Abstract
Aims: We performed this retrospective study to evaluate the value of clinicopathological factors and a novel molecular marker stathmin in predicting treatment response to neoadjuvant chemotherapy (NCT) with docetaxel-containing regimens in patients with locally advanced breast cancer. Methods: Fifty-four consecutive locally advanced patients receiving docetaxel-containing NCT between January 2006 and July 2010 in Zhejiang Cancer Hospital were included. The expression levels of estrogen receptor (ER), progesterone receptor (PgR), epidermal growth factor receptor-2 (HER-2), and p53 were detected by immunohistochemistry, while expression of stathmin mRNA was measured by Quanti-Gene assay. Results: The overall clinical objective response (cOR) rate was 75.9% (41/54) in breast. A total of 34 patients (63.0%) experienced pathological OR (pOR), with pathological complete remission (pCR) rate of 20.4% (11/54) in breast and 16.7% (9/54) in both breast and axilla. In univariate analysis, there were associations of pOR in both breast and axilla with age (p=0.054), ER status (p=0.059), subtypes (p=0.062), p53 (p=0.030), and stathmin expression (three terciles) (p=0.039). Mean expression of stathmin in pOR group was 0.410, compared with that in no response group of 0.556 (p=0.051 by Student's t-test). Similarly, a lower expression of stathmin might represent a higher pCR rate (p=0.061). Moreover, the LOWESS smoothing plot showed the same trend, that is, that tumor with a lower level of stathmin expression had a higher probability of response to docetaxel-containing NCT. After multivariate adjustment, both ER and stathmin remained significant with hazard ratio of 4.58 (95% CI: 1.11–18.94, p=0.036) and 2.94 (95% CI: 1.26–6.86, p=0.012), respectively. Conclusions: In conclusion, ER and stathmin were independent predictive factors for NCT with docetaxel-containing regimens.
Introduction
It has been well known that breast cancer is a heterogeneous disease and has differential responses to the same treatment. Chemotherapy resistance is caused by multiple mechanisms, and it is likely that response to a certain chemotherapeutic regimen/drug could be predicted by markers involved in chemoresistant-related mechanisms. Neoadjuvant chemotherapy (NCT) provides an attractive clinical setting to identify predictive markers of chemoresistance. In the last decade many efforts have been made in an attempt to find molecular predictors of response to anticancer agents, however, thus far, only a few markers are successfully identified and effectively utilized in routine clinical practice (Nagasaki and Miki, 2008; Beasley and Olson, 2010).
Docetaxel, a taxane derived from the European yew tree, Taxus baccata, could promote microtubule stabilization and prevent depolymerization, and thus cause cell cycle arrest in the mitotic phase. There is evidence suggesting that negative hormone receptor (HR), overexpression or amplification of epidermal growth factor receptor 2 (HER2), high proliferation activity, and mutated p53 might predict chemo-response (Bertheau et al., 2007; Li et al., 2011; Chen et al., 2011). However, the consensus on biological markers specific to response to taxane-containing regimens has not been established yet. Stathmin is a newly discovered microtubule-destabilizing protein, which plays an important role in regulating microtubule system dynamic balance through phosphorylation and dephosphorylation in the different stages of cell cycle (Cassimeris, 2002). A variety of malignant tumors has high levels of stathmin expression (Jeon et al., 2010). Preclinical experiments have showed that, after downregulation of expression of stathmin, the proliferation of malignant cells could be inhibited; while overexpression of stathmin, which is frequently observed in breast tumors, would interfere with the combination between taxanes and microtubules, resulting in significant reduction in taxane response in breast cancer cells (Alli et al., 2007b). More interestingly, targeting stathmin expression with the RNA interference technique could induce microtubule polymerization and promote G2/M progression, respectively, and consequently recover the response of stathmin-overexpressing breast cancer cells to paclitaxel and vinblastine (Alli et al., 2007a). It remains unclear whether the expression of stathmin in breast cancer can predict response to docetaxel-containing chemotherapy in a clinical setting.
The purpose of this study was to investigate the potential value of stathmin, and other clinicopathological and molecular makers such as age, tumor size before NCT, estrogen receptor (ER), progesterone receptor (PgR), HER2, and p53, in predicting treatment response to neoadjuvant docetaxel-containing chemotherapy in Chinese patients with locally advanced breast cancer.
Materials and Methods
Study patients
The present study included 54 patients with histological confirmation of locally advanced breast cancer without prior systemic or local treatment. All these patients, recruited from January 2006 to July 2010 in Zhejiang Cancer Hospital, were pathologically diagnosed as invasive breast cancer by core needle biopsy, and absence of disseminated disease were measured by bilateral mammogram (bilateral breast magnetic resonance imaging when necessary), chest computed tomography scan, abdominal ultrasound, and bone scan. All the patients provided informed consent for their information to be used for the current research. This study was approved by the Ethical Committee of Zhejiang Cancer Hospital.
The general characteristics of patients are shown in Table 1. The mean age of patients was 48 years (ranging from 33 to 63 years). NCT with docetaxel-containing regimens was administered to all these patients for 2–4 cycles. The regimens included TE (docetaxel 75 mg/m2 on day 1, epirubicin 75 mg/m2 on day 1; every 3 weeks), TEC (docetaxel 75 mg/m2 on day 1, epirubicin 75 mg/m2 on day 1, cyclophosphamide 500 mg/m2 on day 1; every 3 weeks), and TC (docetaxel 75 mg/m2 on day 1, cyclophosphamide 500 mg/m2 on day 1; every 3 weeks). Evaluation of effectiveness of neoadjuvant chemotherapy was performed after 2 cycles. If disease progressed, surgery was subsequently opted; otherwise, NCT with prior regimen continued. After 4 cycles of NCT, all the patients received surgery.
Table 1.
Distribution of Characteristics of Breast Cancer Patients in Different Response Groups
| |
|
Clin. (breast) |
|
Path. (breast) |
|
Path. (breast+axilla) |
|
Path. (breast+axilla) |
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
cOR |
cNR |
|
pOR |
pNR |
|
pOR |
pNR |
|
pCR |
Non-pCR |
|
||||||||
| Characteristics | Total n=54 | n | % | n | % | p | n | % | n | % | p | n | % | n | % | p | n | % | n | % | p |
| Age (years) | 0.340 | 0.091 | 0.054 | 0.068 | |||||||||||||||||
| <50 | 27 | 22 | 81.5 | 5 | 18.5 | 20 | 74.1 | 7 | 25.9 | 19 | 70.4 | 8 | 29.6 | 7 | 25.9 | 20 | 74.1 | ||||
| ≥50 | 27 | 19 | 70.4 | 8 | 29.6 | 14 | 51.9 | 13 | 48.1 | 12 | 44.4 | 15 | 55.6 | 2 | 7.4 | 25 | 92.6 | ||||
| Tumor size (baseline) | 0.949 | 0.432 | 0.653 | 0.512 | |||||||||||||||||
| ≤5cm | 37 | 28 | 75.7 | 9 | 24.3 | 22 | 59.5 | 15 | 40.5 | 22 | 59.5 | 15 | 40.5 | 7 | 18.9 | 30 | 81.1 | ||||
| >5cm | 17 | 13 | 76.5 | 4 | 23.5 | 12 | 70.6 | 5 | 29.4 | 9 | 52.9 | 8 | 47.1 | 2 | 11.8 | 15 | 88.2 | ||||
| ER | 0.401 | 0.017 | 0.059 | 0.083 | |||||||||||||||||
| Negative | 22 | 18 | 81.8 | 4 | 18.2 | 18 | 81.8 | 4 | 18.2 | 16 | 72.7 | 6 | 27.3 | 6 | 27.3 | 16 | 72.7 | ||||
| Positive | 32 | 23 | 71.9 | 9 | 28.1 | 16 | 50.0 | 16 | 50.0 | 15 | 46.9 | 17 | 53.1 | 3 | 9.4 | 29 | 90.6 | ||||
| PgR | 0.198 | 0.325 | 0.456 | 0.903 | |||||||||||||||||
| Negative | 29 | 20 | 69.0 | 9 | 31.0 | 20 | 69.0 | 9 | 31.0 | 18 | 62.1 | 11 | 37.9 | 5 | 17.2 | 24 | 82.8 | ||||
| Positive | 25 | 21 | 84.0 | 4 | 16.0 | 14 | 56.0 | 11 | 44.0 | 13 | 52.0 | 12 | 48.0 | 4 | 16.0 | 21 | 84.0 | ||||
| HER2 | 0.923 | 0.591 | 0.322 | 0.887 | |||||||||||||||||
| Negative | 41 | 31 | 75.6 | 10 | 24.4 | 25 | 61.0 | 16 | 39.0 | 22 | 53.7 | 19 | 46.3 | 7 | 17.1 | 34 | 82.9 | ||||
| Positive | 13 | 10 | 76.9 | 3 | 23.1 | 9 | 69.2 | 4 | 30.8 | 9 | 69.2 | 4 | 30.8 | 2 | 15.4 | 11 | 84.6 | ||||
| IHC-based subtype | 0.778 | 0.048 | 0.062 | 0.404 | |||||||||||||||||
| Luminal-like (HR+) | 33 | 24 | 72.7 | 9 | 27.3 | 17 | 51.5 | 16 | 48.5 | 16 | 48.5 | 17 | 51.5 | 4 | 12.1 | 29 | 87.9 | ||||
| ERBB2+(HR-HER2+) | 6 | 5 | 83.3 | 1 | 16.7 | 6 | 100.0 | 0 | 0.0 | 6 | 100.0 | 0 | 0.0 | 2 | 33.3 | 4 | 66.7 | ||||
| Basal (HR-HER2-) | 15 | 12 | 80.0 | 3 | 20.0 | 11 | 73.3 | 4 | 26.7 | 9 | 60.0 | 6 | 40.0 | 3 | 20.0 | 12 | 80.0 | ||||
| Stathmin expr. | 0.492 | 0.033 | 0.039 | 0.061 | |||||||||||||||||
| First tercile | 18 | 14 | 77.8 | 4 | 22.2 | 13 | 72.2 | 5 | 27.8 | 13 | 72.2 | 5 | 27.8 | 5 | 27.8 | 13 | 72.2 | ||||
| Second tercile | 18 | 15 | 83.3 | 3 | 16.7 | 14 | 77.8 | 4 | 22.2 | 12 | 66.7 | 6 | 33.3 | 4 | 22.2 | 14 | 77.8 | ||||
| Third tercile | 18 | 12 | 66.7 | 6 | 33.3 | 7 | 38.9 | 11 | 61.1 | 6 | 33.3 | 12 | 66.7 | 0 | 0.0 | 18 | 100.0 | ||||
| p53 | 0.100 | 0.018 | 0.030 | 0.438 | |||||||||||||||||
| Negative | 21 | 14 | 66.7 | 7 | 33.3 | 9 | 42.9 | 12 | 57.1 | 8 | 38.1 | 13 | 61.9 | 2 | 9.5 | 19 | 90.5 | ||||
| Positive | 29 | 25 | 86.2 | 4 | 13.8 | 22 | 75.9 | 7 | 24.1 | 20 | 69.0 | 9 | 31.0 | 5 | 17.2 | 24 | 82.8 | ||||
p-value in bold indicates <0.10.
Clin, clinical; Path, pathological; OR, objective response; NR, on response; pCR, pathological complete remission; expr, expression.
Immunohistochemical evaluation and subtype classification
Immunohistochemical (IHC) assessment of ER, PgR, HER2, and p53 was conducted in paraffin-embedded tumor samples before treatment as previously described. HER2 positive was considered as HER2 3+ by IHC or positive on fluorescence in situ hybridization (FISH), whereas cases with IHC 0-1+ or 2+ without FISH detecting were regarded as HER2 negative. According to molecular profile of breast cancer subtypes, the IHC surrogates for three major subtypes was defined as following: luminal-like (ER+ and/or PgR+), ERBB2+ (ER−, PgR−, HER2+), and basal-like (ER−, PgR−, HER2−, also known as triple-negative) (Carey et al., 2006; Yu et al., 2010).
Tissue sample preparation and measurement of stathmin mRNA levels
Quanti-Gene assays (Panomics, Inc., Freemont, CA) were performed to detect mRNA levels of stathmin in paraffin-embedded tissue samples according to the manufacturer's protocol. Cancer tissue was distinguished from normal tissue by hematoxylin-eosin staining, placed into 1.5 mL Eppendorf tube, treated with 200 μL lysate homogenization and 4 μL proteinase K (50 μg/μL), and followed by 65°C constant temperature incubation for 2 h and centrifugation of cytoplasm of cancer cells. The oligonucleotide probe QuantiGene 2.0 kit to detect the stathmin mRNA was designed and synthesized by the Panomics. The detailed procedure of mRNA measurement has been described elsewhere (Eastham et al., 2008). Peptidylprolyl isomerase B (PPIB) as the internal reference control, each sample was assayed for gene copy number of stathmin and PPIB, and the optical density (OD) value of blank solution and positive control of serial dilution concentration. Relative expression of stathmin mRNA=stathmin OD value/PPIB OD value. Considering the expression of stathmin was a continuous variable, we classified the values into three terciles for convenience.
Evaluation of treatment response
RECIST criteria were used to evaluate clinical response to chemotherapy. No clinical evidence of palpable tumors in the breast was defined as a clinical complete remission (cCR). Reduction in greatest tumor diameter of ≥30% was graded as a partial response (PR). An increase in greatest tumor diameter of >20% or appearance of new disease was considered as progressive disease (PD). Tumors that did not meet the criteria for objective PR or PD were considered as stable disease (SD). According to standard of CR, PR, SD, and PD, each patient was assigned into either the clinical objective response (cOR, cOR=cCR+cPR) group, or into the clinical no response (cNR, cNR=SD+PD) group. The Miller-Payne scoring system was used to assess the pathological response of tumor (Silver et al., 2010). Miller–Payne scoring has five grades: score 1, no or minimal reduction in tumor; score 2, up to 30% reduction; score 3, 30% to 90% reduction; score 4, >90% reduction but with some residual invasive (or axillary) disease; score 5, no residual invasive carcinoma (or axillary) metastasis. A score of 3, 4, or 5, with 5 being a pathological complete remission (pCR), was termed as pathological objective response (pOR). The pCR was defined as the absence of invasive tumor in the final surgical breast and axillary lymph nodes sample. Residual ductal carcinoma in situ could be included in the pCR category. A score of 1 or 2 was termed as pathological no response (pNR). Because of small sample size and low statistical power in pCR analysis, we preferred to analyze the prediction value of markers for pOR (vs. pNR) rather than pCR (vs. non-pCR).
Statistics
Chi-square (χ2) test was used to compare difference among categorical variables (performing Fisher's exact test when χ2 test unavailable). Logistic regression analysis was used to determine independent predictive factors for treatment response. Hazard ratio and its 95% confidence intervals were calculated. Student's t-test or the Mann–Whitney test was used to compare continuous variables between two groups. All statistical tests were two-sided and were carried out at significance level of 0.05 using the SPSS statistical software package (version 12.0; SPSS Company, Chicago, IL).
Results
Table 1 shows the detailed information of clinical and pathological response rates. We evaluated the response to NCT using different evaluation standard. The overall cOR rate was 75.9% (41/54), with cCR rate of 31.5% (17/54). A total of 34 patients (63.0%) experienced pOR in breast, with pCR rate of 20.4% (11/54) in breast and 16.7% (9/54) in both breast and axilla.
Table 1 also lists the distribution of various clinical and pathological characteristics by the response outcomes (cOR in breast, pOR in breast, pOR in both breast and axilla, and pCR in both breast and axilla). For clinical response, no clinicopathological factor could predict response to NCT with docetaxel-containing regimen. However, there were strong associations of good pathological response (pOR in breast, or in both breast and axilla) with younger age, negative ER, and ERBB2+ and basal-like subtypes. Of note, IHC-based p53 expression (indicating a mutated p53 protein) was also related to a higher pOR in breast (p=0.018) and in both breast and axilla (p=0.030). More interestingly, despite the small sample size, we observed the borderline associations of pCR with age and ER status (p=0.068 and 0.083, respectively). Because the data for tumor grade were lacking in many cases, we did not include this variable in our analysis.
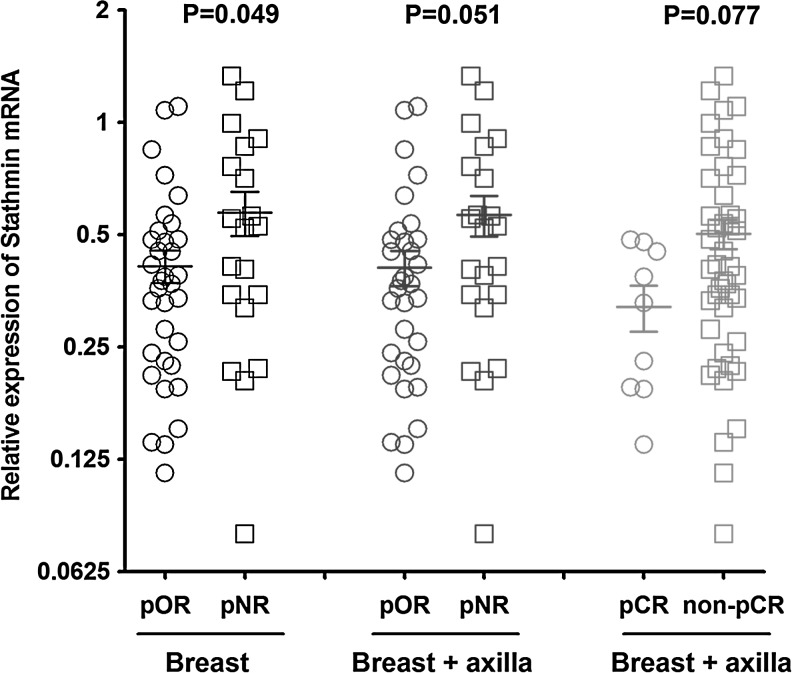
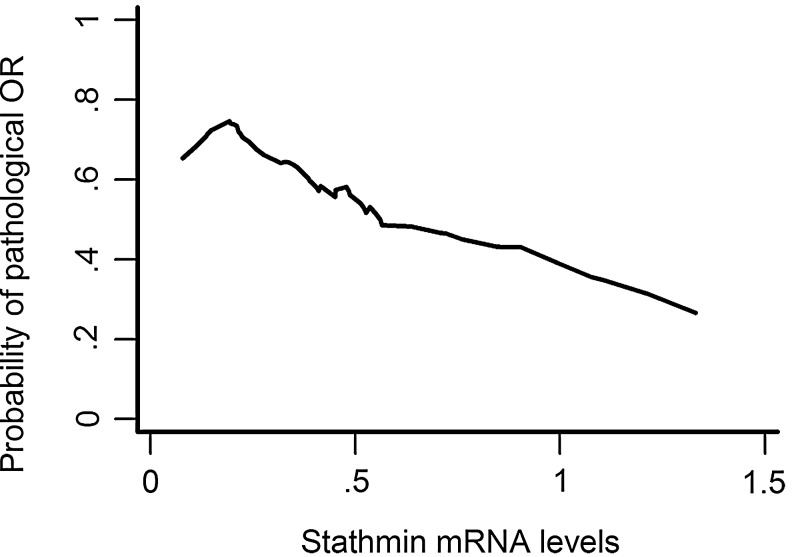
With regard to relationship between expression of stathmin and response to chemotherapy, we observed that lower expression of stathmin was not only significantly related to a higher pOR rate (p=0.033 in breast, and p=0.039 in both breast and axilla) but also tended to predict an increased pCR rate to some extent (p=0.061). Since we arbitrarily divided the expression of stathmin into three categories by three terciles (first/second/third), the association between stathmin expression and tumor response might be observed by chance. To confirm our observations, we further analyzed the distribution of continuous value of stathmin in different pathological response levels (Fig. 1). Mean value of stathmin expression in pOR (in both breast and axilla) group (pCR+pPR) was 0.410 (with standard error of 0.045), compared with that in pNR group (SD+PD) of 0.556 (with standard error of 0.068) (p=0.051 by Student's t-test). Similarly, the tumors with lower expression of stathmin tended to achieve a higher pCR rate (p=0.077 by Mann–Whitney test). Moreover, the LOWESS smoothing plot reconfirmed the prior similar trend, that is, tumor with a lower level of stathmin expression had a higher probability of response to NCT (Fig. 2). Collectively, we proposed that stathmin was a predictor of NCT with docetaxel-containing regimens.
FIG. 1.
Distribution of continuous value of stathmin in different pathological response levels.
FIG. 2.
Relation between continuous stathmin expression and probability of response to NCT based on univariate nonparametric smoothing method (LOWESS smoothing plot). NCT, neoadjuvant chemotherapy.
When multivariate adjustment was performed by putting age (<50 vs. ≥50 years), p53 (IHC-negative vs. positive), ER (IHC-negative vs. positive), and stathmin (first vs. second vs. third tercile) in the logistic regression model, both ER and stathmin were identified as independent predictive factors for NCT, with hazard ratio of 4.58 (95% CI: 1.11 to 18.94, p=0.036) and 2.94 (95% CI: 1.26 to 6.86, p=0.012), respectively (Table 2).
Table 2.
Multivariate Logistic Regression Results
| |
Path. OR (breast) |
Path. OR (breast+axilla) |
||||||
|---|---|---|---|---|---|---|---|---|
| Variables | B | p-Value | HR | 95% CI | B | p-Value | HR | 95% CI |
| Age (<50 vs. >= 50) | 0.50 | 0.494 | 1.65 | 0.39 to 6.97 | 0.91 | 0.209 | 2.48 | 0.60 to 10.18 |
| p53 (Negative vs. Positive) | −1.22 | 0.076 | 0.29 | 0.08 to 1.14 | −0.90 | 0.190 | 0.41 | 0.11 to 1.56 |
| ER (Negative vs. Positive) | 1.86 | 0.029 | 6.44 | 1.21 to 34.19 | 1.52 | 0.036 | 4.58 | 1.11 to 18.94 |
| Stathmin (First vs. Second vs. Third tercile) | 1.13 | 0.021 | 3.10 | 1.18 to 8.14 | 1.08 | 0.012 | 2.94 | 1.26 to 6.86 |
p-value in bold indicates <0.10.
HR, hazard ratio; ER, estrogen receptor.
Discussion
In the present study, we aimed to investigate the value of clinicopathological factors and the novel molecular marker stathmin in predicting response to NCT with docetaxel-containing regimens in locally advanced breast cancer. Four cycles of NCT yielded a pOR (pCR+pPR) rate of 63.0% in breast and a pCR rate of 16.7% in both breast and axilla. Further, patients receiving NCT would achieve a higher pCR rate in those with younger age, negative ER status, ERBB2+ and basal-like subtypes, and IHC-positive p53. We also demonstrated that the lower expression of stathmin corresponded to a higher response rate either in a univariate analysis or in a multivariate analysis.
The evaluation of pathologic response to NCT provides an ideal platform to identify the potential predictive factors of response to anticancer drugs. Outcome of pCR as an intermediate end point could be achieved within weeks after the start of treatment and is therefore an ideal way of comparing anticancer drugs. If achieving a pCR could be predicted even earlier, patients with breast cancer would be saved from having to continue ineffective treatment regimens. Our exploratory study focused on both pOR and pCR rather than on pCR only because of limited case numbers and small events of response.
Many (though not all) investigators suggest that HER2 is an independent predictor of response to taxane-containing NCT (Pritchard et al., 2008). In our study, we did not find a significant correlation between HER2 and pOR or pCR to NCT treatment in a univariate analysis. It might be caused by the small sample size with insufficient statistical power. Although we failed to identify the association between HER2 and chemotherapy, we indeed observed that ERBB2+ subtype rather than HER2+ cancers was an indicator of higher response. In addition, it is interesting that we found that positive p53 immunostaining could well predict response to NCT. Although p53 germ-line mutation is rather infrequent in sporadic and hereditary breast cancer patients, somatically mutated or aberrant p53 occurs at a rate of more than 40% in breast tumor tissues. In the current study, we did not perform p53 mutation detection in breast tumor DNA samples but referred to immunodetectable p53 as mutated p53, since positive p53 immunostaining has been used to indicate p53 mutations that increase protein half-life and lead to intracellular accumulation (Yu et al., 2009). The sensitivity and specificity of IHC for the detection of p53 mutant forms is approximately 80%. Therefore, it is acceptable to treat the immunodetectable p53 results as DNA mutation sequencing results. According to p53 status, we observed a significant association between NCT response and breast cancer with mutated p53 protein. Our results were consistent with previous reports (Di et al., 2004; Bertheau et al., 2007).
Recent studies found that a variety of human tumor tissues showed a relative high expression of stathmin (such as leukemia, lymphoma, breast cancer, gastric cancer, head and neck cancer, liver cancer, and lung cancer) (McGrogan et al., 2008). The downstream target of stathmin protein is microtubule system including tubulin, microtubule, spindle, and other organelles. Stathmin protein can affect anti-microtubule chemotherapy through the following two aspects: (i) changing the combination of chemotherapeutic drugs with microtubules; (ii) arresting tumor cells in G2-M phase (McGrogan et al., 2008). Because the acting sites of stathmin protein and taxane are on the same channel, it is reasonable to infer that the expression of stathmin can be used as indicator of taxane chemosensitivity and efficiency. Alli et al. (2007b) reported that inhibiting stathmin protein expression could increase the sensitivity of breast cancer cells to taxane. McGroga et al. (2008) also concluded that stathmin might present a therapeutic efficiency indicator of taxane in breast cancer. In this study, we displayed that stathmin expression levels were significantly associated with response to chemotherapy, and the result were consistent with most of the previous findings.
In summary, we proposed that ER status and expression of stathmin in tumor were independent predictive factors for NCT with docetaxel-containing regimens; tumor with a lower level of stathmin expression had a higher probability of response to docetaxel-containing NCT. However, our preliminary results need to be validated in other large study cohorts, and the correlation of stathmin with breast cancer prognosis needs further investigation.
Acknowledgments and Funding
This research is supported by grants from Zhejiang Public Welfare Foundation of Applied Research (2010C33017), Zhejiang Provincial Health Department Foundation (2009A028), Zhejiang Provincial Health Department Foundation (2010KY041), and Zhejiang Provincial Education Department Foundation (20061020).
Author Disclosure Statement
No competing financial interests exist.
References
- Alli E. Yang JM. Ford JM, et al. Reversal of stathmin-mediated resistance to paclitaxel and vinblastine in human breast carcinoma cells. Mol Pharmacol. 2007a;71:1233–1240. doi: 10.1124/mol.106.029702. [DOI] [PubMed] [Google Scholar]
- Alli E. Yang JM. Hait WN. Silencing of stathmin induces tumor-suppressor function in breast cancer cell lines harboring mutant p53. Oncogene. 2007b;26:1003–1012. doi: 10.1038/sj.onc.1209864. [DOI] [PubMed] [Google Scholar]
- Beasley GM. Olson JA., Jr What's new in neoadjuvant therapy for breast cancer. Adv Surg. 2010;44:199–228. doi: 10.1016/j.yasu.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Bertheau P. Turpin E. Rickman DS, et al. Exquisite sensitivity of TP53 mutant and basal breast cancers to a dose-dense epirubicin-cyclophosphamide regimen. PLoS Med. 2007;4:e90. doi: 10.1371/journal.pmed.0040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LA. Perou CM. Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14:18–24. doi: 10.1016/s0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- Chen Y. Chen C. Yang B, et al. Estrogen receptor-related genes as an important panel of predictors for breast cancer response to neoadjuvant chemotherapy. Cancer Lett. 2011;302:63–68. doi: 10.1016/j.canlet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Di LA. Chan S. Paesmans M, et al. HER-2/neu as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Breast Cancer Res Treat. 2004;86:197–206. doi: 10.1023/B:BREA.0000036783.88387.47. [DOI] [PubMed] [Google Scholar]
- Eastham LL. Mills CN. Niles RM. PPARalpha/gamma expression and activity in mouse and human melanocytes and melanoma cells. Pharm Res. 2008;25:1327–1333. doi: 10.1007/s11095-007-9524-9. [DOI] [PubMed] [Google Scholar]
- Jeon TY. Han ME. Lee YW, et al. Overexpression of stathmin1 in the diffuse type of gastric cancer and its roles in proliferation and migration of gastric cancer cells. Br J Cancer. 2010;102:710–718. doi: 10.1038/sj.bjc.6605537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XR. Liu M. Zhang YJ, et al. Evaluation of ER, PgR, HER-2, Ki-67, cyclin D1, and nm23-H1 as predictors of pathological complete response to neoadjuvant chemotherapy for locally advanced breast cancer. Med Oncol. 2011;28(Suppl 1):31–38. doi: 10.1007/s12032-010-9676-z. [DOI] [PubMed] [Google Scholar]
- McGrogan BT. Gilmartin B. Carney DN, et al. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Nagasaki K. Miki Y. Molecular prediction of the therapeutic response to neoadjuvant chemotherapy in breast cancer. Breast Cancer. 2008;15:117–120. doi: 10.1007/s12282-008-0031-6. [DOI] [PubMed] [Google Scholar]
- Pritchard KI. Messersmith H. Elavathil L, et al. HER-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol. 2008;26:736–744. doi: 10.1200/JCO.2007.15.4716. [DOI] [PubMed] [Google Scholar]
- Silver DP. Richardson AL. Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KD. Di GH. Yuan WT, et al. Functional polymorphisms, altered gene expression and genetic association link NRH:quinone oxidoreductase 2 to breast cancer with wild-type p53. Hum Mol Genet. 2009;18:2502–2517. doi: 10.1093/hmg/ddp171. [DOI] [PubMed] [Google Scholar]
- Yu KD. Li JJ. Di GH, et al. A straightforward but not piecewise relationship between age and lymph node status in Chinese breast cancer patients. PLoS One. 2010;5:e11035. doi: 10.1371/journal.pone.0011035. [DOI] [PMC free article] [PubMed] [Google Scholar]