Summary
We reviewed the records of eight patients with a dural arteriovenous fistula (DAVF) close to the hypoglossal canal and determined the angioarchitecture of the clinical entity at the anterior condylar confluence.
Eight patients with DAVF received endovascular treatment at our institute over the past five years. Imaging with selective three-dimensional angiography and thin-slice computed tomography were used to identify the fistula and evaluate the drainage pattern.
Based on the angiographic findings, the ascending pharyngeal artery was the main feeder in all cases, and the occipital, middle meningeal, posterior auricular, and posterior meningeal arteries also supplied the DAVF to varying degrees. Contralateral contribution was found in five patients. The main drainage route was the external vertebral plexus via the lateral condylar veins in four patients, the inferior petrosal sinus in three patients, and the internal jugular vein via the connecting emissary veins in one patient. Selective angiography identified the shunt point at the anterior condylar confluence close to the anterior condylar vein. Shunt occlusion with transvenous coil packing was performed in all cases; transarterial feeder embolization was also used in three patients. Two patients treated with tight packing of the anterior condylar vein developed temporary or prolonged hypoglossal palsy.
Based on our results, the main confluence of the shunt is located at the anterior condylar confluence connecting the anterior condylar vein and multiple channels leading to the extracranial venous systems. To avoid postoperative nerve palsy, the side of the anterior condylar vein in the hypoglossal canal should not be densely packed with coils. Evaluating the angioarchitecture using the selective three-dimensional angiography and tomographic imaging greatly helps to determine the target and strategy of endovascular treatment for these DAVF.
Key words: anterior condylar confluence, dural arteriovenous fistula, embolization, hypoglossal canal, hypoglossal nerve paresis
Introduction
Generally, a dural arteriovenous fistula (DAVF) has an arteriovenous (A-V) shunt at the dura or between the dura and skull, and the shunted flow drains into the adjacent dural sinus. The other type of DAVF, which has no sinus connection, such as tentorial, ethmoidal, or spinal DAVF, has direct reflux into the intracranial or intrathecal venous systems.
The nomenclature for the location of the DAVF is usually based on the sinus or the affected veins where the shunted flow concentrates and redistributes to the adjacent veins. These sinuses create a pattern of venous confluence receiving multi-axial venous flow; therefore the shunted flow refluxes into multidirectional veins. A DAVF close to the jugular foramen has been referred to as various entities including the DAVF involving the inferior petrosal sinus1, DAVF of the marginal sinus2,3, hypoglossal DAVF4-6, DAVF of the anterior condylar vein within the hypoglossal canal7, and jugular foramen DAVF8. Although the drainage pattern and the associated symptoms differ for each due to changes in the size of the drainers and occlusion and thrombosis of them, the shunt point is identified in a very limited area. The development of high resolution dimensional techniques for angiography has allowed identification of the shunt point of these DAVF in small venous complex close to the hypoglossal canal. This complex is referred to as the anterior condylar confluence (ACC)9 or petrosal confluence10.
The evaluation of this angioarchitecture greatly aids the identification of the target and strategy of endovascular treatment for these DAVF. Based on analysis of our eight cases and a review of previous reports we propose a new clinical entity, DAVF at the ACC. The anatomic and clinical implications and the pitfalls of treatments are discussed.
Patients
Eight patients with DAVF at the ACC were treated in our university hospital and affiliated hospitals over a five year period (Table 1). Patients included four men and four women (age range: 41 to 80 years; mean 58.8 years). The lesions were located on the left side in six patients and on the right in two. All patients complained of severe tinnitus, which characterizes this disorder. Two patients presented with chemosis and ocular motor palsy and three patients had a mild paresis of the hypoglossal nerve.
Table 1.
| Case | Age/Sex | Symptoms | Arterial supply | Venous drainage | Treatment | Result | Complication |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 80/F | Lt.tinnitus | Lt.APA, OA VA, | Lt.IPS, IJV, ACV, | TVE | Cure | None |
| Rt.APA | LCV | ||||||
|
| |||||||
| 2 | 56/F | Rt.tinnitus, | Rt. APA, VA, | Rt.IPS, IJV, ACV, | TVE+TAE | Cure | None |
| XII & III palsy | Lt. APA, VA | LCV | |||||
|
| |||||||
| 3 | 63/F | Lt.tinnitus, | Lt. APA, ICA, | Lt.IJV, LCV | TAE, TVE+TAE | Cure | None |
| XII palsy | Rt. APA, VA | ||||||
|
| |||||||
| 4 | 68/F | Lt.tinnitus, | Lt. OA, APA, | Lt. LCV, IJV | TVE | Cure | None |
| XII palsy | Rt. APA | ||||||
|
| |||||||
| 5 | 54/M | Lt.tinnitus | Lt. APA, VA | Lt.IJV, ACV, LCV, IPS | TAE, TAE | Cure | None |
|
| |||||||
| 6 | 50/M | Lt.tinnitus | Lt. APA, VA | Lt.ACV | TVE+TAE | Cure | XII palsy |
| (transient) | |||||||
|
| |||||||
| 7 | 41/M | Rt.tinnitus | Rt. APA, VA | Rt.LCV | TVE | Cure | None |
|
| |||||||
| 8 | 58/M | Lt. tinnitus, | Lt. APA, VA, | Lt. IPS, LCV | TVE | Cure | None |
| VI palsy, chemosis | Rt. APA | ||||||
|
| |||||||
| III palsy: oculomotor nerve palsy, XII palsy: hypoglossal nerve palsy, APA: ascending pharyngeal artery, OA: occipital artery, | |||||||
| VA: vertebral artery, IPS: inferior petrosal sinus, IJV: internal jugular vein, ACV: anterior condylar vein, LCV: lateral condylar vein, | |||||||
| PCV: posterior condylar vein | |||||||
Angioarchitecture
The ascending pharyngeal artery was the main feeder in all cases, and the mastoid branches of the occipital artery, middle meningeal artery, and posterior auricular artery also supplied the DAVF to varying degrees. All but three cases had feeders arising from the posterior meningeal branch of the vertebral artery. The extracranial segmental arteries at the upper cervical level were found to anastomose with the posterior meningeal arteries and join the supply of the DAVF in two cases. A contribution from the contralateral ascending pharyngeal and posterior meningeal arteries was present in five patients. The descending clival meningeal arteries from the tentorial artery were found to contribute to the supply in one patient. These feeders concentrated into the anterior condylar vein and the pouch of the anterior condylar confluence (ACC)located just ventrolateral to the hypoglossal canal. Particularly in cases supplied by both of the ascending pharyngeal arteries, the shunt point was located in the ACC, not the anterior condylar vein (ACV) because the selective angiogram showed the different shunt point in the ACC (Case 4).
The main drainage route was the vertebral plexus via the anterior or lateral condylar veins in three patients, the inferior petrosal sinus (IPS) in three patients, and the internal jugular vein via a direct connection from the ACC in two patients. The drainage channel to the vertebral plexus was the anterior condylar vein in four patients, lateral condylar vein in seven patients, and the prevertebral plexus in two patients. A communication to the cavernous sinus via Trolard’s inferior petro-occipital vein and venous plexus of Raktrzik11 was unexpectedly opened after the embolization of (IPS) in one patient. Interestingly, two out of three patients with IPS drainage had similar symptoms of oculomotor or abducens palsy, chemosis, and conjunctival edema as in DAVF of the cavernous sinus. The anatomic scheme of the ACC and surrounding venous structures is shown in Figure 1.
Treatment and Results
Shunt occlusion with transvenous coil packing was performed in seven patients; five also included the use of transarterial feeder embolization. The remaining patient underwent transarterial embolization (TAE)alone. Before embolization, we identified the location of the shunt point on angiographic and tomographic images obtained by intraoperative superselective or three-dimensional angiography from the ascending pharyngeal artery and intraoperative computed tomography obtained by rotating the C-arm of the angiography machine [Dyna CT system (AXIOM Artis, Siemens, Erlangen)]. These images were used to identify the connection of each of the drainage routes and the relationship between the hypoglossal canal and the tip of the microcatheter and to determine the target of embolization.
In all cases the DAVF was completely gone and symptomatically cured. There were no cases with the recurrence of the symptoms within six to 46 postoperative months, and follow-up MRA taken six months showed no recurrent shunt. One case (Case six) treated with tight packing of the anterior condylar vein developed temporary hypoglossal palsy as a postoperative complication. In Case two, packing the condylar vein resulted in prolonged hypoglossal nerve palsy. In contrast, patients one and three with hypoglossal nerve palsy had postoperative improvement due to the intentional avoidance of tight packing of the ACV.
Representative Cases
Case 2: (Figure 2) A 63-year-old woman was diagnosed with brainstem vascular disease after presenting with dysarthria due to paresis of the tongue. A loud bruit was audible at the left retromastoid region and left hypoglossal nerve palsy was noted. Angiography showed DAVF at the ACC and incidental aneurysms of the right internal carotid and middle cerebral arteries. The DAVF was supplied mainly from the bilateral APA, and had small feeders from the mastoid branch of the occipital artery, meningeal branches from the posterior meningeal artery and a segmental artery at the atlanto-axial level, and clival branches of the tentorial artery on the left side.
Figure 2 .
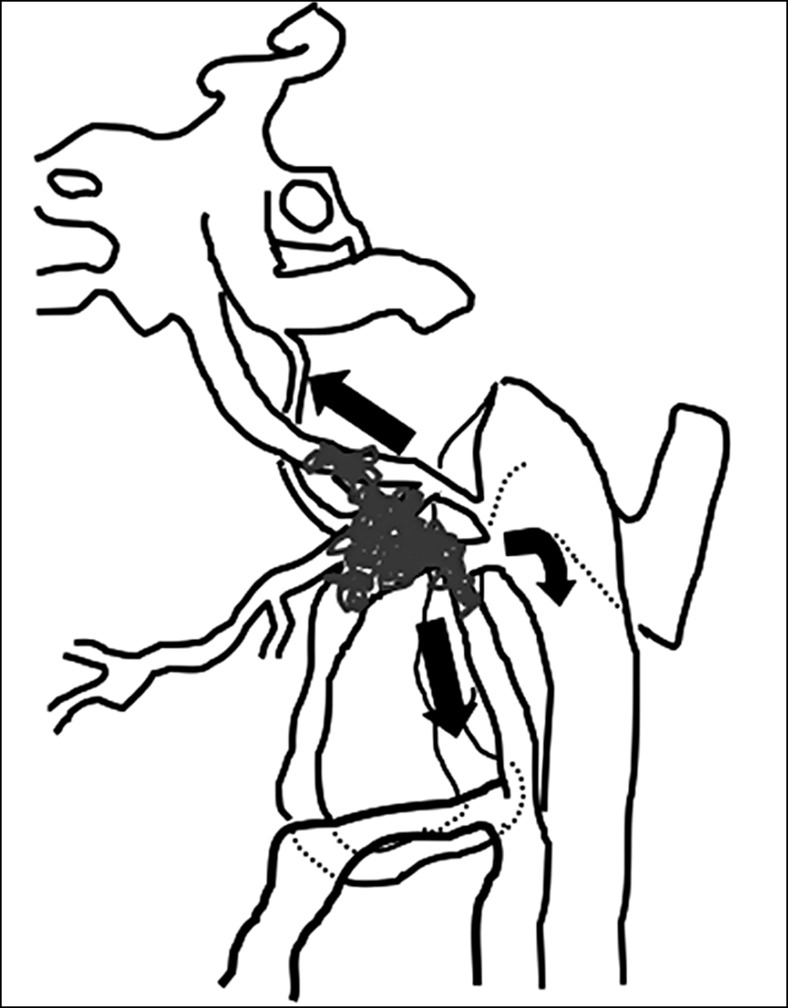
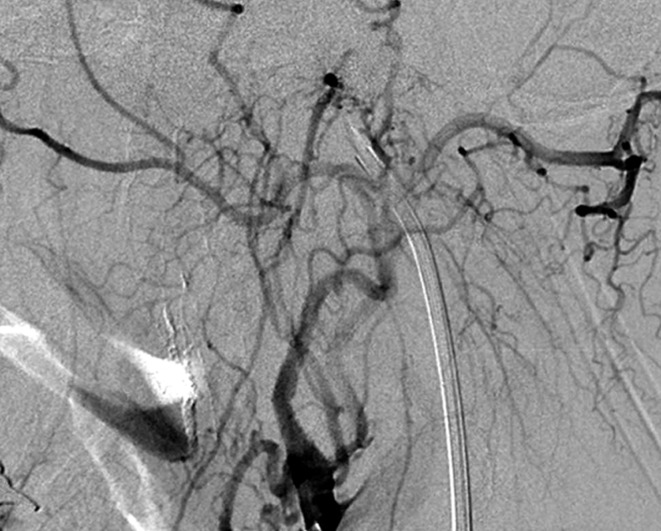
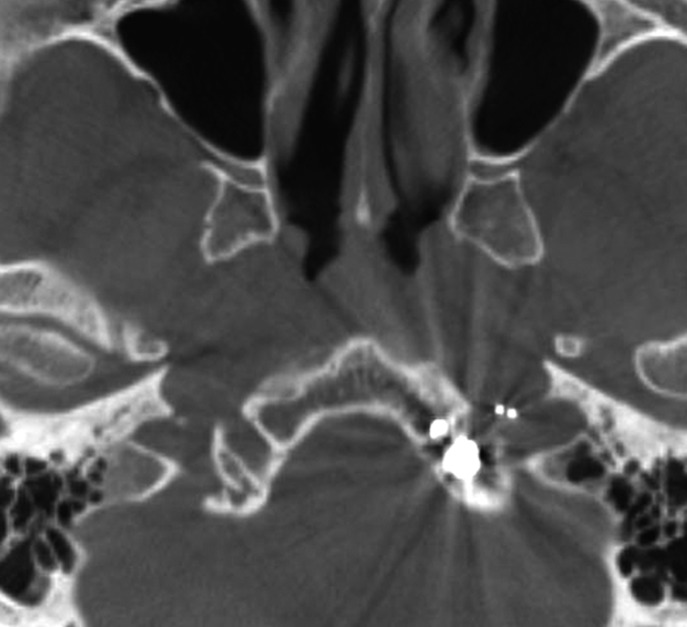
Case 2. Left external carotid artery (ECA) angiogram [A-P (A) and lateral (B) view] and right ECA angiogram (C) showing the DAVF at the anterior condylar confluence (ACC). The shunt point is located at the anterior condylar vein (ACV) (black arrow). Left internal carotid angiography reveals the contribution of the clival branches from the meningohypophyseal trunk (D). Enhanced CT (E) shows that the expanded ACC (white arrow) is clearly visualized at the external side of the enlarged hypoglossal canal. Postoperative plain skull radiogram (F) shows the coil mass at the inferior petrosal sinus and ACC, and partially packed into the ACV. Postoperative angiogram showing complete obliteration of the shunt (G). Scheme of the shunt point and embolized portion (H).
A.
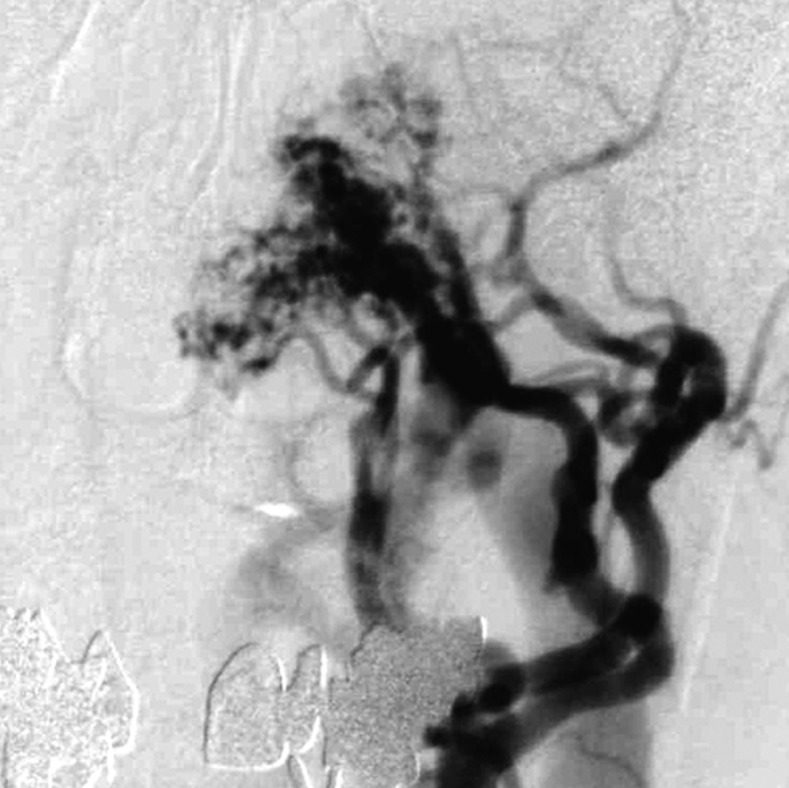
B.
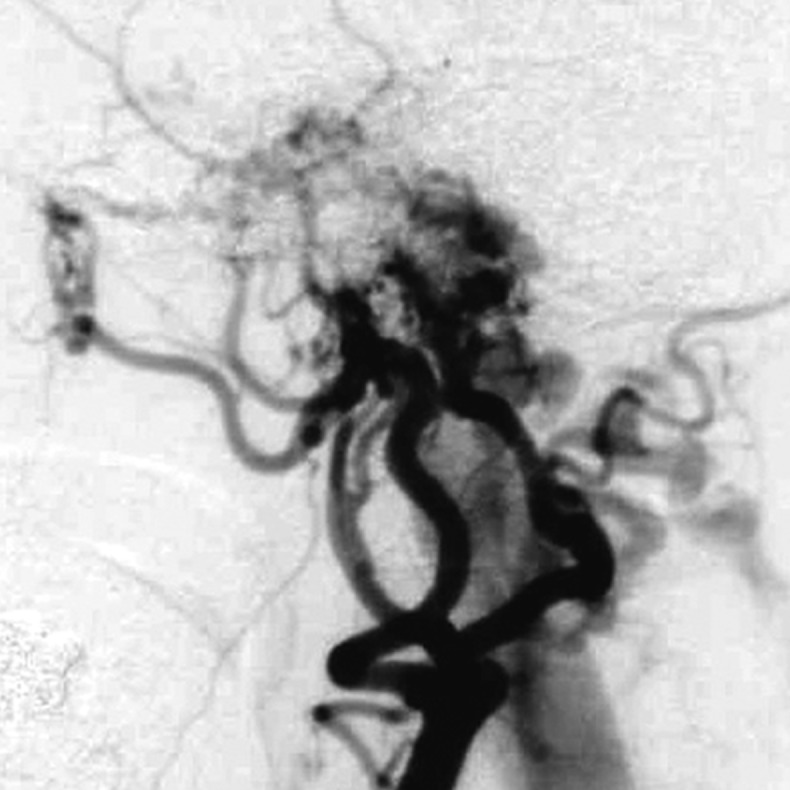
C.
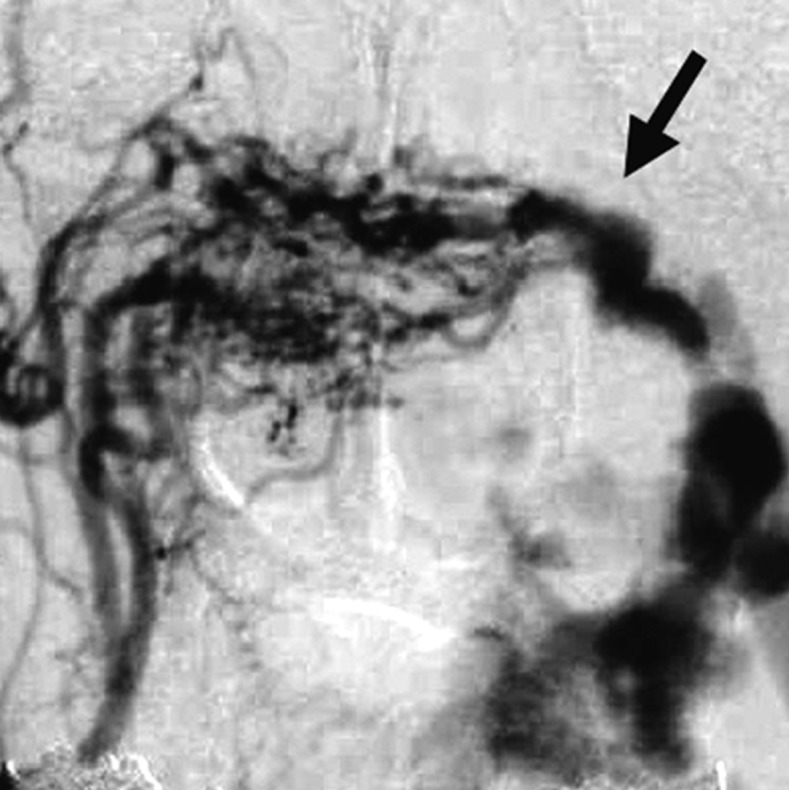
D.
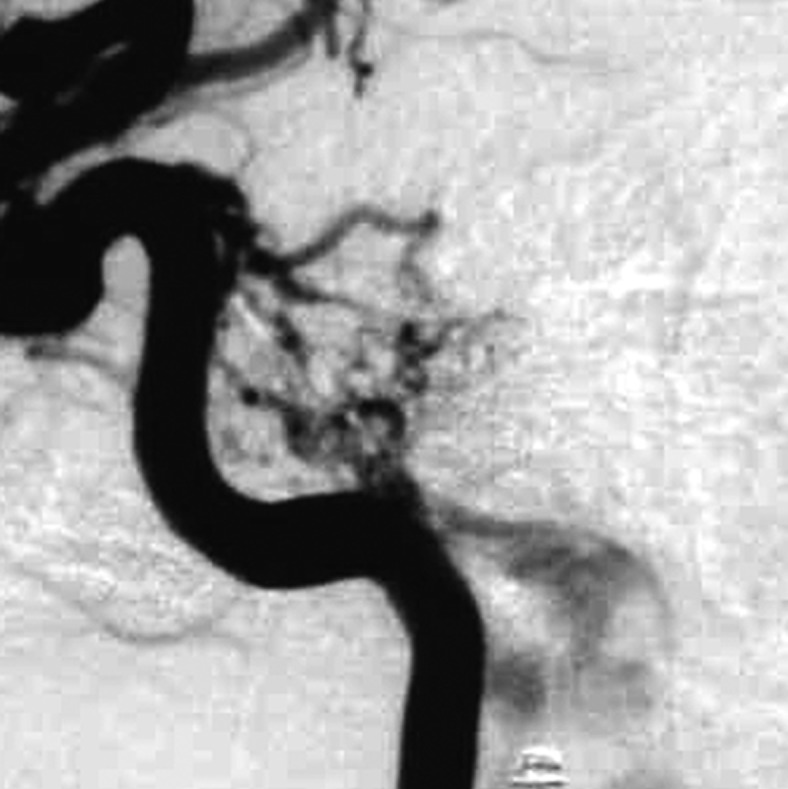
E.
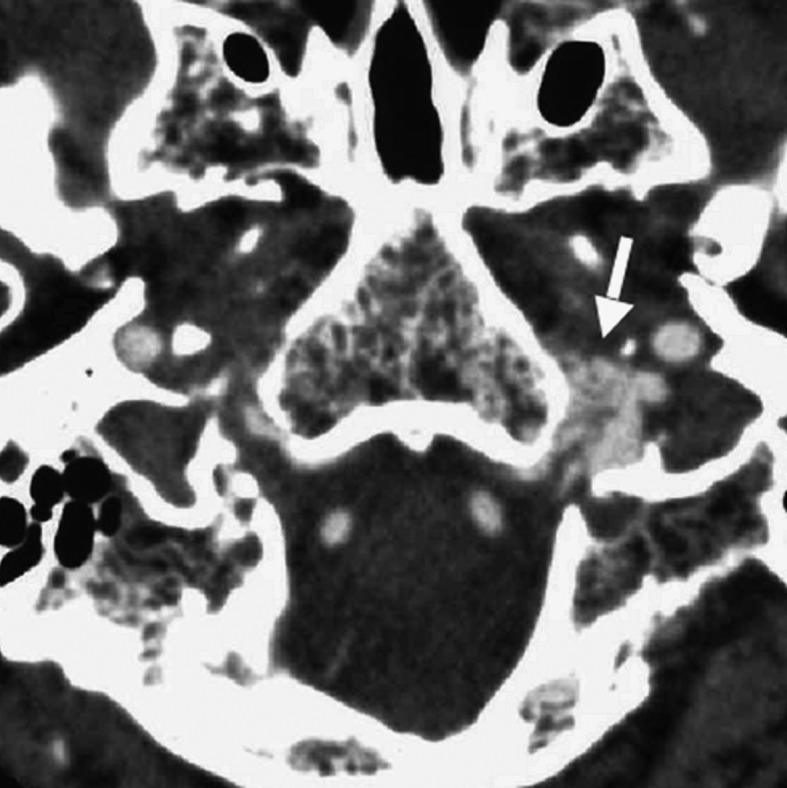
F.
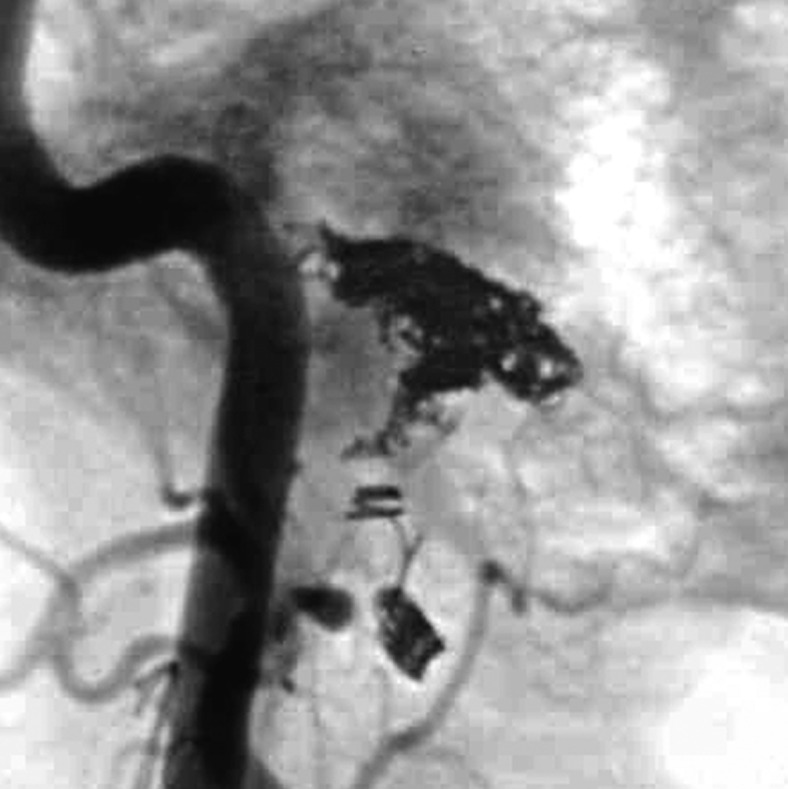
G.
H.
The shunt point was located at the ACV and the caudal side of the IPS. The main draining veins were the vertebral plexus via the lateral condylar vein (LCV) and inferior petrosal sinus (IPS). Because of the relatively high shunt flow, transarterial embolization using particles and coils of the branches from the external carotid arteries was performed in advance. The shunted drainage routes, ACC and LCV, were subsequently embolized transvenously with coils. The coils were placed in the ACC and LCV as well as the ACV. Hypoglossal nerve palsy lasted for six months, even after complete occlusion of the shunt but then gradually improved.
Case 4: (Figure 3) A 58-year-old man with an episode of head trauma one year prior to presentation noted pulsatile tinnitus in the left retromastoid region five months after the trauma. Beginning one month prior to presentation he developed double vision, chemosis and exophthalmos of the left eye. Ophthalmologic examination disclosed left abducens palsy. Angiography showed a DAVF at the ACC supplied by the left APA and the meningeal branch of the vertebral artery draining into the IPS and LCV.
The angioarchitecture surrounding the shunt point was evaluated with selective three-dimensional angiography of the APA. Based on this image, the ACV was occluded. After occlusion of the caudal side of the IPS, the other channel, Trolard’s inferior petro-occipital vein, opened and drained into the cavernous sinus. This route was also packed with coils and finally the LCV was embolized. Although we did not pack the ACV intentionally, the downstream part of the draining veins was effectively embolized, resulting in complete occlusion of the fistula.
The symptoms quickly improved postoperatively with no complications.
Case 3: (Figure 4) A 68-year-old woman developed spastic involuntary movement of the tongue and left tinnitus four months prior to presentation. The angiography showed a DAVF at the ACC fed by bilateral APA and branches of the left occipital artery and draining into the vertebral plexus via the lateral condylar vein and small drainage into the IJV.
Figure 4 .
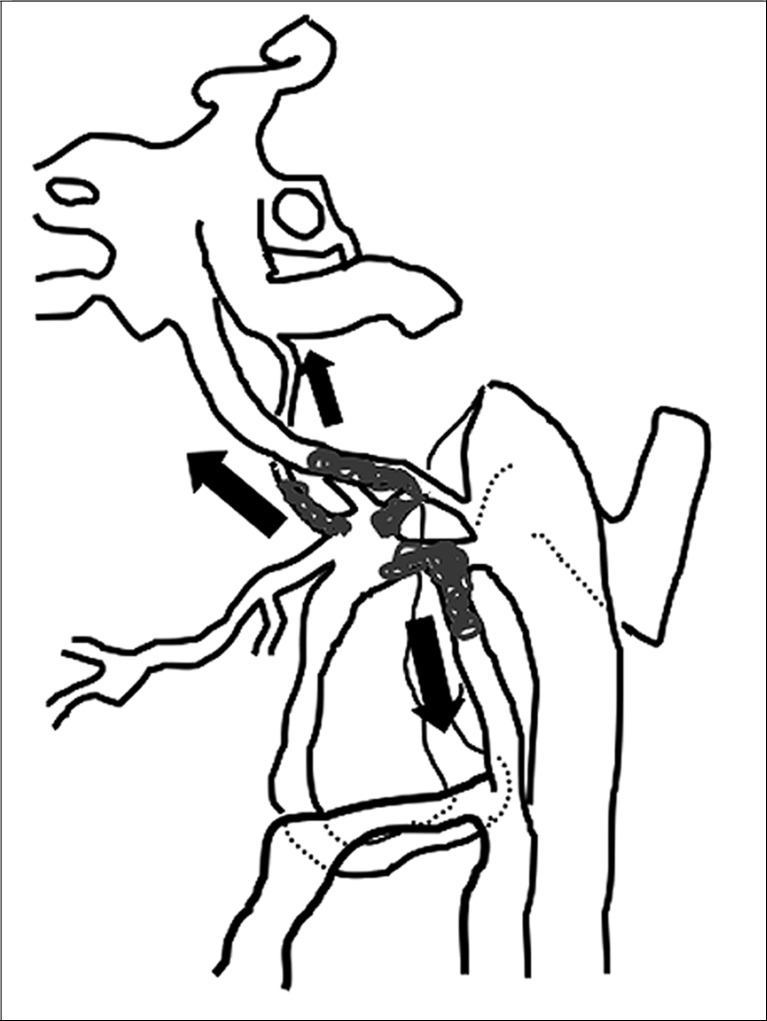
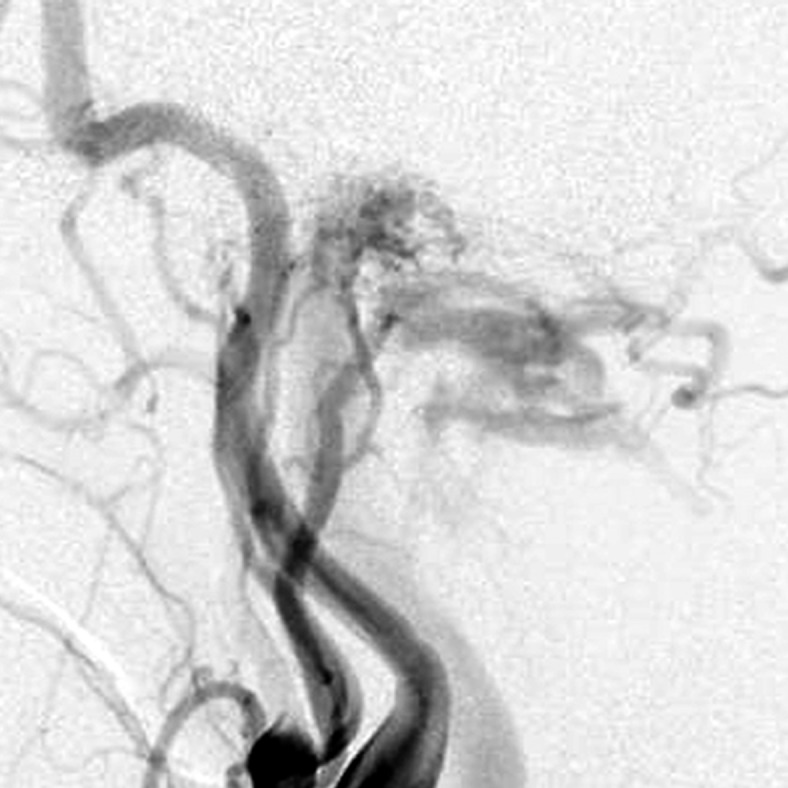
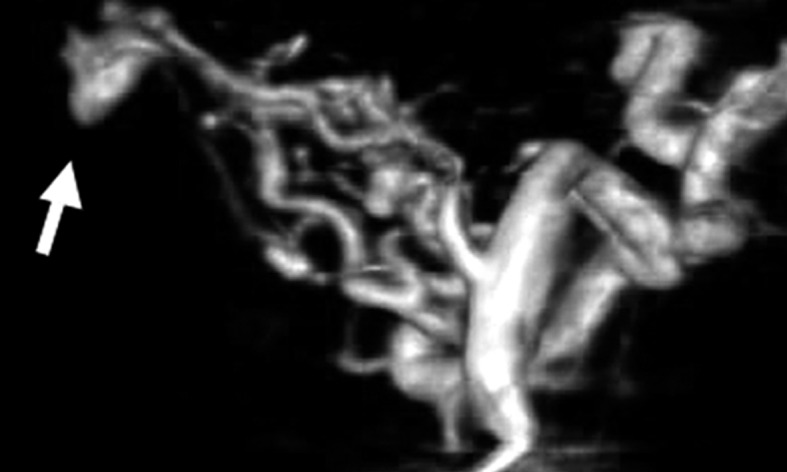
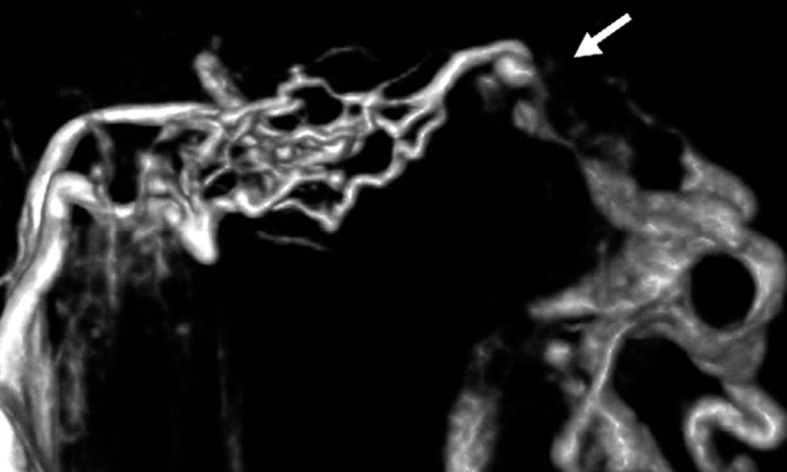
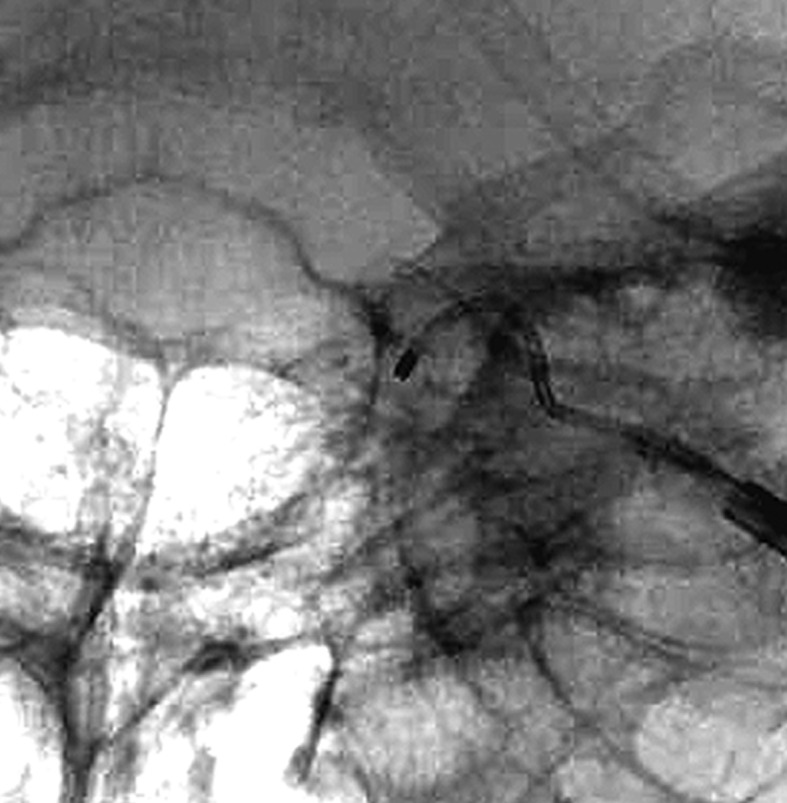
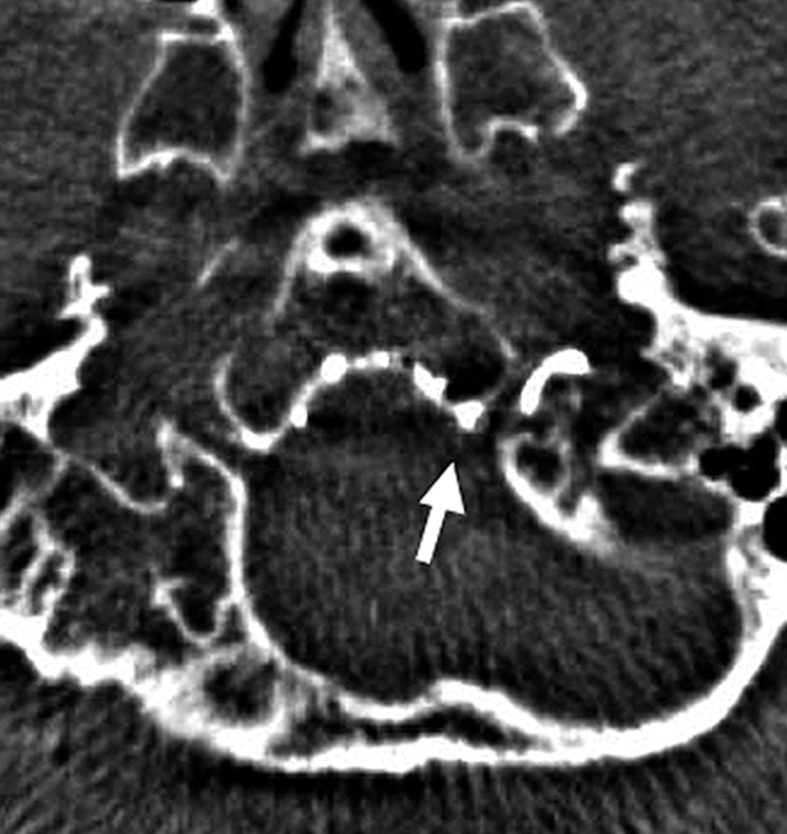
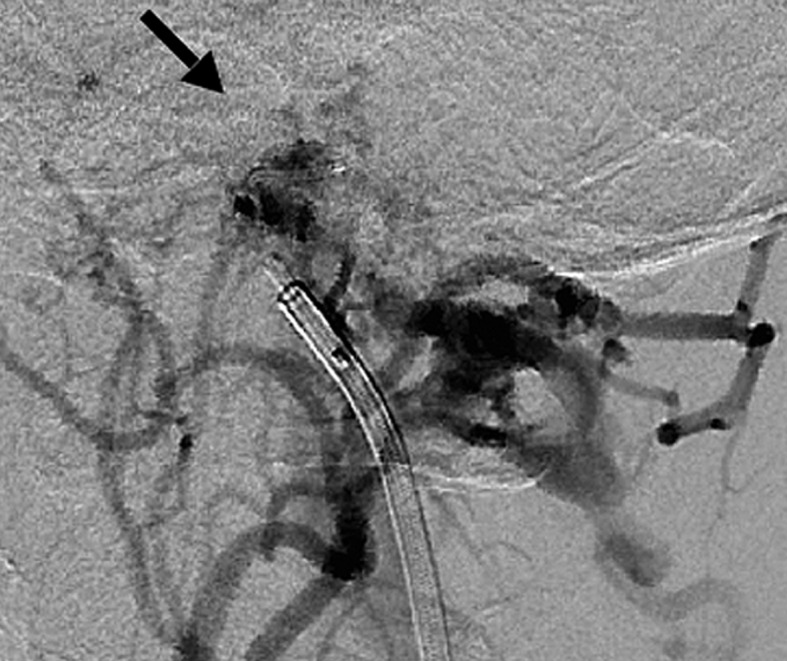
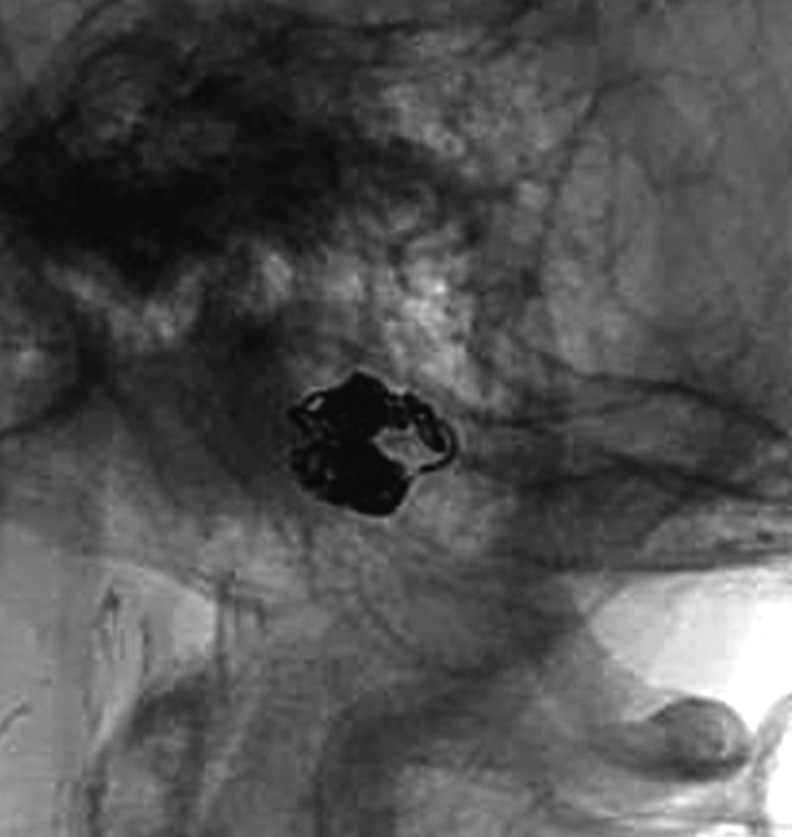
Case 3. Left external carotid artery (ECA) angiogram (A) shows the DAVF at the anterior condylar confluence (ACC). 3D-angiogram of the left occipital artery (B) and right ascending pharyngeal artery (APA) (C) shows the common drainage sac, ACC (white arrow). Intraoperative Dyna-CT (D) discloses the tip of the transvenously introduced microcatheter (E) in the nearest part of the shunt, which is located at the intracranial side of anterior condylar vein in the expanded hypoglossal canal (white arrow). Left ECA angiogram (lateral view) showing the different location from the expected shunt point where the microcatheter tip (black arrow) is located (F). Postoperative left ECA angiogram (G) showing the disappearance of the DAVF and the plain skull radiosram (H) showing the coil mass in the ACC. Scheme of the shunt point and embolized portion (I)
A.
B.
C.
D.
E.
F.
G.
H.
I.
The shunt point was the origin of the left anterior condylar vein. Superselective three-dimensional angiography of the APA showed that all the abnormal feeding arteries were concentrated on the medial side of the left ACV. We inserted the microcatheter transvenously and reached the medial orifice of the shunt. However, the thin slice Dyna-CT showed that the position of the microcatheter was just inside the hypoglossal canal, but we found that the feeders from the left external carotid artery shunted into the more inferolateral part of the shunt point. Based on these findings, the shunt from the bilateral feeders was located in the inferior wall of the ACC.
The embolization of the medial side of the hypoglossal canal, involving the intracranial side of the anterior condylar vein, seemed likely to cause postoperative hypoglossal nerve palsy. Therefore we roughly packed the ACV, withdrew the catheter to the ACC, and embolized at that location. Complete obliteration of the shunt was accomplished with five coils. The tinnitus stopped and the tongue position and movement normalized.
Discussion
Anatomic View
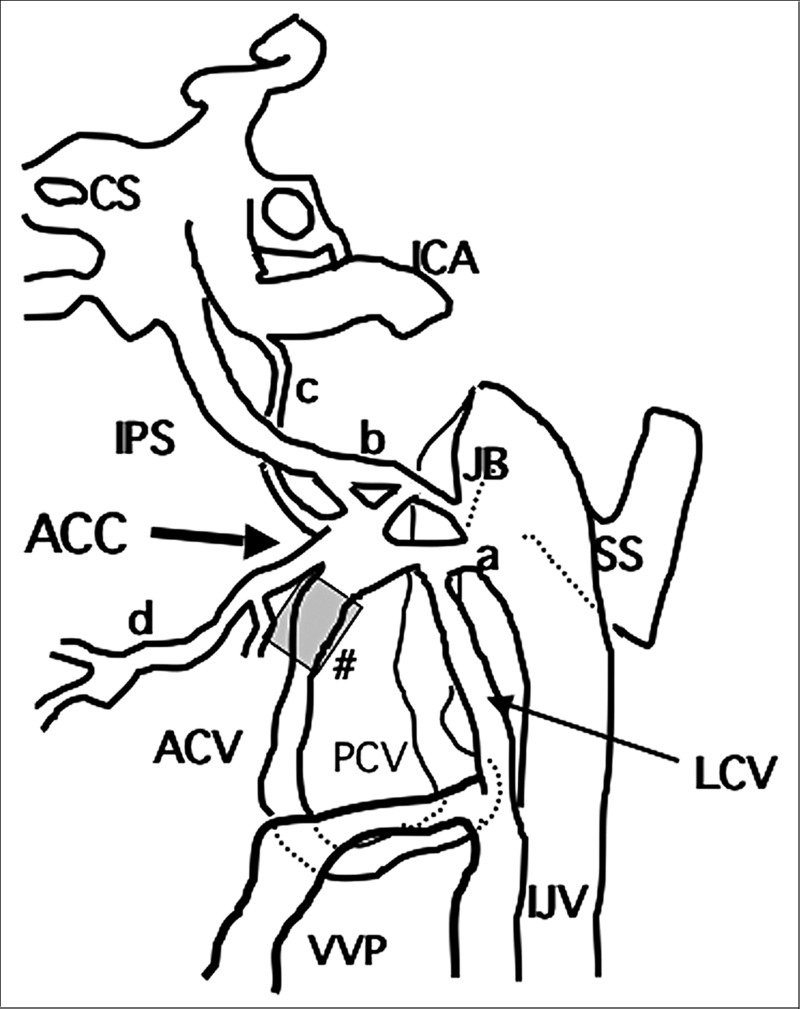
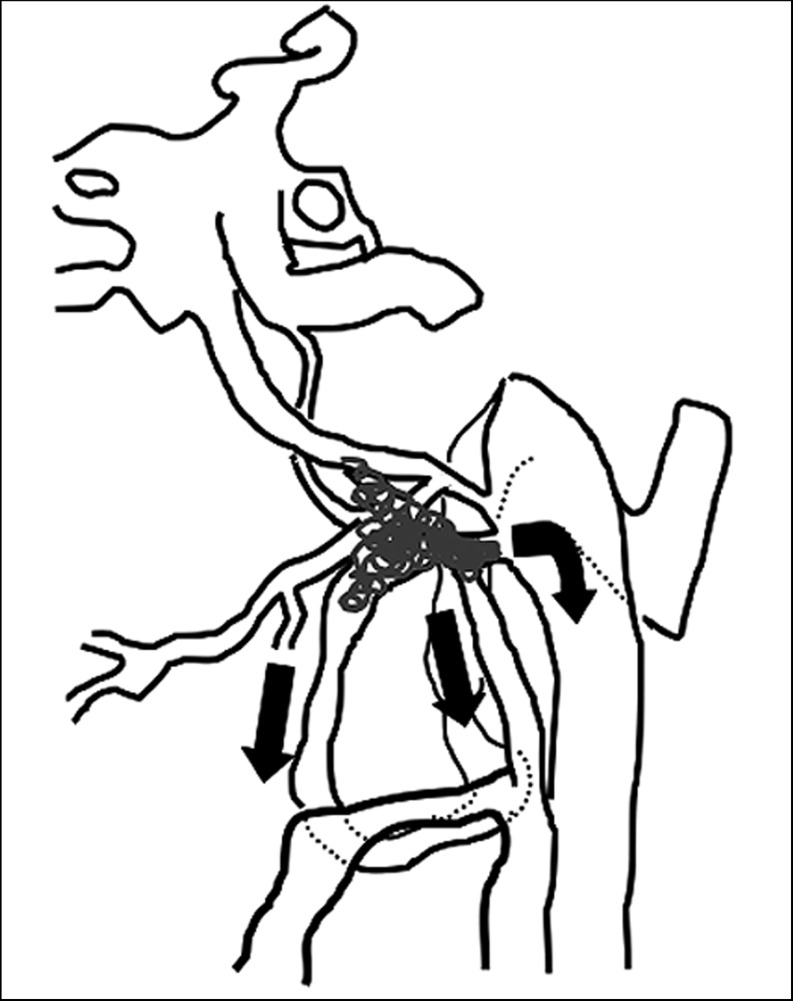
The anterior condylar confluence (ACC) is a major venous crossroad at the base of the skull and is located extracranially in front of the aperture of the hypoglossal vein connection with the anterior condylar vein. This was described by Katsuta et Al.10 as the petrosal confluence in which tributaries from the hypoglossal canal, petroclival fissure and the vertebral venous plexus meet together and then drain into the jugular bulb through multiple channels between cranial nerves IX to XI. The ACC offers multiple connections with the dural venous sinuses of the posterior cranial fossa, the internal jugular vein, and the vertebral venous system.
The size of the ACC is between 3 and 5 mm in the anterior-posterior axis and approximately 2 mm in its ventrodorsal extension. According to the analysis of the ACC and surrounding structures by San Marco Ruiz et Al.9, the following veins regularly contribute to the formation of the ACC: 1) the anterior condylar vein; 2) branches from the internal jugular vein or its bulb; 3) the lateral condylar vein; 4) anastomoses with the inferior petrosal sinus; 5) branches from the internal carotid artery venous plexus of Rektorzik, corresponding to Trolard’s inferior petro-occipital vein11 and 6) branches from the prevertebral venous plexus found on the anterior atlanto-occipital membrane (Figure 1).
Figure 1.
The anatomic scheme of the anterior condylar confluence and surroundung venous complex. ACC: anterior condylar confluence, ACV: anterior condylar vein, CS: cavernous sinus, ICA: internal cerebral artery, IJV: internal jugular vein, IPS: inferior petrosal sinus, JB: jugular bulb, LCV: lateral condylar vein, PCV: posterior condylar vein, SS: sigmoid sinus, VVP: vertebral venous plexus, a: anastomotic channel with IJV or jugular bulb, b: anastomtic channel with IPS, c: anastomotic channel with branches from ICA venous plexus (Trolard’s inferior petro-occipital vein), d: anastomotic channel with prevertebral venous plexus, #: hypoglossal canal.
With respect to the surrounding venous routes, the anterior condylar vein and marginal sinus are the very close structures. The anterior condylar vein constitutes the venous plexus around the hypoglossal nerve and is one of the emissary veins passing through the hypoglossal canal and represents a plexiform connection of the anterior internal vertebral venous plexus and the ACC. In pathological situations such as DAVF, these pathways develop abnormally and function as a shunt.
Clinical Entities Caused by DAVF
The main feeding arteries from the ipsilateral external carotid system, particularly those from the ascending pharyngeal artery (APA), exclusively supply the DAVF. Contralateral APAs also contribute through the anterior margin of the foramen magnum. Posterior meningeal arteries and extracranial dural branches from the segmental arteries from the vertebral artery often join to supply it. These feeders concentrate to the venous plexus of the outlet of the hypoglossal canal and exclusively drain into the ACC.
The drainage routes are usually multiple, although they never reflux into the cortical veins. The main drainage route is the lateral condylar vein which drains into the vertebral plexus or cervical veins. Occasionally there is anterior drainage via the pterygoid plexus, and some cases have the reflux draining rostrally into the cavernous sinus via the inferior petrosal sinus or internal carotid artery venous plexus of Rakrtrzik, which connects with Trolard’s inferior petro-occipital vein. While the jugular bulb is located just posterolateral to the ACC, the jugular vein contributes less as the source of these drainage routes12. There is no exclusive route of intracranial reflux into the cortical or medullary veins. This is completely different from the tentorial, ethmoidal, and spinal DAVFs whose drainage courses to the intracranial venous channels.
Since the ACC is an extracranial structure, there is a question as to the location of the shunt point. We believe that it may be the dural wall at the turning-up point just in the fundus of the hypoglossal canal.
In the situation in which the anterior condylar vein acts as the main drainage route, the shunt point may appear to be the anterior condylar vein. However the true shunt point should be more upstream. The drainage patterns are varied and embolization of only the anterior condylar vein did not stop the fistula in some cases of our series. If we believe the shunt point to be the marginal sinus which connects with the anterior condylar veins, we cannot explain the various drainage channels because it is situated more caudally. Similarly, the IPS should not be the true shunt point, because not all of the shunts could be stopped even if the IPS was embolized first. This type of DAVF is quite similar to the DAVF at the cavernous sinus because of the multiple drainage patterns, the occlusive changes in some of the channels, and the surrounding similar “suboccipital cavernous sinus” network13. According to the new classification by Lasjaunias, this type of DAVF may be included in the “ventral epidural shunt” type, which is the same category as DAVF at the cavernous sinus14. Therefore we advocate that they be called a “DAVF at the ACC” based on the anatomic and hemodynamic characteristics.
Symptomatology
Because this type of AVF shunt is located very close to the petrous bone, patients suffer from severe tinnitus, and a pulsatile bruit is audible at the mastoid process5. Because of the peculiar drainage into the IPS, the symptoms are similar to those of a carotid-cavernous fistula15. Pulsatile compression of the enlarged anterior condylar veins occasionally causes hypoglossal nerve palsy or involuntary movement of the tongue16,17. In common with all of the DAVF, some drainage routes spontaneously occlude to varying degrees and patterns. The development of symptoms depends on the direction of the shunt and the distribution of the arterial pressure. Otherwise, the only symptom is severe tinnitus and reaching a definitive diagnosis tends to be delayed. Therefore the shunt distribution may spread and change in the interim.
Endovascular Treatment
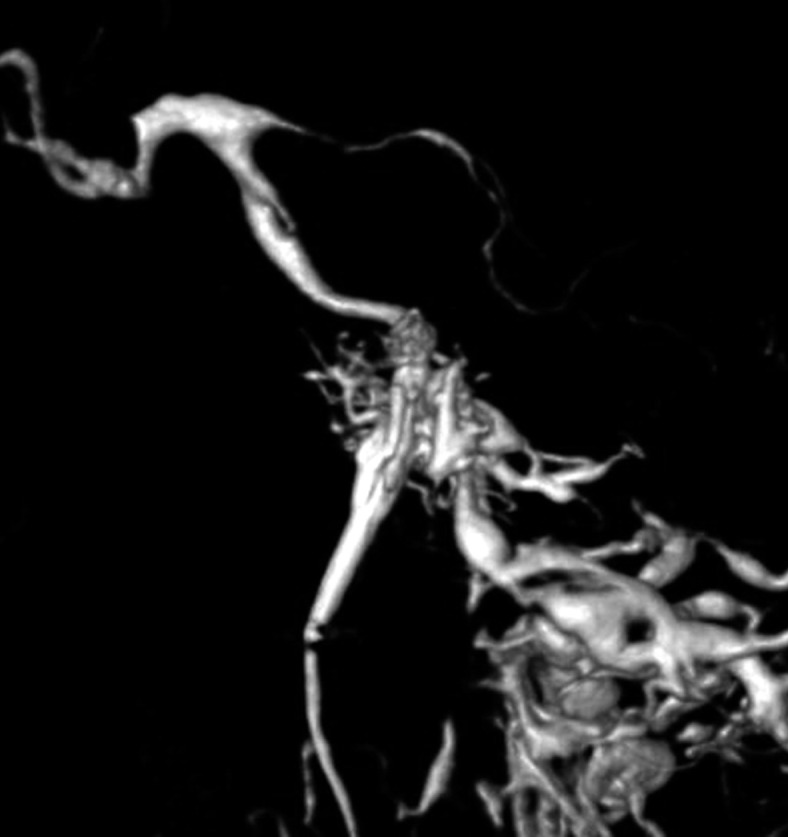
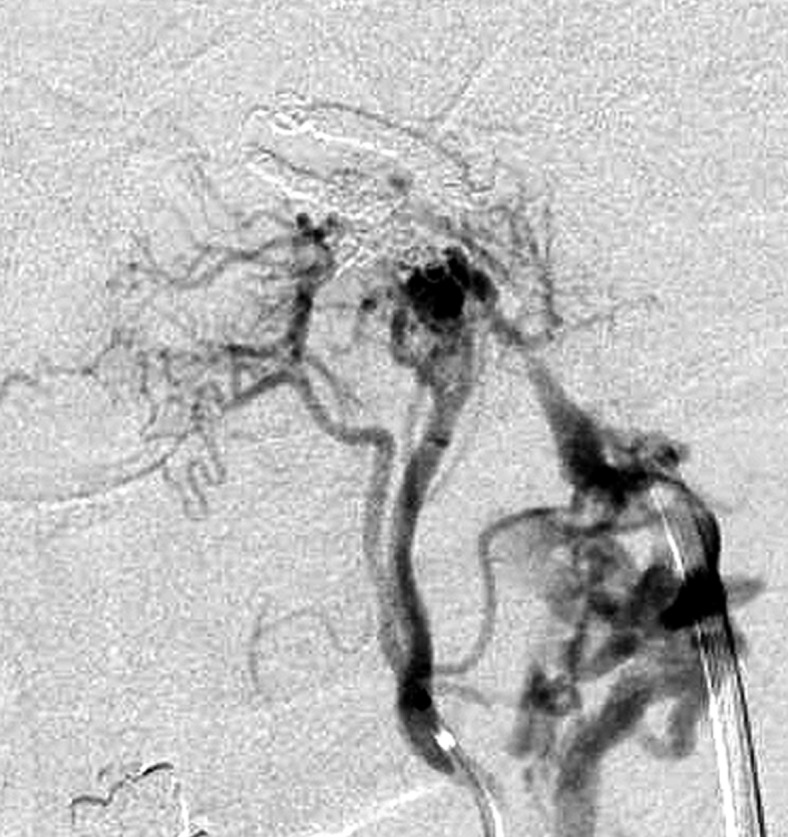
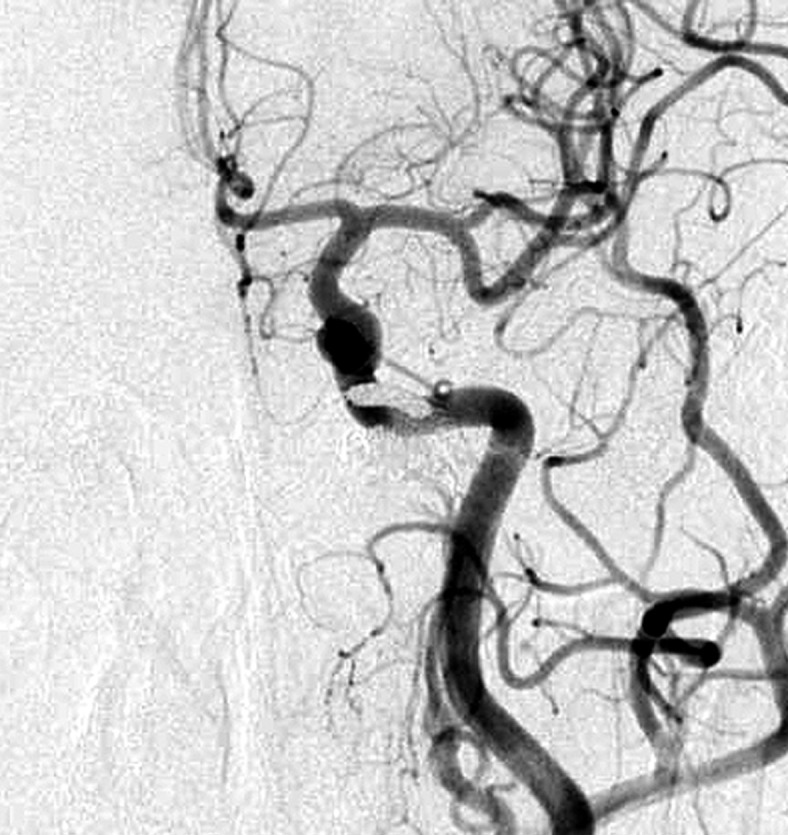
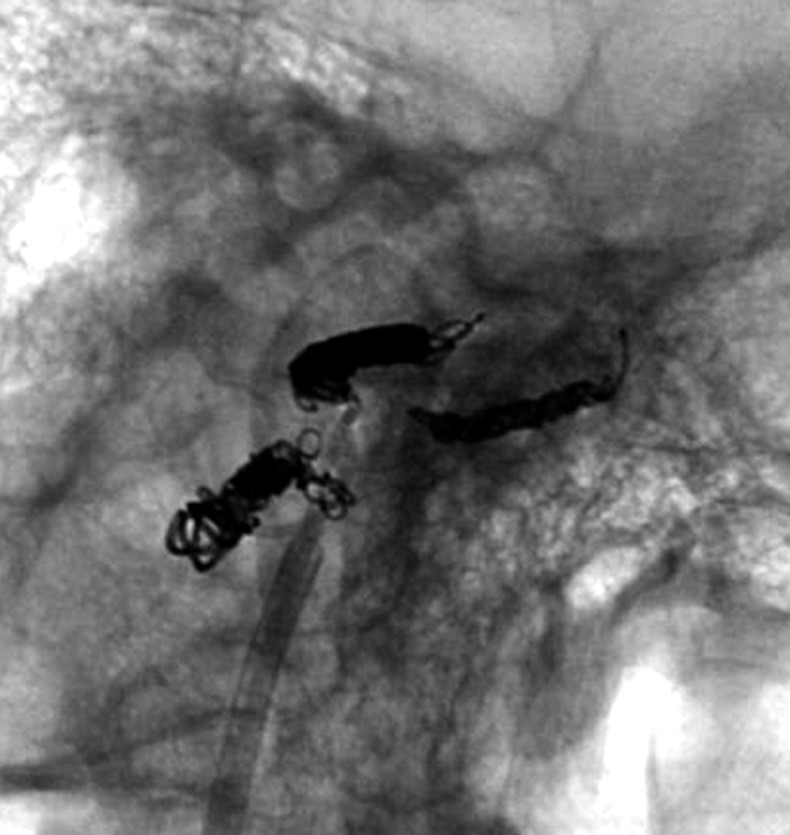
Aggressive treatment of DAVF involves coil packing of the shunt point. However, it is often difficult to identify the shunt point because the feeders and drainers are superimposed in both the carotid and vertebral angiograms. Selective angiography of the main feeding artery, the ascending pharyngeal artery (APA), gives the most useful information. Specifically the three-dimensional-rotational angiogram (3D-DSA) is essential in recognizing the angioarchitecture (Figures 3 and 4).
Figure 3.
Case 4. Selective 3-D angiogram of left ascending pharyngeal artery (APA) showing the DAVF at the ACC (A). Selective APA angiogram after embolization of the draining veins (inferior petrosal sinus and Trolard’s inferior petro-occipital vein) showing the remaining drainage into the vertebral plexus through the lateral condylar vein (LCV) (B). Coils were added into the LCV (C) and total occlusion of the DAVF was achieved (D). Postoperative Dyna-CT shows the coils placed to occlude the IPS at the superolateral side of the hypoglossal canal (E). Scheme of the shunt point and embolized portion (F).
A.
B.
C.
D.
E.
F.
The entrance into the ACC from the jugular vein is unclear in most cases via the transvenous approach. In our experience, it can be found caudal to the confluence of the inferior petrosal sinus and the microcatheter should be advanced medio-inferiorly and turned just posteriorly at this point to insert into the ACC. The selective venogram in the ACC is also useful in identifying the drainage routes.
With respect to methods of embolization, coil packing of the ACC and adjacent structures is the most common method3,4,7,11. However the anterior condylar vein (ACV) should not be tightly packed with coils to avoid hypoglossal nerve palsy. Therefore it is safest to place the coils initially in the downstream portion of each drainage route. In our experience, embolization of the ACC seems to be the best way to stop the shunt, even if the shunt point is close to the vein. Thin slice bone CT imaging across the canal, which clearly shows the position of the catheter tip, is very useful to avoid overpacking the canal. As one of the ways to avoid overpacking the anterior condylar vein in the hypoglossal canal the use of softer embolic materials like as hydrocoils or absorbable agents like as liquid embolic materials may be considered.
Transarterial embolization of the APA should be performed with care to avoid cranial nerve palsy. Therefore, this method is adjunctive and should be used to treat high shunt feeders or persistent fistula after TVE.
The etiology of DAVF in the ACC has not been elucidated because it is a rare disease. However, it will be important to determine the correct strategy of endovascular treatment and to achieve the safest and the most effective embolization based on understanding this pathologic angioarchitecture.
Conclusions
Since DAVF around the hypoglossal canal has many variations in its clinical presentation and drainage pattern, the nomenclature for this disorder has not been defined in previous reports. Such diversity can be well recognized by identifying the ACC and surrounding multiple venous connections, advocated by Trolard11 and systematized by San Millan Ruiz9.
The shunt point of this DAVF is located close to the anterior condylar vein traversing the hypoglossal canal, and imprudent coil packing of this portion may cause hypoglossal nerve palsy. Furthermore, drainage routes are variable in each case because some of the routes are hypoplastic or previously thrombosed, similar to the drainage patterns of cavernous sinus DAVF. We emphasize that this specific anatomical situation, extradurally located DAVF, is very important to identify the correct strategy of endovascular treatments. In particular, it is valuable to identify the exact location of the shunt point and drainage pattern using superselective three-dimensional angiography and tomographic imaging to identify the correct treatment strategy, thereby avoiding dangerous complications.
Abbreviations
- APA
ascending pharyngeal artery
- OA
occipital artery
- VA
vertebral artery
- ACC
anterior condylar confluence
- IPS
inferior petrosal sinus
- IJV
internal jugular vein
- ACV
anterior condylar vein
- LCV
lateral condylar vein
- PCV
posterior condylar vein
- TAE
transarterial embolization
- TVE
transvenous embolization
References
- 1.Barnwell SL, Halbach VV, et al. Dural arteriovenous fistulas involving the inferior petrosal sinus: angiographic findings in six patients. Am J Neuroradiol. 1990;11:511–516. [PMC free article] [PubMed] [Google Scholar]
- 2.McDougall CG, Halbach VV, et al. Dural arteriovenous fistulas of the marginal sinus. Am J Neuroradiol. 1997;18:1565–1572. [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi A, Mizoi K, et al. Marginal sinus dural arteriovenous shunts - the study of the clinical presentation, angiogram, and treatments. Journal of Cerebrovascular Surgery. 1992;20:384–390. [Google Scholar]
- 4.Kiyosue H, Tanoue S, et al. Ocular symptoms associated with a dural arteriovenous fistula involving the hypoglossal canal: selective transvenous coil embolization. Case report. J Neurosurg. 2001;94:630–632. doi: 10.3171/jns.2001.94.4.0630. [DOI] [PubMed] [Google Scholar]
- 5.Komiyama M, Ishiguro T, et al. Intense pulse-synchronous tinnitus caused by dural arteriovenous fistula at the hypoglossal canal. No To Shinkei. 2002;54:830–831. [PubMed] [Google Scholar]
- 6.Okahara M, Kiyosue H, et al. Selective transvenous embolization of dural arteriovenous fistulas involving the hypoglossal canal. Interventional Neuroradiology. 2007;13:59–66. doi: 10.1177/159101990701300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst R, Bulas R, et al. Three cases of dural arteriovenous fistula of the anterior condylar vein within the hypoglossal canal. Am J Neuroradiol. 1999;20:2016–2020. [PMC free article] [PubMed] [Google Scholar]
- 8.Kuwayama N, Akai T, et al. Dural arteriovenous fistulae involving the transverse-sigmoid sinus and foramen magnum. Surg Neurol. 1994;41:389–395. doi: 10.1016/0090-3019(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 9.San Millan Ruiz D, Gailloud P, et al. The craniocervical venous system in relation to cerebral venous drainage. Am J Neuroradiol. 2002;23:1500–1508. [PMC free article] [PubMed] [Google Scholar]
- 10.Katsuta T, Rhoton AL, Jr, et al. The jugular foramen: microsurgical anatomy and operative approaches. Neurosurgery. 1997;41:149–201. doi: 10.1097/00006123-199707000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Trolard P. Anatomie dusysteme veineux de l’encephale et du crane. These de la Faculte de Medecine de Paris. 1868:1–32. (Fre). [Google Scholar]
- 12.Kojima T, Miyachi S. The diagnosis and treatment of dural arteriovenous fistula at the anterior condylar confluence. Noshinkeigeka Sokuho. 2006;16:731–737. [Google Scholar]
- 13.Arnautovic KI, al-Mefty O, et al. Al: The suboccipital cavernous sinus. J Neurosurg. 1997;86:252–262. doi: 10.3171/jns.1997.86.2.0252. [DOI] [PubMed] [Google Scholar]
- 14.Lasjaunias P. Classification of dural arteriovenous shunts (DAVS) based on the craniospinal epidural venous drainage of the central nervous system and adjacent bony structures. Jpn. J Neurosurgery. 2008;17 (in press) [Google Scholar]
- 15.Liu HM, Shih HC, et al. Posterior cranial fossa arteriovenous fistula with presenting as caroticocavernous fistula. Neuroradiology. 2001;43:405–408. doi: 10.1007/s002340100551. [DOI] [PubMed] [Google Scholar]
- 16.Blomquist MH, Barr JD, et al. Isolated unilateral hypoglossal neuropathy caused by dural arteriovenous fistula. Am J Neuroradiol. 1998;19:951–953. [PMC free article] [PubMed] [Google Scholar]
- 17.Combarros O, Alvarez de Arcaya A, et al. Isolated unilateral hypoglossal nerve palsy: nine cases. J Neurol. 1998;245:98–100. doi: 10.1007/s004150050185. [DOI] [PubMed] [Google Scholar]