Abstract
Recent pre-clinical and clinical studies have shown that stem cell-based therapies hold tremendous promise for the treatment of human disease. Mesenchymal stem cells (MSC) are emerging as promising anti-cancer agents which have an enormous potential to be utilized to treat a number of different cancer types. MSC have inherent tumor-trophic migratory properties, which allows them to serve as vehicles for delivering effective, targeted therapy to isolated tumors and metastatic disease. MSC have been readily engineered to express anti-proliferative, pro-apoptotic, anti-angiogenic agents that specifically target different cancer types. Many of these strategies have been validated in a wide range of studies evaluating treatment feasibility or efficacy, as well as establishing methods for real-time monitoring of stem cell migration in vivo for optimal therapy surveillance and accelerated development. This review aims to provide an in depth status of current MSC-based cancer therapies, as well as the prospects for their clinical translation.
Keywords: Mesenchymal stem cells, cancer therapy, migration, delivery vehicles, targeting, molecular imaging
Introduction
Malignant disease is estimated to account for 1 in each 4 human deaths over all age groups in the United States in 2007 [1]. In recent years, the early detection of some cancer types combined with the advent of cancer specific drugs has increased median survival in cancer patients. However, the short half life of a number of cancer specific drugs, their limited delivery to some tumor types and their detrimental effects on vital non-tumor bodily tissues and functions are major hindrances in precluding cure. A number of adult stem or progenitors cells have been isolated from different tissues including brain, heart, and kidney and have emerged as attractive candidates to treat a wide range of diseases (reviewed in [2, 3]. The ability of MSC to develop into various cell types, and the ease with which they can be expanded in culture, have led to a great deal of interest in their use as therapeutic agents to treat a wide range of diseases. They can be isolated from adult human tissues, have the capability for self-renewal and differentiation into mesenchymal lineages-osteocytic, chondrocytic, and adipogenic. They can be expanded and manipulated in vitro, and subsequently re-grafted. Following re-implantation, they have been found to suppress immune system, reintegrate into tissue architecture and give rise to progeny consisting of both stem cells and lineage restricted daughter cell types [2]. Most importantly, MSC exhibit potent pathotropic migratory properties, rendering them attractive for use as targeted delivery vectors in tumor therapy [2, 3]. Besides offering high site-specificity, this treatment modality also efficiently remedies potential problems sprouting from limited biological drug half-life as drug secretion can be engineered to be continuous. This review sheds light on the utilization of engineered MSC in cancer therapy and their hopes and hurdles in translating MSC based therapies to clinics in cancer patients.
MSC: Sources
Stem cells are the natural sources of embriogenetic tissue generation and continuous regeneration throughout adult life. Adult stem cells have been studied extensively and are already a successful source of FDA-approved treatments for a number of diseases including Parkinson’s disease and juvenile diabetes [4]. MSC have been successfully isolated from a number of organs including brain, liver, kidney, lung, bone marrow, muscle, thymus, pancreas, skin, adipose tissue, fetal tissues, umbilical cord, Wharton’s jelly, and placenta [5–8]. MSC possess the potential of converting to tissue types of other lineages, both within or across germ lines [9, 10]. The highest degree of lineage plasticity has been imputed to bone marrow derived MSC, which are capable of giving rise to virtually all cell types following implantation into early blastocysts and are relatively easy to handle in vitro [10, 11]. Most of the preclinical studies to date have been performed with bone marrow derived MSC which might not be the most practical source available for the clinical settings. The harvesting of bone marrow requires an invasive procedure which yields a small number of cells, and the number, differentiation potential, and lifespan of bone marrow-derived MSCs decline with patient age [12–14]. Two alternate sources for harvesting MSCs that have received considerable attention in recent years are adipose tissue and umbilical cord blood. Adipose tissue obtained from subcutaneous tissue represents the most abundant potential source for harvesting MSCs reliably using simple techniques. The expansion potential, differentiation capacity, and immunophenotype of MSCs derived from adipose tissue are nearly identical to those isolated from bone marrow [13]. Umbilical cord blood, obtained after removal of the placenta, is a rich source of hematopoietic stem cells [15, 16] and has been shown to be also a rich source of MSCs [17]. Mononuclear cells can be separated and cultured from the cord blood, and cells in heterogenous adherent layer have been shown to have a fibroblastiod morphology, and express same markers as bone marrow derived MSC, namely CD13, CD29, CD49e, CD54, CD90, but not CD14, CD31, CD34, CD45, CD49d, nor CD106, among others [18]. Umbilical cord blood derived MSC expand at a higher rate as compared to bone marrow and adipose-derived MSCs [13, 19], which may be due in part to higher telomerase activity[20]. All three type of cells differentiate into osteocytes and chondrocytes [13, 18, 21, 22]which is consistent with the properties of MSCs. Most of the pre-clinical studies discussed in this review have been performed bone marrow derived MSC unless mentioned otherwise.
MSC: Migration
A number of studies have shown that MSC migrate to sites of injury, ischemia and tumor microenvironments. The mechanisms by which MSC migrate across endothelium and home to the target tissues are not yet fully understood, however extensive studies have shown that migration of MSC is dependent upon the different cytokine/receptor pairs SDF-1/CXCR4, SCF-c-Kit, HGF/c-Met, VEGF/VEGFR, PDGF/PDGFr, MCP-1/CCR2, and HMGB1/RAGE (reviewed in [23]. Among these cytokine/receptor pairs, Stromal cell-derived factor SDF-1 and its receptor CXC chemokine receptor-4 (CXCR4) are important mediators of stem cell recruitment to tumors. The importance of the interaction between secreted SDF-1 and cell surface CXCR4 for stem cell migration has been displayed by experiments in which the activity of either the receptor or the cytokine has been inhibited [24–26]. Recent studies on gene expression profiles of MSC exposed to conditioned medium (CM) of various tumor cells revealed the downregulation of matrix metalloproteinase-2 (MMP-2) and upregulation of CXCR4 in MSC. This exposure to tumor cell CM enhanced migration of MSC toward tumor cells which was further confirmed by SDF-1 and MMP-2 inhibition studies. These results suggest that the CXCR4 and MMP-2 are involved in the multistep migration processes of MSC tropism to tumors [26]. Another recent study has reported the involvement of a potent pro-inflammatory cytokine, macrophage migration inhibitory factor (MIF) in MSC migration. An activating antibody (CD74Ab) was employed in this study to examine the effect of one MIF receptor, CD74 (major histocompatibility complex class II-associated invariant chain), on MSC motility. Targeting CD74 to regulate migration and homing potentially may be a useful strategy to improve the efficacy of a variety of MSC therapies including cancers [28]. A recent report on MSC behavior indicates that MSC are attracted to sites of irradiation, and that local irradiation might promote specificity of MSC migration and engraftment [29]. Although these findings are not surprising in the light of general stem cell tropism for injured tissues, they do stress the potential synergism between radiotherapy and tumor specific MSC targeting in the clinical arena.
Besides targeting the tumor main burden, MSC and other stem cell types have been shown to track tumor metastases and small intracranial microsatellite deposits of different tumor types, and effectively treat these by either the factors released by stem cells or in loco expression of tumoricidal transgenes that they have been engineered with [30–32]. These findings provide a strong rationale for the development of therapies that capitalize on the tumoritropic properties of MSC by engineering them into carriers for anti-tumor therapy.
MSC for tumor therapy
The unmodified MSC have been shown to have anti-tumor effects both in vitro and in different mouse models of cancer. This is attributed to the factors released by MSCs that have antitumor properties reducing the proliferation of glioma, melanoma, lung cancer, hepatoma, and breast cancer cells [33–36]. Human mesenchymal stem cells (MSCs) injected intravenously (i.v. ) in a mouse model of Kaposi's sarcoma were shown to home to sites of tumorigenesis and potently inhibit tumor growth [37]. MSCs have also been shown to have anti-angiogenic effect both in vitro and in mouse models of melanoma [38]. Direct injection of MSC into subcutaneous melanoma bearing mice induced apoptosis and abrogated tumor growth [38]. MSC have been genetically modified mainly to introduce and over express target exogenous genes for expression/secretion of a desired therapeutic factor for targeted treatment of different cancer types.
MSC delivery of interleukins
Interleukins are cytokines that regulate inflammatory and immune responses and are known to have anti-tumor effects via direct tumoricidal effects or positive modulation of the endogenous immune system [39]. The delivery interleukins via MSC has been utilized in order to improve the anti-cancer immune surveillance by activating cytotoxic lymphocytes and natural killer cells [39]. MSCs engineered to express interleukin (IL)-12 prevented metastasis into the lymph nodes and other internal organs as well as increased tumor cell apoptosis in mice bearing pre-established metastases of melanoma, breast and hepatoma tumors [40]. Similarly, transplantation of IL-18 secreting MSCs was associated with enhanced T cell infiltration and long-term anti-tumor immunity in mice bearing non-invasive and invasive gliomas [41]. In two recent studies, human MSC expressing IL-12 have been shown to have anti-tumor effects in mice beating renal cell carcinomas[42] and cervical tumors [43]. Both studies revealed sustained expression of IL-12 and interferon (IFN)-γ in sera and tumor sites. MSC have also been engineered to express IL-12 and tested in different mouse tumor models of melanoma [44] and glioma [34]. Nakamura et al showed that rat primary MSC can migrate from the contralateral hemisphere to intracranial glioma and that intratumoral injection of MSC engineered to express IL-2 increased animal survival [34]. In a recent study, a significant reduction in glioma volumes was seen as a result of combining peripheral immunization using interferon gamma (IFN-γ) transduced autologous tumor cells with local intratumoral delivery of MSC expressing interleukin 7 (IL-7). This combined treatment modality also resulted in a higher density of intratumoral T-cells in rats receiving combined therapies compared to rats receiving either cytokine alone suggesting that the therapeutic effect is dependent on a T-cell response [45]. MSC have also been engineered with other immune-stimulatory molecules, like CX3CL1 which is a strong T cell chemoattractant. Intravenous or intratracheal delivery of MSC-CX3CL1 was shown to strongly inhibit development of lung metastasis and increase survival of mice bearing lung metastases cells [46, 47].
Interferons
MSCs have been used as delivery agents for a variety of molecules that can inhibit tumor growth and interferon (IFN)-β has been shown to have anti-proliferative and proapoptotic effects [48–50]. However, its in vivo therapeutic efficacy has been limited due to toxicity associated with systemic administration. Human MSCs engineered to express IFN-β, have been used for targeted delivery to metastatic breast and melanoma models [51, 52], gliomas [25] and lung metastasis [25, 53, 54]. Studeny and colleagues engineered human adult MSC stably expressing INF-β and showed their in vivo efficacy against solid melanomas in nude mice [51]. The anti-tumor effects of MSC expressing INF-β in central nervous system (CNS) tumors was provided by seminal work of Nakamizo and colleagues in 2005, who evaluated whether human MSC could still track murine brain tumors when administered through the blood stream [25]. By manipulating MSC to secrete INF-β, this tropism could be exploited for antitumor effect, as in vivo administration of hMSC-INF-β resulted in significantly enhanced murine survival. In a related study, Ren et al [54] evaluated the potential of MSCs expressing IFN-β in a model of prostate cancer lung metastasis. Targeted homing of MSCs producing IFN-β was seen at sites of tumor in the lungs with established pulmonary metastases, and this resulted in suppression of tumor growth. More recent studies have shown the anti-tumor effects of a multifunctional regulatory cytokine, IFN-α when delivered via MSC. IFN-α is frequently used as adjuvant therapeutic to eradicate micrometastatic deposits in patients with a high risk of systemic recurrence [55, 56]. The therapeutic efficacy of MSC expressing IFN-α was evaluated for the treatment of lung metastasis in a mouse model of metastatic melanoma. The systemic administration of MSC expressing IFN-α reduced the growth of melanoma cells and significantly prolonged survival due to increase in tumor cell apoptosis and a decrease in blood vasculature [53].
Prodrugs
A number of prodrug activation schemes, that convert non-toxic prodrugs into toxic anti-metabolites, are available for selective killing of tumor cells. Cytosine deaminase (CD), herpes simplex virus (HSV)-1 Thymidine kinase (TK) and carboxyesterase genes, which confer sensitivity to 5-fluorocytosine 5-FC, ganciclovir (GCV) and camptothecin-11 (CPT-11), respectively are being evaluated in clinical trials [57]. Activation of pro-drugs that are not toxic to MSC and have a bystander tumor-killing effect are appropriate for the use of MSC as “pharmacologic pumps”. This approach was initially explored using cytosine deaminase (CD), which can convert the nontoxic "prodrug" 5-fluorocytosine to the drug, 5-fluorouracil, a chemotherapeutic agent that can readily diffuse out of the producer stem cell and into surrounding cells and is selective toxic to rapidly dividing cells [30]. MSC engineered to express HSV-TK and injected into the tumor or the vicinity of the tumor, infiltrated solid parts as well as the border of rats bearing glioma and ultimately showed high therapeutic efficacy by significant reduction of tumor volumes through bystander-mediated glioma cell killing [58]. In a recent study, the ability of human adipose tissue-derived mesenchymal stem cells (AT-MSCs), engineered to express the suicide gene cytosine deaminase::uracil phosphoribosyltransferase (CD::UPRT) has been explored in mouse models of prostate cancer. CD::UPRT converts the relatively nontoxic 5-fluorocytosine (5-FC) into the highly toxic antitumor 5-fluorouracil (5-FU). Therapeutic AT-MSCs expressing CD::UPRT were effective in significantly inhibiting prostate cancer tumor growth after intravenous administration in mice bearing tumors and treated with 5-FC [59].
Oncolytic viruses
Oncolytic viruses are natural or genetically modified viruses that, upon infection, selectively replicate in and kill neoplastic cells while sparing normal cells [60, 61]. The systemic administration of oncolytic virus (OV) is often inefficient due to clearance of the virus by host defense mechanism and spurious targeting of non-cancer tissues through the bloodstream [62]. Cell mediated OV delivery could shield the virus from host defenses and direct them toward tumors. Different stem cell types, including MSC have also been used as host cells for the replication, transportation and local release of intact conditionally replication oncolytic adenoviruses (CRAd) [63]. Human MSC were shown to support replication of adenovirus bearing thymidine kinase and to have bystander effect against different cancer cell lines [64]. When administered intravenously into murine models of solid ovarian cancer, CRAd-charged MSC resulted in significantly enhanced anti-tumor effect and extended survival as compared to direct delivery of CRAd [65]. Similar results were obtained by extending the former experiment to mice bearing pulmonary metastases of lung carcinoma [66]. MSCs have also been employed to deliver adenovirus which subsequently infected and replicated within malignant cells and eradicated the tumors [65, 66]. MSCs have been utilized to deliver CRAds in a mouse model of intracranial malignant glioma [67]. CRAd-loaded MSC resulted in efficient adenoviral infection of distant glioma cells confirming the ability of MSCs as carriers for oncolytic adenoviral vectors for the treatment of malignant glioma. In a recent study delivery and efficacy of oAV, Delta24-RGD by human MSC has been assessed in mouse models of glioblastomas [68]. MSC-Delta24 were injected into the carotid artery of mice harboring orthotopic glioma xenografts selectively localized to glioma xenografts and released Delta24-RGD, which subsequently infected glioma cells, inhibited glioma growth and resulted in eradication of tumors with significant increase in the median survival of treated animals as compared to controls.
Antiangiogenic agents
Tumor angiogenesis represents a way for cancer cells to function and to thrive for self-sustained growth [69]. The findings that various growth factors and molecules of the extracellular matrix are responsible for tumor-mediated angiogenesis lead to the utilization of targeted anti-angiogenic therapy [70]. Recent phase II clinical studies have provided evidence that delivery of anti-angiogenic drugs through vasculature transiently normalize the abnormal structure and function of the blood vessels and result in reduction of tumor-associated vasogenic brain edema and clinical benefit in most patients [71]. The vessel normalization is associated with a significant decrease in their mean vessel diameter and permeability [72, 73] and increased pericyte coating of small vasculature [74]. MSCs are known to localize to tumor vasculature upon intratumoral implantation thus offering possibilities targeting particularly vascularized tumors [75]. Recent findings demonstrate that MSCs display pericyte markers and can be considered members of the pericyte family and that intratumorally grafted MSC allow could possibly function as tumor pericytes [76]. Different stem cell types have also been used to express antiangiogenic molecules [2]. In a recent study, we have shown that a single administration of stem cell delivered ant-angiogenic repeats of thrombospondin (TSP)-1 markedly reduces tumor vessel-density that results in the inhibition of tumor-progression and increased survival in mice bearing highly malignant human gliomas [77]. This suggests that the use of MSC to deliver anti-angiogenic agents could be exploited for enhanced therapeutic benefit as MSC by themselves would lead to vascular normalization thus enhancing the antitumor immune response and the expression of anti-angiogenic agents, like aaTSP-1 would target tumor associated endothelial cells.
Pro-apoptotic proteins
The delivery of pro-apoptotic proteins such as TRAIL (tumor necrosis factor-related apoptosis induced ligand) via stem cell is a relatively new approach towards tumor cell killing. TRAIL is an endogenous member of the TNF ligand family that binds to its death domain containing receptors DR4 and DR5 and induces apoptosis via activation of caspases preferentially in cancer cells while sparing most other cell types [78]. A number of studies have shown the therapeutic efficacy of different adult stem cell types including MSC engineered to express TRAIL in either cell lines or mouse models of colorectal carcinoma [79], gliomas [80–82], lung breast, squamous and cervical cancer [83] result in induction of apoptosis and a subsequent reduction of tumor cell viability. However, TRAIL is a type II membrane protein and its release into the microenvironment requires additional cleavage from its cell membrane anchoring site. Engineering truly paracrine TRAIL-secreting cells would thus require designing of the TRAIL protein. Previous work from our laboratory has focused on designing a secretable version of TRAIL that consists of fusion between the extracellular domain of TRAIL and the extracellular domain of the hFlt3 ligand which binds to the Flt3 tyrosine kinase receptor. The re- engineered recombinant protein named ‘secretable TRAIL (S-TRAIL) is efficiently secreted into the producer cell’s immediate microenvironment and exhibits higher cytotoxicity on glioma cells than the native TRAIL protein [31, 84, 85]. In a recent study, we have shown that human MSC are resistant to TRAIL mediated apoptosis and when engineered to express S-TRAIL, induce caspase mediated apoptosis in established glioma cell lines as well as glioblastoma stem cells (GBSCs) in vitro. Using highly malignant and invasive human glioma models generated from human GBSCs and employing real time imaging with correlative neuropathology, we have shown that MSC-S-TRAIL migrate extensively to tumors in the brain and have profound anti-tumour effects in vivo (Fig. 2). This study demonstrates the efficacy of therapeutic S-TRAIL and the potential of human MSC to be used as delivery vehicles targeting GBSCs in vivo [31].
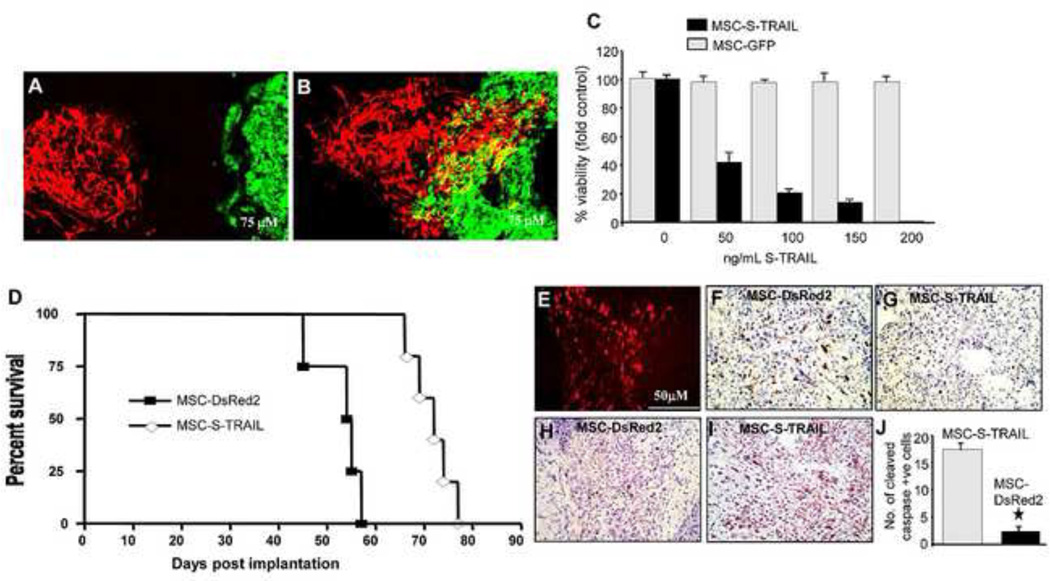
Figure 2. Migration and Therapeutic efficacy of human mesenchymal stem cells expressing S-TRAIL in mice bearing CSC gliomas.
MSC expressing tdTomato were implanted intracranially at a 1 mm distance from established human gliomas expressing GFP-Fluc. (A–B) Photomicrographs showing MSC-tdTomato (red) and gliomas (green) on day 2 (A) and day 10 (B) in brain sections. (C) GBM8 glioma cells were incubated with the conditioned medium from MSC-S-TRAIL and 18 hrs later, GBM8 were analyzed for their viability and casapas-3 activation. Plots showing GBM8 viability incubated with different concentrations of S-TRAIL. (D) Mice bearing established GBM tumors were implanted with MSC-S-TRAIL or control MSC-DsRed2. Survival curves of GBM8-GFP-Fluc-bearing mice treated with MSC-DsRed2 (square) and MSC-S-TRAIL (diamond). (E-J) Photomicrographs showing presence of DsRed2 MSC in brain sections from control mice (E) and Ki67 (F,G) and cleaved caspase-3 (H,I) cells in brain sections from control and MSC-S-TRAIL mice 2 weeks post MSC-implantation. (J) Plot showing the number of cleaved caspase-3 (cells in MSC-S-TRAIL and MSC-DsRed2 treated tumors. Original magnification 10x (B,C); 20X (E-I). Obtained with permission from Proceedings of National Academy of Sciences (PNAS).
Growth factor antagonists
Among the number of molecules that block the activity growth factors, there are limited number of molecules that can be expressed in stem cells and released in the extracellular milieu. NK4 is an antagonist of hepatocyte growth factor (HGF) [86] which is a strong inducer of tumor growth and angiogenesis [87, 88]. MSC engineered to express NK4 in mice bearing lung metastases were studied by Kanehira et al [89]. Systemically administered MSC-NK4 homed specifically to the sites of lung metastatic tumor, efficiently inhibited tumor progression/metastases in the lung and prolonged survival of mice. The anti-metastatic effect of NK4-MSCs in vivo was due to the inhibition of angiogenesis and lymphangiogenesis within the tumor tissues.
Synergistic approaches utilizing MSC based therapeutics with other anti-tumor agents
Given the heterogeneity of tumors in general, it is unlikely that any one effective strategy will provide a satisfactory treatment regimen for tumors. The advent of molecular theragnostics and personalized medicine might largely remedy the differences in nature and therapeutic resistance between different tumors [2, 90], but cannot provide adequate answers to the existence of profound intratumoral heterogeneity, as is observed, for instance, in gliomas [91]. A realist approach would be to combine distinct therapeutic targets, such as those involved in tumor cell growth and apoptosis and the proliferation of tumor associated vasculature to fully eradicate different tumor types.
A recent study has demonstrated that the combined approach using systemic MSC-mediated delivery of TRAIL together with XIAP inhibition suppresses metastatic growth of pancreatic carcinomas [96]. Finally, besides molecular approaches, current clinical treatment regimens such as local radiotherapy might be suited for enhancing stem cell therapy as their effects on irradiated tissue seem to additionally promote the homing of transplanted stem cells [29]. Recent studies have revealed that tumor irradiation enhances the tumor tropism of human umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) by increased IL-8 expression on glioma cells [97]. The sequential treatment with irradiation followed by TRAIL-secreting UCB-MSCs synergistically enhanced apoptosis in glioma cells by upregulating expression of DR5 and subsequently inducing caspase activation. In vivo survival experiments in orthotopic xenografted mice showed that MSC-based TRAIL gene delivery to irradiated tumors had greater therapeutic efficacy than a single treatment. These results suggest that clinically relevant tumor irradiation increases the therapeutic efficacy of MSC-TRAIL by increasing tropism of MSCs and TRAIL-induced apoptosis, which might be a more useful therapeutic strategy for treating tumors in general and gliomas in particular. We have also designed supplementary treatments to augment the antitumor effect of stem cell mediated S-TRAIL therapy by utilizing micro-RNA inhibitors [94] and PI3-Kinase inhibitors [95] in vivo in mouse models of glioblastomas. In both cases, the supplementary treatment augments the response of glioma cells to stem cell delivered TRAIL. These findings offer a preclinical rationale for application of mechanism-based systemically delivered anti-proliferative agents and novel stem cell-based proapoptotic therapies to improve treatment of malignant gliomas.
Encapsulated MSC for Therapy
Due to their ability to provide a physiologic environment that promotes cell survival and prevent immune response while permitting easy in vivo transplantation and cell retention, biodegradable hydrogels and synthetic extracellular matrix (sECM) to encapsulate stem cells have been utilized [98, 99]. A number of different biomaterials such as alginate, agarose and other polymers have been used for encapsulation. In models of intracerebral hypoxia- ischemia and traumatic spinal cord injury, sECM acted as the necessary biomechanical substrate for endogenous neuro-regeneration by increasing their stem cell viability and promoting differentiation into neurons [100–102]. Subsequent studies have again highlighted the utility of biodegradable scaffolds in facilitating stem cell-based therapy in the CNS [103–105]. Recent in vivo studies suggest considerable potential for transplanted biodegradable scaffolds containing stem (and other neuronal) cells in models of bone regeneration. Alginate-poly-L-lysine encapsulated MSCs expressing bone morphogenetic protein-2, a potent cytokine for bone formation, were found to induce bone formation [106]. Chondrogenesis could be enhanced in targeted cell population in vivo after Sox-9 delivery from alginate/chitosan polysaccharide encapsulated MSC engineered to express Sox-9, [107]. Recent studies with FDA-approved viscous bovine collagen–based material (i.e., Contigen), that was earlier shown to permit an enhanced and more durable effect from a gene product secreted by the MSC ([108], have shown that contigen embedded MSC expressing IL-12 have substantial anti-tumor activity in mice bearing breast tumors [109].
In vivo imaging of MSC fate, anti-tumor agent pharmacokinetics and therapeutic efficacy
The clinical translation of MSC based therapies will depend on how successfully the robust surveillance systems are designed to simultaneously monitor the long term fate of MSC, the pharmacokinetics of MSC delivered therapeutics and ultimately the therapeutic efficacy of MSC in vivo. Several strategies can be pursued to visualize stem cell behavior in vivo.
Optical Imaging
Our laboratory has previously engineered different stem cell types including human MSC to stably express the bioluminescent enzyme firefly luciferase (Fluc) [95, 110–112]. After implanting stem cells into the cerebral hemispheres of nude mice contralateral to pregrafted tumors, NSC migration could be followed non-invasively in real-time along their migratory path towards tumors [113]. A similar study was designed with MSC modified to secrete both S-TRAIL and Fluc [31]. Utilizing hybrid (fluorescence and bioluminescence) reporter constructs, dual bioluminescence and intravital imaging in vivo, the entire process of tumor formation, MSC migration, MSC dispersion throughout the tumor and MSC killing of glioma cells was monitored non-invasively in a longitudinal fashion. This allows not only the imaging in real-time of gross MSC migration, but also to visualize tumor penetration by MSC at the single cell level. In the same study, we also evaluated whether MSC survival was influence by the tumors and if intratumoral MSC implantation had any effects on the progression of tumors. Utilizing dual bioluminescence and fluorescence imaging, we showed that MSC survival is increased by the presence of glioma tumors in the brain (Fig. 3A). This can be attributed to the secretion of a number of bioactive growth factors and cytokines, such as VEGF, transforming growth factor-h, or interleukin (IL)-10 [114, 115] secreted by the tumor microenvironment which have been shown to exert a profound immunosuppressive activity on antigen-presenting cells (APC) and T-effector cells. A number of studies have shown enhancement of tumor growth and development, potentially through immunomodulatory and pro-angiogenic properties of MSC, while others have shown no apparent effect of MSC or have demonstrated inhibition of tumor growth and extended survival [116–119]. In a similar set up, we also showed that MSC have no significant influence on the progression of gliomas in the brain (Fig. 3B).
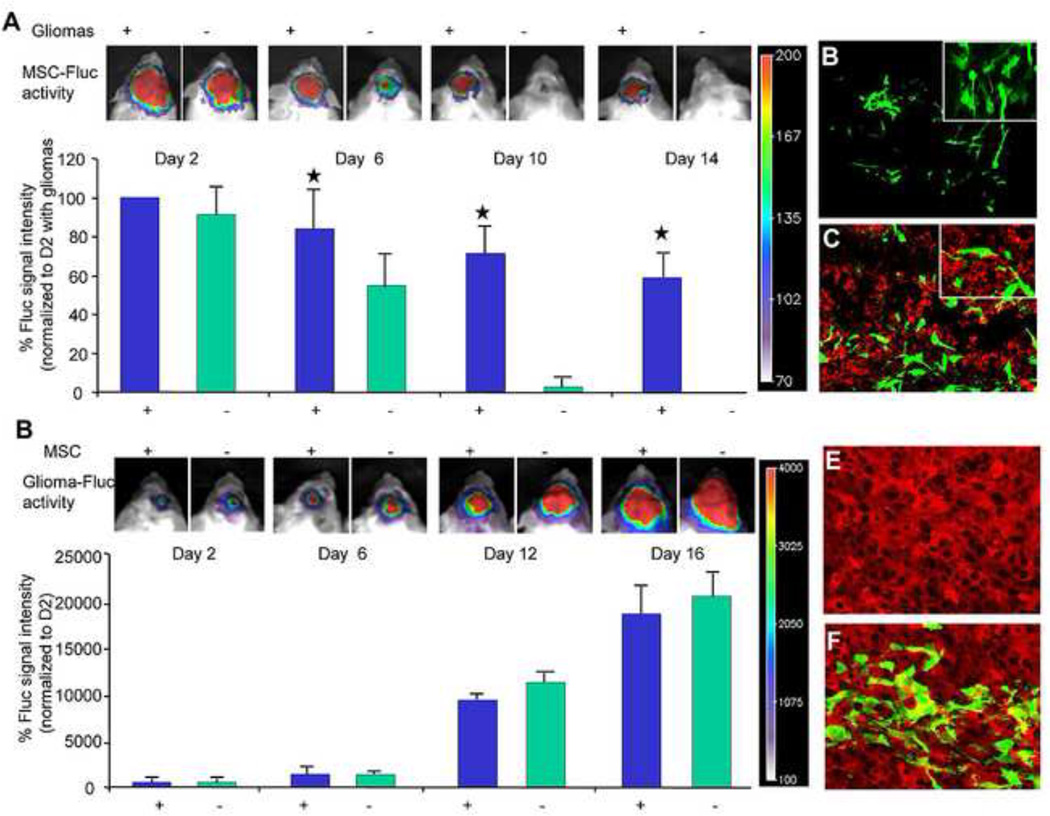
Figure 3. Human MSC survive longer in tumor bearing mice and do not influence tumor growth.
(A) Fluc bioluminescence intensities of MSC-GFP-Fluc implanted intraparenchymally either alone or mixed with Gli36-EGFRvIII human glioma cells. One representative image of mice with MSC-GFP-Fluc implanted with (+) or without (−) glioma cells is shown. (B–C) Photomicrographs on brain sections from mice 16 days post-implantation showing presence of GFP positive MSC in normal brain (B) and the presence of Ki67 positive glioma cells and GFP positive MSC in glioma bearing brains (C). (D) Fluc bioluminescence intensities of intraparenchymally implanted mice with Gli36-EGFRvIII-FD human glioma cells or a mix of Gli36-EGFRvIII-FD and MSC-GFP. One representative image of mice with Gli36-EGFRvIII-FD implanted with (+) or without (−) MSC-GFP is shown. (E–F) Photomicrographs on brain sections from mice 16 days post-implantation showing expression of DsRed2 in glioma cells (E) and the presence of GFP positive MSC with in mice bearing gliomas (F). Original magnification × 20 (B–C; E–F). Obtained with permission from Proceedings of National Academy of Sciences (PNAS).
Recently we have shown that stem cells engineered to express anti-angiogenic, aaTSP-1 targets the vascular-component of gliomas [77]. In this study, the changes in vessel density, stem cell migration, and changes in tumor volumes were monitored simultaneously in real time in vivo. Although, MSC and other stem cell types like NSC are promising therapeutic delivery vehicles, pre-clinical and clinical applications of stem cell-based therapy would benefit significantly from the ability to simultaneously determine therapeutic efficacy and pharmacokinetics of therapies delivered by engineered stem cells. In a recent study, we have engineered and screened numerous fusion variants that contained therapeutic (TRAIL) and diagnostic (luciferase) domains designed to allow simultaneous investigation of multiple events in stem cell-based therapy in vivo. When various stem cell lines were engineered with the optimized molecule, SRLOL2TR, diagnostic imaging showed marked differences in the levels and duration of secretion between stem cell lines, while the therapeutic activity of the molecule showed the different secretion levels translated to significant variability in tumor cell killing [110]. In vivo, simultaneous diagnostic and therapeutic monitoring revealed that stem cell-based delivery significantly improved pharmacokinetics and anti-tumor effectiveness of the therapy compared to intravenous or intratumoral delivery [110]. As a treatment for highly malignant brain tumor xenografts, tracking SRLOL2TR showed stable stem cell-mediated delivery that significantly regressed peripheral and intracranial tumors. Together, the integrated diagnostic and therapeutic properties of SRLOL2TR answer critical questions necessary for successful utilization of stem cells as novel therapeutic vehicles.
Other Imaging Techniques
Given the need for stem cell imaging techniques in larger animals or humans (where bioluminescent imaging is precluded by limited depth of tissue penetration), magnetic resonance imaging (MRI) and positron emission tomography (PET) have been evaluated for feasibility of stem cell tracking. Superparamagnetic particles have been conjugated to different stem cell types that have successfully demonstrated feasibility of imaging migration and peri- or intratumoral localization by MRI in mice [27, 90, 120–124]. MSCs transduced with either adenoviruses and retroviruses expressing the HSV1-tk PET reporter gene suggest that engineered MSCs can be noninvasively imaged with 9-(4-18Ffluoro-3-[hydroxymethyl]butyl)guanine (18F-FHBG) after their transplantation in rats [125]. Recently, the reporter gene imaging of implanted human MSCs by using clinical positron emission tomography (PET)-computed tomography (CT) scanning suggests that human MSC can be translated in large animals [126]. In one study, embryonic stem cells transduced with thymidine kinase and injected directly into murine hearts could be imaged with PET at high spatial resolution using the thymidine kinase-specific PET reporter probe [18F]-FHBG [122]. Interestingly, teratomas formed in this study by embryonic stem cells could effectively be treated by systemic administration of ganciclovir. This indicates that the ganciclovir-thymidine kinase therapeutic regimen could be toxic to thymidine kinase expressing, non-immortalized stem cells themselves, and thus serve as a reporter-suicide for additional control in the prevention of tumor formation [122]. A few recent studies have explored the non-invasive tracking of MSC migration and sodium iodide symporter (NIS) transgene expression in real time prior to therapy in a mouse model of breast [127] and heapatocellular cancer [128] by SPECT and PET imaging respectively. SPECT imaging performed in mice injected with MSC-NIS and (99m) TcO(4) revealed non-specificity of hNIS gene expression at earlier time points, however, at later time points, this expression depleted in non-target tissues and persisted at the tumor site. Based on these imaging/biodistribution data, mice received a therapeutic dose of (131) I 14D following MSC-NIS injection which resulted in a significant reduction in tumor growth as compared to controls [127]. This study reveals that the ability to non-invasively track MSC migration and transgene expression in real time prior to therapy is a major advantage for developing efficient stem cell based therapies. In conclusion, imaging surveillance of stem cell biodistribution and fate will be vital to successful implementation of stem cell delivery in cancer treatment. Several technologies are currently feasible for stem cell tracking, including bioluminescence, MRI and PET imaging, and are likely to contribute to future translational research.
Prospects and caveats on the way to the clinics
The ability MSCs to preferentially migrate towards local and disseminated malignant disease, interact with different tissue environments in addition to their easy availability, non immunogenic nature, relative ease of manipulation in vitro without requiring immortalization present them as most attractive candidates for cell based therapies in humans. The clinical translation of umbilical cord blood derived MSC will be limited by their unreliable and often low isolation efficiency and requires allogeneic transfer. In contrast, allogeneic transfer is not necessary for adipose or bone marrow-derived MSCs, in which case an autograft can easily be harvested from any patient. The advantage of using autologous stem cells is mainly their immunological compatibility, which has been shown to have a profound effect on cell survival after transplantation. The safety of the grafted progenitors is a major concern in clinical setting. Importantly, non-immortalized adult stem cells do not confer the same danger as immortalized adult stem cells and may be used without posing risk to the patient. A number of clinical trials utilizing MSCs particularly for myocardial damage [129, 130] and prevention of graft versus host disease [13, 131, 132] have not reported any major adverse events from allogeneic transplants. There are also a number of ongoing clinical trials which are utilizing MSC for cancer therapy (Table 1), however most of these trials do not use engineered MSC and the results of any adverse effect from such trials is still awaited. If MSC are engineered to secrete environmentally hostile compounds, they might pose a problem after eradication of their target malignant process [91]. It would therefore be desirable to selectively eradicate MSC when malignant transformation is suspected by incorporating activatable cellular suicide genes into transplanted MSC or to selectively turn off gene expression. Possible mechanisms that allow for such controls are stem cell-conferred prodrug converting enzymes and transgenes that require additional in vivo cues for expression and the use of tetracyclin-regulatable promoters to turn off gene expression. It is important to mention that a few reports have implicated MSCs in promoting the growth of certain cancers. The endogenous expression of IL-6 and CCL5 by MSCs, have been shown to increase the growth and metastasis of breast cancer cells, respectively [116, 117]. However, there have not been significant subsequent reports on the promotion of tumor growth by MSC in recent years. A thorough understanding of MSC biology and fate in tumor models that recapitulate more closely clinical settings are critical when developing MSC based therapies for clinical translation in cancer patients.
Table 1.
Ongoing Clinical trials using Mesenchymal Stem Cells for Cancer
| NCT ID | Title | Condition | Intervention | Phase | Enrollment |
|---|---|---|---|---|---|
| NCT00823316 | Safety and efficacy study of Umbilical Cord blood- derived Mesenchymal Stem Cells to promote engraftment of unrelated Hematopoietic Stem Cell transplantation |
Acute Leukemia | Human umbilical cord blood-derived mesenchymal stem cells |
Phase I Phase II |
This study is ongoing, but not recruiting participants. |
| NCT00790413 | Haploidentical Stem Cell transplantation in Neuroblastoma |
Neuroblastoma | Co-transplantation of mesenchymal stem cells |
Phase 0 | This study is currently recruiting participants |
| NCT00361049 | Donor Mesenchymal Stem Cell infusion in treating patients with acute or chronic Graft-Versus-Host disease after undergoing a donor Stem Cell transplant |
Cancer | Phase I | ||
| NCT01275612 | Mesenchymal Stem Cells In Cisplatin-induced acute renal failure in patients with solid organ cancers |
Solid Tumors | Mesenchymal stromal cell infusion |
Phase I | currently recruiting participants |
| NCT01045382 | MSC and HSC co-infusion in mismatched mini- transplants |
Leukemia | Mesenchymal stem cells |
Phase II | Not yet open for participant recruitment |
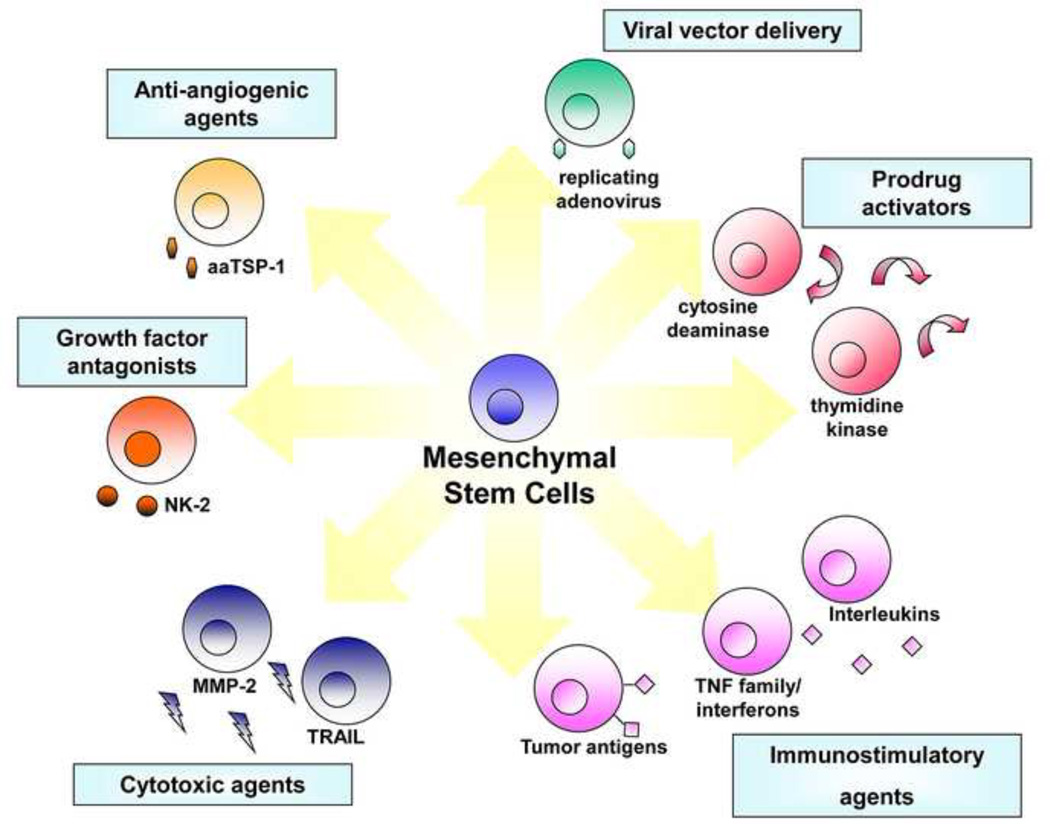
Figure 1. Transgene strategies potentiating MSC for tumor therapy.
Tailored to the specific molecular profiles associated with individual tumor types, stem cells can be designed with a variety of different anti-tumor effects.
Acknowledgements
This work was supported in part by National Institutes of Health (CA138922-01A2; KS), American Cancer Society (KS) and James McDonnel Foundation (KS).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Corsten MF, Shah K. Therapeutic stem-cells for cancer treatment: hopes and hurdles in tactical warfare. Lancet Oncol. 2008;9(4):376–384. doi: 10.1016/S1470-2045(08)70099-8. [DOI] [PubMed] [Google Scholar]
- 3.Teo AK, Vallier L. Emerging use of stem cells in regenerative medicine. Biochem J. 2010;428(1):11–23. doi: 10.1042/BJ20100102. [DOI] [PubMed] [Google Scholar]
- 4.Smith S, Neaves W, Teitelbaum S. Adult stem cell treatments for diseases? Science. 2006;313(5786):439. doi: 10.1126/science.1129987. [DOI] [PubMed] [Google Scholar]
- 5.Momin EN, et al. Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther. 2010;5(4):326–344. doi: 10.2174/157488810793351631. [DOI] [PubMed] [Google Scholar]
- 6.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 7.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21(1):105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 8.Fukuchi Y, et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22(5):649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 9.Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med. 2001;7(4):393–395. doi: 10.1038/86439. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 11.Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 12.Bentzon JF, et al. Tissue distribution and engraftment of human mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene. Biochem Biophys Res Commun. 2005;330(3):633–640. doi: 10.1016/j.bbrc.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 13.Kern S, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 14.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82(4):583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 15.Rubinstein P, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92(22):10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyrsch A, et al. Umbilical cord blood from preterm human fetuses is rich in committed and primitive hematopoietic progenitors with high proliferative and self-renewal capacity. Exp Hematol. 1999;27(8):1338–1345. doi: 10.1016/s0301-472x(99)00059-4. [DOI] [PubMed] [Google Scholar]
- 17.Prindull G, et al. CFU-F circulating in cord blood. Blut. 1987;54(6):351–359. doi: 10.1007/BF00626017. [DOI] [PubMed] [Google Scholar]
- 18.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin HS, et al. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7(11):581–588. doi: 10.1053/bbmt.2001.v7.pm11760145. [DOI] [PubMed] [Google Scholar]
- 20.Chang YJ, et al. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006;24(3):679–685. doi: 10.1634/stemcells.2004-0308. [DOI] [PubMed] [Google Scholar]
- 21.Bieback K, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22(4):625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 22.Lee OK, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 23.Momin EN, et al. The Oncogenic Potential of Mesenchymal Stem Cells in the Treatment of Cancer: Directions for Future Research. Curr Immunol Rev. 2010;6(2):137–148. doi: 10.2174/157339510791111718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imitola J, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamizo A, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65(8):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 26.Son BR, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24(5):1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- 27.Song M, et al. MRI tracking of intravenously transplanted human neural stem cells in rat focal ischemia model. Neurosci Res. 2009;64(2):235–239. doi: 10.1016/j.neures.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Barrilleaux BL, et al. Activation of CD74 inhibits migration of human mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2010;46(6):566–572. doi: 10.1007/s11626-010-9279-1. [DOI] [PubMed] [Google Scholar]
- 29.Francois S, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24(4):1020–1029. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 30.Aboody KS, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97(23):12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasportas LS, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci U S A. 2009;106(12):4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei J, et al. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell. 2004;5(5):477–488. doi: 10.1016/s1535-6108(04)00116-3. [DOI] [PubMed] [Google Scholar]
- 33.Maestroni GJ, Hertens E, Galli P. FactoRs) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci. 1999;55(4):663–667. doi: 10.1007/s000180050322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura K, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11(14):1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 35.Qiao C, et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32(1):8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Qiao L, et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18(4):500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 37.Khakoo AY, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203(5):1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otsu K, et al. Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 2009;113(18):4197–4205. doi: 10.1182/blood-2008-09-176198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada H, Pollack IF. Cytokine gene therapy for malignant glioma. Expert Opin Biol Ther. 2004;4(10):1609–1620. doi: 10.1517/14712598.4.10.1609. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, et al. A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol Ther. 2008;16(4):749–756. doi: 10.1038/mt.2008.3. [DOI] [PubMed] [Google Scholar]
- 41.Xu X, et al. Evaluating dual activity LPA receptor pan-antagonist/autotaxin inhibitors as anticancer agents in vivo using engineered human tumors. Prostaglandins Other Lipid Mediat. 2009;89(3–4):140–146. doi: 10.1016/j.prostaglandins.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao P, et al. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. 2010;290(2):157–166. doi: 10.1016/j.canlet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 43.Seo SH, et al. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther. 2011 doi: 10.1038/gt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagg J, et al. Marrow stromal cells for interleukin-2 delivery in cancer immunotherapy. Hum Gene Ther. 2004;15(6):597–608. doi: 10.1089/104303404323142042. [DOI] [PubMed] [Google Scholar]
- 45.Gunnarsson S, et al. Intratumoral IL-7 delivery by mesenchymal stromal cells potentiates IFNgamma-transduced tumor cell immunotherapy of experimental glioma. J Neuroimmunol. 2010;218(1–2):140–144. doi: 10.1016/j.jneuroim.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Xin H, et al. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25(7):1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 47.Xin H, et al. Intratracheal delivery of CX3CL1-expressing mesenchymal stem cells to multiple lung tumors. Mol Med. 2009;15(9–10):321–327. doi: 10.2119/molmed.2009.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chawla-Sarkar M, Leaman DW, Borden EC. Preferential induction of apoptosis by interferon (IFN)-beta compared with IFN-alpha2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res. 2001;7(6):1821–1831. [PubMed] [Google Scholar]
- 49.Johns TG, et al. Antiproliferative potencies of interferons on melanoma cell lines and xenografts: higher efficacy of interferon beta. J Natl Cancer Inst. 1992;84(15):1185–1190. doi: 10.1093/jnci/84.15.1185. [DOI] [PubMed] [Google Scholar]
- 50.Wong VL, et al. Growth-inhibitory activity of interferon-beta against human colorectal carcinoma cell lines. Int J Cancer. 1989;43(3):526–530. doi: 10.1002/ijc.2910430331. [DOI] [PubMed] [Google Scholar]
- 51.Studeny M, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–3608. [PubMed] [Google Scholar]
- 52.Studeny M, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeteddelivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96(21):1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 53.Ren C, et al. Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells. 2008;26(9):2332–2338. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ren C, et al. Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther. 2008;15(21):1446–1453. doi: 10.1038/gt.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grander D, Einhorn S. Interferon and malignant disease--how does it work and why doesn't it always? Acta Oncol. 1998;37(4):331–338. doi: 10.1080/028418698430548. [DOI] [PubMed] [Google Scholar]
- 56.Lens M, et al. Cutaneous melanoma: interferon alpha adjuvant therapy for patients at high risk for recurrent disease. Dermatol Ther. 2006;19(1):9–18. doi: 10.1111/j.1529-8019.2005.00051.x. [DOI] [PubMed] [Google Scholar]
- 57.Danks MK, et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007;67(1):22–25. doi: 10.1158/0008-5472.CAN-06-3607. [DOI] [PubMed] [Google Scholar]
- 58.Miletic H, et al. Bystander killing of malignant glioma by bone marrow-derived tumor-infiltrating progenitor cells expressing a suicide gene. Mol Ther. 2007;15(7):1373–1381. doi: 10.1038/sj.mt.6300155. [DOI] [PubMed] [Google Scholar]
- 59.Cavarretta IT, et al. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther. 2010;18(1):223–231. doi: 10.1038/mt.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24(52):7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 61.Parato KA, et al. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5(12):965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 62.Nakashima H, Kaur B, Chiocca EA. Directing systemic oncolytic viral delivery to tumors via carrier cells. Cytokine Growth Factor Rev. 2010;21(2–3):119–126. doi: 10.1016/j.cytogfr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Power AT, Bell JC. Cell-based delivery of oncolytic viruses: a new strategic alliance for a biological strike against cancer. Mol Ther. 2007;15(4):660–665. doi: 10.1038/sj.mt.6300098. [DOI] [PubMed] [Google Scholar]
- 64.Pereboeva L, et al. Approaches to utilize mesenchymal progenitor cells as cellular vehicles. Stem Cells. 2003;21(4):389–404. doi: 10.1634/stemcells.21-4-389. [DOI] [PubMed] [Google Scholar]
- 65.Komarova S, et al. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5(3):755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 66.Stoff-Khalili MA, et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat. 2007 doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 67.Sonabend AM, et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26(3):831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 68.Yong RL, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69(23):8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jain RK, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 70.Samant RS, Shevde LA. Recent Advances in Anti-Angiogenic Therapy of Cancer. Oncotarget. 2011;2(3) doi: 10.18632/oncotarget.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kadambi A, et al. Vascular endothelial growth factor (VEGF)-C differentially affects tumor vascular function and leukocyte recruitment: role of VEGF-receptor 2 and host VEGF-A. Cancer Res. 2001;61(6):2404–2408. [PubMed] [Google Scholar]
- 73.Tong RT, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 74.Hormigo A, Gutin PH, Rafii S. Tracking normalization of brain tumor vasculature by magnetic imaging and proangiogenic biomarkers. Cancer Cell. 2007;11(1):6–8. doi: 10.1016/j.ccr.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bexell D, et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17(1):183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bexell D, Scheding S, Bengzon J. Toward brain tumor gene therapy using multipotent mesenchymal stromal cell vectors. Mol Ther. 2010;18(6):1067–1075. doi: 10.1038/mt.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Eekelen M, et al. Human stem cells expressing novel TSP-1 variant have anti-angiogenic effect on brain tumors. Oncogene. 2010;29(22):3185–3195. doi: 10.1038/onc.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256(1):58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- 79.Mueller LP, et al. TRAIL-transduced multipotent mesenchymal stromal cells (TRAIL-MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther. 2010 doi: 10.1038/cgt.2010.68. [DOI] [PubMed] [Google Scholar]
- 80.Kim SK, et al. PEX-producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin Cancer Res. 2005;11(16):5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- 81.Ehtesham M, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62(24):7170–7174. [PubMed] [Google Scholar]
- 82.Ehtesham M, et al. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62(20):5657–5663. [PubMed] [Google Scholar]
- 83.Loebinger MR, et al. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69(10):4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shah K, et al. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005;57(1):34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 85.Shah K, et al. Inducible release of TRAIL fusion proteins from a proapoptotic form for tumor therapy. Cancer Res. 2004;64(9):3236–3242. doi: 10.1158/0008-5472.can-03-3516. [DOI] [PubMed] [Google Scholar]
- 86.Matsumoto K, Nakamura T. NK4 (HGF-antagonist/angiogenesis inhibitor) in cancer biology and therapeutics. Cancer Sci. 2003;94(4):321–327. doi: 10.1111/j.1349-7006.2003.tb01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao Y, et al. Molecular mechanisms and therapeutic development of angiogenesis inhibitors. Adv Cancer Res. 2008;100:113–131. doi: 10.1016/S0065-230X(08)00004-3. [DOI] [PubMed] [Google Scholar]
- 88.Cao Y, Cao R, Hedlund EM. R Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med. 2008;86(7):785–789. doi: 10.1007/s00109-008-0337-z. [DOI] [PubMed] [Google Scholar]
- 89.Kanehira M, et al. Targeted delivery of NK4 to multiple lung tumors by bone marrow-derived mesenchymal stem cells. Cancer Gene Ther. 2007;14(11):894–903. doi: 10.1038/sj.cgt.7701079. [DOI] [PubMed] [Google Scholar]
- 90.Ozdemir V, et al. Shifting emphasis from pharmacogenomics to theragnostics. Nat Biotechnol. 2006;24(8):942–946. doi: 10.1038/nbt0806-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noble M, et al. Can neural stem cells be used as therapeutic vehicles in the treatment of brain tumors? Nat Med. 2000;6(4):369–370. doi: 10.1038/74610. [DOI] [PubMed] [Google Scholar]
- 92.Calin GA, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65(14):6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 94.Corsten MF, et al. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer Res. 2007;67(19):8994–9000. doi: 10.1158/0008-5472.CAN-07-1045. [DOI] [PubMed] [Google Scholar]
- 95.Bagci-Onder T, et al. A dual PI3K/mTOR inhibitor, PI–103, cooperates with stem cell delivered TRAIL in experimental glioma models. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- 96.Mohr A, et al. Targeting of XIAP combined with systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand inhibits metastatic growth of pancreatic carcinoma cells. Stem Cells. 2010;28(11):2109–2120. doi: 10.1002/stem.533. [DOI] [PubMed] [Google Scholar]
- 97.Kim SM, et al. Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells. 2010;28(12):2217–2228. doi: 10.1002/stem.543. [DOI] [PubMed] [Google Scholar]
- 98.Morris PJ. Immunoprotection of therapeutic cell transplants by encapsulation. Trends Biotechnol. 1996;14(5):163–167. doi: 10.1016/0167-7799(96)10020-2. [DOI] [PubMed] [Google Scholar]
- 99.Rihova B, et al. Immunocompatibility and biocompatibility of cell delivery systems. Adv Drug Deliv Rev. 2000;42(1–2):65–80. doi: 10.1016/s0169-409x(00)00054-5. [DOI] [PubMed] [Google Scholar]
- 100.Pan L, et al. Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. J Neurosci Res. 2009;87(14):3207–3220. doi: 10.1002/jnr.22142. [DOI] [PubMed] [Google Scholar]
- 101.Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat Biotechnol. 2002;20(11):1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- 102.Teng YD, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cui FZ, et al. Hyaluronic acid hydrogel immobilized with RGD peptides for brain tissue engineering. J Mater Sci Mater Med. 2006;17(12):1393–1401. doi: 10.1007/s10856-006-0615-7. [DOI] [PubMed] [Google Scholar]
- 104.Ma W, et al. CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp Neurol. 2004;190(2):276–288. doi: 10.1016/j.expneurol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 105.Potter W, Kalil RE, Kao WJ. Biomimetic material systems for neural progenitor cell-based therapy. Front Biosci. 2008;13:806–821. doi: 10.2741/2721. [DOI] [PubMed] [Google Scholar]
- 106.Ding HF, et al. Biologic effect and immunoisolating behavior of BMP-2 gene-transfected bone marrow-derived mesenchymal stem cells in APA microcapsules. Biochem Biophys Res Commun. 2007;362(4):923–927. doi: 10.1016/j.bbrc.2007.08.094. [DOI] [PubMed] [Google Scholar]
- 107.Babister JC, et al. Genetic manipulation of human mesenchymal progenitors to promote chondrogenesis using "bead-in-bead" polysaccharide capsules. Biomaterials. 2008;29(1):58–65. doi: 10.1016/j.biomaterials.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 108.Eliopoulos N, et al. Human-compatible collagen matrix for prolonged and reversible systemic delivery of erythropoietin in mice from gene-modified marrow stromal cells. Mol Ther. 2004;10(4):741–748. doi: 10.1016/j.ymthe.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 109.Eliopoulos N, et al. Neo-organoid of marrow mesenchymal stromal cells secreting interleukin-12 for breast cancer therapy. Cancer Res. 2008;68(12):4810–4818. doi: 10.1158/0008-5472.CAN-08-0160. [DOI] [PubMed] [Google Scholar]
- 110.Hingtgen SD, et al. A novel molecule integrating therapeutic and diagnostic activities reveals multiple aspects of stem cell-based therapy. Stem Cells. 2010;28(4):832–841. doi: 10.1002/stem.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shah K, et al. Imaging neural stem cell fate in mouse model of glioma. Chapter 5. Curr Protoc Stem Cell Biol. 2009;(Unit 5A 1) doi: 10.1002/9780470151808.sc05a01s8. [DOI] [PubMed] [Google Scholar]
- 112.Shah K, et al. Novel bimodal viral vectors and in vivo imaging reveal the fate of human neural stem cells in experimental glioma model. J Neurosci. 2007 doi: 10.1523/JNEUROSCI.0296-08.2008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang Y, et al. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum Gene Ther. 2003;14(13):1247–1254. doi: 10.1089/104303403767740786. [DOI] [PubMed] [Google Scholar]
- 114.Maeurer MJ, et al. Host immune response in renal cell cancer: interleukin-4 (IL-4) and IL-10 mRNA are frequently detected in freshly collected tumor-infiltrating lymphocytes. Cancer Immunol Immunother. 1995;41(2):111–121. doi: 10.1007/BF01527407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vieweg J, et al. Reversal of tumor-mediated immunosuppression. Clin Cancer Res. 2007;13(2 Pt 2):727s–732s. doi: 10.1158/1078-0432.CCR-06-1924. [DOI] [PubMed] [Google Scholar]
- 116.Hall B, et al. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86(1):8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 117.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 118.Lu YR, et al. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 2008;7(2):245–251. doi: 10.4161/cbt.7.2.5296. [DOI] [PubMed] [Google Scholar]
- 119.Yu JM, et al. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008;17(3):463–473. doi: 10.1089/scd.2007.0181. [DOI] [PubMed] [Google Scholar]
- 120.Brekke C, et al. Cellular multiparametric MRI of neural stem cell therapy in a rat glioma model. Neuroimage. 2007;37(3):769–782. doi: 10.1016/j.neuroimage.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 121.Delcroix GJ, et al. Mesenchymal and neural stem cells labeled with HEDP-coated SPIO nanoparticles: in vitro characterization and migration potential in rat brain. Brain Res. 2009;1255:18–31. doi: 10.1016/j.brainres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 122.Sweeney TJ, et al. Visualizing the kinetics of tumor-cell clearance in living animals. Proc Natl Acad Sci U S A. 1999;96(21):12044–12049. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Waerzeggers Y, et al. Multimodal imaging of neural progenitor cell fate in rodents. Mol Imaging. 2008;7(2):77–91. [PubMed] [Google Scholar]
- 124.Zhu W, et al. Superparamagnetic iron oxide labeling of neural stem cells and 4.7T MRI tracking in vivo and in vitro. J Huazhong Univ Sci Technolog Med Sci. 2007;27(1):107–110. doi: 10.1007/s11596-007-0130-1. [DOI] [PubMed] [Google Scholar]
- 125.Roelants V, et al. Comparison between adenoviral and retroviral vectors for the transduction of the thymidine kinase PET reporter gene in rat mesenchymal stem cells. J Nucl Med. 2008;49(11):1836–1844. doi: 10.2967/jnumed.108.052175. [DOI] [PubMed] [Google Scholar]
- 126.Willmann JK, et al. Imaging gene expression in human mesenchymal stem cells: from small to large animals. Radiology. 2009;252(1):117–127. doi: 10.1148/radiol.2513081616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dwyer RM, et al. Mesenchymal Stem Cell (Msc) Mediated Delivery of the Sodium Iodide Symporter (Nis) Supports Radionuclide Imaging and Treatment of Breast Cancer. Stem Cells. 2011 doi: 10.1002/stem.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Knoop K, et al. Image-guided, Tumor Stroma-targeted (131)I Therapy of Hepatocellular Cancer After Systemic Mesenchymal Stem Cell-mediated NIS Gene Delivery. Mol Ther. 2011 doi: 10.1038/mt.2011.93. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ikeda N, et al. Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke. 2005;36(12):2725–2730. doi: 10.1161/01.STR.0000190006.88896.d3. [DOI] [PubMed] [Google Scholar]
- 130.Kurozumi K, et al. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9(2):189–197. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 131.Li Y, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59(4):514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 132.Liu H, et al. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129(Pt 10):2734–2745. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]