Abstract
Aim: The aim of this study was to detect endothelial nitric oxide synthase (eNOS) Glu298Asp gene variants in a random sample of the Egyptian population, compare it with those from other populations, and attempt to correlate these variants with serum levels of nitric oxide (NO). The association of eNOS genotypes or serum NO levels with the incidence of acute myocardial infarction (AMI) was also examined. Methods: One hundred one unrelated healthy subjects and 104 unrelated AMI patients were recruited randomly from the 57357 Hospital and intensive care units of El Demerdash Hospital and National Heart Institute, Cairo, Egypt. eNOS genotypes were determined by polymerase chain reaction–restriction fragment length polymorphism. Serum NO was determined spectrophotometrically. Results: The genotype distribution of eNOS Glu298Asp polymorphism determined for our sample was 58.42% GG (wild type), 33.66% GT, and 7.92% TT genotypes while allele frequencies were 75.25% and 24.75% for G and T alleles, respectively. No significant association between serum NO and specific eNOS genotype could be detected. No significant correlation between eNOS genotype distribution or allele frequencies and the incidence of AMI was observed. Conclusion: The present study demonstrated the predominance of the homozygous genotype GG over the heterozygous GT and homozygous TT in random samples of Egyptian population. It also showed the lack of association between eNOS genotypes and mean serum levels of NO, as well as the incidence of AMI.
Introduction
Since its identification as the endothelium-derived relaxing factor in 1987 (Ignarro et al., 1987), nitric oxide (NO) has rapidly gained fame as among the most important signaling molecules in the cardiovascular system. NO is produced in the endothelium by the enzyme endothelial nitric oxide synthase (eNOS) and is involved in signaling for vasorelaxation, platelet aggregation, proliferation of vascular smooth muscle cells, and other mechanisms of cardiovascular homeostasis (Strijdom et al., 2009). Several polymorphisms have been identified in the eNOS gene, but the Glu298Asp (rs1799983) polymorphism in exon 7 was the only common variation that leads to amino acid substitution in the mature protein (Hingorani et al., 1999). In this polymorphism the guanine at position 894 is substituted by thymine leading to a change in the amino acid at position 298 from glutamate to aspartate. A study suggested a functional effect of the Glu298Asp polymorphism (Casas et al., 2006).
Several reports described the genotype distribution of eNOS Glu298Asp polymorphism among different populations. However, few were focused on Egyptians utilizing only a limited number of cardiovascular patients (Nagib El-Kilany et al., 2004; Motawi et al., 2011). The present study aimed to detect eNOS Glu298Asp gene polymorphisms in a random sample of the Egyptian population, to compare eNOS genotype distribution in Egyptians with those from other populations, and to compare eNOS Glu298Asp genotypes and serum levels of NO of healthy controls with those of myocardial infarction (MI) Egyptian patients, and to explore functional correlations of eNOS genotypes with serum levels of NO.
Materials and Methods
Study population
One hundred one random, unrelated healthy subjects were recruited from the volunteers attending the blood bank at the 57357 Hospital in Cairo, Egypt. The number of female volunteers was 32 (age range 18 and 62 years) and men was 69 (age range 19 and 54 years). On the other hand, 104 random, unrelated MI patients (35 women, with an age range 34 and 55 years, and 69 men between 35 and 55 years) were recruited from the intensive care unit of El Demerdash Hospital, Cairo, Egypt, and the National Heart Institute, Imbaba, Giza. Patients were included if they had a diagnosis of an acute single or multivessel coronary artery disease (CAD) verified by clinical presentation, electrocardiogram changes, and/or cardiac marker elevation.
Written informed consent was obtained from all participants in the study. Information on personal and family medical history and health-relevant behaviors, including exercise and diet, were obtained by a routine questionnaire filled in by blood donors at the time of venesection. Exclusion criteria for both groups included any concomitant acute or chronic severe diseases, such as renal failure, hepatic insufficiency, or diabetes mellitus.
Specimen collection
Fasting blood samples (4 mL) were collected into two sets of tubes: the first set was ethylenediaminetetraacetic acid (EDTA)–coated vacuum tubes stored at 4°C for DNA extraction; the second set was non-EDTA–coated vacuum tubes. After centrifugation for 10 min at 1000 rpm, serum was stored at −20°C in 0.25 mL aliquots. These aliquots were used for NO determination.
Purification of DNA from human blood by spin protocol
DNA extraction and purification was performed using QIAamp DNA Blood Mini Kits (Qiagen, Hilden, Germany). The purified DNA was free of protein, nucleases, and other contaminants or inhibitors and was used directly in the polymerase chain reaction (PCR) (Greenspoon et al., 1998; Fahle and Fischer, 2000).
Screening for Glu298Asp variant of the eNOS gene by PCR-restriction fragment length polymorphism
The presence of the missense Glu298Asp variant was determined by PCR-restriction fragment length polymorphism analysis. A set of primers was designed to amplify the 457–base pair (bp) fragment encompassing the missense Glu298Asp variant (the forward and reverse primers 5′-TGAGGGTCACACAGGTTCCT-3′ and 5′-TCCCTGAGGAGGGCATGAGGCT-3′, respectively). The produced PCR fragments were digested with the restriction enzyme Ban II, separated by 2% agarose gel electrophoresis, and visualized by ethidium bromide staining. The mutant allele (T) has no Ban II cutting site while the wild-type allele (G) has a Ban II cutting site producing two DNA fragments, 320- and 137-bp (Shimasaki et al., 1998). A representative example of a gel showing different Glu298Asp variants is shown in Figure 1.
FIG. 1.
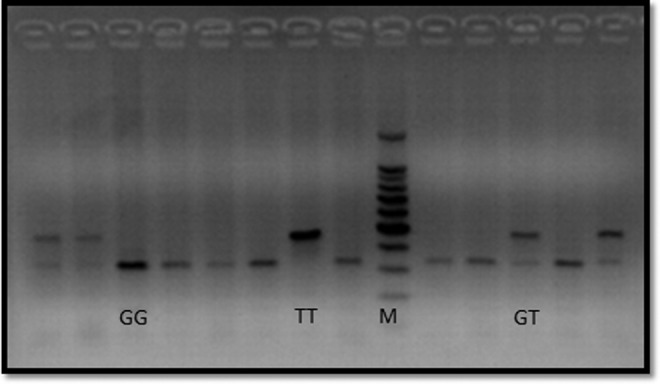
A representative gel of outcomes of eNOS polymorphisms. The PCR amplification products were digested with 10 U of the restriction endonuclease Ban II, before loading onto a 2% low melting agarose gel. M, 100-bp DNA ladder marker. eNOS, endothelial nitric oxide synthase; PCR, polymerase chain reaction.
Assay of serum NO
The determination of serum NO was based on the initial conversion of nitrate to nitrite by vanadium (III) chloride (VCl3) (Cox, 1980) followed by colorimetric detection of nitrite as an azo dye product of Griess (Bryan and Grisham, 2007). The Griess reaction depends on a two-step diazotization reaction in which acidified NO2− produces a nitrosylating agent that reacts with sulfanilic acid to produce the diazonium ion. This ion is then coupled to N-1-naphthyl-ethylenediamine to form a chromophoric azo-derivative that absorbs light at 540 nm.
Statistical analysis
Statistical analyses were performed using SPSS (originally, Statistical Package for the Social Sciences) version 16.0. Variables in two or three groups were compared using the Mann–Whitney U-test or the Kruskal–Wallis test. Testing correlations were done by the Spearman test. Statistical significance was accepted at p<0.05.
Results
Control population
eNOS Glu298Asp genotypes
This study included 101 healthy random volunteers divided into 32 female and 69 male subjects. The genotype distribution and allele frequencies of eNOS genotypes among all, female, and male subjects are summarized in Table 1. No significant differences in the eNOS genotype distribution pattern (Mann–Whitney test, p=0.1209) or in the allele frequencies (Mann–Whitney test, p=0.0909) between female and male subjects were observed.
Table 1.
Genotype Distribution and Allele Frequencies of Endothelial Nitric Oxide Synthase Glu298Asp and Serum Nitric Oxide Levels Among All, Male, and Female Control Subjects
| All subjects (n=101) | Male subjects (n=69) | Female subjects (n=32) | ||
|---|---|---|---|---|
| Agea | 28.6+0.92 | 28.4+0.96 | 29.0+2.0 | |
| Genotype distribution | GG (%) | 59 (58.4%) | 37 (53.6%) | 22 (68.8%) |
| GT (%) | 34 (33.7%) | 25 (36.2%) | 9 (28.1%) | |
| TT (%) | 8 (7.9%) | 7 (10.2%) | 1 (3.1%) | |
| Allele frequencies | G (%) | 152 (75.3%) | 99 (71.7%) | 53 (82.8%) |
| T (%) | 50 (24.7%) | 39 (28.3%) | 11 (17.2%) | |
| Serum NO (μM)a | 30.3+1.4 | 30.0+1.5 | 30.8+2.8 |
Results are mean±SEM.
NO, nitric oxide; SEM, standard error of the mean.
Serum NO levels
A comparison between the mean serum NO concentrations of female versus male subjects revealed a nonsignificant difference (Mann–Whitney test, p=0.8267; Table 1). Also no statistical significance was detected when comparing serum NO concentrations of the different age groups (<20, 21–30, 31–40, and >40 years old) in all subjects or in women versus men using one-way analysis of variance or Tukey's multiple comparison tests (p≥0.05; data are not shown).
Association of serum NO levels and eNOS Glu298Asp genotypes
Table 2 summarizes the mean serum concentrations of NO for various eNOS genotypes. The mean serum NO concentration in the subjects with the TT genotype appeared to be slightly higher relative to those in either GT or GG, but on comparing the serum NO concentrations among different genotypes using the Kruskal–Wallis, no significant difference was found (p=0.50). Dunn's multiple comparison tests also did not show any significant difference between any of the genotypes among either male or female subjects (all p-values >0.05). The same observations can also be applied for the results of dominant and recessive models of eNOS Glu298Asp genotypes (Table 2).
Table 2.
Mean Serum Nitric Oxide Concentrations for Various Endothelial Nitric Oxide Synthase Glu298Asp Genotypes in Control Subjects
| eNOS genotype | Serum NO concentrations (μM) | p-Value | |
|---|---|---|---|
| GG (n=59) | 29.5+1.8 | 0.50 | |
| GT (n=34) | 30.6+2.4 | ||
| TT (n=8) | 34.8+5.0 | ||
| Dominant model | GG + GT (n=93) | 29.9+1.4 | 0.26 |
| TT (n=8) | 34.8+5.0 | ||
| Recessive model | GG (n=59) | 30.3+1.4 | 0.52 |
| GT + TT (n=42) | 31.4+2.1 |
eNOS, endothelial nitric oxide synthase.
MI patients versus controls
eNOS Glu298Asp genotypes
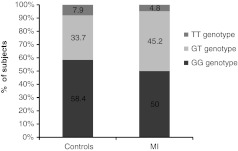
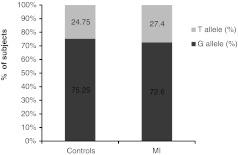
Comparisons of the genotype distribution and allele frequencies of eNOS genotypes in control subjects and acute myocardial infarction (AMI) patients are displayed in Figures 2 and 3, respectively. No significant difference in the eNOS genotype distribution pattern (Mann–Whitney test, p=0.3665) or in the allele frequencies (Mann–Whitney test, p=0.5420) was observed.
FIG. 2.
Genotype distribution pattern of eNOS Glu298Asp gene among control (healthy) subjects and MI patients. MI, myocardial infarction.
FIG. 3.
Allele frequencies of eNOS Glu298Asp gene in control (healthy) subjects and MI patients.
Serum NO levels
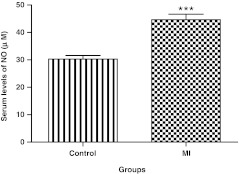
The results displayed in Figure 4 showed that MI patients had a significant 47.6% increase in serum NO levels as compared with the control subjects (Mann–Whitney test, p≤0.0001).
FIG. 4.
Serum levels of NO in the control group and MI patients. Results are expressed as mean±SEM. ***Significant difference from the control group at p≤0.0001. NO, nitric oxide; SEM, standard error of the mean.
Association of serum NO levels and eNOS Glu298Asp genotypes
Similar to results in controls, no significant correlation between serum NO levels and eNOS Glu298Asp genotypes in MI patients was obtained (data not shown).
Discussion
The Glu298Asp polymorphism is the only coding region variant identified in eNOS, and mechanistic studies indicate a functional effect of this substitution (Casas et al., 2006). A meta-analysis of the Glu298Asp polymorphism in 19 different population studies (9252 subjects) reported that the wild-type GG is the predominant genotype representing 67%, while the GT and TT genotypes are present in 28% and 4% of the subjects, respectively (Zintzaras et al., 2006).
In the present study, it was found that the wild-type GG genotype is prevalent in 58.4% of the control subjects while GT and TT are present in 33.7% and 7.9%, respectively. The allele frequencies of the G and T alleles were 75.3% and 24.7%, respectively. An earlier study conducted using only 10 Egyptian healthy subjects showed genotype frequencies of GG (50%), GT (40%), and TT (10%) (Nagib El-Kilany et al., 2004).
These results are generally comparable to a study on healthy Caucasians (n=171), which showed that the GG is the genotype found in highest frequency (50.3%), the GT frequency was 39.6%, and the TT was 8.2% (Walch et al., 2008). Analogous genotype distributions were also demonstrated in other studies for populations of European origin: Germany (n=190; GG 50.5%, GT 40%, and TT 9.5%) (Krex et al., 2006), Turkish (n=150; GG 49.3%, GT 41.3%, and TT 9.3%) (Afrasyap and Ozturk, 2004), English (n=331; GG 47.8%, GT 42%, and TT 10.2%) (Hingorani et al., 1999), and in the European HapMap-CEU study (n=120; GG 40.0%, GT 51.7%, and TT 8.3%). The allele frequencies in all these studies ranged from 65.8% to 71.1% for the G allele and from 29.0% to 34.2% for the T allele.
A remarkably different genotype distribution appeared in Asians where the wild-type GG predominates in around 75% of the population while the homozygous Asp variant (TT genotype) is nearly absent. Representative examples of studies in Japan (n=513; GG 84.4%, GT 17.4%, and TT 0%) (Kato et al., 1999), Korea (n=411; GG 97.6%, GT 19.5%, and TT 0.9%) (Moon et al., 2002), and in the HapMap projects (HapMap-HCP: n=90; GG 77.8%, GT 22.2%, and TT 0%) and (HapMap-JPT: n=88; GG 86.4%, GT 13.6%, and TT 0%). Our genotyping results were also distant from those of black African population: South African (n=42; GG 78.6%, GT 19.0%, and TT 2.4%) (Hillermann et al., 2005) and African Americans (n=60; GG 70.4%, GT 23.9%, and TT 5.6%) (Li et al., 2004).
As for the MI patients in the present study, it was found that the wild-type GG genotype is prevalent in 50% of the MI patients while GT and TT are present in 45.2% and 4.8%, respectively. The allele frequencies of the G and T alleles were 72.6% and 27.4%, respectively, showing nonsignificant differences in the eNOS genotype or allele distribution pattern between the control (i.e., healthy) subjects and the MI patients.
Our results positively coincide with the results of a study made by Aras et al. (2002) who found that the eNOS gene polymorphism has no significant effect on the risk and extent of CAD in the Turkish population where the eNOS (GG, GT, and TT) genotypes were present in 60 (51.3%), 48 (41.0%), and 9 (7.7%) of the 117 healthy control subjects; in 89 (43.4%), 87 (42.4%), and 29 (14.2%) of the CAD patients; and in 43 (53.8%), 28 (35.0%), and 5 (11.2%) of the 80 premature MI patients, respectively. Moreover, Jeerooburkhan et al. (2001) reported that Glu2983Asp polymorphism does not influence the risk of ischemic heart disease in a large cohort of middle-aged British men. In an Australian population, Cai et al. (1999) and Liyou et al. (1998) found no association of eNOS Glu298Asp polymorphism with the occurrence or severity of CAD or number of significantly stenosed vessels in white Australians. Similarly, in the ECTIM study (INSERM, Paris, France), investigators found no association of eNOS Glu298Asp polymorphism with MI in a case–control study of 531 MI patients and 610 control subjects recruited in France and Northern Ireland (Poirier et al., 1999). Also, there was no association in the case of the Taiwanese population (Wang et al., 2001) and the Austrian population (Schmoelzer et al., 2003).
In contrast, Hingorani et al. (1999) observed an excess of homozygotes for the Asp298 variant among patients with angiograph-proven CAD. In a case–control study with Japanese individuals, Shimasaki et al. (1998) observed an association of the Glu298Asp gene variation with the risk of MI; they found that eNOS Glu298Asp T allele carriers had a 1.7-fold increased risk of an MI. In addition Chang et al. (2003) and Kerkeni et al. (2006) found a significant correlation between this polymorphism and CAD. Our results are also in contrast with those of Motawi et al. (2011) who found higher frequency of Glu298Asp polymorphism in the Egyptian CAD group as compared with controls.
These conflicting findings may be due in part to differences in the number and populations studied and different methods of case ascertainment (Spence et al., 2004). These findings further support the previously reported role of ethnicity in determining the prevalence of genetic polymorphisms and their subsequent putative impacts in a given population (Meroufel et al., 2009).
Interest in the measurement of serum NO concentration is increasing since it has been reported that NO levels are influenced by several diseases including diabetes, heart failure, sepsis, and liver cirrhosis; however, little is known about the normal range and the physiological changes of serum NO concentrations in healthy population (Ghasemi et al., 2008). In the present study, the mean serum NO in healthy controls was 30.3 μM, which is consistent with the mean serum NO reported for the Turkish population, that is, 32.6 μM (Afrasyap and Ozturk, 2004). A large study utilizing 1983 Iranian healthy subjects showed that the mean serum NO was 24.4 μM (Ghasemi et al., 2008). The mean serum NO was 55 μM in Japanese population (Higashino et al., 2007), and 53.11 μM in Korean population (Moon et al., 2002). In African Americans, the mean serum NO concentration was reported to be 8.8 μM in subjects with dominant eNOS genotype (GG) and 9.9 μM in subjects with recessive eNOS genotypes (GT + TT) (Li et al., 2004).
In the present study, there was a lack of association between eNOS genotypes and the serum NO concentrations in control subjects. These results were consistent with studies done on healthy volunteers from the Korean (Moon et al., 2002), Turkish (Afrasyap and Ozturk, 2004), and African American (Li et al., 2004) populations, but inconsistent with one Korean study (Yoon et al., 2000). In the first Korean study (Moon et al., 2002), a lack of association between the eNOS Glu298Asp polymorphism genotypes and the serum NO concentration was evident in 411 Korean subjects. There were also nonsignificant differences in serum NO concentrations between subjects having the wild-type (GG) eNOS genotype and the subjects having genotypes GT or TT. However, in an earlier Korean study (Yoon et al., 2000), plasma NO was significantly dependent on the genotypes of the Glu298Asp polymorphism in 128 healthy subjects with the T-allele carriers having higher plasma NO concentrations. In the Turkish population, the average serum NO concentrations were found to be 30.3 μM, 29.1 μM, and 36.3 μM for GG, GT, and TT genotypes, respectively (Afrasyap and Ozturk, 2004).
Besides, our results showed a significant increase in the serum levels of NO in the MI patients over the control subjects. The reason for this finding maybe attributed to the fact that AMI results in increased myocardial inducible nitric oxide synthase expression and NO production and higher nitrotyrosine levels, leading to myocardial dysfunction and increased mortality (Feng et al., 2001; Jones and Bolli, 2006). There was no association between eNOS genotypes and the serum levels of NO in the AMI patients. These results coincide with those obtained for South Indian AMI patients (Angeline et al., 2010). To evaluate the exact causal relationships between the eNOS polymorphisms and serum NO levels, more studies on gene expression, protein level, the activity of eNOS, and the nature of their dependence on genotypes are required.
In conclusion, the most noticeable findings of this study are as follows:
1. The genotype distribution of eNOS Glu298Asp polymorphism in the random sample of Egyptian population was 58.4% GG (wild type), 33.7% GT, and 7.9% TT genotypes while the allele frequencies were 75.3% and 24.7% for the G and T alleles, respectively. Further confirmatory studies would be required using a larger sample size.
2. The eNOS Glu298Asp genotype distribution pattern in Egyptians appears to be similar to that reported in Caucasians and totally different from that of Asians and black Africans.
3. No sex-related differences in the eNOS Glu298Asp genotype distributions or the allele frequencies were detected in the collected samples.
4. There was a lack of significance between genotypes of eNOS Glu298Asp polymorphism in the control subjects and AMI patients.
5. The mean serum NO concentration in the random sample of Egyptian population was 30.3 μM, which is close to the Turkish population, but lower than Asian population in the reported studies.
6. The mean serum NO concentration in AMI patients is significantly higher than that of the control subjects.
7. No age- or sex-related differences in mean serum NO concentrations were observed in control subjects.
8. There was a lack of association between the genotypes of eNOS Glu298Asp in the studied subjects and their mean serum NO levels.
Acknowledgment
This study was supported by the Science and Technology Development Fund (STDF) grant No. 2951.
Author Disclosure Statement
No competing financial interests exist.
References
- Afrasyap L. Ozturk G. NO level and endothelial NO synthase gene polymorphism (Glu298Asp) in the patients with coronary artery disease from the Turkish population. Acta Biochim Biophys Sin (Shanghai) 2004;36:661–666. doi: 10.1093/abbs/36.10.661. [DOI] [PubMed] [Google Scholar]
- Angeline T. Isabel W. Tsongalis GJ. Endothelial nitric oxide gene polymorphisms, nitric oxide production and coronary artery disease risk in a South Indian population. Exp Mol Pathol. 2010;89:205–208. doi: 10.1016/j.yexmp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Aras O. Hanson NQ. Bakanay SM, et al. Endothelial nitric oxide gene polymorphism (Glu298Asp) is not associated with coronary artery disease in Turkish population. Thromb Haemost. 2002;87:347–349. [PubMed] [Google Scholar]
- Bryan NS. Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H. Wilcken DE. Wang XL. The Glu-2983Asp (894G3T) mutation at exon 7 of the endothelial nitric oxide synthase gene and coronary artery disease. J Mol Med. 1999;77:511–514. doi: 10.1007/s001099900020. [DOI] [PubMed] [Google Scholar]
- Casas JP. Cavalleri GL. Bautista LE, et al. Endothelial nitric oxide synthase gene polymorphisms and cardiovascular disease: a HuGE review. Am J Epidemiol. 2006;164:921–935. doi: 10.1093/aje/kwj302. [DOI] [PubMed] [Google Scholar]
- Chang K. Baek SH. Seung KB, et al. The Glu298Asp polymorphism in the endothelial nitric oxide synthase gene is strongly associated with coronary spasm. Coron Artery Dis. 2003;14:293–299. doi: 10.1097/01.mca.0000073080.69657.71. [DOI] [PubMed] [Google Scholar]
- Cox RD. Determination of nitrate and nitrite at parts per billion level by chemiluminescence. Anal Chem. 1980;52:331–333. [Google Scholar]
- Fahle GA. Fischer SH. Comparison of six commercial DNA extraction kits for recovery of cytomegalovirus DNA from spiked human specimens. J Clin Microbiol. 2000;38:3860–3863. doi: 10.1128/jcm.38.10.3860-3863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q. Lu X. Jones DL, et al. Increased inducible nitric oxide synthase expression contributes to myocardial dysfunction and higher mortality after myocardial infarction in mice. Circulation. 2001;104:700–704. doi: 10.1161/hc3201.092284. [DOI] [PubMed] [Google Scholar]
- Ghasemi A. Zahedi Asl S. Mehrabi Y, et al. Serum nitric oxide metabolite levels in a general healthy population: relation to sex and age. Life Sci. 2008;83:326–331. doi: 10.1016/j.lfs.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Greenspoon SA. Scarpetta MA. Drayton ML, et al. QIAamp spin columns as a method of DNA isolation for forensic casework. J Forensic Sci. 1998;43:1024–1030. [PubMed] [Google Scholar]
- Higashino H. Miya H. Mukai H, et al. Serum nitric oxide metabolite (NO(x)) levels in hypertensive patients at rest: a comparison of age, gender, blood pressure and complications using normotensive controls. Clin Exp Pharmacol Physiol. 2007;34:725–731. doi: 10.1111/j.1440-1681.2007.04617.x. [DOI] [PubMed] [Google Scholar]
- Hillermann R. Carelse K. Gebhardt GS. The Glu298Asp variant of the endothelial nitric oxide synthase gene is associated with an increased risk for abruptio placentae in pre-eclampsia. J Hum Genet. 2005;50:415–419. doi: 10.1007/s10038-005-0270-8. [DOI] [PubMed] [Google Scholar]
- Hingorani AD. Liang CF. Fatibene J, et al. A common variant of the endothelial nitric oxide synthase (Glu298—>Asp) is a major risk factor for coronary artery disease in the UK. Circulation. 1999;100:1515–1520. doi: 10.1161/01.cir.100.14.1515. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ. Buga GM. Wood KS, et al. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerooburkhan N. Jones LC. Bujac S, et al. Genetic and environmental determinants of plasma nitrogen oxides and risk of ischemic heart disease. Hypertension. 2001;38:1054–1061. doi: 10.1161/hy1101.092967. [DOI] [PubMed] [Google Scholar]
- Jones SP. Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kato N. Sugiyama T. Morita H, et al. Lack of evidence for association between the endothelial nitric oxide synthase gene and hypertension. Hypertension. 1999;33:933–936. doi: 10.1161/01.hyp.33.4.933. [DOI] [PubMed] [Google Scholar]
- Kerkeni M. Addad F. Chauffert M, et al. Hyperhomocysteinemia, endothelial nitric oxide synthase polymorphism, and risk of coronary artery disease. Clin Chem. 2006;52:53–58. doi: 10.1373/clinchem.2005.057950. [DOI] [PubMed] [Google Scholar]
- Krex D. Fortun S. Kuhlisch E, et al. The role of endothelial nitric oxide synthase (eNOS) genetic variants in European patients with intracranial aneurysms. J Cereb Blood Flow Metab. 2006;26:1250–1255. doi: 10.1038/sj.jcbfm.9600284. [DOI] [PubMed] [Google Scholar]
- Li R. Lyn D. Lapu-Bula R, et al. Relation of endothelial nitric oxide synthase gene to plasma nitric oxide level, endothelial function, and blood pressure in African Americans. Am J Hypertens. 2004;17:560–567. doi: 10.1016/j.amjhyper.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Liyou N. Simons L. Friedlander Y, et al. Coronary artery disease is not associated with the E298D variant of the constitutive, endothelial nitric oxide synthase gene. Clin Genet. 1998;54:528–529. doi: 10.1111/j.1399-0004.1998.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Meroufel D. Médiène-Benchekor S. Dumont J, et al. Relationship between endothelial nitric oxide synthase gene polymorphisms and the risk of myocardial infarction in the Algerian population. Egypt. J Med Hum Genet. 2009;10:89–95. [Google Scholar]
- Moon J. Yoon S. Kim E, et al. Lack of evidence for contribution of Glu298Asp (G894T) polymorphism of endothelial nitric oxide synthase gene to plasma nitric oxide levels. Thromb Res. 2002;107:129–134. doi: 10.1016/s0049-3848(02)00208-6. [DOI] [PubMed] [Google Scholar]
- Motawi T. Shaker O. Taha M, et al. Endothelial nitric oxide synthase and angiotensinogen gene polymorphism in coronary artery diseases in Egypt. Angiology. 2011;62:191–197. doi: 10.1177/0003319710373094. [DOI] [PubMed] [Google Scholar]
- Nagib El-Kilany GE. Nayel E. Hazzaa S. Nitric oxide synthase gene G298 allele. Is it a marker for microvascular angina in hypertensive patients? Cardiovasc Radiat Med. 2004;5:113–118. doi: 10.1016/j.carrad.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Poirier O. Mao C. Mallet C, et al. Polymorphisms of the endothelial nitric oxide synthase gene: no consistent association with myocardial infarction in the ECTIM study. Eur J Clin Invest. 1999;29:284–290. doi: 10.1046/j.1365-2362.1999.00451.x. [DOI] [PubMed] [Google Scholar]
- Schmoelzer I. Renner W. Paulweber B, et al. Lack of association between the Glu298Asp polymorphism of the endothelial nitric oxide synthase with manifest coronary artery disease, carotid atherosclerosis and forearm vascular reactivity in two Austrian populations. Eur J Clin Invest. 2003;33:191–198. doi: 10.1046/j.1365-2362.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- Shimasaki Y. Yasue H. Yoshimura M, et al. Association of the missense Glu298Asp variant of the endothelial nitric oxide synthase gene with myocardial infarction. J Am Coll Cardiol. 1998;31:1506–1510. doi: 10.1016/s0735-1097(98)00167-3. [DOI] [PubMed] [Google Scholar]
- Spence MS. Paul G. Patterson CC, et al. Endothelial nitric oxide synthase gene polymorphism and ischemic heart disease. Am Heart J. 2004;148:847–851. doi: 10.1016/j.ahj.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Strijdom H. Chamane N. Lochner A. Nitric oxide in the cardiovascular system: a simple molecule with complex actions. Cardiovasc J Afr. 2009;20:303–310. [PMC free article] [PubMed] [Google Scholar]
- Walch K. Kolbus A. Hefler-Frischmuth K. Polymorphisms of the endothelial nitric oxide synthase gene in premenopausal women with polycystic ovary syndrome. Maturitas. 2008;61:256–259. doi: 10.1016/j.maturitas.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Wang CL. Hsu LA. Ko YS, et al. Lack of association between the Glu298Asp variant of the endothelial nitric oxide synthase gene and risk of coronary artery disease among Taiwanese. J Formos Med Assoc. 2001;100:736–740. [PubMed] [Google Scholar]
- Zintzaras E. Kitsios G. Stefanidis I. Endothelial NO synthase gene polymorphisms and hypertension: a meta-analysis. Hypertension. 2006;48:700–710. doi: 10.1161/01.HYP.0000238124.91161.02. [DOI] [PubMed] [Google Scholar]