Abstract
The ability of cytokines to direct the immune response to vaccination, infection and tumors has motivated their use in therapy to augment or shape immunity. To avoid toxic side effects associated with systemic cytokine administration, several approaches have been developed using particle-encapsulated cytokines to deliver this cargo to specific cell types and tissues. Initial work used cytokine-loaded particles to deliver proinflammatory cytokines to phagocytes to enhance antimicrobial and antitumor responses. These particles have also been used to create a cytokine depot at a local site to supplement prophylactic or antitumor vaccines or injected directly into solid tumors to activate immune cells to eliminate established tumors. Finally, recent advances have revealed that paracrine delivery of cytokines directly to T cells has the potential to enhance T-cell mediated therapies. The studies reviewed here highlight the progress in the last 30 years that has established the potential of particle-mediated cytokine immunotherapy.
Keywords: cancer, cytokine, immunotherapy, influenza, liposome, nanoparticle, PLGA, polymer, vaccine
Cytokines are potent modulating agents involved in immune homeostasis, regulating the inflammatory response, promoting effective control of pathogens and enforcing tolerogenic mechanisms. Administration of cytokines represents a mechanism to manipulate the immune system for therapy of cancer, autoimmune disorders or infectious disease and their adjuvant properties can increase vaccine efficacy. The advent of recombinant DNA technology has allowed the production of cytokines at levels that make their clinical use viable and approaches utilizing these proteins to modify disease processes have been remarkably successful in animal models. These therapies have translated into some notable successes in humans, but systemic cytokine administration often results in harmful side effects that limit their efficacy. Like many other therapeutic proteins, when cytokines are administered intravenously (iv.), they are rendered ineffective by protein degradation and excretion mechanisms, or binding to nonspecific receptors. A second issue with systemic administration is that, physiologically, the majority of cytokines act in a local paracrine fashion thereby avoiding the toxicity and nonspecific effects associated with high systemic concentrations. Coupling the rapid clearance of cytokine from serum with the high concentrations needed to mimic paracrine delivery drives the need for repeated administration of toxic doses of cytokine to achieve a therapeutic outcome [1–5]. As a result, there has been a concerted effort focused on strategies to deliver cytokines specifically to cells or tissues of interest. One approach has been to use lipidand polymer-based particles (which have been well-established as drug delivery vehicles) to encapsulate cytokines such as IFN-γ, TNF-α, IL-2, IL-12, type I interferons, GM-CSF, IL-1α, IL-4, IL-6, IL-7, IL-15, IL-18, IL-21 and leukemia inhibitory factor (LIF) for a wide range of applications (Table 1). This diverse set of cytokines has a range of effects on innate and adaptive responses as illustrated in Figures 1 & 2, respectively. Encapsulation and delivery via these vehicles has two distinct advantages:
-
▪
The vehicle protects the cytokine from neutralization in vivo;
-
▪
When the vehicles are targeted to specific cell types, the delayed release of cytokine from the vehicle allows sustained paracrine delivery of the cytokine.
Table 1.
Summary of administered cytokine-loaded particles.
| Particle type | Systemic administration |
Anticancer vaccination |
Intratumoral injection |
Prophylactic vaccination |
Direct attachment to T cells |
|---|---|---|---|---|---|
| Liposomes | MAF [82–85] IL-1α [86] TNF-α [86,94–96,98] IFN-γ [80,89,91,92] IL-2 [97] |
Murine models: IFN-γ [100,101] IL-2 [65,102–106] Human trials: IL-2 [107,108,201] |
Murine tumors: IL-2 [109,110,113] Human tumors: IL-12 [125] |
Murine models: IFN-γ [127] IL-6 [128,129,131] IL-12 [136,137] IL-4 [136] IL-2 [65,139,141–143] IL-15 [140] Human trials: IL-2 [144,145] |
IL-15sa [149] IL-21 [149] |
| Polymeric particles | IL-2 [87] IL-12 [88] IFN-γ [90] |
Murine models: GM-CSF [99] |
Murine tumors: IL-2 [111,112] IL-12 [114–120] GM-CSF [114,116] TNF-α [117–120] IL-18 [120] Human tumors: IL-2 [121] IL-12 [122–124] |
Murine models: IL-12 [138] |
IL-2 [145] IL-6 [146,147] LIF [146,147] |
All the studies cited in the paper are organized by particle type and administration method. Within each cell the cytokines used and the appropriate citation number are listed. For cancer vaccines and prophylactic vaccines, these studies are divided between preclinical murine data and data from human trials. Within the intratumoral injection studies, data is distinguished between use of murine versus human xenograft tumors in murine hosts.
IL-15sa: IL-15 super agonist; LIF: Leukemia-inhibitory factor; MAF: Macrophage-activating factor.
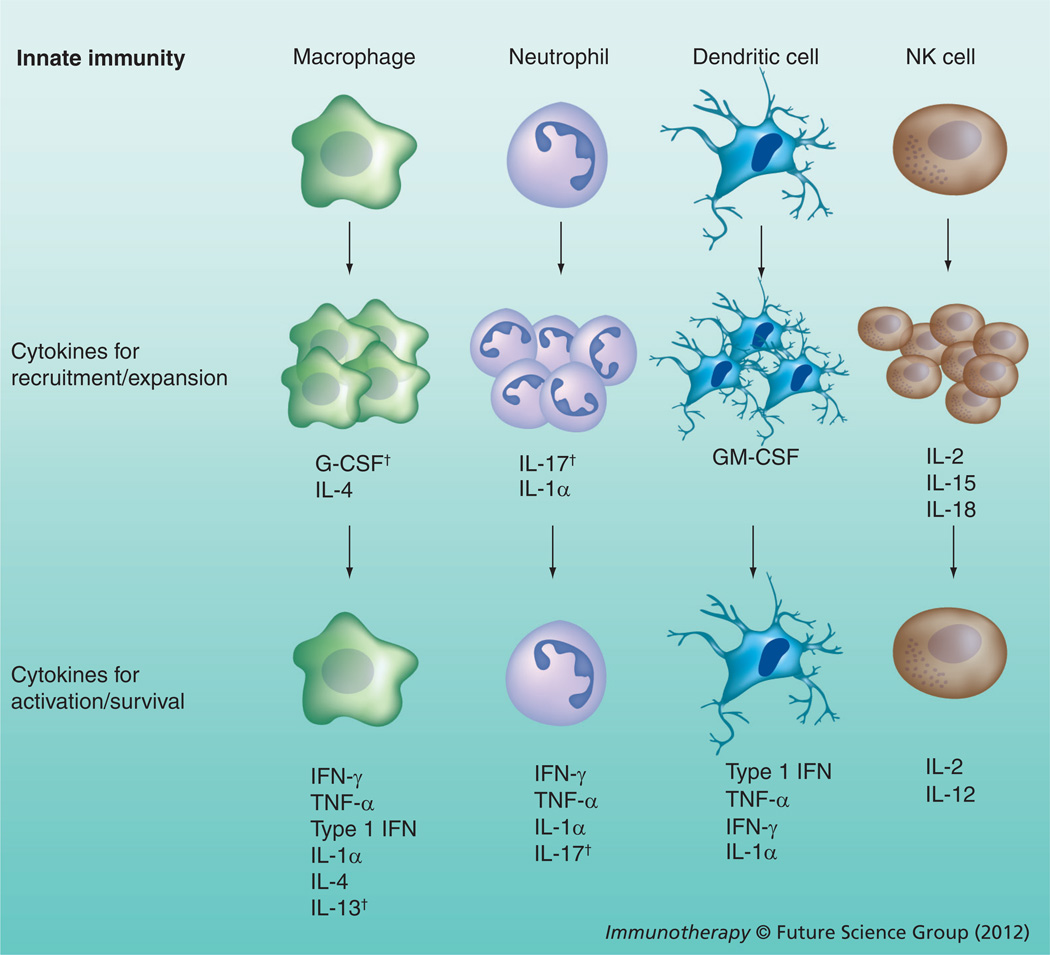
Figure 1. Roles of particle-encapsulated cytokines in the regulation of innate immune cells.
Cytokines first serve to recruit such innate cell populations as macrophages, neutrophils, dendritic cells and NK cells to a site of inflammation, where cytokines then provide the cellular cues for these populations to expand. As these populations arrive and expand, a different set of cytokines activates and matures these cells, inducing effector mechanisms that include killing the pathogen, killing infected cells, antigen presentation to adaptive immune cells and production of cytokines that supplement either innate or adaptive immune cells.
†Indicates cytokines not yet studied in particle formulations.
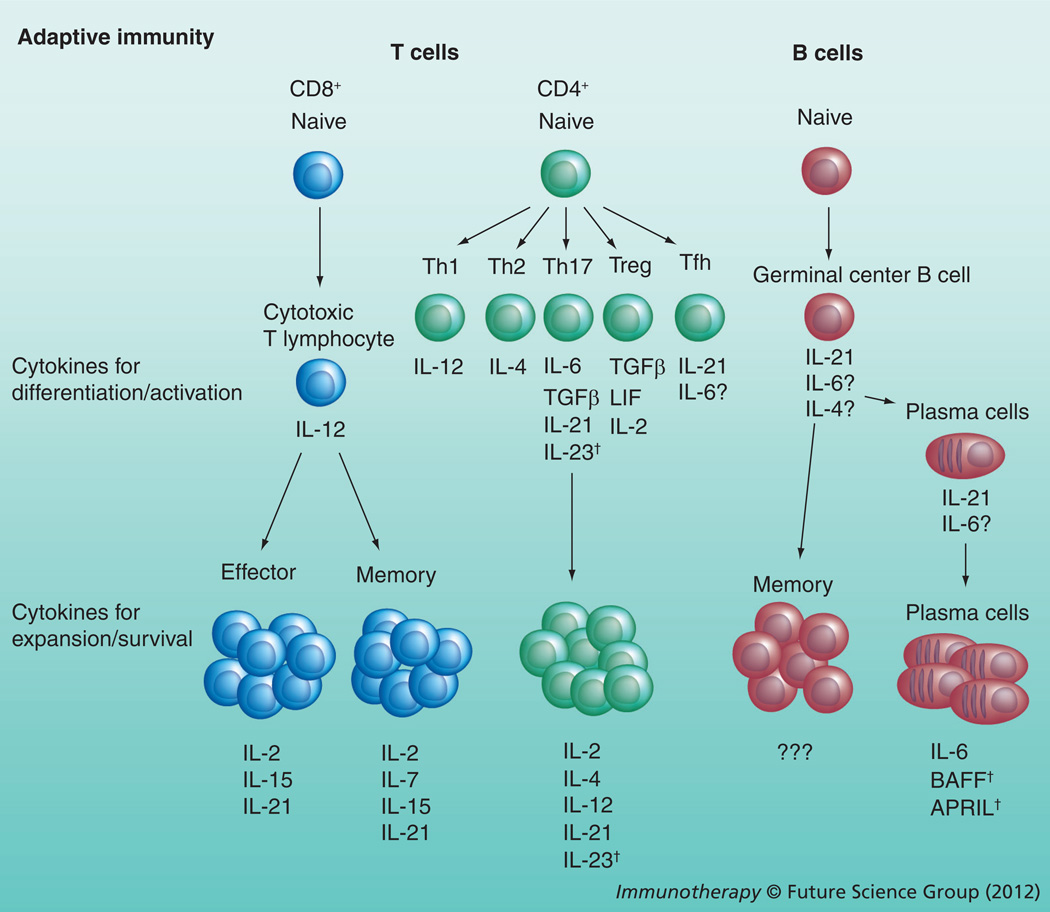
Figure 2. Roles of particle-encapsulated cytokines in the regulation of adaptive immune cells.
Initial exposure of adaptive immune cells to cytokines during an inflammatory response serves to activate these populations and induce differentiation toward different subsets. These cytokine cues are especially important for the differentiation of the different CD4+ T-cell subsets. Once these adaptive immune cells differentiate, a different set of cytokines is required for expansion and survival of each subset. For example, IL-7 is required for CD8+ memory T cells but not CD8+ effector T cells and the cytokines required for plasma cell homeostasis include IL-6, BAFF and APRIL, while the cytokines required for memory B cells are not known.
†Indicates cytokines not yet studied in particle formulations.
Liposomes are vesicles composed of one or more lipid membranes surrounding an aqueous lumen and are typically synthesized using the lipid film rehydration method shown in Figure 3A. Liposomal formulations have been used to encapsulate and deliver therapeutics (e.g., small molecules, proteins and oligonucleotides) for over 40 years [6,7], some of which are now in clinical use [8]. More recent efforts have utilized these vesicles for the delivery of antigens and adjuvants as a means to induce immune responses [9–12]. The biocompatibility of liposomes makes them ineffective adjuvants alone, however liposomes can be altered to directly activate innate immune cells by changing their surface chemistry or adding pathogen-associated molecular patterns (PAMPs) to their surface [13,14]. Several liposomal adjuvant formulations are currently in clinical trials [15–21]. A drawback of liposomes when trying to target a specific tissue or cell type is that their physicochemical properties results in their rapid clearance by the MPS and instability in plasma, which combine to limit their circulation times. To prevent the opsonization that initiates clearance by the MPS and extend the liposome half-life in circulation, polyethylene glycol (PEG) was chemically attached to the liposomes (stealth liposomes) to provide a dense, hydrated brush on the liposome surface [8,22]. However, liposomes are also limited by an inability to engineer their degradation to control the release of the therapeutic cargo, which has prompted the exploration of synthetic polymer systems capable of encapsulating and releasing therapeutics.
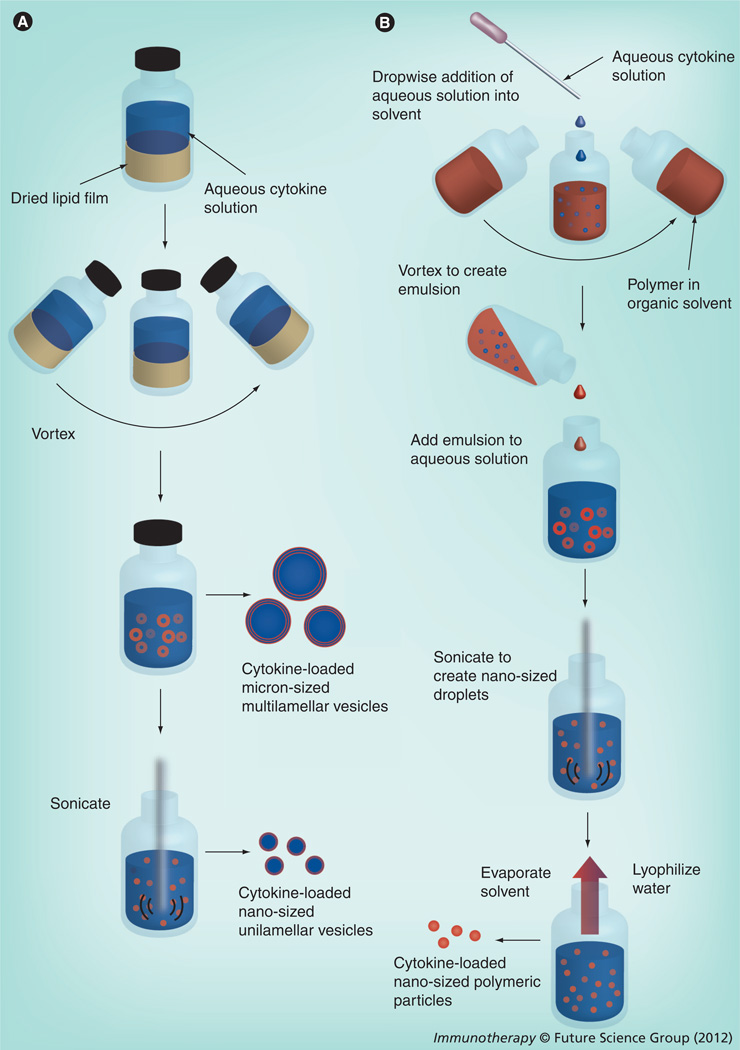
Figure 3. Common methods to synthesize cytokine-loaded liposomes and polymeric particles (see image on opposite page).
(A) Liposomes are most commonly synthesized via film rehydration. In this process, a solution of organic solvent containing lipids is evaporated to leave a lipid film on the interior of a glass vial or flask. This film is rehydrated with an aqueous solution containing a cytokine along with a variety of buffers and salts. Physical agitation (e.g., vortexing and sonication) is used to induce budding of liposomes from the lipid film surface, which results in micron-sized multilamellar vesicles encapsulating the cytokine. The vesicles can then be made unilamellar and nanosized (100–200 nm) by sonication or freeze/thaw. (B) The water–oil–water method is one of the most common methods to generate polymeric particles containing a cytokine. An aqueous solution containing the cytokine is added dropwise to an organic solvent containing the dissolved polymer. During this addition, mechanical agitation (vortex, sonication) is used to create a water/oil emulsion. This emulsion is then added to an aqueous solution that usually contains polyvinyl alcohol, which serves as an emulsion stabilizer. This new emulsion is then sonicated to create nano-sized droplets of polymer and cytokine. The solvent is then removed by evaporation and the remaining aqueous solution is lyophilized to remove the water and yield a dry powder containing nano-sized polymeric particles loaded with cytokine.
In the last 30 years, a wide range of biocompatible and biodegradable polymeric delivery systems, ranging from gels to nanoparticles, have improved stability, circulation times and release kinetics compared with lipid formulations, and have been used to improve the ability to encapsulate and deliver therapeutic proteins [23–26]. Similar to liposomes, polymeric micro- and nano-particles have been utilized successfully to deliver antigen, which in the context of their inherent adjuvant activity [27,28] or incorporation of PAMPs to their surface [29,30], makes them potent vaccine formulations. However, early studies of these novel particles revealed that the processes required for their formation and degradation can induce protein denaturation and thus compromise the integrity of the cargo [31–33]. While polymeric particle synthesis techniques vary widely, particles are most commonly composed of the US FDA-approved polylactic-coglycolic acid (PLGA) and are synthesized using the water–oil–water emulsion technique as illustrated in Figure 3B. Formation of polymer particles by this method requires that the protein be exposed to mechanical agitation and an organic solvent–water interface, while degradation of these polymers usually results in a low pH environment around the protein before release. These shortcomings have driven additional work to optimize the conditions of encapsulation and release to prevent denaturation of a range of proteins – making polymeric particles a viable tool for cytokine delivery.
At the same time that advances in lipid- and polymer-based particle delivery systems have occurred, the field of cytokine biology has transitioned from describing immunological phenomena to a complex understanding of how cytokines and their receptors coordinate the immune system, which has made them therapeutic targets [34]. In mouse models, the administration or deletion of cytokines and their receptors has led to significant advances in basic immunology that have provided insights into how to modify inflammatory processes. For example, multiple cytokine antagonists are used clinically in humans, primarily in the context of treating autoimmune disorders. Although IL-2, G-CSF and type I interferons are in clinical use, the administration of cytokines to manipulate the immune response has been challenging to translate into human patients. The ability to combine advances in particle technology with a better understanding of cytokine biology will improve the feasibility of cytokine therapy. Indeed, the use of liposomes and polymer particles to encapsulate cytokines has shown promise as a therapeutic for treatment of cancer and infectious disease and as a means to increase vaccine efficacy [35–37]. The aim of this article is to outline the different methods used to engineer carriers to encapsulate and deliver bioactive cytokines and review the ability of cytokine-loaded particles to achieve therapeutic outcomes.
Characterization & optimization of cytokine-loaded particle formulations
In vitro characterization & optimization
Liposomes
Approximately 30 years ago, Fidler and coworkers demonstrated the ability of liposomes to encapsulate and deliver macrophage-activating factor (MAF) to macrophages in vitro. This pioneering method of delivery was shown to be more effective than free MAF at activating monocytes or macrophages to kill tumor or virus-infected cells [38–41]. This work was expanded to show that coencapsulating MAF or IFN-γ with immunomodulatory peptides such as muramyl dipeptide in the same liposome further enhanced macrophage activity [42–44]. The finding that the effects of liposomal IFN-γ were dependent on its release and binding to extracellular membrane receptors emphasized that encapsulated cytokines must be released by their carrier outside the target cell to be effective [45]. Subsequent studies revealed that IFN-γ is encapsulated in liposomes primarily by electrostatic association with anionic lipids in the membrane and that the addition of cholesterol to the membrane increases liposome stability, which slows cytokine release [46–48]. Liposomal formulations that contained type I interferons and TNF-α have also been developed [49–52], with Eppstein and coworkers showing that anionic, multilamellar liposomes (MLVs) were most effective in encapsulating type I interferons compared with unilamellar and multilamellar liposomes with neutral or cationic surface charge. Further work showed that liposomal IFN-α was capable of antimitogenic and antiviral activity [50,51] and was more effective than free IFN-α at inhibiting proliferation of IFN-α-sensitive cell lines [53,54]. Similar studies with TNF-α demonstrated that anionic liposomes containing cholesterol were more capable of encapsulating and maintaining the bioactivity of the cytokine [49,52]. Finally, work by Anderson and coworkers showed that the composition of liposomes could be tailored to deliver bioactive IL-1α, IL-2, IL-6 or GM-CSF [55], which played an important role in helping to expand the array of cytokines used in future liposome formulations.
Polymer particles
Encapsulation of cytokines in polymeric particles was first described by Hora et al., when they used poly(DL-lactide-co-glycolide) to deliver IL-2 [56]. As highlighted above, protein denaturation during the encapsulation process is a limitation of these formulations and optimizing the conditions to minimize loss of bioactivity became a priority. Cleland and coworkers found that IFN-γ was best stabilized during encapsulation in PLGA by adding detergents and sugars to a buffered solution at pH 5 [31]. Importantly, they also showed that in these particles, the slow release of IFN-γ caused a loss of bioactivity due to the low pH induced by the acidic byproducts that result from degradation of PLGA [57]. However, the structural stability required for the bioactivity of many cytokines is different and several studies have compared the functionality of different formulations of IL-2, IFN-α, IL-12, GM-CSF and IL-18 after encapsulation and release from the polymer particles [58–62]. The natural method of cytokine delivery to target cells can also be considered in these formulations. For example, particles with surface-attached TNF-α were designed to mimic the naturally occurring cell membrane-bound TNF-α [63]. Despite the harsh conditions associated with the processes of encapsulation and release from polymeric particles, these optimized formulations provided an additional strategy for particle-mediated cytokine delivery.
In vivo characterization of cytokine-loaded particles
In order to appreciate the limitations and potential of particle-encapsulated cytokines in vivo, it is important to understand that the cytokine release rate, residence time at the site of injection, biodistribution and bioactivity of cytokine varies for each formulation. Indeed, there is literature that establishes that the route of administration and surface charge of liposomes has a critical role in determining which tissues and cells received the cytokine cargo. Thus, compared with free cytokines, the iv. injection of liposomes containing IFN-γ, IFN-α, IL-2 or TNF-α enhances the plasma residence time of the cytokine [64–67]. Similarly, for intraperitoneal [64,68], intramuscular [69], subcutaneous [64,70–72] or intranasal [64,73] administration of liposome and polymeric particles, these carriers can act as depots and increase the cytokine residence time at the injection site. Changing the surface chemistry of these particles by the addition of PEG or charge also influenced the residence time in plasma and localization to different tissues. The outer PEG brush of nano-sized stealthy liposomes extended cytokine circulation kinetics compared with MLVs that are often tens of microns in diameter [65,67]. In addition, it was shown that liposome surface charge influenced the distribution of the particles and hence the cargo. Thus, anionic charge increased delivery to alveolar macrophages in the lung [74–76], while a neutral or cationic charge targeted delivery to the liver and spleen [64,74]. In vivo, the composition of the leukocyte populations in the blood, spleen and peritoneum, or at the site of injection were shown to change after particle-mediated administration of IL-2 [65,72], GM-CSF [66,71], TNF-α [66,77] and IL-7 [70]. A similar delivery strategy for IFN-γ or IL-1α was shown to activate alveolar, peritoneal, liver and splenic macrophages in vivo by ex vivo assays for killing tumor cells [73,76,78], killing the intracellular parasite Leishmania [79,80], or by promoting cytokine production [81]. These initial studies clearly demonstrate the release of bioactive cytokine in vivo and reveal that the route of administration can dictate the cell populations receiving the encapsulated cytokine and can thus be used to target different immune cells.
Systemic administration of cytokine-loaded liposomes & polymeric particles
Targeting the MPS for antitumor & antimicrobial activities
Initial efforts to use liposomes or particles to encapsulate cytokines for therapy took advantage of the ability of the MPS to clear particles from the bloodstream following iv. injection to target proinflammatory cytokines to macrophages. Fidler and coworkers used MAF encapsulated in micron-sized anionic MLV liposomes to target alveolar macrophages and activate them to kill tumor metastases in the lungs [82–85]. Similar studies that used iv. injection of liposomal IL-1α and TNF-α to activate macrophages also induced resistance to metastatic tumors [86]. Interestingly, the intraperitoneal injection of particle-encapsulated IL-2 [87] or IL-12 [88] – two cytokines that primarily stimulate a T-cell mediated immune response –also decreased hepatic and subcutaneous cancer metastases, respectively, which demonstrated that this strategy is also capable of inducing adaptive immune responses. In all cases, the liposomal preparations were more effective at promoting the eradication of the tumor nodules in the lungs and improving survival time than administration of free cytokine, despite using fewer and smaller cytokine doses.
Similar to the studies with cancer, encapsulated IFN-γ was shown to promote resistance to viruses [89], bacteria [90,91] and parasites [80] in vivo. In prophylactic scenarios, IFN-γ encapsulated in liposomes or polymer particles administered systemically prior to infection could enhance innate antimicrobial effector mechanisms and promote resistance to challenge [80,89–91]. In addition, liposomes containing IFN-γ and muramyl tripeptide phosphatidylethanolamide, either individually or together, were used to treat mice infected with Listeria monocytogenes [92] or Leishmania [80], two pathogens that infect macrophages. In these studies, compared with free cytokine, the use of particles to passively target cytokines to the MPS resulted in a lower bacterial or parasite burden in the spleen and liver, which provides a proof-of-concept that may be broadly applicable to combat other pathogens that affect the MPS.
Avoiding the MPS for therapeutic treatment
Although the ability of the MPS to clear particles from the blood is useful for delivery to macrophages, it severely reduces the half-life of therapeutics and hinders the ability to target other sites. Consequently, significant effort has gone into improving the circulation kinetics of drug delivery vehicles as well as providing new ways to target them to sites such as tumors or lymphocytes in secondary lymphoid tissues [8]. The most common approaches combine the use of nanometer-sized stealthy particles with passive targeting to sites with leaky vasculature, especially tumors [93]. Yuyama and coworkers made use of stealthy, thermosensitive liposomes to deliver TNF-α to a tumor and then used heat to rupture the liposomes and release the cytokine locally [94]. Other studies used a similar approach in conjunction with either liposomal chemotherapy [95] or radiation therapy [96] to treat tumor xenografts. In addition, the ability of stealthy liposomal IL-2 to shrink tumors was compared with free IL-2 or PEGylated IL-2 [97] and revealed that stealthy liposomes enhanced tumor shrinkage with significantly decreased toxic side effects when compared with treatment with free cytokine. Finally, the ability of stealthy liposomal TNF-α to permeate the leaky vasculature in the brain, associated with experimental cerebral malaria, showed that TNF-α bound to the liposomal surface was more effective at reducing parasite burden and preventing experimental cerebral malaria than either liposome-encapsulated TNF-α or free TNF-α [98]. The ability of stealthy liposomes to avoid clearance by the MPS enhances their use to deliver encapsulated cytokines to tissues with leaky vasculature, including tumors or sites of infection and inflammation.
Cytokine depots for vaccination & cancer therapy
Cytokine depots for anticancer vaccines
Fundamental studies of the immune response to tumors have demonstrated that effector cells capable of recognizing tumor-specific antigens and eradicating malignant cells reside in the tumor architecture. However, this microenvironment is immunosuppressive and antagonizes the ability of these cells to limit tumor growth. While traditional chemotherapeutic and surgical techniques have become increasingly effective in treating cancer, the persistence and metastasis of tumor cells remains a major problem. Therefore, using immunotherapy to promote an antitumor response while generating protective immunological memory provides one method to improve cancer therapy and survival. The use of IL-2 or IL-12 in mice induces an effective antitumor immune response, but clinical trials using systemic administration of these cytokines have been limited by patient toxicity. An alternative approach has focused on engineering autologous tumor cells to secrete proinflammatory cytokines (e.g., IFN-γ and IL-2) and injecting them at the tumor site to initiate the antitumor immune response. These methods are rarely feasible for patients as they are technically challenging and require large primary tumors capable of generating enough engineered cells to be effective. Consequently, several groups have replaced engineered cells with cytokine depots composed of liposomes or polymeric particles and have either administered these particles with tumor-based antigens as a cancer vaccine strategy or directly injected these particles into tumors to activate an antitumor immune response.
One approach to treating cancer involves the use of vaccines to aid in the eradication of cancer cells that remain after tumor resection or that have metastasized. Cytokine-loaded particles can enhance the tumor-specific immune response using the antigen provided by irradiated tumor cells or tumor antigens co-encapsulated with the cytokine. After immunization, the humoral and cellular immune responses were measured and mice were then challenged with tumor cells and monitored for tumors and survival. In a model of melanoma, polymer particles encapsulating GM-CSF increased leukocyte infiltration to the site of injection as well as the fraction of mice without tumors [99]. Compared with free IFN-γ, liposomal formulations increased both the fraction of mice that survived and the number of mice without melanoma tumors, and were comparable with administration of tumor cells engineered to express IFN-γ [100,101]. In a variety of models, administration of liposomal IL-2 increased the fraction of mice that survived and remained tumor-free following a challenge with tumor cells and provided equal or better protection than irradiated tumor cells engineered to express IL-2 [65,102–106]. A mechanistic understanding of these events is provided by studies which showed that liposomal IL-2 increased both the humoral response [102,105,106] and the cytolytic capacity of CD8+ T cells in the spleen and draining lymph node [102,103,106] and that this resistance was dependent on CD4+ and CD8+ T cells [105].
The efficacy of the cancer vaccines supplemented with liposomal IL-2 demonstrated in mouse tumor models provided the foundation to move these formulations into the clinic. Studies of tumor rejection in a mouse lymphoma model by administration of liposomes co-encapsulating a tumor-specific idiotype and IL-2 [105] served as a basis for human studies by Neelapu and coworkers [107,108]. In their first study, the tumor-specific idiotype was isolated from each patient with advanced-stage follicular lymphoma and incorporated into liposomes containing IL-2. Approximately 6 months after completion of chemotherapy, patients still in clinical remission were given five doses of vaccine at months 0, 1, 2, 3 and 5. These vaccinations proved capable of generating sustained, tumor-specific CD4+ and CD8+ T cells in all ten patients and tumor-specific antibody responses in four out of the ten patients. After 50 months, six out of ten patients remained in continuous remission [107]. In their second clinical trial, Neelapu and coworkers collected tumor samples from 11 patients that had either partially responded to chemotherapy or had progressive disease. Total membrane proteins were isolated from the tumors and encapsulated in liposomes with IL-2 to speed time to vaccination. After immunizations, no patients had evidence of autoimmunity and 5 demonstrated tumor-specific T-cell responses. Of the 11 patients, one achieved complete remission for up to 44 months after vaccination [108]. The second set of clinical trials is currently underway as a Phase II trial using liposomal IL-2 to supplement vaccine therapy in patients with stage III melanoma. This trial also examines the effect of IFN-α-2b on the antibody and cellular responses to melanoma [201]. Together, these three trials have demonstrated that cancer vaccines supplemented with liposomal IL-2 are well tolerated by patients and are effective in inducing tumor-specific T-cell responses. The completed trials show that these vaccines can be useful in patients that are in clinical remissions, but strategies using vaccines to treat established tumors in patients with progressive disease must still be optimized.
Intratumoral injection of cytokine depots for anticancer therapy
While the vaccine studies described above administer tumor antigens with cytokine-loaded particles, these particle formulations have also been used to treat primary tumors using perior intra-tumoral injection. In these models, the primary tumor serves as the source of antigen and the cytokine depot provided by the particles activates leukocytes in the tumor microenvironment to promote the immune response capable of eradicating the primary tumor and metastasized tumor cells. Initial work used peritumor injections of liposomal IL-2 and demonstrated enhanced antitumor responses compared with free IL-2, as measured by tumor progression (metastases or tumor weight), numbers of activated macrophages and survival [109,110]. Similarly, the local injection of polymeric microparticles loaded with IL-2 for the treatment of brain or liver tumors, showed that this approach was more effective at treating tumors and protecting against rechallenge than tumor cells engineered to express IL-2 [111,112]. In one series of studies to look at effects of IL-2 on metastasis, liposomes containing IL-2 were injected directly into metastasized nonimmunogenic B16 melanomas that were surgically resected 21 days later and animals were then monitored for survival [113]. Compared with treatment with soluble IL-2, liposomal IL-2 treatment resulted in higher survival rates, slower tumor growth rates prior to resection and increased recruitment of T cells and granulocytes into the tumor. In addition, liposomal IL-2 also provided a protective response against B16 rechallenge at day 56. Consistent with the biology of IL-2 as a T-cell growth factor, the resistance to rechallenge is a clear indicator of the ability of liposomal IL-2 to engage the adaptive immune response and induce T-cell memory.
Studies using cytokine-loaded particles for cancer therapy were expanded to include IL-12 alone and in combination with TNF-α, GM-CSF or IL-18. As shown in Figure 2, IL-12 activates both CD4+ and CD8+ T cells to make IFN-γ, which subsequently activates innate immune cells to kill tumor cells (Figure 1). Initial experiments used poly(lactic acid) polymer particles to encapsulate IL-12 and compared the antitumor response to encapsulated PEG–IL-2 or GM-CSF. These particles were injected directly into established Line-1 murine lung carcinoma tumors and IL-12 particles were shown to prevent tumor growth and metastasis and provide protective immunity against rechallenge [114]. In a follow-up study, it was shown that treatment of established tumors with IL-12-loaded particles prior to surgical resection promoted the development of systemic antitumor immunity that was better able to prevent disease recurrence and metastasis than surgery alone [115]. Furthermore, particles containing IL-12 plus GM-CSF induced higher systemic levels of IFN-γ and IL-12 and were more effective than either cytokine alone at promoting resistance to primary and metastatic tumors [116]. Interestingly, the ability of these particles to eradicate the primary tumor was dependent on CD4+ and CD8+ T cells, while effects on metastasis were dependent on NK/NKT cells [116]. The approach of combining cytokines in the same particle was then expanded to include other cytokines and intratumoral injections of various combinations of IL-12, TNF-α, IL-18 and GM-CSF were compared in order to determine the most effective antitumor therapy [117–120]. The combination of IL-12 and TNF-α was found to be the most effective in promoting control of tumors associated with induction of tumor-specific T cells that produced IFN-γ and recruitment of polymorphonuclear cells and CD8+ T cells into the tumor [117,118]. Furthermore, this immune response was capable of impairing the growth of untreated tumors [119] and also led to the induction of a long-lived memory population that provided resistance to tumor rechallenge [117–119]. The ability to generate a memory T-cell population using these approaches suggests that cytokines such as IL-7, IL-15 and IL-21 that influence T-cell homeostasis may not only be useful for therapy applications, but that these particles can also provide tools to better understand cytokine immunobiology.
While cytokine depots have shown the most promise for anticancer therapies, the challenge for these formulations is to translate their efficacy in model systems into clinical applications. Importantly, studies by Bankert and others advanced particle-encapsulated IL-2 and IL-12 beyond murine tumors by studying the effect of intratumoral injection into human tumor xenografts in SCID mice [121–125]. Initial studies injected IL-2-loaded polymer particles with human tumor cells and resulted in murine NK cells mediating suppression of tumor growth [121]. In similar experiments, when mice were administered IL-12-loaded particles with peripheral blood lymphocytes and tumor cells from the same patient, tumor engraftment was completely suppressed [122]. Importantly, the presence of IL-12 released from the particles induced the transferred peripheral blood lymphocytes to produce IFN-γ. These studies were then advanced by the implantation of nondisrupted pieces of human tumor (lung, breast or ovarian) directly from biopsy tissue into SCID mice [123–125]. When lipid- or polymer-based particles encapsulating rhIL-12 were injected into these tumors, they induced human CD4+ and CD8+ T cells to make IFN-γ, which upregulated the inducible nitrous oxide synthase in human leukocytes that is capable of suppressing tumor growth [123]. Further phenotypic analysis of these human tumor-associated leukocytes showed that the majority of the cells were CD4+ effector memory T cells that had been reactivated by rhIL-12 [124]. These studies clearly demonstrate the ability of intratumoral injection of cytokine-loaded particles to overcome the suppressive tumor microenvironment and activate an antitumor immune response in human tumors and have provided a foundation for moving this type of therapy into human trials.
Cytokine depots to supplement vaccination against pathogens
While the aim of cancer immunotherapy is to induce an adaptive cellular immune response to eliminate tumors and limit metastasis, the basis for the long-term protection provided by the majority of vaccines against pathogens is a long-lived antibody response. Indeed, encapsulated cytokines as adjuvants can augment the humoral immune response in vaccinations against viral and bacterial pathogens. For example, particles encapsulating IFN-γ injected with antigens from influenza [126,127] or Yersinia pestis [128], promoted the development of high-affinity antigen-specific antibody titers (IgA, IgG1 and IgG2a) [126–128] as well as increased resistance to challenge with live virus [126,127]. Importantly, Griffin and coworkers showed that when IFN-γ was coencapsulated with Y. pestis antigen in polymeric particles, antigen-specific antibody titers and T-cell responses remained elevated for more than a year [128]. The cytokine IL-6 also has a role in B-cell maturation and increasing high affinity antibody titers [129–131]. Liposomal IL-6 has been used to augment antibody responses in a variety of settings including model antigens with or without other adjuvants [129], HIV subunits [131] or challenge with Y. pestis [130]. It should be noted that IL-6 has an important role in the differentiation of naive CD4+ T helper cells to T follicular helper cells (Tfh) [132], which are required for B-cell maturation and antibody class switching [133]. At present, it is unclear whether the increased antibody responses observed when IL-6 was delivered via these particle formulations was a result of increased Tfh differentiation and/or direct stimulation effects on plasma cells.
As highlighted in Figure 2, there are multiple subsets of T helper cells that are associated with specific classes of immunity required to control different pathogens. Similar to the ability of IL-6 to promote the generation of Tfh, other cytokines that can direct the T-helper cell response (Figure 2) have also been encapsulated in liposomes or polymeric particles and tested as adjuvants for vaccination against pathogens. While IL-12 is critical in directing the Th1 response and resistance to intracellular infection [134,135], IL-4 is critical for a Th2 response and immunity to helminths. Liposomes loaded with antigens derived from bovine herpes virus plus IL-12 or IL-4 and injected subcutaneously increased the number IFN-γ+ and IL-4+ splenocytes, respectively, and IL-12 also increased IgG1 and IgG2a antibody titers [136]. Different administration routes were then tested to optimize this formulation for the highest antibody and cellular response in the lungs [137]. Similar results were obtained using polymeric particles to encapsulate IL-12, the adjuvant AS01B and subunit antigens for vaccination against Mycobacterium tuberculosis. Subcutaneous administration of this formulation resulted in elevated IgG2a serum antibody titers, an increased number of IFN-γ-secreting cells in the spleen and draining lymph nodes and increased resistance to bacterial challenge [138]. These studies have demonstrated that sustained delivery of IL-12 by liposomes or particles enhances the Th1 response that drives the protective response to vaccination against intracellular pathogens and opens the door for the use of cytokine-loaded particles to promote other T-helper cell subsets for resistance to such pathogens as helminths (Th2) and fungi (Th17).
Given the important role of IL-2 and IL-15 as growth factors for effector and memory T cells, it makes sense that these cytokines might be useful in the context of vaccination against viral and bacterial pathogens. Indeed, intranasal administration of particles co-encapsulating IL-2 and polysaccharide antigens derived from Pseudomonas aeruginosa or Aerobacter levanicum resulted in increases in antigen-specific antibody-secreting cell numbers and IgA titers in the lungs and increased protection following lethal challenge [139]. A similar study used liposomal IL-15 plus tetanus toxoid and demonstrated a modest increase in toxoid-specific antibody titers compared with administration of free IL-15 [140]. For influenza, multiple studies have shown that co-encapsulating a viral antigen and IL-2 in liposomes increases influenza-specific antibody titers [65,141–143] and provides better protection against challenge with influenza [141,143]. Kedar and coworkers optimized these formulations and demonstrated that liposomal antigen combined with separate liposomal IL-2 induced the highest antibody titers and that the antibodies that were generated crossreacted with a wide spectrum of influenza A strains [141–143]. One interpretation of these data is that for effective influenza vaccination, the antigen and cytokine require cellular pathways where the antigen is processed intracellularly and the cytokine must be released prior to uptake and bind to cytokine receptors on the cell surface. It is important to note that for effective vaccines against different pathogens, the antigen may also need to be released prior to cell uptake, making co-encapsulation with the cytokine the optimal formulation.
The studies discussed above provided a strong proof-of-concept that motivated translation into the clinical setting and similar formulations containing a trivalent preparation of antigens from three influenza strains encapsulated in liposomes and liposomal IL-2 (INFLUSOME-VAC) have advanced to human trials [144,145]. In young adults, INFLUSOME-VAC induced higher seroconversion rates against two strains compared with the commercial split virion vaccine and against all three strains compared with the commercial subunit vaccine [145]. Similarly, in elderly patients INFLUSOME-VAC induced higher seroconversion rates for two strains when compared with the commercial vaccine [144]. These studies have demonstrated that vaccine formulations containing a single cytokine can be used to enhance the protective humoral and cellular response against either cancer or a pathogen. Such formulations have been shown to be more effective than conventional vaccines in human patients and implies that cytokine-loaded particles may be useful to improve current vaccines and for the development of new approaches to protect against pathogens like HIV and malaria that have evaded current attempts at vaccination.
Direct attachment of cytokine-loaded particles to T cells for paracrine delivery
As highlighted in Figure 2 and at various points in this article, T-cell populations represent the basic underpinning of adaptive immunity and have central roles as effector cells and memory cells and are involved in the maintenance of peripheral tolerance. It has been a long-term goal to manipulate these populations for immunotherapy, especially in the treatment of cancer. While the studies discussed above have shown that administration of cytokine-loaded particles in vivo can influence T cells, two groups have developed methods to enhance the specificity of particle-mediated cytokine delivery to these cells. Fahmy and Steenblock first designed IL-2-loaded PLGA particles to act as artificial APCs, where the particle surface was functionalized with streptavidin to allow for the attachment of biotinylatedαCD3 andαCD28 [146]. These beads were then capable of binding CD3 and CD28 on the T-cell surface to activate T cells, while the release of the IL-2-promoted T-cell expansion. To determine whether these particles could be used to induce naive CD4+ T cells to differentiate into a specific T-helper cell lineage, the same particles were targeted to CD4 using anti-CD4 antibody and encapsulated either IL-6 or LIF; cytokines known to induce T-helper 17 (Th17) cells or Tregs, respectively. By attaching these particles to naive CD4+ T cells and culturing them in vitro in the presence of TGF-β and IL-2, the particles were able to release their cytokines and induce the appropriate CD4+ T-cell subset [147,148]. The ability of CD4-targeted, LIF-loaded particles attached to T cells in vitro to induce Tregs in vivo was demonstrated by improved tolerance of an allograft transplant in a murine host [147,148]. These data illustrate the effectiveness of targeting cytokine-loaded particles to specific surface molecules on CD4+ T cells to deliver signals capable of altering the differentiation of naive CD4+ T cells in vivo for therapeutic applications.
The second approach to functionalize T cells used novel lipid particles partially composed of maleimide-functionalized lipid, which allowed for the chemical conjugation of the particles to free thiols in proteins presented on the surface of CD8+ T cells [149]. Irvine and coworkers used this methodology to target IL-15 super agonist (IL-15sa) and IL-21, to tumor antigen-specific CD8+ T cells in vitro. Importantly, the combination of IL-15sa and IL-21 has been shown to augment CD8+ effector and memory cell populations [150]. These functionalized T cells were then transferred into mice bearing B16 melanoma tumors and the transferred cells and attached lipid particles were enriched in the tumor – indicating that the particles did not inhibit the ability of T cells to migrate. Compared with systemically administered IL-15sa and IL-21, the particle-mediated paracrine delivery of IL-15sa and IL-21 resulted in an 80-fold increase in expansion of tumor-specific CD8+ T cells in mice bearing established tumors and showed complete arrest of tumor growth and 100% survival after 30 days [49]. While these data validate this approach to directly target cytokine to T cells, it is limited to cell transfer models as efforts to target drug delivery vehicles to specific cell types in vivo has proven to be a significant challenge. However, in the setting of cancer therapy, there are many studies in which autologous NK and T cells are expanded and activated in vitro before being given back to patients. Indeed, recent studies in human patients have shown that autologous CD8+ T cells engineered to be specific for the B-cell antigen CD19 were effective in the treatment of chronic lymphoid leukemia [151]. Combining this type of therapy with the direct attachment of particles loaded with cytokines capable of supplementing the expansion and efficacy of these T cells could increase the likelihood of successful treatment in a growing number of cancer or infectious models.
Conclusion & future perspective for particle-mediated cytokine therapy
The utility of particle systems – whether lipid or polymer based – to deliver immunomodulatory cytokines in vivo has been investigated for several decades. These studies have demonstrated the ability to encapsulate and deliver a wide range of cytokines that affect both the innate and adaptive immune response for purposes ranging from cancer therapy, to vaccine supplementation, to treatment of pathogens. Investigators in this field have spent significant time developing novel ways to administer these cytokine-loaded particles to induce the most effective immune response. While accumulation of systemically delivered particle-encapsulated therapeutics in the MPS can be undesirable, this in vivo clearance system serves as an efficient means to deliver particles loaded with proinflammatory cytokines to innate immune cells to enhance the immune response against cancer or a pathogen. At present, the most promising means to administer these cytokine-loaded particles is by using them as cytokine depots in either vaccine or anticancer therapies. A number of studies in mice have demonstrated the potential of delivering a wide variety of cytokines in vivo using these strategies in multiple experimental systems. These studies have begun to be translated into the clinic as liposomal IL-2 formulations have been used in clinical trials to supplement vaccines for cancer therapy as well as for prophylactic vaccines against influenza. It is likely that more liposomal formulations with cytokines other than IL-2 will soon follow and polymeric particle formulations, which are further down the pipeline, are making their way into the clinic through recent clinical trials using polymer-encapsulated chemotherapeutics. The most recently devised method to deliver cytokines is the attachment of cytokine-loaded particles directly to T cells in vitro to influence T-cell differentiation and expansion in vivo. The field of particle-mediated delivery of therapeutics is continuously working to improve the design of novel lipid- and polymer-based particles [8,152,153] to increase their usefulness in future clinical applications, while the rapid progress in the understanding of cytokine biology will help to identify which particle-encapsulated cytokines should be used to enhance the desired immune response.
Executive summary.
Characterization & optimization of cytokine-loaded particle formulations
-
▪In vitro characterization and optimization:
-
-Liposomes were optimized to encapsulate macrophage-activating factor, IFN-γ, type I interferon, TNF-α, IL-1α, IL-2, IL-6 and GM-CSF, and were subsequently shown to be more effective than free cytokine at inducing the appropriate cellular responses to the cytokine.
-
-Work encapsulating cytokines in polymeric particles began tailoring each formulation to maintain cytokine bioactivity from encapsulation through release.
-
-
-
▪In vivo characterization of cytokine-loaded particles:
-
-Properties of cytokine-loaded particles were tuned to control biodistribution and release of the cytokine in vivo.
-
-The delivery of bioactive cytokine was assayed by inducing cells to kill tumor cells, infected cells or produce cytokines.
-
-
Systemic administration of cytokine-loaded liposomes & polymeric particles
-
▪Targeting the MPS for antitumor and antimicrobial activities:
-
-Intravenous injection of cytokine-loaded particles targeted to the MPS induce tumor killing.
-
-Prophylactic, systemic administration of cytokine-loaded particle can enhance protection against infectious challenge.
-
-
-
▪Avoiding the MPS for therapeutic treatment:
-
-Engineering stealthy particles by attaching polyethylene glycol to the surface allows increased accumulation of the particles and encapsulated cytokine to tissues with leaky vasculature (e.g., inflamed tissue and solid tumors).
-
-
Cytokine depots for vaccination & cancer therapy
-
▪Cytokine depots for anticancer vaccines:
-
-Particles loaded with GM-CSF, IFN-γ or IL-2 administered with tumor antigens were more effective at eliciting tumor-specific T-cell responses that serve to protect mice from subsequent tumor challenge.
-
-In clinical trials, cancer vaccines supplemented with liposomal IL-2 have been well tolerated by patients and were shown to induce tumor specific T-cell responses in patients either with progressive disease or in clinical remission.
-
-
-
▪Intratumoral injection of cytokine depots for anticancer therapy:
-
-Intratumoral injections of particles loaded with IL-2 or combinations of IL-12 and TNF-α, GM-CSF or IL-18 served to activate immune cells in the tumor microenvironment, resulting in decreased growth of the primary tumor, eradication of tumor metastasis and resistance to tumor rechallenge.
-
-Human tumor xenografts – including nondisrupted tumors from tissue biopsy – in SCID mice were injected with IL-12-loaded particles, which activated human leukocytes in the tumor to suppress tumor growth.
-
-
-
▪Cytokine depots to supplement vaccination against pathogens:
-
-Supplementing prophylactic vaccines against pathogens with particles loaded with a variety of cytokines was shown to enhance high-affinity antibody titers, cellular responses and resistance to challenge with the pathogen.
-
-Influenza vaccines containing liposomal IL-2 have shown increased efficacy compared with the commercial vaccine in both young and old adults in clinical trials.
-
-
Direct attachment of cytokine-loaded particles to T cells for paracrine delivery
-
▪
Cytokine-loaded particles can be engineering for direct attachment to T cells in vitro, allowing for paracrine delivery of the encapsulated cytokines to the T cells.
-
▪
Transfer of T cells labeled with cytokine-loaded particles in vivo showed enhanced functionality of the cytokine when compared with systemic treatment with soluble cytokine.
Acknowledgments
The authors received grants from the NIH (NIH 5-T32-AR-007442-25 and NIH AI-042334).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Atkins MB, Robertson MJ, Gordon M, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 1997;3(3):409–417. [PubMed] [Google Scholar]
- 2.Bennett CL, Vogelzang NJ, Ratain MJ, Reich SD. Hyponatremia and other toxic effects during a Phase I trial of recombinant human gamma interferon and vinblastine. Cancer Treat. Rep. 1986;70(9):1081–1084. [PubMed] [Google Scholar]
- 3.Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol. Pathol. 1999;27(1):58–63. doi: 10.1177/019262339902700112. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270(5238):908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Engl. J. Med. 1987;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 6.Gregoriadis G. The carrier potential of liposomes in biology and medicine (second of two parts) N. Engl. J. Med. 1976;295(14):765–770. doi: 10.1056/NEJM197609302951406. [DOI] [PubMed] [Google Scholar]
- 7.Gregoriadis G. The carrier potential of liposomes in biology and medicine (first of two parts) N. Engl. J. Med. 1976;295(13):704–710. doi: 10.1056/NEJM197609232951305. [DOI] [PubMed] [Google Scholar]
- 8. Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. ▪▪ Seminal review of the progress of liposomal formulations toward clinical applications.
- 9.Allison AC, Gregoria G. Liposomes as immunological adjuvants. Nature. 1974;252(5480):252–252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 10.Alving CR. Liposomes as carriers of antigens and adjuvants. J. Immunol. Methods. 1991;140(1):1–13. doi: 10.1016/0022-1759(91)90120-5. [DOI] [PubMed] [Google Scholar]
- 11.Gregoriadis G, Gursel I, Gursel M, McCormack B. Liposomes as immunological adjuvants and vaccine carriers. J. Control. Release. 1996;41(1–2):49–56. [Google Scholar]
- 12.Henriksen-Lacey M, Korsholm KS, Andersen P, Perrie Y, Christensen D. Liposomal vaccine delivery systems. Expert Opin Drug Deliv. 2011;8(4):505–519. doi: 10.1517/17425247.2011.558081. [DOI] [PubMed] [Google Scholar]
- 13.Christensen D, Agger EM, Andreasen LV, et al. Liposome-based cationic adjuvant formulations (CAF): past, present, and future. J. Liposome Res. 2009;19(1):2–11. doi: 10.1080/08982100902726820. [DOI] [PubMed] [Google Scholar]
- 14.Moon JJ, Suh H, Bershteyn A, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat. Mater. 2011;10(3):243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decoster L, Wauters I, Vansteenkiste JF. Vaccination therapy for non-small-cell lung cancer: review of agents in Phase III development. Ann Oncol. 2011 doi: 10.1093/annonc/mdr564. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 16.Wu YL, Park K, Soo RA, et al. INSPIRE: A Phase III study of the BLP25 liposome vaccine (L-BLP25) in Asian patients with unresectable stage III non-small cell lung cancer. BMC Cancer. 2011 doi: 10.1186/1471-2407-11-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butts C, Maksymiuk A, Goss G, et al. Updated survival analysis in patients with stage IIIb or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): Phase IIB randomized, multicenter, open-label trial. J. Cancer Res. Clin. Oncol. 2011;137(9):1337–1342. doi: 10.1007/s00432-011-1003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohyanagi F, Horai T, Sekine I, et al. Safety of BLP25 liposome vaccine (L-BLP25) in Japanese patients with unresectable stage III NSCLC after primary chemoradiotherapy: preliminary results from a Phase I/II study. Jpn. J. Clin. Oncol. 2011;41(5):718–722. doi: 10.1093/jjco/hyr021. [DOI] [PubMed] [Google Scholar]
- 19.Butts C, Murray RN, Smith CJ, et al. A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin. Lung Cancer. 2010;11(6):391–395. doi: 10.3816/CLC.2010.n.101. [DOI] [PubMed] [Google Scholar]
- 20.Sangha R, North S. L-BLP25: a MUC1-targeted peptide vaccine therapy in prostate cancer. Expert Opin Biol. Ther. 2007;7(11):1723–1730. doi: 10.1517/14712598.7.11.1723. [DOI] [PubMed] [Google Scholar]
- 21.Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin. Cancer Res. 2007;13(15 Pt 2):S4652–S4654. doi: 10.1158/1078-0432.CCR-07-0213. [DOI] [PubMed] [Google Scholar]
- 22.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomedicine. 2006;1(3):297–315. [PMC free article] [PubMed] [Google Scholar]
- 23.Christian DA, Cai S, Bowen DM, et al. Polymersome carriers: from self-assembly to siRNA and protein therapeutics. Eur. J. Pharm. Biopharm. 2009;71(3):463–474. doi: 10.1016/j.ejpb.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gombotz WR, Pettit DK. Biodegradable polymers for protein and peptide drug delivery. Bioconjug. Chem. 1995;6(4):332–351. doi: 10.1021/bc00034a002. [DOI] [PubMed] [Google Scholar]
- 25.Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263(5580):797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 26.Lee KY, Yuk SH. Polymeric protein delivery systems. Progress in Polymer Science. 2007;32(7):669–697. [Google Scholar]
- 27.Torres MP, Wilson-Welder JH, Lopac SK, et al. Polyanhydride microparticles enhance dendritic cell antigen presentation and activation. Acta Biomater. 2011;7(7):2857–2864. doi: 10.1016/j.actbio.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulery BD, Kumar D, Ramer-Tait AE, et al. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLoS ONE. 2011;6(3):e17642. doi: 10.1371/journal.pone.0017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demento SL, Bonafe N, Cui W, et al. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J. Immunol. 2010;185(5):2989–2997. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demento SL, Siefert AL, Bandyopadhyay A, Sharp FA, Fahmy TM. Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends Biotechnol. 2011;29(6):294–306. doi: 10.1016/j.tibtech.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cleland JL, Jones AJ. Stable formulations of recombinant human growth hormone and interferon-gamma for microencapsulation in biodegradable microspheres. Pharm. Res. 1996;13(10):1464–1475. doi: 10.1023/a:1016063109373. ▪▪ Provides a model for optimizing polymer particle formulations to maintain cytokine viability.
- 32.Taluja A, Youn YS, Bae YH. Novel approaches in microparticulate PLGA delivery systems encapsulating proteins. J. Mater. Chem. 2007;17(38):4002–4014. [Google Scholar]
- 33.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm. Res. 2000;17(10):1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 34.Oppenheim JJ. Cytokines: past, present, and future. Int. J. Hematol. 2001;74(1):3–8. doi: 10.1007/BF02982543. [DOI] [PubMed] [Google Scholar]
- 35.Salem ML, Gillanders WE, Kadima AN, et al. Review: novel nonviral delivery approaches for interleukin-12 protein and gene systems: curbing toxicity and enhancing adjuvant activity. J. Interferon Cytokine Res. 2006;26(9):593–608. doi: 10.1089/jir.2006.26.593. [DOI] [PubMed] [Google Scholar]
- 36.Shaker MA, Younes HM. Interleukin-2: evaluation of routes of administration and current delivery systems in cancer therapy. J. Pharm. Sci. 2009;98(7):2268–2298. doi: 10.1002/jps.21596. [DOI] [PubMed] [Google Scholar]
- 37.Younes HM, Amsden BG. Interferon-gamma therapy: evaluation of routes of administration and delivery systems. J. Pharm. Sci. 2002;91(1):2–17. doi: 10.1002/jps.10007. [DOI] [PubMed] [Google Scholar]
- 38.Fidler IJ, Raz A, Fogler WE, Hoyer LC, Poste G. The role of plasma membrane receptors and the kinetics of macrophage activation by lymphokines encapsulated in liposomes. Cancer Res. 1981;41(2):495–504. [PubMed] [Google Scholar]
- 39.Kleinerman ES, Schroit AJ, Fogler WE, Fidler IJ. Tumoricidal activity of human monocytes activated in vitro by free and liposome-encapsulated human lymphokines. J. Clin. Invest. 1983;72(1):304–315. doi: 10.1172/JCI110970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poste G, Kirsh R, Fogler WE, Fidler IJ. Activation of tumoricidal properties in mouse macrophages by lymphokines encapsulated in liposomes. Cancer Res. 1979;39(3):881–892. [PubMed] [Google Scholar]
- 41.Sone S, Poste G, Fidler IJ. Rat alveolar macrophages are susceptible to activation by free and liposome-encapsulated lymphokines. J. Immunol. 1980;124(5):2197–2202. [PubMed] [Google Scholar]
- 42.Koff WC, Fidler IJ, Showalter SD, et al. Human monocytes activated by immunomodulators in liposomes lyse herpesvirus-infected but not normal cells. Science. 1984;224(4652):1007–1009. doi: 10.1126/science.6426057. [DOI] [PubMed] [Google Scholar]
- 43.Saiki I, Fidler IJ. Synergistic activation by recombinant mouse interferon-gamma and muramyl dipeptide of tumoricidal properties in mouse macrophages. J. Immunol. 1985;135(1):684–688. [PubMed] [Google Scholar]
- 44.Saiki I, Sone S, Fogler WE, et al. Synergism between human recombinant gamma-interferon and muramyl dipeptide encapsulated in liposomes for activation of antitumor properties in human blood monocytes. Cancer Res. 1985;45(12 Pt 1):6188–6193. [PubMed] [Google Scholar]
- 45.Eppstein DA, Marsh YV, van der Pas M, Felgner PL, Schreiber AB. Biological activity of liposome-encapsulated murine interferon gamma is mediated by a cell membrane receptor. Proc. Natl Acad. Sci. USA. 1985;82(11):3688–3692. doi: 10.1073/pnas.82.11.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldbach P, Dumont S, Kessler R, Poindronb P, Stamm A. Preparation and characterization of interferon-γ-containing liposomes. Int. J. Pharm. 1995;123(1):33–39. [Google Scholar]
- 47.Storm G, Van Slooten ML, Boerman O, et al. Liposomes as sustained release system for human interferon-gamma: biopharmaceutical aspects. Biochim. Biophys. Acta. 2001;1530(2–3):134–145. doi: 10.1016/s1388-1981(00)00174-8. [DOI] [PubMed] [Google Scholar]
- 48.van Slooten ML, Visser AJ, van Hoek A, et al. Conformational stability of human interferon-gamma on association with and dissociation from liposomes. J. Pharm. Sci. 2000;89(12):1605–1619. doi: 10.1002/1520-6017(200012)89:12<1605::aid-jps12>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 49.Debs RJ, Duzgunes N, Brunette EN, et al. Liposome-associated tumor necrosis factor retains bioactivity in the presence of neutralizing anti-tumor necrosis factor antibodies. J. Immunol. 1989;143(4):1192–1197. [PubMed] [Google Scholar]
- 50.Eppstein DA, Marsh YV. Partial dissociation of antiviral and antimitogenic activities of murine interferon after its incorporation into liposomes. J. Interferon Res. 1983;3(2):161–168. doi: 10.1089/jir.1983.3.161. [DOI] [PubMed] [Google Scholar]
- 51.Eppstein DA, Stewart WE., 2nd Binding and capture of human interferon-alpha by reverse evaporation vesicles, multilamellar vesicles, and small unilamellar vesicles. J. Interferon Res. 1981;1(4):495–504. doi: 10.1089/jir.1981.1.495. [DOI] [PubMed] [Google Scholar]
- 52.Nii A, Fan D, Fidler IJ. Cytotoxic potential of liposomes containing tumor necrosis factor-alpha against sensitive and resistant target cells. J. Immunother. (1991) 1991;10(1):13–19. doi: 10.1097/00002371-199102000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Killion JJ, Fishbeck R, Bar-Eli M, Chernajovsky Y. Delivery of interferon to intracellular pathways by encapsulation of interferon into multilamellar liposomes is independent of the status of interferon receptors. Cytokine. 1994;6(4):443–449. doi: 10.1016/1043-4666(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 54.Shin DM, Fidler IJ, Bucana CD, et al. Superior antiproliferative effects mediated by interferon-alpha entrapped in liposomes against a newly established human lung cancer cell line. J. Biol. Response Mod. 1990;9(4):355–360. [PubMed] [Google Scholar]
- 55.Anderson PM, Hanson DC, Hasz DE, Halet MR, Blazar BR, Ochoa AC. Cytokines in liposomes: preliminary studies with IL-1, IL-2, IL-6, GM-CSF and interferon-gamma. Cytokine. 1994;6(1):92–101. doi: 10.1016/1043-4666(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 56.Hora MS, Rana RK, Nunberg JH, Tice RT, Gilley RM, Hudson ME. Controlled release of interleukin-2 from biodegradable microspheres. Nat. Biotechnol. 1990;8(8):755–758. doi: 10.1038/nbt0890-755. [DOI] [PubMed] [Google Scholar]
- 57.Yang J, Cleland JL. Factors affecting the in vitro release of recombinant human interferon-gamma (rhIFN-gamma) from PLGA microspheres. J. Pharm. Sci. 1997;86(8):908–914. doi: 10.1021/js960480l. [DOI] [PubMed] [Google Scholar]
- 58.Lagarce F, Garcion E, Faisant N, et al. Development and characterization of interleukin-18-loaded biodegradable microspheres. Int. J. Pharm. 2006;314(2):179–188. doi: 10.1016/j.ijpharm.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 59.Liu LS, Liu SQ, Ng SY, et al. Controlled release of interleukin-2 for tumour immunotherapy using alginate/chitosan porous microspheres. J. Control. Release. 1997;43(1):65–74. [Google Scholar]
- 60.Sanchez A, Tobio M, Gonzalez L, Fabra A, Alonso MJ. Biodegradable micro- and nanoparticles as long-term delivery vehicles for interferon-alpha. Eur. J. Pharm. Sci. 2003;18(3–4):221–229. doi: 10.1016/s0928-0987(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 61.Sharma A, Harper CM, Hammer L, et al. Characterization of cytokine-encapsulated controlled-release microsphere adjuvants. Cancer Biother. Radiopharm. 2004;19(6):764–769. doi: 10.1089/cbr.2004.19.764. [DOI] [PubMed] [Google Scholar]
- 62.Thomas TT, Kohane DS, Wang A, Langer R. Microparticulate formulations for the controlled release of interleukin-2. J. Pharm. Sci. 2004;93(5):1100–1109. doi: 10.1002/jps.20009. [DOI] [PubMed] [Google Scholar]
- 63.Bryde S, Grunwald I, Hammer A, et al. Tumor necrosis factor (TNF)-functionalized nanostructured particles for the stimulation of membrane TNF-specific cell responses. Bioconjug. Chem. 2005;16(6):1459–1467. doi: 10.1021/bc0501810. [DOI] [PubMed] [Google Scholar]
- 64.Anderson PM, Katsanis E, Sencer SF, et al. Depot characteristics and biodistribution of interleukin-2 liposomes: importance of route of administration. J. Immunother. (1991) 1992;12(1):19–31. [PubMed] [Google Scholar]
- 65.Kedar E, Gur H, Babai I, et al. Delivery of cytokines by liposomes: hematopoietic and immunomodulatory activity of interleukin-2 encapsulated in conventional liposomes and in long-circulating liposomes. J. Immunother. 2000;23(1):131–145. doi: 10.1097/00002371-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 66.Kedar E, Palgi O, Golod G, Babai I, Barenholz Y. Delivery of cytokines by liposomes. III. Liposome-encapsulated GM-CSF and TNF-alpha show improved pharmacokinetics and biological activity and reduced toxicity in mice. J. Immunother. 1997;20(3):180–193. doi: 10.1097/00002371-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 67.van der Veen AH, Eggermont AM, Seynhaeve AL, van Tiel ST, ten Hagen TL. Biodistribution and tumor localization of stealth liposomal tumor necrosis factor-alpha in soft tissue sarcoma bearing rats. Int. J. Cancer. 1998;77(6):901–906. doi: 10.1002/(sici)1097-0215(19980911)77:6<901::aid-ijc17>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 68.Bonetti A, Kim S. Pharmacokinetics of an extended-release human interferon alpha-2b formulation. Cancer Chemother. Pharmacol. 1993;33(3):258–261. doi: 10.1007/BF00686225. [DOI] [PubMed] [Google Scholar]
- 69.Eppstein DA, Stewart WE., 2nd Altered pharmacological properties of liposome-associated human interferon-alpha. J. Virol. 1982;41(2):575–582. doi: 10.1128/jvi.41.2.575-582.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bui T, Faltynek C, Ho RJ. Differential disposition of soluble and liposome-formulated human recombinant interleukin-7: effects on blood lymphocyte population in guinea pigs. Pharm. Res. 1994;11(5):633–641. doi: 10.1023/a:1018955708443. [DOI] [PubMed] [Google Scholar]
- 71.Pettit DK, Lawter JR, Huang WJ, et al. Characterization of poly(glycolide-co-D,L-lactide)/poly(D,L-lactide) microspheres for controlled release of GM-CSF. Pharm. Res. 1997;14(10):1422–1430. doi: 10.1023/a:1012176823155. [DOI] [PubMed] [Google Scholar]
- 72.Koten JW, Van Luyn MJ, Cadee JA, et al. IL-2 loaded dextran microspheres with attractive histocompatibility properties for local IL-2 cancer therapy. Cytokine. 2003;24(3):57–66. doi: 10.1016/s1043-4666(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 73.Goldbach P, Dumont S, Kessler R, Poindron P, Stamm A. In situ activation of mouse alveolar macrophages by aerosolized liposomal IFN-gamma and muramyl tripeptide. Am. J. Physiol. 1996;270(3 Pt 1):L429–L434. doi: 10.1152/ajplung.1996.270.3.L429. [DOI] [PubMed] [Google Scholar]
- 74.Eppstein DA. Altered pharmacologic properties of liposome-associated human interferon-alpha. II. J. Interferon Res. 1982;2(1):117–125. doi: 10.1089/jir.1982.2.117. [DOI] [PubMed] [Google Scholar]
- 75.Fidler IJ, Raz A, Fogler WE, et al. Design of liposomes to improve delivery of macrophage-augmenting agents to alveolar macrophages. Cancer Res. 1980;40(12):4460–4466. [PubMed] [Google Scholar]
- 76.Fogler WE, Raz A, Fidler IJ. In situ activation of murine macrophages by liposomes containing lymphokines. Cell Immunol. 1980;53(1):214–219. doi: 10.1016/0008-8749(80)90440-2. [DOI] [PubMed] [Google Scholar]
- 77.Debs RJ, Fuchs HJ, Philip R, et al. Immunomodulatory and toxic effects of free and liposome-encapsulated tumor necrosis factor alpha in rats. Cancer Res. 1990;50(2):375–380. [PubMed] [Google Scholar]
- 78.Fidler IJ, Fogler WE, Kleinerman ES, Saiki I. Abrogation of species specificity for activation of tumoricidal properties in macrophages by recombinant mouse or human interferon-gamma encapsulated in liposomes. J. Immunol. 1985;135(6):4289–4296. [PubMed] [Google Scholar]
- 79.Hockertz S, Franke G, Kniep E, Lohmann-Matthes ML. Mouse interferon-gamma in liposomes: pharmacokinetics, organ-distribution, and activation of spleen and liver macrophages in vivo. J. Interferon Res. 1989;9(5):591–602. doi: 10.1089/jir.1989.9.591. [DOI] [PubMed] [Google Scholar]
- 80.Hockertz S, Franke G, Paulini I, Lohmann-Matthes ML. Immunotherapy of murine visceral leishmaniasis with murine recombinant interferon-gamma and MTP-PE encapsulated in liposomes. J. Interferon Res. 1991;11(3):177–185. doi: 10.1089/jir.1991.11.177. [DOI] [PubMed] [Google Scholar]
- 81.Mullerad J, Cohen S, Voronov E, Apte RN. Macrophage activation for the production of immunostimulatory cytokines by delivering interleukin 1 via biodegradable microspheres. Cytokine. 2000;12(11):1683–1690. doi: 10.1006/cyto.2000.0775. [DOI] [PubMed] [Google Scholar]
- 82.Fidler IJ. Therapy of spontaneous metastases by intravenous injection of liposomes containing lymphokines. Science. 1980;208(4451):1469–1471. doi: 10.1126/science.7384789. [DOI] [PubMed] [Google Scholar]
- 83.Fidler IJ, Barnes Z, Fogler WE, et al. Involvement of macrophages in the eradication of established metastases following intravenous injection of liposomes containing macrophage activators. Cancer Res. 1982;42(2):496–501. [PubMed] [Google Scholar]
- 84.Fidler IJ, Schroit AJ. Synergism between lymphokines and muramyl dipeptide encapsulated in liposomes – in situ activation of macrophages and therapy of spontaneous cancer metastases. J. Immunol. 1984;133(1):515–518. [PubMed] [Google Scholar]
- 85.Pak CC, Fidler IJ. Liposomal delivery of biological response modifiers to macrophages. Biotherapy. 1991;3(1):55–64. doi: 10.1007/BF02175099. [DOI] [PubMed] [Google Scholar]
- 86.Saito M, Fan D, Lachman LB. Antitumor effects of liposomal IL1 α and TNF α against the pulmonary metastases of the B16F10 murine melanoma in syngeneic mice. Clin. Exp. Metastasis. 1995;13(4):249–259. doi: 10.1007/BF00133480. [DOI] [PubMed] [Google Scholar]
- 87.Loeffler CM, Platt JL, Anderson PM, et al. Antitumor effects of interleukin 2 liposomes and anti-CD3-stimulated T-cells against murine MCA-38 hepatic metastasis. Cancer Res. 1991;51(8):2127–2132. [PubMed] [Google Scholar]
- 88.Shah AU, D’Souza MJ. Sustained-release interleukin-12 microspheres in the treatment of cancer. Drug Dev. Ind. Pharm. 1999;25(9):995–1004. doi: 10.1081/ddc-100102262. [DOI] [PubMed] [Google Scholar]
- 89.Saravolac EG, Kournikakis B, Gorton L, Wong JP. Effect of liposome-encapsulation on immunomodulating and antiviral activities of interferon-gamma 1. Antiviral Res. 1996;29(2–3):199–207. doi: 10.1016/0166-3542(95)00832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Segura S, Gamazo C, Irache JM, Espuelas S. Gamma interferon loaded onto albumin nanoparticles: in vitro and in vivo activities against Brucella abortus. Antimicrob. Agents Chemother. 2007;51(4):1310–1314. doi: 10.1128/AAC.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.ten Hagen TL, van Vianen W, Bakker-Woudenberg IA. Modulation of nonspecific antimicrobial resistance of mice to Klebsiella pneumoniae septicemia by liposome-encapsulated muramyl tripeptide phosphatidylethanolamine and interferon-gamma alone or combined. J. Infect. Dis. 1995;171(2):385–392. doi: 10.1093/infdis/171.2.385. [DOI] [PubMed] [Google Scholar]
- 92.Melissen PM, van Vianen W, Bidjai O, van Marion M, Bakker-Woudenberg IA. Free versus liposome-encapsulated muramyl tripeptide phosphatidylethanolamide (MTPPE) and interferon-γ (IFN-γ) in experimental infection with Listeria monocytogenes. Biotherapy. 1993;6(2):113–124. doi: 10.1007/BF01877424. [DOI] [PubMed] [Google Scholar]
- 93.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 94.Yuyama Y, Tsujimoto M, Fujimoto Y, Oku N. Potential usage of thermosensitive liposomes for site-specific delivery of cytokines. Cancer Lett. 2000;155(1):71–77. doi: 10.1016/s0304-3835(00)00410-9. [DOI] [PubMed] [Google Scholar]
- 95.ten Hagen TL, Seynhaeve AL, van Tiel ST, Ruiter DJ, Eggermont AM. Pegylated liposomal tumor necrosis factor-alpha results in reduced toxicity and synergistic antitumor activity after systemic administration in combination with liposomal doxorubicin (Doxil) in soft tissue sarcoma-bearing rats. Int. J. Cancer. 2002;97(1):115–120. doi: 10.1002/ijc.1578. [DOI] [PubMed] [Google Scholar]
- 96.Kim DW, Andres ML, Li J, et al. Liposome-encapsulated tumor necrosis factor-alpha enhances the effects of radiation against human colon tumor xenografts. J. Interferon Cytokine Res. 2001;21(11):885–897. doi: 10.1089/107999001753289497. [DOI] [PubMed] [Google Scholar]
- 97.Kedar E, Braun E, Rutkowski Y, Emanuel N, Barenholz Y. Delivery of cytokines by liposomes. II. Interleukin-2 encapsulated in long-circulating sterically stabilized liposomes: immunomodulatory and anti-tumor activity in mice. J. Immunother. Emphasis Tumor Immunol. 1994;16(2):115–124. doi: 10.1097/00002371-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 98.Postma NS, Crommelin DJ, Eling WM, Zuidema J. Treatment with liposome-bound recombinant human tumor necrosis factor-alpha suppresses parasitemia and protects against Plasmodium berghei k173-induced experimental cerebral malaria in mice. J. Pharmacol. Exp. Ther. 1999;288(1):114–120. [PubMed] [Google Scholar]
- 99.Golumbek PT, Azhari R, Jaffee EM, et al. Controlled release, biodegradable cytokine depots: a new approach in cancer vaccine design. Cancer Res. 1993;53(24):5841–5844. [PubMed] [Google Scholar]
- 100.van Slooten ML, Kircheis R, Koppenhagen FJ, Wagner E, Storm G. Liposomes as cytokine-supplement in tumor cell-based vaccines. Int. J. Pharm. 1999;183(1):33–36. doi: 10.1016/s0378-5173(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 101.van Slooten ML, Storm G, Zoephel A, et al. Liposomes containing interferon-gamma as adjuvant in tumor cell vaccines. Pharm. Res. 2000;17(1):42–48. doi: 10.1023/a:1007514424253. [DOI] [PubMed] [Google Scholar]
- 102.Johnston D, Reynolds SR, Bystryn JC. Interleukin-2/liposomes potentiate immune responses to a soluble protein cancer vaccine in mice. Cancer Immunol. Immunother. 2006;55(4):412–419. doi: 10.1007/s00262-005-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koppenhagen FJ, Kupcu Z, Wallner G, et al. Sustained cytokine delivery for anticancer vaccination: liposomes as alternative for gene-transfected tumor cells. Clin. Cancer Res. 1998;4(8):1881–1886. [PubMed] [Google Scholar]
- 104.Krup OC, Kroll I, Bose G, Falkenberg FW. Cytokine depot formulations as adjuvants for tumor vaccines. I. Liposome-encapsulated IL-2 as a depot formulation. J. Immunother. 1999;22(6):525–538. doi: 10.1097/00002371-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 105. Kwak LW, Pennington R, Boni L, et al. Liposomal formulation of a self lymphoma antigen induces potent protective antitumor immunity. J. Immunol. 1998;160(8):3637–3641. ▪ Provides a thorough examination of the development of cytokine-loaded particles for cancer vaccines.
- 106.Popescu MC, Robb RJ, Batenjany MM, et al. A novel proteoliposomal vaccine elicits potent antitumor immunity in mice. Blood. 2007;109(12):5407–5410. doi: 10.1182/blood-2006-08-039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Neelapu SS, Baskar S, Gause BL, et al. Human autologous tumor-specific T-cell responses induced by liposomal delivery of a lymphoma antigen. Clin. Cancer Res. 2004;10(24):8309–8317. doi: 10.1158/1078-0432.CCR-04-1071. ▪▪ Clinical trial of liposomal IL-2 for cancer vaccine in patients in clinical remission.
- 108. Neelapu SS, Gause BL, Harvey L, et al. A novel proteoliposomal vaccine induces antitumor immunity against follicular lymphoma. Blood. 2007;109(12):5160–5163. doi: 10.1182/blood-2006-12-063594. ▪▪ Clinical trial of liposomal IL-2 as a cancer vaccine in patients with progressive disease.
- 109.Anderson PM, Katsanis E, Leonard AS, et al. Increased local antitumor effects of interleukin 2 liposomes in mice with MCA-106 sarcoma pulmonary metastases. Cancer Res. 1990;50(6):1853–1856. [PubMed] [Google Scholar]
- 110.Konno H, Yamashita A, Tadakuma T, Sakaguchi S. Inhibition of growth of rat hepatoma by local injection of liposomes containing recombinant interleukin-2. Antitumor effect of IL-2 liposome. Biotherapy. 1991;3(3):211–218. doi: 10.1007/BF02171684. [DOI] [PubMed] [Google Scholar]
- 111.Hanes J, Sills A, Zhao Z, et al. Controlled local delivery of interleukin-2 by biodegradable polymers protects animals from experimental brain tumors and liver tumors. Pharm. Res. 2001;18(7):899–906. doi: 10.1023/a:1010963307097. [DOI] [PubMed] [Google Scholar]
- 112.Rhines LD, Sampath P, DiMeco F, et al. Local immunotherapy with interleukin-2 delivered from biodegradable polymer microspheres combined with interstitial chemotherapy: a novel treatment for experimental malignant glioma. Neurosurgery. 2003;52(4):872–879. doi: 10.1227/01.neu.0000053211.39087.d1. discussion 879–880. [DOI] [PubMed] [Google Scholar]
- 113.Neville ME, Robb RJ, Popescu MC. In situ vaccination against a non-immunogenic tumour using intratumoural injections of liposomal interleukin 2. Cytokine. 2001;16(6):239–250. doi: 10.1006/cyto.2001.0963. [DOI] [PubMed] [Google Scholar]
- 114.Egilmez NK, Jong YS, Sabel MS, et al. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res. 2000;60(14):3832–3837. [PubMed] [Google Scholar]
- 115.Sabel MS, Hill H, Jong YS, et al. Neoadjuvant therapy with interleukin-12-loaded polylactic acid microspheres reduces local recurrence and distant metastases. Surgery. 2001;130(3):470–478. doi: 10.1067/msy.2001.115839. [DOI] [PubMed] [Google Scholar]
- 116.Hill HC, Conway TF, Jr, Sabel MS, et al. Cancer immunotherapy with interleukin 12 and granulocyte-macrophage colony-stimulating factor-encapsulated microspheres: coinduction of innate and adaptive antitumor immunity and cure of disseminated disease. Cancer Res. 2002;62(24):7254–7263. [PubMed] [Google Scholar]
- 117.Sabel MS, Skitzki J, Stoolman L, et al. Intratumoral IL-12 and TNF-alpha-loaded microspheres lead to regression of breast cancer and systemic antitumor immunity. Ann. Surg. Oncol. 2004;11(2):147–156. doi: 10.1245/aso.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 118.Arora A, Su G, Mathiowitz E, et al. Neoadjuvant intratumoral cytokine-loaded microspheres are superior to postoperative autologous cellular vaccines in generating systemic anti-tumor immunity. J. Surg. Oncol. 2006;94(5):403–412. doi: 10.1002/jso.20572. [DOI] [PubMed] [Google Scholar]
- 119.Sabel MS, Arora A, Su G, et al. Synergistic effect of intratumoral IL-12 and TNF-alpha microspheres: systemic anti-tumor immunity is mediated by both CD8+ CTL and NK cells. Surgery. 2007;142(5):749–760. doi: 10.1016/j.surg.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 120.Sabel MS, Su G, Griffith KA, Chang AE. Intratumoral delivery of encapsulated IL-12, IL-18 and TNF-alpha in a model of metastatic breast cancer. Breast Cancer Res. Treat. 2010;122(2):325–336. doi: 10.1007/s10549-009-0570-3. [DOI] [PubMed] [Google Scholar]
- 121.Egilmez NK, Jong YS, Iwanuma Y, et al. Cytokine immunotherapy of cancer with controlled release biodegradable microspheres in a human tumor xenograft/SCID mouse model. Cancer Immunol. Immunother. 1998;46(1):21–24. doi: 10.1007/s002620050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Egilmez NK, Jong YS, Hess SD, et al. Cytokines delivered by biodegradable microspheres promote effective suppression of human tumors by human peripheral blood lymphocytes in the SCID-Winn model. J. Immunother. 2000;23(2):190–195. doi: 10.1097/00002371-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 123.Hess SD, Egilmez NK, Bailey N, et al. Human CD4+ T cells present within the microenvironment of human lung tumors are mobilized by the local and sustained release of IL-12 to kill tumors in situ by indirect effects of IFN-gamma. J. Immunol. 2003;170(1):400–412. doi: 10.4049/jimmunol.170.1.400. [DOI] [PubMed] [Google Scholar]
- 124.Broderick L, Yokota SJ, Reineke J, et al. Human CD4+ effector memory T cells persisting in the microenvironment of lung cancer xenografts are activated by local delivery of IL-12 to proliferate, produce IFN-gamma, and eradicate tumor cells. J. Immunol. 2005;174(2):898–906. doi: 10.4049/jimmunol.174.2.898. [DOI] [PubMed] [Google Scholar]
- 125. Simpson-Abelson MR, Purohit VS, Pang WM, et al. IL-12 delivered intratumorally by multilamellar liposomes reactivates memory T cells in human tumor microenvironments. Clin. Immunol. 2009;132(1):71–82. doi: 10.1016/j.clim.2009.03.516. ▪ Using cytokine-loaded particles to reactivate memory T cells in intact human tumor xenografts.
- 126.Cao M, Sasaki O, Yamada A, Imanishi J. Enhancement of the protective effect of inactivated influenza virus vaccine by cytokines. Vaccine. 1992;10(4):238–242. doi: 10.1016/0264-410x(92)90158-g. [DOI] [PubMed] [Google Scholar]
- 127.van Slooten ML, Hayon I, Babai I, et al. Immunoadjuvant activity of interferon-gamma-liposomes co-administered with influenza vaccines. Biochim Biophys Acta. 2001;1531(1–2):99–110. doi: 10.1016/s1388-1981(01)00092-0. [DOI] [PubMed] [Google Scholar]
- 128.Griffin KF, Conway BR, Alpar HO, Williamson ED. Immune responses to V antigen of Yersinia pestis co-encapsulated with IFN-gamma: effect of dose and formulation. Vaccine. 1998;16(5):517–521. doi: 10.1016/s0264-410x(97)80005-9. [DOI] [PubMed] [Google Scholar]
- 129.Duits AJ, van Puijenbroek A, Vermeulen H, et al. Immunoadjuvant activity of a liposomal IL-6 formulation. Vaccine. 1993;11(7):777–781. doi: 10.1016/0264-410x(93)90265-y. [DOI] [PubMed] [Google Scholar]
- 130.Griffin KF, Eyles JE, Spiers ID, Alpar HO, Williamson ED. Protection against plague following immunisation with microencapsulated V antigen is reduced by co-encapsulation with IFN-gamma or IL-4, but not IL-6. Vaccine. 2002;20(31–32):3650–3657. doi: 10.1016/s0264-410x(02)00396-1. [DOI] [PubMed] [Google Scholar]
- 131.Lachman LB, Shih LC, Rao XM, et al. Cytokine-containing liposomes as adjuvants for HIV subunit vaccines. AIDS Res. Hum. Retroviruses. 1995;11(8):921–932. doi: 10.1089/aid.1995.11.921. [DOI] [PubMed] [Google Scholar]
- 132.Arnold CN, Campbell DJ, Lipp M, Butcher EC. The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur. J. Immunol. 2007;37(1):100–109. doi: 10.1002/eji.200636486. [DOI] [PubMed] [Google Scholar]
- 133.Crotty S. Follicular helper CD4 T cells (TFH) Annu. Rev. Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 134.Afonso LC, Scharton TM, Vieira LQ, et al. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263(5144):235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 135.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260(5107):496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 136.Arnold CN, Campbell DJ, Lipp M, Butcher EC. Effect of IL-4 and IL-12 liposomal formulations on the induction of immune response to bovine herpesvirus type-1 glycoprotein D. Vaccine. 1997;15(16):1753–1760. doi: 10.1016/s0264-410x(97)00111-4. [DOI] [PubMed] [Google Scholar]
- 137.Baca-Estrada ME, Foldvari M, Snider M. Induction of mucosal immune responses by administration of liposome-antigen formulations and interleukin-12. J. Interferon Cytokine Res. 1999;19(5):455–462. doi: 10.1089/107999099313893. [DOI] [PubMed] [Google Scholar]
- 138.Ha SJ, Park SH, Kim HJ, et al. Enhanced immunogenicity and protective efficacy with the use of interleukin-12-encapsulated microspheres plus AS01B in tuberculosis subunit vaccination. Infect. Immun. 2006;74(8):4954–4959. doi: 10.1128/IAI.01781-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Abraham E, Shah S. Intranasal immunization with liposomes containing IL-2 enhances bacterial polysaccharide antigen-specific pulmonary secretory antibody response. J. Immunol. 1992;149(11):3719–3726. [PubMed] [Google Scholar]
- 140.Gursel M, Gregoriadis G. Interleukin-15 acts as an immunological co-adjuvant for liposomal antigen in vivo. Immunol. Lett. 1997;55(3):161–165. doi: 10.1016/s0165-2478(97)02699-0. [DOI] [PubMed] [Google Scholar]
- 141.Babai I, Barenholz Y, Zakay-Rones Z, et al. A novel liposomal influenza vaccine (INFLUSOME-VAC) containing hemagglutinin-neuraminidase and IL-2 or GM-CSF induces protective anti-neuraminidase antibodies cross-reacting with a wide spectrum of influenza A viral strains. Vaccine. 2002;20(3–4):505–515. doi: 10.1016/s0264-410x(01)00326-7. [DOI] [PubMed] [Google Scholar]
- 142.Babai I, Samira S, Barenholz Y, Zakay-Rones Z, Kedar E. A novel influenza subunit vaccine composed of liposome-encapsulated haemagglutinin/neuraminidase and IL-2 or GM-CSF. II. Induction of Th1 and Th2 responses in mice. Vaccine. 1999;17(9–10):1239–1250. doi: 10.1016/s0264-410x(98)00347-8. [DOI] [PubMed] [Google Scholar]
- 143.Mbawuike IN, Wyde PR, Anderson PM. Enhancement of the protective efficacy of inactivated influenza A virus vaccine in aged mice by IL-2 liposomes. Vaccine. 1990;8(4):347–352. doi: 10.1016/0264-410x(90)90093-2. [DOI] [PubMed] [Google Scholar]
- 144. Ben-Yehuda A, Joseph A, Barenholz Y, et al. Immunogenicity and safety of a novel IL-2-supplemented liposomal influenza vaccine (INFLUSOME-VAC) in nursing-home residents. Vaccine. 2003;21(23):3169–3178. doi: 10.1016/s0264-410x(03)00251-2. ▪▪ Clinical trial using liposomal IL-2 in flu vaccines.
- 145. Ben-Yehuda A, Joseph A, Zeira E, et al. Immunogenicity and safety of a novel liposomal influenza subunit vaccine (INFLUSOME-VAC) in young adults. J. Med. Virol. 2003;69(4):560–567. doi: 10.1002/jmv.10345. ▪▪ Clinical trial using liposomal IL-2 in flu vaccines.
- 146.Steenblock ER, Fahmy TM. A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Mol. Ther. 2008;16(4):765–772. doi: 10.1038/mt.2008.11. [DOI] [PubMed] [Google Scholar]
- 147. Park J, Gao W, Whiston R, et al. Modulation of CD4+ T lymphocyte lineage outcomes with targeted, nanoparticle-mediated cytokine delivery. Mol Pharm. 2011;8(1):143–152. doi: 10.1021/mp100203a. ▪▪ Direct attachment of cytokine-loaded polymer particles to naive CD4+ T cells to dictate cell lineage.
- 148.Gao W, Thompson L, Zhou Q, et al. Treg versus Th17 lymphocyte lineages are cross-regulated by LIF versus IL-6. Cell Cycle. 2009;8(9):1444–1450. doi: 10.4161/cc.8.9.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 2010;16(9):1035–1041. doi: 10.1038/nm.2198. ▪▪ Direct attachment of cytokine-loaded lipid particles to supplement activated CD8+ T cells in vivo.
- 150.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 2005;201(1):139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 153.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 2011;10(7):521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
Website
- 201.Vaccine therapy plus interleukin-2 with or without interferon alfa-2b in treating patients with stage III melanoma. http://clinicaltrials.gov/show/NCT00004104.