-CSD melanomas peaks somewhere around 50 yr of age and decreases with older age. They arise primarily on the intermittently sun-exposed skin of the trunk and proximal extremities on individuals with multiple melanocytic nevi. The lesions tend to be pigmented and microscopically are composed of larger, slightly pigmented melanocytes that are arranged primarily in nests and display upward intraepidermal scatter in their radial growth phase. On a molecular level, these melanomas, as well as the melanocytic nevi associated with this phenotype, are characterized by a high frequency of BRAF mutations (about 70%). The definition using the degree of solar elastosis is provisional, as it likely to represent a complex trait influenced by cumulative UV exposure, age, anatomical site, and constitutional factors (likely but not limited to skin pigmentation and tanning ability). A recent study showed evidence that a low level of solar elastosis is independently associated with BRAF mutation status, but it remains unclear what the underlying mechanisms are. In general, the non-CSD category strongly overlaps but is not identical with SSM. ‘Non-CSD melanoma’ is a broader concept that includes all melanomas arising on skin without marked chronic sun-induced damage, including those that would be classified histopathologically as NM. Previous studies suggested that the category of NM, while clinically a well-recognized phenomenon, identifies variants of melanoma on a subordinate taxonomic level (Broekaert et al., 2010). Biologically, NMs represent lesions with an accelerated transition from RGP to VGP, but they are heterogenous, as they can be seen in non-CSD, CSD, acral, and mucosal categories.
The association with melanocytic nevi, either directly adjacent to melanoma or elsewhere on the body, is a striking feature of non-CSD melanoma. The nevi arise primarily in the first two decades of life, with the melanomas starting to appear later, consistent with a model in which they require additional genetic alterations to occur. As described above, nevus number is a heritable trait. Thus, the finding of multiple, mutant BRAF-driven melanocytic neoplasms—nevi and melanomas—developing relatively early in life and on areas of the skin with comparatively little cumulative sun exposure implies a constitutional susceptibility to this class of lesions (Curtin et al., 2005). One aspect of this heritability has been linked to constitutional variation of the melanocortin 1 receptor MC1R. Within the category of non-CSD melanomas, germline polymorphisms in MC1R have been associated with BRAF mutations in several studies (Fargnoli et al., 2008; Landi et al., 2006), raising the possibility that altered signaling downstream of the melanocortin receptor may be one of several possible factors contributing to this susceptibility. Of note, the association of BRAF mutations with MC1R variants appears strictly confined to the setting of non-CSD melanomas. As MC1R variants are even more common in people with CSD melanomas and because CSD melanomas are driven by mutations other than BRAF, analyses of data sets that include CSD melanomas may miss the association with BRAF mutation or even detect an inverse association (Scherer et al., 2010). This suggests further, unexplored heterogeneity within the category of non-CSD melanoma, one in which BRAF is associated with variation of MC1R, and another in which wild-type MC1R is associated with NRAS and other, yet to be discovered, oncogenic alterations.
The fact that the BRAF-driven melanocytic neoplasms appear to be initiated during a window early in life is intriguing and deserves further study. A possible explanation of this period of vulnerability for BRAF-mutated neoplasms could be related to the massive numeric increase of melanocytes during the expansion of the skin surface during the growth of the body in the first two decades. During childhood and adolescence, the surface areas of the trunk and extremities expand relatively more than the head, which is proportionally larger during early life. Assuming that the relative densities of melanocytes remain constant during this growth period, melanocytes (or their progenitors) must proliferate substantially at rates proportional to the relative surface expansion of the respective body areas. It is attractive to speculate that this prolonged period of homeostatic proliferation may set up a state of vulnerability for the effects of mutant BRAF. According to this model, some of the variation of nevus density across body areas (higher on trunk and extremities compared to the head) could be related to the differences in surface expansion and concomitant melanocytic proliferation.
Acral melanomas
These melanomas originate from the glabrous skin of the palms, soles, and the nail apparatus and occur at similar incidences across all world populations. Their anatomical distribution and epidemiology suggest that UV radiation is not a causal factor. Molecularly, BRAF mutations are observed at lower frequency than for non-CSD melanomas, with about 20% having mutations in KIT and about 50% likely to have mutations in yet to be discovered oncogenes. Acral melanomas are characterized by an unusual degree of genomic instability that is already present in their very earliest manifestation and results in frequent focal amplifications. The cyclin D1 locus on chromosome 11q13 is the most frequently amplified site and occurs in 40% of cases (Sauter et al., 2002).
As a result of a shared anatomical attribute of acral skin and mucosa—both lack hair follicles—the melanocytes at these sites may differ from melanocytes of non-glabrous skin in ways which might explain the characteristic patterns of genomic instability observed for these classes of melanomas. Hair follicles are the only known niche for melanocyte stem cells (unless this function is performed by some other anatomical compartment in these sites). If no stem cell compartment exists in these sites, then melanocytes would be expected to have exceptionally long life spans and, by consequence, subjected to more replicative stress and telomere exhaustion. Such conditions would favor chromosomal damage over other types of genomic aberrations.
Mucosal melanomas
These melanomas originate from the non-stratified epithelia of the oropharynx, paranasal sinuses, tarsal conjunctiva, and anogenital area. Similar to acral melanoma, mucosal melanomas show a high degree of genomic instability with frequent amplifications. However, the genomic regions recurrently affected by these amplifications differ from those found in acral melanomas. Cyclin D1 amplifications are less common than in acral melanoma and, instead, amplification of the CDK4 locus on chromosome 12q are frequently found (Curtin et al., 2005; van Dijk et al., 2003; Muthusamy et al., 2006).
Melanomas arising from melanocytes not associated with epithelia
As outlined above, melanocytic neoplasms can arise in the dermis or the uvea, without any involvement of epithelial structures such as the epidermis, hair follicle, or mucosa. These neoplasms fall into the categories of intradermal melanocytic neoplasms such as blue nevi and related lesions (nevus of Ota, nevus of Ito) and melanomas arising from such nevi, as well as uveal melanoma. In all types, mutations of G-protein alpha subunits of the Gq family, GNAQ, and GNA11, are found in the majority of cases, and these mutations are virtually absent in melanocytic neoplasms arising from epithelia-associated melanocytes. These findings, together with the considerations about melanocyte development and migration outlined above, suggest that they represent a distinct class of melanocytic neoplasms, possibly arising from a different type of melanocyte. As described above, dermal melanocytes are more dependent on endothelin and WNT signaling than KIT signaling and can be expanded in number by activating these pathways. WNT receptors as well as endothelin receptors both signal through Gαq subunits. Furthermore, GRM1 receptor expression in mouse melanocytes results in the formation of blue nevi in mice (Pollock et al., 2003). GRM1 also signals through Gαq family members. It is therefore likely that the high frequency of mutations in signaling components of these pathways reflects the dependence of these melanocytes on specific signaling pathways that utilize G-protein-coupled receptors signaling via Gαq. The anatomical distribution of these lesions reaches from primary melanocytomas of the central nervous system to segmental melanocytic proliferations involving the first branch of the trigeminal nerve (nevus of Ota) or cervical nerves (nevus of Ota) to the discrete lesions of blue nevi. This pattern may be due to the acquisition of Gαq mutations during different time points of melanocyte development and migration, where mutations arising early in this migration result in localized tumors of the central nervous system, mutations arising during migrating melanoblasts result in segmentally distributed lesions, whereas blue nevi and related neoplasms arise from mutations of distal progeny of these melanocytes. Future studies will have to determine whether peripheral nerves harbor an active pool of melanocyte stem cells that provides melanocytes for the skin and can give rise to specific neoplasms, if transformed by mutations in Gαq family members.
Uveal melanomas
Uveal melanomas originate from melanocytes of the choroid, the ciliary body, or the iris. They are composed of spindle and/or epithelioid, highly pigmented melanocytes. They have a unique pattern of metastasis with a propensity to bypass the lymph nodes and metastasize directly to the liver. On a molecular level, they are characterized by mutations in GNAQ or GNA11 with no mutations in BRAF, NRAS, or KIT. Additional mutations in the histone de-ubiquitinase BAP1 arise later during progression followed by loss of chromosome 3, eliminating the remaining wild-type BAP1 allele. Loss of BAP1 coincides with metastatic potential in uveal melanoma (Harbour et al., 2010).
Blue nevi and melanomas arising from blue nevi
These neoplasms arise within the dermis and typically spare epithelial structures, in contrast to the majority of melanocytic neoplasms arising on the skin or mucosa, which typically arise within the epithelium.
Melanocytomas
These melanocytic neoplasms of the central nervous system closely resemble blue nevi and also show frequent mutations in GNAQ and probably GNA11. They can pose differential diagnostic problems to melanoma metastases, and the detection of Gαq mutations may help to establish the diagnosis.
Conclusions
Melanoma represents a group of distinct disease types. Several subtypes are emerging that show differences in their anatomical distribution, age of onset, relationship to UV radiation, and patterns of somatic mutations. Future studies have to refine the boundaries between these subtypes, possibly distinguish additional subtypes, and fill in their respective progression pathways from precursor lesions to manifest melanoma. Although incomplete, the subtypes outlined above already provide practical guidance for the management of patients and for the interpretation of research results. Because of the striking differences between some of the subtypes, it appears obvious that they have to be regarded separately when considering guidelines or recommendations for prevention, therapy, and prognostication of melanoma, as what may apply to one type may not for the other. This effect has been highlighted by the advent of molecularly targeted therapy but is clearly not restricted to this area. Most, if not all, current treatment guidelines are formulated for melanoma as if it were a single disease entity.
As we have highlighted, many important questions remain to be answered in how specific environmental factors interact with melanocytes and how these interactions may vary depending on the developmental state of the host and the type of melanocyte, its differentiation, anatomical localization in the tissue, and its surrounding environment.
Acknowledgements
David C. Whiteman is supported by a Future Fellowship from the Australian Research Council. William J. Pavan is supported by the National Human Genome Research Institute’s Intramural Research Program. Boris C. Bastian is supported by grants from the National Cancer Institute (CA025874, CA142873, CA131524).
References
- Ackerman AB. Malignant melanoma: a unifying concept. Hum. Pathol. 1980;11:591–595. doi: 10.1016/s0046-8177(80)80069-4. [DOI] [PubMed] [Google Scholar]
- Ackerman AB, David KM. A unifying concept of malignant melanoma: biologic aspects. Hum. Pathol. 1986;17:438–440. doi: 10.1016/s0046-8177(86)80030-2. [DOI] [PubMed] [Google Scholar]
- Adameyko I, Lallemend F. Glial versus melanocyte cell fate choice: Schwann cell precursors as a cellular origin of melanocytes. Cell. Mol. Life Sci. 2010;67:3037–3055. doi: 10.1007/s00018-010-0390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adameyko I, Lallemend F, Aquino JB, Pereira JA, Topilko P, Müller T, Fritz N, Beljajeva A, Mochii M, Liste I. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139:366–379. doi: 10.1016/j.cell.2009.07.049. [DOI] [PubMed] [Google Scholar]
- Albino AP, Le Strange R, Oliff AI, Furth ME, Old LJ. Transforming ras genes from human melanoma: a manifestation of tumour heterogeneity? Nature. 1984;308:69–72. doi: 10.1038/308069a0. [DOI] [PubMed] [Google Scholar]
- Albino AP, Nanus DM, Mentle IR, Cordon-Cardo C, McNutt NS, Bressler J, Andreeff M. Analysis of ras oncogenes in malignant melanoma and precursor lesions: correlation of point mutations with differentiation phenotype. Oncogene. 1989;4:1363–1374. [PubMed] [Google Scholar]
- Alexeev V, Yoon K. Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J. Invest. Dermatol. 2006;126:1102–1110. doi: 10.1038/sj.jid.5700125. [DOI] [PubMed] [Google Scholar]
- Aoki H, Motohashi T, Yoshimura N, Yamazaki H, Yamane T, Panthier JJ, Kunisada T. Cooperative and indispensable roles of endothelin 3 and KIT signalings in melanocyte development. Dev. Dyn. 2005;233:407–417. doi: 10.1002/dvdy.20340. [DOI] [PubMed] [Google Scholar]
- Aoki H, Yamada Y, Hara A, Kunisada T. Two distinct types of mouse melanocyte: differential signaling requirement for the maintenance of non-cutaneous and dermal versus epidermal melanocytes. Development. 2009;136:2511–2521. doi: 10.1242/dev.037168. [DOI] [PubMed] [Google Scholar]
- Aoki H, Hara A, Motohashi T, Osawa M, Kunisada T. Functionally distinct melanocyte populations revealed by reconstitution of hair follicles in mice. Pigment Cell Melanoma Res. 2011;24:125–135. doi: 10.1111/j.1755-148X.2010.00801.x. [DOI] [PubMed] [Google Scholar]
- Ball NJ, Yohn JJ, Morelli JG, Norris DA, Golitz LE, Hoeffler JP. Ras mutations in human melanoma: a marker of malignant progression. J. Invest. Dermatol. 1994;102:285–290. doi: 10.1111/1523-1747.ep12371783. [DOI] [PubMed] [Google Scholar]
- Bastiaens M, ter Huurne J, Gruis N, Bergman W, Westendorp R, Vermeer BJ, Bouwes Bavinck JN. The melanocortin-1-receptor gene is the major freckle gene. Hum. Mol. Genet. 2001;10:1701–1708. doi: 10.1093/hmg/10.16.1701. [DOI] [PubMed] [Google Scholar]
- Bastian BC, Kashani-Sabet M, Hamm H, Godfrey T, Moore DH, Brocker EB, LeBoit PE, Pinkel D. Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Res. 2000;60:1968–1973. [PubMed] [Google Scholar]
- Bastian BC, Olshen AB, LeBoit PE, Pinkel D. Classifying melanocytic tumors based on DNA copy number changes. Am. J. Pathol. 2003;163:1765–1770. doi: 10.1016/S0002-9440(10)63536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille V, Sasieni P, Grulich A, Swerdlow A, McCarthy W, Hersey P, Newton Bishop JA, Cuzick J. Solar keratoses: a risk factor for melanoma but negative association with melanocytic naevi. Int. J. Cancer. 1998;78:8–12. doi: 10.1002/(sici)1097-0215(19980925)78:1<8::aid-ijc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Bauer J, Kilic E, Vaarwater J, Bastian BC, Garbe C, de Klein A. Oncogenic GNAQ mutations are not correlated with disease-free survival in uveal melanoma. British Journal of Cancer. 2009;101:813–815. doi: 10.1038/sj.bjc.6605226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. 2008;14:6821–6828. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- Beral V, Robinson N. The relationship of malignant melanoma, basal and squamous skin cancers to indoor and outdoor work. Br. J. Cancer. 1981;44:886–891. doi: 10.1038/bjc.1981.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DT, Demenais F, Iles MM. Genome-wide association study identifies three loci associated with melanoma risk. Nat. Genet. 2009;41:920–925. doi: 10.1038/ng.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss JM, Ford D, Swerdlow AJ. Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case-control studies. Int. J. Cancer. 1995;62:367–376. doi: 10.1002/ijc.2910620402. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Trinkle LS, Nordlund JJ. Neural-tube-derived melanocyte subsets undergo commitment to their distinct lineages in culture. Cell Differ. Dev. 1990;30:129–145. doi: 10.1016/0922-3371(90)90081-7. [DOI] [PubMed] [Google Scholar]
- Broekaert SMC, Roy R, Okamoto I, et al. Genetic and morphologic features for melanoma classification. Pigment Cell Melanoma Res. 2010;23:763–770. doi: 10.1111/j.1755-148X.2010.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buac K, Xu M, Cronin J, Weeraratna AT, Hewitt SM, Pavan WJ. NRG1/ERBB3 signaling in melanocyte development and melanoma: inhibition of differentiation and promotion of proliferation. Pigment Cell Melanoma Res. 2009;22:773–784. doi: 10.1111/j.1755-148X.2009.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulliard J-L. Site-specific risk of cutaneous malignant melanoma and pattern of sun exposure in New Zealand. Int. J. Cancer. 2000;85:627–632. doi: 10.1002/(sici)1097-0215(20000301)85:5<627::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Bulliard JL, Cox B, Elwood JM. Latitude gradients in melanoma incidence and mortality in the non-Maori population of New Zealand. Cancer Causes Control. 1994;5:234–240. doi: 10.1007/BF01830242. [DOI] [PubMed] [Google Scholar]
- Bulliard JL, Cox B, Elwood JM. Comparison of the site distribution of melanoma in New Zealand and Canada. Int. J. Cancer. 1997;72:231–235. doi: 10.1002/(sici)1097-0215(19970717)72:2<231::aid-ijc5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Carli P, Massi D, Santucci M, Biggeri A, Gianotti B. Cutaneous melanoma histologically associated with a nevus and melanoma de novo have a different profile of risk: results from a case-control study. J. Am. Acad. Dermatol. 1999;40:549–557. doi: 10.1016/s0190-9622(99)70436-6. [DOI] [PubMed] [Google Scholar]
- Catala M, Ziller C, Lapointe F, Le Douarin NM. The developmental potentials of the caudal most part of the neural crest are restricted to melanocytes and glia. Mechanisms of Development. 2000;95:77–87. doi: 10.1016/s0925-4773(00)00349-x. [DOI] [PubMed] [Google Scholar]
- Chen YT, Dubrow R, Holford TR, Zheng T, Barnhill RL, Fine J, Berwick M. Malignant melanoma risk factors by anatomic site: a case-control study and polychotomous logistic regression analysis. Int. J. Cancer. 1996;67:636–643. doi: 10.1002/(SICI)1097-0215(19960904)67:5<636::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Choi W, Wolber R, Gerwat W, Mann T, Batzer J, Smuda C, Liu H, Kolbe L, Hearing VJ. The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J. Cell Sci. 2010;123(Pt 18):3102–3111. doi: 10.1242/jcs.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SF. Stem cells for epidermal melanocytes–a challenge for students of dermatopathology. Am. J. Dermatopathol. 2009;31:331–341. doi: 10.1097/DAD.0b013e31819cd0cb. [DOI] [PubMed] [Google Scholar]
- Crombie IK. Racial differences in melanoma incidence. Br. J. Cancer. 1979a;40:185–193. doi: 10.1038/bjc.1979.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie IK. Variation of melanoma incidence with latitude in North America and Europe. Br. J. Cancer. 1979b;40:774–781. doi: 10.1038/bjc.1979.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic Activation of KIT in Distinct Subtypes of Melanoma. J. Clin. Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Davison JM, Rosenbaum E, Barrett TL, Goldenberg D, Hoque MO, Sidransky D, Westra WH. Absence of V599E BRAF mutations in desmoplastic melanomas. Cancer. 2005;103:788–792. doi: 10.1002/cncr.20861. [DOI] [PubMed] [Google Scholar]
- Deichmann M, Krahl D, Thome M, Wüst K, Hassanzadeh J, Helmke B. The oncogenic B-raf V599E mutation occurs more frequently in melanomas at sun-protected body sites. Int. J. Oncol. 2006;29:139–145. [PubMed] [Google Scholar]
- Demunter A, Ahmadian MR, Libbrecht L, Stas M, Baens M, Scheffzek K, Degreef H, De Wolf-Peeters C, van Den Oord JJ. A novel N-ras mutation in malignant melanoma is associated with excellent prognosis. Cancer Res. 2001;61:4916–4922. [PubMed] [Google Scholar]
- Dhomen N, Da Rocha Dias S, Hayward R, et al. Inducible expression of (V600E) Braf using tyrosinase-driven Cre recombinase results in embryonic lethality. Pigment Cell Melanoma Res. 2010;23:112–120. doi: 10.1111/j.1755-148X.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- Diffey BL, Gies HP. The confounding influence of sun exposure in melanoma. Lancet. 1998;351:1101–1102. doi: 10.1016/s0140-6736(05)79381-8. [DOI] [PubMed] [Google Scholar]
- van Dijk M, Sprenger S, Rombout P, Marres H, Kaanders J, Jeuken J, Ruiter D. Distinct chromosomal aberrations in sinonasal mucosal melanoma as detected by comparative genomic hybridization. Genes Chromosom. Cancer. 2003;36:151–158. doi: 10.1002/gcc.10156. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Dupin E, Le Douarin NM. Development of melanocyte precursors from the vertebrate neural crest. Oncogene. 2003;22:3016–3023. doi: 10.1038/sj.onc.1206460. [DOI] [PubMed] [Google Scholar]
- Edlundh-Rose E, Egyházi S, Omholt K, Månsson-Brahme E, Platz A, Hansson J, Lundeberg J. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res. 2006;16:471–478. doi: 10.1097/01.cmr.0000232300.22032.86. [DOI] [PubMed] [Google Scholar]
- van Elsas A, Zerp SF, van der Flier S, Krüse KM, Aarnoudse C, Hayward NK, Ruiter DJ, Schrier PI. Relevance of ultraviolet-induced N-ras oncogene point mutations in development of primary human cutaneous melanoma. Am. J. Pathol. 1996;149:883–893. [PMC free article] [PubMed] [Google Scholar]
- Elwood JM, Gallagher RP. Body site distribution of cutaneous malignant melanoma in relationship to patterns of sun exposure. Int. J. Cancer. 1998;78:276–280. doi: 10.1002/(SICI)1097-0215(19981029)78:3<276::AID-IJC2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- English DR, Armstrong BK. Melanocytic nevi in children. Am. J. Epidemiol. 1994;139:390–401. doi: 10.1093/oxfordjournals.aje.a117011. [DOI] [PubMed] [Google Scholar]
- Eom HS, Kim MS, Hur SY, Yoo NJ, Lee SH. Somatic mutation of GNAQ gene is rare in common solid cancers and leukemias. Acta Oncol. 2009;48:1082–1084. doi: 10.1080/02841860902882444. [DOI] [PubMed] [Google Scholar]
- Falchi M, Bataille V, Hayward NK, Duffy DL, Newton Bishop JA, Pastinen T, Cervino A, et al. Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nat. Genet. 2009;41:915–919. doi: 10.1038/ng.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargnoli MC, Pike K, Pfeiffer RM, Tsang S, Rozenblum E, Munroe DJ, et al. C1R variants increase risk of melanomas harboring BRAF mutations. J. Invest. Dermatol. 2008;128:2485–2490. doi: 10.1038/jid.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch KR, McGowan KA, van Raamsdonk CD, Fuchs H, Lee D, Puech A, Herault Y, Threadgill DW, Hrabe de Angelis M, Barsh GS. Genetics of dark skin in mice. Genes Dev. 2003;17:214–228. doi: 10.1101/gad.1023703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming ID, Barnawell JR, Burlison PE, Rankin JS. Skin cancer in black patients. Cancer. 1975;35:600–605. doi: 10.1002/1097-0142(197503)35:3<600::aid-cncr2820350309>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Franceschi S, Levi F, Randimbison L, La Vecchia C. Site distribution of different types of skin cancer: new aetiological clues. Int. J. Cancer. 1996;67:24–28. doi: 10.1002/(SICI)1097-0215(19960703)67:1<24::AID-IJC6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Fritschi L, Mchenry P, Green A, Mackie R, Green L, Siskind V. Naevi in schoolchildren in Scotland and Australia. Br. J. Dermatol. 1994;130:599–603. doi: 10.1111/j.1365-2133.1994.tb13106.x. [DOI] [PubMed] [Google Scholar]
- Garcia RJ, Ittah A, Mirabal S, Figueroa J, Lopez L, Glick AB, Kos L. Endothelin 3 induces skin pigmentation in a keratin-driven inducible mouse model. J. Invest. Dermatol. 2008;128:131–142. doi: 10.1038/sj.jid.5700948. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Blog FB, Szabo G. Effects of aging and chronic sun exposure on melanocytes in human skin. J. Invest. Dermatol. 1979;73:141–143. doi: 10.1111/1523-1747.ep12581580. [DOI] [PubMed] [Google Scholar]
- Goel VK, Lazar AJ, Warneke CL, Redston MS, Haluska FG. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J. Invest. Dermatol. 2006;126:154–160. doi: 10.1038/sj.jid.5700026. [DOI] [PubMed] [Google Scholar]
- Green A. A theory of site distribution of melanomas: Queensland, Australia. Cancer Causes Control. 1992;3:513–516. doi: 10.1007/BF00052747. [DOI] [PubMed] [Google Scholar]
- Green A, MacLennan R, Siskind V. Common acquired naevi and the risk of malignant melanoma. Int. J. Cancer. 1985;35:297–300. doi: 10.1002/ijc.2910350303. [DOI] [PubMed] [Google Scholar]
- Green A, MacLennan CR, Youl P, Martin N. Site distribution of cutaneous melanoma in Queensland. Int. J. Cancer. 1993;53:232–236. doi: 10.1002/ijc.2910530210. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson DF, Sulem P, Stacey SN, Goldstein AM, Rafnar T, Sigurgeirsson B, Benediktsdottir KR, et al. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat. Genet. 2008;40:886–891. doi: 10.1038/ng.161. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Onken MD, Roberson EDO, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SL, MacKie RM, MacLennan R. Development of melanocytic nevi in the first three years of life. J. Natl. Cancer Inst. 2000;92:1436–1438. doi: 10.1093/jnci/92.17.1436. [DOI] [PubMed] [Google Scholar]
- Hinds MW. Anatomic distribution of malignant melanoma of the skin among non-Caucasians in Hawaii. Br. J. Cancer. 1979;40:497–499. doi: 10.1038/bjc.1979.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly EA, Kelly JW, Shpall SN, Chiu SH. Number of melanocytic nevi as a major risk factor for malignant melanoma. J. Am. Acad. Dermatol. 1987;17:459–468. doi: 10.1016/s0190-9622(87)70230-8. [DOI] [PubMed] [Google Scholar]
- Holman CD, Armstrong BK. Pigmentary traits, ethnic origin, benign nevi, and family history as risk factors for cutaneous malignant melanoma. J. Natl Cancer Inst. 1984a;72:257–266. [PubMed] [Google Scholar]
- Holman CD, Armstrong BK. Cutaneous malignant melanoma and indicators of total accumulated exposure to the sun: an analysis separating histogenetic types. J. Natl Cancer Inst. 1984b;73:75–82. [PubMed] [Google Scholar]
- Jouneau A, Yu YQ, Pasdar M, Larue L. Plasticity of cadherin-catenin expression in the melanocyte lineage. Pigment Cell Res. 2000;13:260–272. doi: 10.1034/j.1600-0749.2000.130408.x. [DOI] [PubMed] [Google Scholar]
- Kelly JW, Rivers JK, MacLennan R, Harrison S, Lewis AE, Tate BJ. Sunlight: a major factor associated with the development of melanocytic nevi in Australian schoolchildren. J. Am. Acad. Dermatol. 1994;30:40–48. doi: 10.1016/s0190-9622(94)70005-2. [DOI] [PubMed] [Google Scholar]
- Kruger S, Garbe C, Buttner P, Stadler R, Guggenmoos-Holzmann I, Orfanos CE. Epidemiologic evidence for the role of melanocytic nevi as risk markers and direct precursors of cutaneous malignant melanoma. J. Am. Acad. Dermatol. 1992;26:920–926. doi: 10.1016/0190-9622(92)70133-z. [DOI] [PubMed] [Google Scholar]
- Kunisada T, Yoshida H, Yamazaki H, Miyamoto A, Hemmi H, Nishimura E, Shultz LD, Nishikawa S, Hayashi S. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- Küsters-Vandevelde HVN, Klaasen A, Küsters B, Groenen PJTA, van Engen-van Grunsven IACH, van Dijk MRCF, Reifenberger G, Wesseling P, Blokx WAM. Activating mutations of the GNAQ gene: a frequent event in primary melanocytic neoplasms of the central nervous system. Acta Neuropathol. 2009 doi: 10.1007/s00401-009-0611-3. http://www.ncbi.nlm.nih.gov/pubmed/19936769. [DOI] [PMC free article] [PubMed]
- Lachiewicz AM, Berwick M, Wiggins CL, Thomas NE. Epidemiologic support for melanoma heterogeneity using the surveillance, epidemiology, and end results program. J. Invest. Dermatol. 2008;128:1340–1342. doi: 10.1038/jid.2008.18. [DOI] [PubMed] [Google Scholar]
- Laennec RTH. Sur les melanoses. Bull. Faculte Med. Paris. 1812;1:23–26. [Google Scholar]
- Lamba S, Felicioni L, Buttitta F, Bleeker FE, Malatesta S, Corbo V, Scarpa A, et al. Mutational profile of GNAQQ209 in human tumors. PLoS One. 2009;4:e6833. doi: 10.1371/journal.pone.0006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster HO. Some geographical aspects of the mortality from melanoma in Europeans. Med. J. Aust. 1956;1:1082–1087. [PubMed] [Google Scholar]
- Landi MT, Bauer J, Pfeiffer RM, Elder DE, Hulley B, Minghetti P, Calista D, Pinkel D, Kanetsky PA, Bastian BC. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313 doi: 10.1126/science.1127515. 512–2. [DOI] [PubMed] [Google Scholar]
- Lang J, MacKie RM. Prevalence of exon 15 BRAF mutations in primary melanoma of the superficial spreading, nodular, acral, and lentigo maligna subtypes. J. Invest. Dermatol. 2005;125:575–579. doi: 10.1111/j.0022-202X.2005.23833.x. [DOI] [PubMed] [Google Scholar]
- LeBoit P, Burg G, Weedon D, Sarasain A. World Health Organization Classification of Tumours. Lyon: IARC Press; 2006. Pathology and Genetics of Skin Tumours. [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Lee JA, Merrill JM. Sunlight and the aetiology of malignant melanoma: a synthesis. Med. J. Aust. 1970;2:846–851. doi: 10.5694/j.1326-5377.1970.tb63213.x. [DOI] [PubMed] [Google Scholar]
- Lee JAH, Strickland D. Malignant melanoma: social status and outdoor work. Br. J. Cancer. 1980;41:757–763. doi: 10.1038/bjc.1980.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Choi JW, Kim YS. Frequencies of BRAF and NRAS mutations are different in histologic types and sites of origin of cutaneous melanoma: a meta-analysis. Br. J. Dermatol. 2010;164:776–784. doi: 10.1111/j.1365-2133.2010.10185.x. [DOI] [PubMed] [Google Scholar]
- Lewis MG. Malignant melanoma in Uganda. (The relationship between pigmentation and malignant melanoma on the soles of the feet) Br. J. Cancer. 1967;21:483–495. doi: 10.1038/bjc.1967.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Fukunaga-Kalabis M, Yu H, Xu X, Kong J, Lee JT, Herlyn M. Human dermal stem cells differentiate into functional epidermal melanocytes. J. Cell Sci. 2010;123:853–860. doi: 10.1242/jcs.061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Kelly JW, Trivett M, Murray WK, Dowling JP, Wolfe R, Mason G, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J. Invest. Dermatol. 2007;127:900–905. doi: 10.1038/sj.jid.5700632. [DOI] [PubMed] [Google Scholar]
- Magnus K. Incidence of malignant melanoma of the skin in Norway, 1955–1970: variations in time and space and solar radiation. Cancer. 1973;32:1275–1286. doi: 10.1002/1097-0142(197311)32:5<1275::aid-cncr2820320537>3.0.co;2-8. KW - N1 - N2 -. [DOI] [PubMed] [Google Scholar]
- Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, Ono T, Albertson D, Pinkel D, Bastian BC. Determinants of BRAF mutations in primary melanoma. J. Natl Cancer Inst. 2003;95:1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- McGovern VJ. Melanoblastoma. Med. J. Aust. 1952;i:139–142. doi: 10.5694/j.1326-5377.1952.tb75022.x. [DOI] [PubMed] [Google Scholar]
- McGovern VJ, Mihm MC, Bailly C, Booth JC, Clark WH, Cochran AJ, Hardy EG, et al. The classification of malignant melanoma and its histologic reporting. Cancer. 1973;32:1446–1457. doi: 10.1002/1097-0142(197312)32:6<1446::aid-cncr2820320623>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- McGowan KA, Li JZ, Park CY, Beaudry V, Tabor HK, Sabnis AJ, Zhang W, et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat. Genet. 2008;40:963–970. doi: 10.1038/ng.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medic S, Ziman M, Soyer HP. PAX3 expression in normal skin melanocytes and melanocytic lesions (naevi and melanomas) PLoS One. 2010;5:e9977. doi: 10.1371/journal.pone.0009977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RE. The effect of prolonged solar radiation on melanocytes of the human epidermis. J. Invest. Dermatol. 1963;41:199–212. doi: 10.1038/jid.1963.97. [DOI] [PubMed] [Google Scholar]
- Muthusamy V, Hobbs C, Nogueira C, Cordon-Cardo C, McKee PH, Chin L, Bosenberg MW. Amplification of CDK4 and MDM2 in malignant melanoma. Genes Chromosomes Cancer. 2006;45:447–454. doi: 10.1002/gcc.20310. [DOI] [PubMed] [Google Scholar]
- Newton-Bishop JA, Chang YM, Iles MM, Taylor JC, Bakker B, Chan M, Leake S, et al. Melanocytic nevi, nevus genes, and melanoma risk in a large case-control study in the United Kingdom. Cancer Epidemiol. Biomarkers Prev. 2010;19(8):2043–2054. doi: 10.1158/1055-9965.EPI-10-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Yoshida H, Kunisada T, Nishikawa SI. Regulation of E- and P-cadherin expression correlated with melanocyte migration and diversification. Dev. Biol. 1999;215:155–166. doi: 10.1006/dbio.1999.9478. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa SI. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- North JP, Kageshita T, Pinkel D, Leboit PE, Bastian BC. Distribution and significance of occult intraepidermal tumor cells surrounding primary melanoma. J. Invest. Dermatol. 2008;128:2024–2030. doi: 10.1038/jid.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Zens MS, Stukel TA, Sacerdote C, Chang YM, Armstrong BK, Bataille V, et al. Nevus density and melanoma risk in women: a pooled analysis to test the divergent pathway hypothesis. Int. J. Cancer. 2009;124:937–944. doi: 10.1002/ijc.24011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CM, Carroll HJ, Whiteman DC. Familial melanoma: a meta-analysis and estimates of attributable fraction. Cancer Epidemiol. Biomarkers Prev. 2010a;19:65–73. doi: 10.1158/1055-9965.EPI-09-0928. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Carroll HJ, Whiteman DC. Estimating the attributable fraction for melanoma: a meta-analysis of pigmentary characteristics and freckling. Int. J. Cancer. 2010b;127(10):2430–2445. doi: 10.1002/ijc.25243. [DOI] [PubMed] [Google Scholar]
- Omholt K, Karsberg S, Platz A, Kanter L, Ringborg U, Hansson J. Screening of N-ras codon 61 mutations in paired primary and metastatic cutaneous melanomas: mutations occur early and persist throughout tumor progression. Clin. Cancer Res. 2002;8:3468–3474. [PubMed] [Google Scholar]
- Onken MD, Worley LA, Long MD, Duan S, Council ML, Bowcock AM, Harbour JW. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2008;49:5230–5234. doi: 10.1167/iovs.08-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdecamp K, Nakayama A, Nguyen MT, Hodgkinson CA, Pavan WJ, Arnheiter H. Melanocyte development in vivo and in neural crest cell cultures: crucial dependence on the Mitf basic-helix-loop-helix-zipper transcription factor. Development. 1997;124:2377–2386. doi: 10.1242/dev.124.12.2377. [DOI] [PubMed] [Google Scholar]
- Palmer JS, Duffy DL, Box NF, Aitken JF, O’Gorman LE, Green AC, Hayward NK, Martin NG, Sturm RA. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am. J. Hum. Genet. 2000;66:176–186. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2002;25:25. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, Zhu H, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat. Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- Poynter JN, Elder JT, Fullen DR, Nair RP, Soengas MS, Johnson TM, Redman B, Thomas NE, Gruber SB. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16:267–273. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- Purdue MP, From L, Armstrong BK, Kricker A, Gallagher RP, McLaughlin JR, Klar NS, Marrett LD. Etiologic and other factors predicting nevus-associated cutaneous malignant melanoma. Cancer Epidemiol. Biomarkers Prev. 2005;14:2015–2022. doi: 10.1158/1055-9965.EPI-05-0097. [DOI] [PubMed] [Google Scholar]
- Quevedo WC, Jr, Szabó G, Virks J, Sinesi SJ. Melanocyte populations in UV-irradiated human skin. J. Invest. Dermatol. 1965;45:295–298. doi: 10.1038/jid.1965.131. [DOI] [PubMed] [Google Scholar]
- Quevedo WC, Szabó G, Virks J. Influence of age and UV on the populations of dopa-positive melanocytes in human skin. J. Invest. Dermatol. 1969;52:287–290. [PubMed] [Google Scholar]
- Rawles ME. Origin of pigment cells from the neural crest in the mouse embryo. Physiol. Zool. 1947;20:248–266. doi: 10.1086/physzool.20.3.30151958. [DOI] [PubMed] [Google Scholar]
- Rieger E, Soyer HP, Garbe C, et al. Overall and site-specific risk of malignant melanoma associated with nevus counts at different body sites: a multicenter case-control study of the German central malignant-melanoma registry. Int. J. Cancer. 1995;62:393–397. doi: 10.1002/ijc.2910620406. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi: 10.1371/journal.pgen.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha G, Potter L, Daforno P, Pringle JH. Cutaneous melanoma subtypes show different BRAF and NRAS mutation frequencies. Clin. Cancer Res. 2006;12:4499–4505. doi: 10.1158/1078-0432.CCR-05-2447. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Niu C, Makino R, Kudo C, Sun C, Watanabe H, Matsunaga J, et al. RAF point mutations in primary melanoma show different prevalences by subtype. J. Invest. Dermatol. 2004;123:177–183. doi: 10.1111/j.0022-202X.2004.22722.x. [DOI] [PubMed] [Google Scholar]
- Sauter ER, Yeo UC, von Stemm A, Zhu W, Litwin S, Tichansky DS, Pistritto G, et al. Cyclin D1 is a candidate oncogene in cutaneous melanoma. Cancer Res. 2002;62:3200–3206. [PubMed] [Google Scholar]
- Scherer D, Rachakonda PS, Angelini S, Mehnert F, Sucker A, Egberts F, Hauschild A, Hemminki K, Schadendorf D, Kumar R. Association between the germline MC1R variants and somatic BRAF/NRAS mutations in melanoma tumors. J. Invest. Dermatol. 2010;130:2844–2848. doi: 10.1038/jid.2010.242. [DOI] [PubMed] [Google Scholar]
- Schouwey K, Delmas V, Larue L, Zimber-Strobl U, Strobl LJ, Radtke F, Beermann F. Notch1 and Notch2 receptors influence progressive hair graying in a dose-dependent manner. Dev. Dyn. 2007;236:282–289. doi: 10.1002/dvdy.21000. [DOI] [PubMed] [Google Scholar]
- Skender-Kalnenas TM, English DR, Heenan PJ. Benign melanocytic lesions: risk markers or precursors of cutaneous melanoma? J. Am. Acad. Dermatol. 1995;33:1000–1007. doi: 10.1016/0190-9622(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Staricco RJ, Pinkus H. Quantitative and qualitative data on the pigment cells of adult human epidermis. J. Invest. Dermatol. 1957;28:33–45. doi: 10.1038/jid.1957.4. [DOI] [PubMed] [Google Scholar]
- Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, Pleasance ED, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens NG, Liff JM, Weiss NS. Plantar melanoma: is the incidence of melanoma of the sole of the foot really higher in blacks than whites? Int. J. Cancer. 1990;45:691–693. doi: 10.1002/ijc.2910450421. [DOI] [PubMed] [Google Scholar]
- Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, Manolescu A, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat. Genet. 2007;39:1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- Sviderskaya EV, Easty DJ, Lawrence MA, Sánchez DP, Negulyaev YA, Patel RH, Anand P, Korchev YE, Bennett DC. Functional neurons and melanocytes induced from immortal lines of postnatal neural crest-like stem cells. FASEB J. 2009;23:3179–3192. doi: 10.1096/fj.08-123596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow AJ. Incidence of malignant melanoma of the skin in England and Wales and its relationship to sunshine. Br. Med. J. 1979;2:1324–1327. doi: 10.1136/bmj.2.6201.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G. The number of melanocytes in human epidermis. Br. Med. J. 1954;1:1016–1017. doi: 10.1136/bmj.1.4869.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G. The regional anatomy of the human integument with special reference to the distribution of hair follicles, sweat glands and melanocytes. Philos. Trans. R. Soc. B-Biol. Sci. 1967;252:447–485. [Google Scholar]
- Teppo L, Pakkanen M, Hakulinsen T. Sunlight as a risk factor of malignant melanoma of the skin. Cancer. 1978;41:2018–2027. doi: 10.1002/1097-0142(197805)41:5<2018::aid-cncr2820410550>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Thomas NE, Edmiston SN, Alexander A, Millikan RC, Groben PA, Hao H, Tolbert D, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol. Biomarkers Prev. 2007;16:991–997. doi: 10.1158/1055-9965.EPI-06-1038. [DOI] [PubMed] [Google Scholar]
- Tsao H, Bevona C, Goggins W, Quinn T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma – A population-based estimate. Arch. Dermatol. 2003;139:282–288. doi: 10.1001/archderm.139.3.282. [DOI] [PubMed] [Google Scholar]
- Uehara S, Izumi Y, Kubo Y, Wang CC, Mineta K, Ikeo K, Gojobori T, et al. Specific expression of Gsta4 in mouse cochlear melanocytes: a novel role for hearing and melanocyte differentiation. Pigment Cell Melanoma Res. 2009;22:111–119. doi: 10.1111/j.1755-148X.2008.00513.x. [DOI] [PubMed] [Google Scholar]
- Vagero D, Ringback G, Kiviranta H. Melanoma and other tumours of the skin among office, other indoor workers and outdoor workers in Sweden 1961–1979. Br. J. Cancer. 1986;53:507–512. doi: 10.1038/bjc.1986.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Fitch KR, Fuchs H, de Angelis MH, Barsh GS. Effects of G-protein mutations on skin color. Nat. Genet. 2004;36:961–968. doi: 10.1038/ng1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O’Brien JM, Simpson EM, Barsh GS, Bastian BC. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, et al. Mutations in GNA11 in uveal melanoma. N. Eng. J. Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ‘t Veer LJ, Burgering BM, Versteeg R, Boot AJ, Ruiter DJ, Osanto S, Schrier PI, Bos JL. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol. Cell. Biol. 1989;9:3114–3116. doi: 10.1128/mcb.9.7.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, Pinkel D, Bastian BC. Improving melanoma classification by integrating genetic and morphologic features. PLoS Med. 2008;5:e120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsmuth RC, Turner F, Barrett JH, Gaut R, Randerson-Moor JA, Bishop DT, Bishop JA. The effect of sun exposure in determining nevus density in UK adolescent twins. J. Invest. Dermatol. 2005;124:56–62. doi: 10.1111/j.0022-202X.2004.23548.x. [DOI] [PubMed] [Google Scholar]
- Wagner SN, Ockenfels HM, Wagner C, Höfler H, Goos M. Ras gene mutations: a rare event in nonmetastatic primary malignant melanoma. J. Invest. Dermatol. 1995;104:868–871. doi: 10.1111/1523-1747.ep12607039. [DOI] [PubMed] [Google Scholar]
- Weinstock MA, Colditz GA, Willett WC, Stampfer MJ, Bronstein BR, Mihm MC, Speizer FE. Moles and site-specific cutaneous malignant melanoma in women. J. Natl Cancer Inst. 1989;81:948–952. doi: 10.1093/jnci/81.12.948. [DOI] [PubMed] [Google Scholar]
- Weyers W, Euler M, Diaz-Cascajo C, Schill WB, Bonczkowitz M. Classification of cutaneous malignant melanoma: a reassessment of histopathologic criteria for the distinction of different types. Cancer. 1999;86:288–299. doi: 10.1002/(sici)1097-0142(19990715)86:2<288::aid-cncr13>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int. J. Cancer. 1998;77:843–848. doi: 10.1002/(sici)1097-0215(19980911)77:6<843::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Parsons PG, Green AC. Determinants of melanocyte density in adult human skin. Arch. Dermatol. Res. 1999;291:511–516. doi: 10.1007/s004030050446. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J. Natl Cancer Inst. 2003;95:806–812. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Brown RM, Purdie DM, Hughes MC. Melanocytic nevi in very young children: The role of phenotype, sun exposure, and sun protection. J. Am. Acad. Dermatol. 2005;52:40–47. doi: 10.1016/j.jaad.2004.07.053. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Stickley M, Watt P, Hughes MC, Davis MB, Green AC. Anatomic site, sun exposure, and risk of cutaneous melanoma. J. Clin. Oncol. 2006;24:3172–3177. doi: 10.1200/JCO.2006.06.1325. [DOI] [PubMed] [Google Scholar]
- Whiteman DC, Bray CA, Siskind V, Hole D, MacKie RM, Green AC. A comparison of the anatomic distribution of cutaneous melanoma in two populations with different levels of sunlight: the west of Scotland and Queensland, Australia 1982–2001. Cancer Causes Control. 2007;18:485–491. doi: 10.1007/s10552-007-0123-1. [DOI] [PubMed] [Google Scholar]
- Wilson YM, Richards KL, Ford-Perriss ML, Panthier JJ, Murphy M. Neural crest cell lineage segregation in the mouse neural tube. Development. 2004;131:6153–6162. doi: 10.1242/dev.01533. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Passeron T, Watabe H, Yasumoto K, Rouzaud F, Hoashi T, Hearing VJ. The effects of dickkopf 1 on gene expression and Wnt signaling by melanocytes: mechanisms underlying its suppression of melanocyte function and proliferation. J. Invest. Dermatol. 2007;127:1217–1225. doi: 10.1038/sj.jid.5700629. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Passeron T, Hoashi T, Watabe H, Rouzaud F, Yasumoto K, Hara T, et al. Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/beta-catenin signaling in keratinocytes. FASEB J. 2008;22:1009–1020. doi: 10.1096/fj.07-9475com. [DOI] [PubMed] [Google Scholar]
- Zhu G, Duffy DL, Eldridge A, et al. A major quantitative-trait locus for mole density is linked to the familial melanoma gene CDKN2A: a maximum-likelihood combined linkage and association analysis in twins and their sibs. Am. J. Hum. Genet. 1999;65:483–492. doi: 10.1086/302494. [DOI] [PMC free article] [PubMed] [Google Scholar]