Abstract
This review represents the focus of a symposium that was presented at the “Alcoholism and Stress: A Framework for Future Treatment Strategies” conference in Volterra, Italy on May 3–6, 2011 and organized / chaired by Dr. Brendan M. Walker. The primary goal of the symposium was to evaluate and disseminate contemporary findings regarding the emerging role of kappa-opioid receptors (KORs) and their endogenous ligands dynorphins (DYNs) in the regulation of escalated alcohol consumption, negative affect and cognitive dysfunction associated with alcohol dependence, as well as DYN / KOR mediation of the effects of chronic stress on alcohol reward and seeking behaviors. Dr. Glenn Valdez described a role for KORs in the anxiogenic effects of alcohol withdrawal. Dr. Jay McLaughlin focused on the role of KORs in repeated stress-induced potentiation of alcohol reward and increased alcohol consumption. Dr. Brendan Walker presented data characterizing the effects of KOR antagonism within the extended amygdala on withdrawal-induced escalation of alcohol self-administration in dependent animals. Dr. Georgy Bakalkin concluded with data indicative of altered DYNs and KORs in the prefrontal cortex of alcohol dependent humans that could underlie diminished cognitive performance. Collectively, the data presented within this symposium identified the multifaceted contribution of KORs to the characteristics of acute and chronic alcohol-induced behavioral dysregulation and provided a foundation for the development of pharmacotherapeutic strategies to treat certain aspects of alcohol use disorders.
Keywords: Alcohol, Anxiety, Central Nucleus of the Amygdala, Cognitive Dysfunction, Decision Making, Dependence, Depression, Dynorphin, Extended amygdala, Impulsivity, Kappa Opioid Receptor, Negative Affect, Negative Reinforcement, norbinaltorphimine (nor-BNI), Nucleus Accumbens, Opioid, Orbitofrontal Cortex, Prefrontal Cortex, Place Preference, Self-administration, Stress, Withdrawal
Alcohol use disorders, comprising alcohol abuse and dependence, are a pervasive problem, with rates in the United States climbing from 7.41% in 1991–1992 to 8.5% in 2004 (Grant et al., 2004) for person 18 years of age and older. Furthermore, factors related to alcohol consumption have been shown to be the third leading cause of preventable death (Mokdad, Marks, Stroup, and Gerberding, 2004) and societal costs associated with alcohol use disorders have been estimated to be at least $148 billion per year (Harwood, Fountain, and Livermore, 1998). Clearly, an impetus for research and treatment of alcohol use disorders exists and progress in this area would be extremely beneficial to the society at large.
Acute alcohol stimulates the release of the endogenous opioid peptides β-endorphin (βEND), enkephalin (ENK), and dynorphin (DYN; Gianoulakis et al., 1996; Marinelli et al., 2003; Marinelli et al., 2004; Dai et al., 2005; Marinelli et al., 2005; Marinelli et al., 2006; Lam et al., 2008; Jarjour et al., 2009) and nonselective antagonists for opioid receptors reduce alcohol consumption in humans (Volpicelli et al., 1992; Mason et al., 1994) and alcohol consumption and self-administration in rats (Gonzales and Weiss, 1998; Stromberg et al., 2001; Coonfield et al., 2002; Shoemaker et al., 2002; Walker and Koob, 2008a; Walker and Ehlers, 2009; Nealey et al., 2011). Selective antagonists of the μ- and δ-opioid receptor (MOR and DOR, for which the endogenous ligands are βEND and ENK, respectively) have been shown to reduce alcohol self-administration (Stromberg et al., 1998; Hyytia and Kiianmaa, 2001), whereas antagonists selective for the κ-opioid receptor (KOR, for which DYN is the endogenous ligand) generally show no effect on nondependent alcohol self-administration (Williams and Woods, 1998; Doyon et al., 2006; Walker and Koob, 2008a; Logrip et al., 2008; Walker et al., 2010), but see Mitchell (2005). Thus, evidence suggests that the MOR and DOR are viable targets to reduce the positive reinforcing effects of alcohol in nondependent cohorts, whereas DYN / KOR systems do not appear to be involved in the positive reinforcing effects of alcohol. However, evidence has been accumulating that DYN / KOR systems contribute to the negative reinforcing effects of alcohol (Walker and Koob, 2008b) based, in part, on the pro-depressive effects of DYN / KOR system activation (Todtenkopf et al., 2004; Carlezon et al., 2005), the anti-depressant properties of KOR antagonists (Pliakas et al., 2001; Mague et al., 2003; Carr et al., 2010) and involvement of KORs with the dysphoria produced by stress (McLaughlin et al., 2003; Land et al., 2008).
Because of the intricate relationship between alcohol and the endogenous opioid peptide systems (EOS), it is extremely important to determine if the EOS is altered by chronic alcohol exposure and, if so, whether those neuroadaptations contribute to the excessive alcohol consumption (Rimondini et al., 2003; O’Dell et al., 2004; Walker and Koob, 2007), increased negative affect (Brown and Schuckit, 1988; Schuckit et al., 1997a; Walker et al., 2010) or dysregulated cognitive processing associated with decision-making and behavioral control (Crews and Boettiger, 2009; Boettiger et al., 2009; Love et al., 2009) that are characteristic of alcohol dependence. Of additional interest is whether the activation of KORs following exposure to chronic stressors (either independent of, or associated with, dependence) contributes to maladaptive behavioral responses such as increased drug reward and drug-seeking behavior (McLaughlin et al., 2003; McLaughlin et al., 2006; Carey et al., 2007; Valdez et al., 2007). In the present symposium, Drs. Walker, Valdez, Bakalkin and McLaughlin present data that address these four lines of inquiry, respectively. Because the effects of repeated stress could be a precipitating factor for excessive alcohol consumption and hasten the transition to dependence or reflective of the repeated cycles of withdrawal that are associated with dependence, this review will lead with Dr. Valdez and McLaughlin’s components, followed by Dr. Walker and Bakalkin’s data and interspersed discussion related to chronic alcohol exposure and alcohol dependence.
Stress, Alcohol Use Disorders and Negative Affect
There is significant comorbidity between alcohol use disorders / alcohol dependence and affective disorders (Regier et al., 1990; Grant and Harford, 1995; Schuckit et al., 1997b), with a substantial percentage of those classified as alcoholic also experiencing major depression (Roy et al., 1991) or an anxiety disorder (Schuckit et al., 1997b). Indeed, it has been suggested that some individuals may use alcohol to “self– medicate” symptoms of anxiety and depression (Gilligan et al., 1987; Khantzian, 1990; Markou et al., 1998), which can lead to escalated alcohol use and relapse behaviors. Importantly, symptoms of negative affect can stem from different etiologies that may be alcohol independent or alcohol–induced (Schuckit et al., 1997a; Hasin and Grant, 2002). These symptoms of negative affect appear to be intensified depending on the presence of physiological signs of withdrawal (i.e., depending on the level of dependence) and can persist for extended periods after such withdrawal has dissipated (Brown and Schuckit, 1988). However, there are currently no pharmacological treatments for the negative affective consequences of excessive alcohol use (Heilig and Koob, 2007), and antidepressant treatment has produced mixed results in reducing alcohol consumption in dependent individuals (Torrens et al., 2005). Recent evidence has implicated the DYN / KOR system involvement with negative affective phenotypes (Todtenkopf et al., 2004; Carlezon et al., 2005) and the dysphoria produced by stress (Land et al., 2008; McLaughlin et al., 2003).
The presence of physiological withdrawal symptoms can be indicative of an organism’s level of dependence and differentiate the extent of certain negative affective characteristics in alcohol dependent populations (Schuckit et al., 1997b). When modeling dependence in animals, it follows that an ideal appraisal should assess multiple symptoms / behaviors in order to establish the appropriate conditions for later comparisons. For example, it has been shown that use of ethanol vapor exposure to induce dependence clearly produces physiological withdrawal symptoms (Schulteis et al., 1995; Roberts et al., 1996), as well as increased negative affective states resembling anhedonia, anxiety and depression (Baldwin et al., 1991; Rassnick et al., 1993; Schulteis et al., 1995; Valdez et al., 2002; Zhao et al., 2007; Walker et al., 2010). However, it must be noted that other animal models of alcohol abuse that do not produce physiological withdrawal per se can show signs of altered affective states that are characterized by anxiogenic responses when alcohol is unavailable (e.g., Holter et al., 1998). One of the most common measures of anxiety-like behavior in rodents is the elevated plus-maze (EPM), which examines exploration of an unfamiliar environment as a conflict with a safe environment (Pellow et al., 1985; Pellow, 1986). Rats chronically exposed to alcohol show decreased open arm exploration in the EPM, an indication of an anxiety-like state, when tested during withdrawal (Baldwin et al., 1991; Rassnick et al., 1993; Rasmussen et al., 2001; Valdez et al., 2002; 2004). However, the role of DYN / KOR systems in regard to alcohol withdrawal-related stress has yet to be fully characterized.
Dr. Valdez presented data indicating that kappa opioid mechanisms play a role in the regulation of stress-related behavior due to withdrawal from alcohol (Valdez, unpublished observations). Following chronic access to an alcohol liquid diet (average intake =14.6 +/−1.3 g/kg for 28 days), rats received injections of the KOR antagonist norbinaltorphimine (nor-BNI, 0.0, 10.0 or 20.0 mg/kg, i.p.) 24 hours prior to testing in the elevated plus maze. Rats fed the alcohol diet showed a significant decrease in open arm exploration during acute withdrawal, an effect attenuated by injections of nor-BNI when compared to control. Nor-BNI did not affect total arm entries, an indication of locomotor behavior, suggesting that this effect was unlikely due to a general change in motor activity. A parallel study found that nor-BNI (20 mg/kg) also reversed anxiety-like behavior in rats injected with the kappa agonist (trans)-3,4-dichloro-N-methyl-N-[2- (1-pyrrolidinyl)-cyclohexyl]benzeneacet-amide (U50,488, 10 mg/kg i.p.), suggesting that similar mechanisms are involved in the regulation of alcohol withdrawal- and kappa agonist-induced changes in behavior. These data suggest that the activation of kappa opioid receptors is involved in the regulation of stress-related behavior during withdrawal.
In conclusion, anxiety and depression appear to be critical factors contributing to relapse in many alcoholics. With regard to animal models, the reinstatement procedure has been used in the field of drug abuse research for over 20 years and is considered to be a valid model of relapse (Katz and Higgins, 2003). Exposure to a stressor such as footshock (Le et al., 2000; Shaham et al., 2003; Liu and Weiss, 2002) or social defeat (Funk et al., 2005) has been shown to reinstate alcohol-seeking behavior in rats. Although the role of kappa opioids in alcohol consumption is already being investigated by Dr. Walker, the role of kappa opioids in alcohol-seeking has yet to be fully explored. Recent evidence suggests that kappa opioids may work via stress-related mechanisms to reinstate drug seeking (Valdez et al., 2007), and decreasing kappa opioid activity may provide a novel method for preventing stress-induced relapse.
Stress-Induced Potentiation of Alcohol Reward
Consistent with Dr. Valdez’ presentation, other recent evidence suggests that kappa opioids may be a key mediator in the stress-related effects of drugs of abuse. For example, similar to stress or cocaine, priming injections of kappa agonists induce reinstatement of cocaine seeking in animals with previous cocaine experience (Beardsley, Howard, Shelton, and Carroll, 2005; McLaughlin et al., 2006; Valdez et al., 2007). Continuing investigations initiated in previously published work (Sperling et al., 2010), McLaughlin and colleagues hypothesized that signaling involving the KOR contributes to forced swim stress (FSS)-induced potentiation of alcohol reward and consumption, as well as reinstatement of alcohol-seeking behavior.
To examine the hypothesis, male C57BL/6J and prodynorphin gene-disrupted (Dyn −/−) or wild type littermate mice were exposed to repeated FSS. C57BL/6J mice were chosen for this work because of established strong responses generally to swim stress (Lucki et al., 2001) and drugs of abuse (Miner, 1997; Orsini et al., 2005), and the availability of prodynorphin gene-disrupted mice on the C57BL/6J background to further these studies (Carey et al., 2009). Biased alcohol conditioned place preference (CPP) and two-bottle free choice (TBC) assays were used to measure the effects of stress-induced KOR signaling on alcohol reward and self-administration, respectively. Additionally, the mediating effect of KOR signaling on stress-induced reinstatement of extinguished alcohol-seeking behavior was assessed with a modified conditioned place preference assay. To confirm the role of the KOR in the resulting behaviors, the KOR agonists U50,488 (10 mg/kg) or Salvinorin A (3 mg/kg), and antagonist nor-BNI (10 mg/kg) were administered prior to parallel testing. Data were then compared to salinetreated, control mice.
C57BL/6J and wild-type mice exposed to repeated FSS 5 min prior to daily place conditioning with alcohol (0.8 g/kg) demonstrated a potentiated alcohol-CPP and increased the consumption of 10% (v/v) alcohol in the TBC assay in a nor-BNI sensitive manner (as reported in Sperling et al., 2010). Dyn −/− mice did not demonstrate significant stress-induced increases in alcohol consumption (Sperling et al., 2010). Additionally, preliminary results presented suggested that exposure to FSS resulted in the reinstatement of an extinguished alcohol CPP response that was prevented by nor- BNI pretreatment. Although the CPP paradigm has been widely used to measure drug reward in rodents (Carr et al., 1989; Bardo et al., 1995; Tzschentke, 1998), early evidence suggested that the alcohol-preferring C57BL/6 mouse demonstrated poor alcohol place conditioning (Cunningham et al., 1992), similar to the situation originally encompassing alcohol place conditioning in rats (for review, see Walker and Ettenberg, 2007). However, the use of a biased design (e.g., place conditioning in which the alcohol / environment pairing occurs in the initially non-preferred environment) instead of an unbiased design (counterbalanced for initial preference) has proved conducive for the establishment of alcohol place preferences, possibly due to the ability to detect smaller preferences (Gremel, Gabriel, and Cunningham, 2006). A number of groups have now used this approach to obtain successful alcohol-CPP with C57BL/6 mice. For example, Kelley (1997) produced significant alcohol CPP with two daily place conditioning sessions in the initially non-preferred chamber. Similarly, Nocjar et al. (1999) utilized a biased place conditioning design and an additional sixteen alcohol / environment conditioning sessions to obtain a CPP response with C57BL/6 mice. The successful use of a biased design was further confirmed in subsequent studies (Middaugh and Bandy, 2000; Gremel et al., 2006; Sakoori and Murphy, 2008; Sperling et al., 2010).
Because the data presented by Dr. McLaughlin was specifically focusing on stress-induced anxiogenic modalities, the use of a biased design (with an apparatus that was reported to be unbiased) would seem to be particularly appropriate since these conditions lend themselves to the removal of a negative state which is interpreted by the organism as a positive event (analogous to negative reinforcement; Carr et al., 1989). Conversely, if one wanted to evaluate the positive, euphorogenic, properties of alcohol (analogous to positive reinforcement), they would be inclined to use an unbiased procedure which controls for initial preference and avoids assigning subjects to alcohol / environment pairings in a manner that would preferentially assess the removal of a negative state (Carr et al., 1989; Walker and Ettenberg, 2007). Of interest to the biased / unbiased conditioning procedure discussion is the fact that the ability to form place preferences using either of these subject assignment procedures is dependent on the apparatus itself and whether it is biased or unbiased (i.e., whether the subjects prefer the apparatus environments in an equal or a skewed manner). Indeed, it has been shown that successful use of an unbiased conditioning procedure requires an unbiased apparatus (e.g., Walker and Ettenberg, 2007), whereas a biased design can be used with either an unbiased or biased apparatus (Cunningham et al., 2003; Tzschentke, 2007). These reports support the further use of the CPP paradigm to study the rewarding effects of alcohol and suggest that the ultimate goal / modality of interest should provide the basis for the appropriate selection of the place preference experimental design.
Pretreatment with U50,488 90 min prior to daily alcohol place conditioning substituted for FSS exposure, resulting in a potentiation of alcohol-CPP, and increased alcohol consumption (Sperling et al., 2010). This is admittedly paradoxical, given that acute KOR activation prevents the rewarding effects induced by reinforcing drugs such as cocaine and alcohol (Shippenberg and Herz, 1986; Herz, 1997; Shippenberg et al., 2007). Consistent with established theory, acute KOR activation with U50,488 has been shown to decrease alcohol self-administration (Lindholm et al., 2001) and alcohol- CPP (Logrip et al., 2009). However, repeated KOR agonist treatment has been demonstrated to paradoxically enhance the dopamine signaling associated with the perception of reward (Heidbreder et al., 1998; Dalman and O’Malley, 1999; Thompson et al., 2000). Repeated KOR agonist treatment produces a deficiency in dopamine transporter activity (Thompson et al., 2000; Collins et al., 2001) without altering total expression levels of transporter protein (Thompson et al., 2000). Notably, repeated administration of the KOR agonist U69,593 increased stimulated extracellular levels of mesolimbic dopamine in the rat nucleus accumbens (Fuentealba et al., 2006). These mechanistic findings are consistent with a behavioral report where repeated infusion of U50,488 produced a dose-dependent, nor-BNI sensitive leftward shift in the cocaine-choice dose–effect curve of rhesus monkeys self-administering cocaine, but not food (Negus, 2004). The author speculates that chronic activation of kappa opioid systems, either from exogenous or endogenous sources, may enhance the relative reinforcing efficacy of cocaine. This effect was directly observed with CPP studies demonstrating U50,488 administration which both suppressed and potentiated cocaine-CPP in a time-dependent, nor-BNI-sensitive manner (McLaughlin et al., 2006). Overall, a growing set of evidence suggests that KOR signaling may bi-directionally modulate the reinforcing effects of drugs of abuse in a time-dependent manner, although the mechanism of the KOR-mediated potentiation has yet to be fully determined.
Interestingly, treatment with the KOR agonist U50,488, but not Salvinorin A, induced reinstatement of alcohol CPP. While the U50,488-induced reinstatement was prevented by pretreatment with nor-BNI, the underlying mechanism mediating the difference in activity between the two KOR agonists is less clear but potentially important. While both KOR ligands are known to be full agonists (Chavkin et al., 2004), the present results are potentially consistent with the ability of these KOR agonists to activate extracellular regulated kinase (ERK) 1/2 mitogen-activated protein (MAP) kinase. Salvinorin A has been shown to be ~40% less effective than U50,488 at inducing internalization and down-regulation of the human KOR (Wang et al., 2005), suggesting a mechanistic basis for both the difference in effect observed here and the potentiation effect described earlier. The suggestion that distinct, equally potent, receptor ligands could have the capacity to recruit one intracellular signaling pathway over the other, termed biased agonism, functional selectivity, or differential agonism (Urban et al., 2007), has raised new possibilities for distinguishing receptor ligands by the unique signaling properties that they may possess (see Future Directions section for additional discussion). This work has been mostly limited to demonstrations in cell culture, where KOR agonists are well known to activate MAP kinase (Belcheva et al., 1998; Jordan et al., 2000). Collectively, however, these data help to reconcile discrepancies in the effects of KOR ligands. For instance, some reports suggest that KOR internalization is required for activation of p38 (Bruchas et al., 2006) and ERK1/2 (Ignatova et al., 1999) while others suggest KOR internalization is not required (Li et al.,1999; Jordan et al., 2000), or that ERK1/2 MAP activation requires PKC (Belcheva et al., 1998). Given the data obtained from studies of Gs-coupled GPCRS demonstrating two distinct temporal mechanisms for the GPCR activation of ERK1/2 (Ahn et al., 2004; Shenoy et al., 2006), it is feasible that a similar dual mechanism regulates the ERK1/2 MAP kinase activation by KOR. As demonstrated by the present results, the signaling differences arising from this dual mechanism may have significant behavioral consequences. Supporting this, recent work in the opioid field has shown that ligand-directed trafficking of the delta-opioid receptor resulted in distinct adaptive responses in tolerance to specific DOR agonist effects in vivo (Pradhan et al., 2010). Likewise, a recent review concluded that KOR ligands may differ in their ability to activate p38 MAP kinase in a manner that correlates with observed differences in producing dysphoric or hallucinogenic effects behaviorally (Bruchas and Chavkin, 2010). Despite this, surprisingly, there are few studies to date that have clearly examined this possibility in regards to treating drug- or alcohol-seeking behaviors. Further studies highlighting the importance of downstream signaling resulting from KOR activation, e.g. p38 or ERK1/2 MAP kinase, using KOR agonists capable of agonist-selective signaling or biased agonism, in mediating drug-seeking behavior, may suggest both additional insights into the neurobiology of stress and alcoholism, and new therapeutic approaches for treatment.
In conclusion, as many physiological and behavioral processes are modulated by the endogenous KOR system, understanding and determining the importance of the precise intracellular signaling proteins involved will be a major challenge, as not all drugs produce the same effects. Targeting the precise cascades of therapeutic drug action will be an important task. For now, the ongoing goal is to acknowledge and investigate this diversity, and assay its potential role in the development of effective therapeutics for drug and alcohol dependence. From a therapeutic perspective, the results suggest that developing KOR agonists that do not elicit a late phase-activation of ERK1/2 MAP kinase could eliminate the unwanted side effect of paradoxical potentiation of cocaine reward.
Dependence-Induced Escalation in Operant Self-Administration
From a theoretical perspective, one approach to the analysis of behaviors and underlying neuroadaptations associated with chronic alcohol exposure would be to apply the conceptual framework of the Opponent–Process Theory of Motivation (Solomon and Corbit, 1974). Essentially, this theory states that in order to maintain homeostasis, an increase in hedonic state will be followed by a compensatory decrease in hedonic state. Furthermore, after repeated stimulations, the positive hedonic state is reduced while the negative affective component is enhanced to compensate for the continued perturbation of the affective system produced by chronic alcohol exposure (see Figure 1). These opponent–processes have been linked to allostatic mechanisms (Koob and Le Moal, 2001) and are hypothesized to reflect a new set–point whereby an individual would be required to continue ingesting drugs of abuse to maintain a normal emotional state. It follows that cessation of alcohol intake would result in negative affective states as symptoms of withdrawal that could drive an organism to excessively seek and use alcohol. Based on the neuroanatomical distribution of KORs in motivational and affective circuitry, KOR agonists can oppose the effects of MOR agonists (Di Chiara and Imperato, 1988). In accordance with the Opponent–Process Theory, if alcohol-mediated MOR or DOR stimulation (Marinelli et al., 2004; 2005; Lam et al., 2008; Jarjour et al., 2009) produces positive hedonic states (Amalric et al., 1987; Shippenberg et al., 1987), then a compensatory mechanism could be increased DYN and/or function of KORs, stimulation of which produces negative hedonic states (Mucha and Herz, 1985). Under conditions of chronic alcohol use (see Figure 1), the predicted response of the EOS would be attenuated MOR signaling and exacerbated DYN / KOR system activity, both of which are supported in the literature (Gianoulakis et al., 1996; Przewlocka et al., 1997; Turchan et al., 1999; Chen and Lawrence, 2000; Lindholm et al., 2000; Saland et al., 2004; Saland et al., 2004; Lindholm et al., 2007). Alterations in the EOS following chronic drug exposure have, in part, been associated with increased CREB-mediated transcriptional genetic regulation (Carlezon et al., 1998; 2005). Therefore, if DYN systems were upregulated or there was increased signaling via KORs following chronic alcohol exposure sufficient to produce escalated alcohol self-administration (Roberts et al., 2000; O’Dell et al., 2004; Walker and Koob, 2007), KOR antagonists should be able to reduce the negative affective states associated with withdrawal and reduce the excessive alcohol self-administration. Indeed, Dr. Walker previously demonstrated that both systemic and central administration of a KOR antagonist was able to selectively reduce escalated operant self-administration of alcohol in dependent Wistar rats while leaving nondependent alcohol self-administration intact (Walker and Koob, 2008a; Walker et al., 2011). The selective effects of KOR antagonism in dependent organisms strongly implicate the recruitment of DYN systems during the transition to dependence, rather than DYN involvement with the acute reinforcing effects of alcohol.
Figure 1. Opioidergic Compensatory Responses Prior to and Following Chronic Alcohol Exposure.
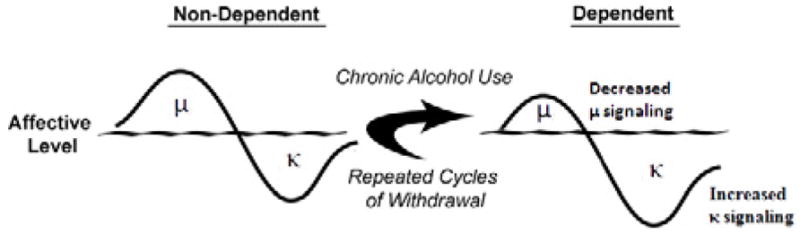
In non-dependent organisms, alcohol-induced μ-opioid receptor-related positive affective states precede compensatory κ-opioid receptor mediated negative affective states. Following chronic alcohol exposure, μ-opioid receptor signaling is attenuated and through multiple mechanisms, κ-opioid receptor signaling is increased; both of which can combine to produce a phenotype indicative of negative motivational and affective states.
Alcohol Dependence and Extended Amygdala KORs
The extended amygdala (Alheid and Heimer, 1988) is a network of functionally interconnected nuclei related to motivation and emotion that includes areas such as the nucleus accumbens shell (AcbSh) and the central nucleus of the amygdala (CeA). Substantial evidence supports the concept of chronic alcohol–induced alterations in DYN or KOR expression that can impact areas such as the AcbSh and CeA. Chronic alcohol–exposed animals have increased prodynorphin mRNA levels in the nucleus accumbens (Acb et al., 1997), increased expression of DYN B in the Acb (Lindholm et al., 2000), altered KOR mRNA expression in the Acb and ventral tegmental area (Rosin et al., 1999), as well as decreased indices of dopaminergic function in the Acb (Carroll et al., 2006; Healey et al., 2008). KORs are neuroanatomically positioned on dopamine (DA) terminals in the AcbSh (Di Chiara and Imperato, 1988) so as to reduce DA release. In an elegant study measuring DA release within the Acb of naive and chronic alcohol– treated animals following pharmacological challenges with selective KOR antagonists (Lindholm et al., 2007), it was shown that only the chronic alcohol–treated animals showed increased DA release following administration of a selective KOR antagonist. This implied that the chronic alcohol–treated animals had upregulated DYN transmission that was exposed by treatment with the antagonist. Chronic alcoholinduced KOR-mediated attenuation of dopaminergic function is not inconsequential, as deficiencies in dopaminergic transmission in limbic areas have been posited to be the neurobiological basis of depression (Nestler and Carlezon, 2006). Support for the CeA as a potential site of DYN action comes from evidence showing that dense populations of KORs are in the CeA (Mansour et al., 1987) and, in support of the Opponent Process Theory, acute alcohol administration initially increases βEND, which is then followed by a significant increase in DYN A (Lam et al., 2008). This pattern of EOS release has also observed in the ventral tegmental area (the source of mesocorticolimbic DA) in which alcohol increased βEND thirty min after the administration, but that increase in βEND dissipated and was followed by an increase in DYN A two hr following the alcohol administration (Jarjour et al., 2009).
To functionally assess the hypothesis that neuroadaptations in the EOS of the extended amygdala contribute to escalated operant alcohol self-administration in dependent Wistar rats, Dr. Walker presented data on the effects of KOR, MOR, DOR, and general opioid receptor antagonism (i.e., nalmefene) in the AcbSh (Nealey et al., 2011) and KOR antagonism in the CeA (Walker, unpublished results) on alcohol self-administration in nondependent and alcohol-dependent rats. One caveat to consider is that nalmefene’s binding affinity is higher for KOR and MOR than DOR (for review, see Walker and Koob, 2008). Consistent with previous research (June et al., 1998; Hyytia and Kiianmaa, 2001; June et al., 2004), intra-accumbens MOR / DOR antagonism (using a CTOP / naltrindole cocktail) and nalmefene showed efficacy for reducing ethanol self-administration in nondependent animals. In dependent animals, KOR antagonism (nor- BNI) in both the AcbSh (Nealey et al., 2011) and CeA (Walker, unpublished results) selectively reduced dependence-induced excessive self-administration and nalmefene was shown to be significantly more potent, but there were no changes in the response to CTOP / naltrindole. Taken together, a KOR mechanism was implicated in the increased potency of nalmefene, however it is extremely important to understand that this interpretation is not stating that MOR / DOR antagonism was ineffective in dependent animals, as the MOR / DOR antagonists were able to dose-dependently reduce self-administration. Instead, the KOR mechanism was identified as a pharmacotherapeutic target with a theoretically distinct basis that putatively involves negative reinforcement mechanisms rather than positive reinforcement mechanisms as the MOR / DOR does (Walker and Koob, 2008a; Nealey et al., 2011).
Negative Reinforcement in Alcohol-Dependent Populations
As alcoholism is considered to be a chronic relapsing disorder, it is critical that the distinction between the positive and negative reinforcing effects of alcohol is understood (although positive reinforcement is well understood, the term negative reinforcement is often misused, see below). Negative reinforcement specifically refers to the removal of a negative state that is interpreted as a reinforcing event (i.e., the removal of bad is good). In dependence, alcohol can remove a negative withdrawal state (hypothesized to include upregulated DYN producing increased KOR stimulation) which leads to an increased probability of alcohol use when in withdrawal. Within this context, KOR stimulation would be considered a punisher (i.e., the application of a negative stimulus), not a negative reinforcer as some have posited (Shippenberg et al., 2007). It is the hypothesized removal of the DYN / KOR-induced negative affect that makes alcohol a negative reinforcer and a dependent individual more likely to excessively consume alcohol. Furthermore, because KOR antagonists appear to attenuate the negative affective states characterizing withdrawal, they too can be considered negative reinforcers and it is the negative reinforcing properties of KOR antagonists that will likely lead to their acceptance by alcohol-dependent populations and result in dramatically increased medication compliance (i.e., people should want to take the medication because it will be able to remove a negative state). However, unlike alcohol, the use of KOR antagonists is not positively reinforcing and does not produce euphoria. Thus, targeting the KOR with antagonists or functional antagonists (see below) will result in removal of the negative aspects of dependence without the production of positive, euphorogenic states that could themselves lead to abuse.
The precise nature of the changes that occur during the transition to an alcohol dependent state remain to be established. As determined by the results presented by Dr. Walker, antagonism of KORs in the AcbSh and CeA functionally impacts ethanol-dependent animals in an exclusive manner. The AcbSh contains terminal regions of the mesolimbic DA system which have been implicated as a signaling system for biologically relevant information through which natural reinforcers (e.g., see Hull et al., 1999; Carelli, 2002; Kelley et al., 2002; Kelley, Baldo, Pratt, and Will, 2005) and drugs of abuse (see Koob, 2000; Maldonado, 2003; Di Chiara et al., 2004; Ikegami and Duvauchelle, 2004) exert their behavioral effects. Dysregulation of mesolimbic DA system functioning has been posited as a basis for disorders of affect, such as depression (Nestler and Carlezon, 2006) and self-medication of disorders of affect has been hypothesized to be a major contributor to excessive alcohol and drug use and relapse (Markou et al., 1998). Considerable work has focused on KOR modulation of DA transmission, but KORs within the AcbSh can presynaptically modulate other neurotransmitters (e.g., glutamate, GABA and serotonin; Fields et al., 2007; Land et al., 2009). Therefore, the specific neurotransmitter system(s) that would be impacted by upregulated DYN or increased KOR signaling remain to be established.
Certain properties of the ligands mentioned above should be made apparent in this review and will hopefully clarify some of the confusion that currently exists in the literature. The first relates to the classification of nalmefene as a general opioid receptor antagonist. Hormonal and intracellular signaling data established with nalmefene have led to the suggestion that nalmefene could be a partial agonist at the KOR (Bart et al., 2005). In regards to the results presented by Dr. Walker, if nalmefene was determined to be a partial agonist at the KOR, based on the fact that DYN levels have been shown to be upregulated following chronic ethanol exposure, then nalmefene could still serve as a functional antagonist at the KOR due to competition with endogenous DYN and, if bound, would produce less of an effect than DYN itself. Thus, the net effect would be decreased signaling via the KOR. Partial agonists at the KOR, as opposed to antagonists, could be of special importance in the alcohol dependence field because of evidence showing that withdrawal in alcohol dependent individuals can be characterized by increased seizure probability and that KOR stimulation in the hippocampus protects against the production of certain types of seizures (Loacker et al., 2007; Schunk et al., 2010)
The second relates to the appropriate pretreatment times when using nor-BNI and revolve around controversial specificity of nor-BNI for the KOR immediately following administration. Evidence from mice tested in nociceptive assays has suggested that nor-BNI has mild affinity for the MOR immediately after administration that appears to last at least 2 h (Broadbear et al., 1994), but has dissipated within 24 hrs. Because of this, some researchers posit the use of an extended nor-BNI pretreatment duration; however, the transient MOR affinity of nor-BNI that has been observed in mice (e.g., Broadbear et al., 1994) was not replicated using rats (Picker et al., 1996). Furthermore, Dr. Walker’s data using Wistar rats has confirmed that there are no observed differences in the effects of nor-BNI when administered immediately or 24 hours prior to ethanol self-administration sessions in nondependent and ethanol-dependent rats (Walker and Koob, 2008a; Walker et al., 2011). Specifically, if nor-BNI has MOR affinity that is functionally-relevant to alcohol consumption when assessing motivational circuitry and behaviors, then one would predict that nondependent ethanol self-administration should be impacted if nor-BNI treatment occurred immediately prior to self-administration sessions. However, as observed using a central route of administration (Walker and Koob, 2008a), infusions of nor-BNI immediately prior to self-administration sessions did not impact nondependent ethanol self-administration. That lack of acute nor-BNI effects in nondependent animals following ICV infusion was confirmed in animals receiving site-specific AcbSh (Nealey et al., 2011) and CeA (Walker, unpublished results) nor-BNI infusions and additionally supported by the fact that subtype-selective antagonists targeting the MOR / DOR did reduce nondependent self-administration of alcohol. Thus, following systemic, ICV, intra-AcbSh and intra-CeA infusions at two separate test locations (i.e., The Scripps Research Institute, La Jolla, CA and Washington State University, Pullman, WA), there were no behavioral indications that the effects of nor-BNI involved a MOR mechanism of action in Wistar rats. One consideration related to this issue is the differential EOS peptide and receptor expression found in various rodent strains (Jamensky and Gianoulakis, 1997; e.g., Gieryk et al., 2010) that could subserve the presence or absence of MOR-related effects following acute nor-BNI administration. For example, the dose-response curve for morphine conditioned place preference is shifted to the right in Wistar rats when compared to another commonly used strain of outbred rats (Shoaib et al., 1995), the basis of which could be interpreted as a differential MOR expression patterns. Taken together, statements positing ubiquitous pharmacological effects of nor-BNI, irrespective of genetic differences in the EOS, should be made with caution and could unnecessarily complicate pharmacotherapeutic development efforts to treat alcohol use disorders.
The contribution of the EOS to alcohol reinforcement and consumption has been evaluated through targeted mutations of genes (i.e., knockouts) coding for the peptide precursors of βEND (proopiomelanocortin; Pomc), ENK (proenkephalin; Penk) and DYN (prodynorphin; Pdyn) and their associated receptors (MOR (Oprm1), DOR (Oprd1) and KOR (Oprk1), respectively). Although recent human studies have identified important associations between human PDYN and OPRK1 genes and alcohol dependence (Xuei et al., 2006; Edenberg et al., 2008), knockout (KO) studies focusing on DYN / KOR systems that have been conducted using C57BL/6 mice (Kovacs et al., 2005; e.g., Blednov et al., 2006; Sperling et al., 2010; Femenia and Manzanares, 2011), while confirming that DYN / KOR systems are involved in alcohol reinforcement and reward, have produced inconsistent results thus far. For example, Pdyn KO mice have been shown to consume less (Blednov et al., 2006; similar to KOR KO mice, Kovacs et al., 2005), more (Femenia and Manzanares, 2011), or the same amount of alcohol (Sperling et al., 2010) than wild-type controls which make comparisons to KOR agonist / antagonist effects difficult. These discrepant results parallel those seen in studies targeting Pomc / Oprm1 genes (e.g., Grisel et al., 1999; Roberts et al., 2000). Interpretation of data involving developmental KO mice is made difficult by the possibility of long-term compensatory responses to missing, yet critical, components of the EOS and the fact that Pdyn-derived peptides include ENK, as well as DYN (Brownstein, 1980). This is exemplified by a recent study that measured EOS gene regulation in response to Pdyn KO (Femenia and Manzanares, 2011). Behaviorally, the study observed enhanced alcohol intake and CPP with altered expression patterns for genes coding for tyrosine hydroxylase, dopamine transporter, Oprk1, Oprm1 and Oprd1 in alcohol reward-related areas of the brain. The wide-ranging alterations in EOS gene expression patterns produced by Pdyn KO complicates the attribution of specific genes to altered alcohol consumption profiles and highlights the complex interplay of factors within the EOS. However, it is notable that, consistent with the effect of KOR antagonists on FSS-induced potentiation of alcohol CPP, Dr. McLaughlin’s group did not observe the potentiating effects of repeated FSS on alcohol reward and consumption in Pdyn KO mice (Sperling et al., 2010). In addition, the results presented by Drs. Walker, McLaughlin and Valdez are consistent with each other both theoretically and experimentally, with the identification of methodological differences providing substantial insight into those studies that do prove to be inconsistent (e.g., the difference in timing of KOR agonist administration providing a basis for either suppression or potentiation of alcohol CPP). Future studies using various strategies to conditionally modify a gene of interest with enhanced systems-level specificity will allow for normal development, provide the basis for restricted excision or overexpression of genes in adult rodents and may allow for enhanced convergence of pharmacological and genetic manipulations.
In conclusion, KORs in the extended amygdala are involved in the escalated self-administration indicative of dependence. Furthermore, KOR antagonists or partial agonists could be of great utility as a treatment for alcohol dependence. Understanding the precise role of KORs through pharmacological and genetic manipulations within different brain circuitry will help to delineate the behavioral domains that should be focused on when tailoring treatment plans in clinical settings and additionally provide a basis for future investigations designed to understand the molecular basis of escalated self-administration and negative affect associated with alcohol dependence.
Alcohol Dependence, Cognitive Dysfunction and Behavioral Dysregulation
Several lines of evidence demonstrate the critical role of the EOS in alcohol dependence. Pharmacological and genetic manipulations with the opioid receptors alter alcohol drinking in animals. In clinics, naltrexone, the non-selective opioid antagonist reduces alcohol drinking and relapse rates in subgroups of alcoholics. Polymorphisms in OPRK1 and PDYN genes that give rise to DYNs, the endogenous KOR ligands (Chavkin et al., 1982), are associated with alcohol dependence (Xuei et al., 2006; Edenberg et al., 2008).
Besides regulation of neurotransmission in reward circuits, the EOS may have a role in specific cognitive processes relevant for control of addictive behavior including craving, decision-making and impulsivity (O’Malley et al., 2002; Bencherif et al., 2004; Boettiger et al., 2009; Love et al., 2009; Koob and Volkow, 2010). In alcoholics, effects of naltrexone in response to alcohol cues, and during decision-making involve the orbitofrontal cortex (OFC; O’Malley et al., 2002; Myrick et al., 2008; Love et al., 2009; Koob and Volkow, 2010), while MOR binding in the dorsolateral prefrontal cortex (dl- PFC) is functionally related to alcohol craving (Bencherif et al., 2004). Processes relevant for motivated behavior such as reward pursuit, and risk taking and the development of substance use disorders are related to functions of the EOS. Several impulse-control disorders including pathological gambling and binge eating may be relieved by naltrexone, suggesting a role of endogenous opioids in impulsivity (Boettiger et al., 2009). Thus, the molecular dysregulation of the EOS system may contribute to impaired neurocognitive function and reduced regulation of alcohol / drug seeking and consumption.
Dr. Bakalkin focused on two primary questions. First, does the EOS undergo molecular adaptations in the brains of human alcoholics? Second, what are the molecular mechanisms through which these adaptations may alter brain function and alcohol-associated behaviors? To characterize the molecular adaptations underlying alcohol dependence, Dr. Bakalkin’s group analyzed brain tissue from human alcohol-dependent subjects for the expression of EOS genes coding for both the receptors (OPRM1, OPRD1 and OPRK1) and precursor peptides (POMC, PENK and PDYN) in the dl-PFC (Brodmann area 9), OFC (Brodmann area 47) and the hippocampus (dentate gyrus); areas involved in cognitive control of addiction (Taqi et al., 2011b; Taqi et al., 2011a; Bazov et al., 2011). The motor cortex (MC) was included as a control area not involved in alcohol dependence. Postmortem human samples were collected from 14 alcoholics and 14 controls at the Tissue Resource Center, University of Sydney (http://www.braindonors.org; http://rp-host.www.pathology.med.usyd.edu.au/trc/index.php; Sheedy et al., 2008). Alcoholics met DSM-4 criteria and consumed greater than 80 g of alcohol per day for the majority of their adult lives. Controls had either abstained from alcohol or were social drinkers who consumed less than 20 g of alcohol per day. Cases with a history of poly-drug abuse or with neurological complications were excluded. Of six opioid genes studied, POMC was expressed at low levels and was excluded from further analysis.
The comparison of alcoholics and controls demonstrated a significant increase for PDYN mRNA in the dl-PFC, and OPKK1 mRNA in the OFC in alcoholics (Bazov et al., 2011; Taqi et al., 2011b). The levels of the other opioid mRNAs did not differ significantly between alcoholics and controls. Analysis of dynorphins by RIA confirmed the expression data. Levels of both dynorphin A (DYN A) and dynorphin B (DYN B) were significantly elevated in the dl-PFC and hippocampus of alcoholics. Changes in the MC were not significant. Importantly, the levels of PDYN mRNA significantly correlated with those of DYN peptides. Analysis of age, postmortem interval, brain pH, storage time, agonal factor score, RNA quality index and smoking history as covariates failed to influence differences between the two groups. Preliminary experiments demonstrated that DYNs are also upregulated in the insula, another cortical area, whereas not affected in the caudate and putamen, all three brain areas involved in alcohol dependence.
Analysis of between-area associations of EOS parameters that were significantly altered in alcoholics identified high significant correlation between the dl-PFC DYN A and OFC OPRK1 in the control group, but not in alcoholics, and significant differences between the groups in the correlation coefficient (Bazov et al., 2011). The correlation suggests a functional DYN / KOR - mediated link between these two areas under normal but not pathological conditions. This speculation is consistent with the hypothesis that the proper functioning of the cerebral cortex requires the successful dynamic interactions between distributed cortical networks of dl-PFC and OFC, and that disrupted dynamics of the dl-PFC - OFC interaction contributes to addictive disorders (Moghaddam and Homayoun, 2008). Mechanistically, the PDYN expressing dl-PFC neurons may project to the OFC where released DYNs may regulate KOR expression. This is in line with animal data showing that KOR expression may be regulated through activation of this receptor (D’Addario et al., 2004; Collins et al., 2001).
Thus the DYN / KOR system including PDYN mRNA and DYNs in the dl-PFC, DYNs in the hippocampus, and OPRK1 mRNA in the OFC, was found to be upregulated in human alcoholics (Bazov et al., 2011; Taqi et al., 2011b; Taqi et al., 2011a). These alterations were observed in brain regions involved in cognitive control of addictive behavior and may represent molecular adaptations developed after many years of alcohol exposure and withdrawal. No significant differences between alcoholics and controls in expression of OPRM1, OPRD1 and PENK genes were evident. In the dl-PFC, hippocampus and insula, DYNs may have a role in regulation of executive and intellectual functions, learning and memory, and emotions in alcoholics, hereas their elevation may impair these cognitive processes. While this hypothesis has not been addressed yet, several studies indirectly support this view (Jiang et al., 1989; Nguyen et al., 2005; Sandin et al., 1998b). In pharmacological experiments, spatial learning and memory were impaired by synthetic DYN B administered into hippocampus (Sandin et al., 1998a). Aged mice and rats perform worse in learning and memory tests, and have higher Pdyn expression and DYN levels than young animals (Jiang et al., 1989). Deletion of Pdyn increases the acquisition and retention of spatial performance in the aged mutant mice compared to wild-type animals (Nguyen et al., 2005). Consistently, postmortem human study found elevated DYN A levels in the PFC of patients with Alzheimer’s disease, and a correlation between these levels and neuropathological scores (Yakovleva et al., 2007).
PDYN gives rise to α-neoendorphin, DYN A and DYN B (cleaved from big dynorphin), all of which are endogenous KOR ligands (Merg et al., 2006). In addition, DYN A can induce effects that are not blocked by opioid antagonists (Faden and Jacobs, 1983; Dubner and Ruda, 1992; Caudle and Mannes, 2000; Singh et al., 2003; Lai et al., 2001; Tan-No et al., 2001; Tan-No et al., 2005; Hauser et al., 2005; Lai et al., 2006). These non-canonical non-opioid effects are generally excitatory, may lead to neurodegeneration and pathological behavior such as chronic pain and paralysis. The non-opioid effects were observed in cell culture toxicity and in vivo animal models. Incubation of cultured mouse striatal neurons with DYN A caused significant activation of proapoptotic caspase-3 and the subsequent death of neurons (Singh et al., 2003) that was not blocked by naloxone. Thus, DYN A is intrinsically neurotoxic peptide which actions are not mediated through opioid receptors.
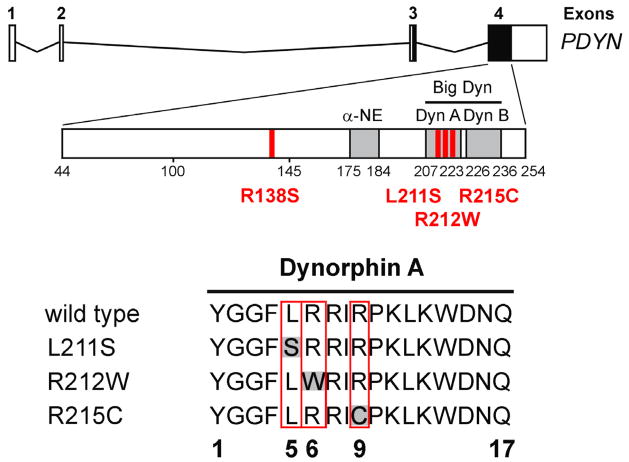
Much of the knowledge about the function of genes and proteins in human brain has come from analysis of a small number of rare mutations. Mutations that cause a disease may represent entry points into novel signaling pathways and molecular mechanisms. Such mutations in the human PDYN gene have been discovered in our recent study, thus providing the first human evidence for pathogenic effects of DYNs (Bakalkin et al., 2010). Spinocerebellar ataxia, or SCA, is a dominant genetic neurodegenerative disorder (Bakalkin et al., 2010; Verbeek et al., 2004). Four missense mutations that cause SCA 23, a new type of SCA, have been identified. Remarkably, three of them including L211S, R212W and R215C are located in the pathogenic DYN A (see Figure 2) and at least two of the mutations can result in a 10–20 fold elevation of DYN A production in recombinant cell lines expressing either wild-type or mutant Pdyn. This may occur due to slower conversion of mutant peptides to ENK or enhancement of the intrinsic neurotoxicity of DYN A as evident from the time lapse imaging analysis of their effects on viability of striatal neurons in culture. Because a subset of the SCA23 subjects are heterozygotes (they carry one wild-type and one mutant PDYN allele), the DYN A mutants are dominant over the wild-type peptide. Generalized pathological changes in the SCA23 subjects demonstrate a fundamental role of DYNs in regulation of neuronal functions and neurodegeneration in the human brain. This role has not been previously established with opioid agonists and antagonists, and Pdyn mutant animals.
Figure 2. Human PDYN missense mutations.
PDYN exons 3 and 4 encode PDYN, which gives rise to the opioid peptides α-neoendorphin (α-NE), dynorphin A (Dyn A), dynorphin B (Dyn B), and big dynorphin (Big Dyn; which encompasses Dyn A and Dyn B). Analysis of DNA sequencing identified four missense mutations, two of which resulted in mutations of Dyn A that were associated with altered Dyn A production.
Large-scale pathological changes induced by mutant peptides suggest that wild type DYNs upregulated in alcoholics may cause dysfunctions or even neurodegeneration of neuronal networks in which these peptides are expressed. Indeed, DYNs are selectively elevated in the areas characterized by substantial cell loss and / or decrease in gray matter volume including dl-PFC, insula, and hippocampus in alcoholics (Bakalkin et al., 2010; Verbeek et al., 2004; Kril et al., 1997; Harper et al., 1985; Sullivan and Pfefferbaum, 2005).
The overall conclusions from Dr. Bakalkin’s presentation were that the EOS undergoes adaptive changes associated with alcohol dependence in human brain (Bazov et al., 2011; Taqi et al., 2011b; Taqi et al., 2011a). The most consistent changes are observed for the PDYN gene and its peptide products DYNs. DYNs were upregulated in the dl-PFC, hippocampus and insula may contribute to impairment of cognitive control of alcohol consumption. Analysis of the dominant genetic neurodegenerative disorder SCA23, identified mutations in DYN A that cause this neurological disease by inducing profound neurodegeneration in the human brain (Bakalkin et al., 2010). Based on these findings we hypothesize that both the opioid-receptor mediated and non-opioid neurodegenerative mechanisms may underlie the behavioral effects of upregulated DYNs in alcoholics. It is essential to develop pharmacological tools to block these non-canonical pathogenic DYN effects, and to apply these tools for further analysis of the DYN A-driven non-opioid neurotoxic mechanism in alcoholism. Because PDYN polymorphism is associated with alcoholism (Xuei et al., 2006), it is important to establish whether genetic variations in this gene contribute to cognitive impairment associated with alcohol dependence and abuse, and associated neurodegeneration.
Conclusions
Drs. Valdez, McLaughlin, Walker and Bakalkin presented data regarding the multifaceted role of DYN and the KOR as a basis for conditions associated with alcohol use disorders, especially alcohol dependence. A number of emerging targets were presented in the symposium that included the KOR as a mediator of negative affect associated with withdrawal and the effects of repeated stress, as well as dependence-induced escalation of responding and neurocognitive deficits (some of which appear to be opioid receptor independent). Furthermore, the emerging concept of biased agonism could become increasingly important and has raised new possibilities for distinguishing receptor ligands by the unique signaling properties that they may possess. Collectively, the data presented in this symposium highlight the increasingly important role of the KOR as a pharmacotherapeutic target for the treatment of alcoholism and identify a number of important directions for future research.
Acknowledgments
Support for this research was provided by R01AA020394 awarded to BMW, R15AA018213 awarded to GRV and R13AA017581 to Dr. Marisa Roberto to support the “Alcoholism and Stress: A Framework for Future Treatment Strategies” conference in Volterra, Italy on May 3–6, 2011 from the National Institute on Alcohol Abuse and Alcoholism, WSU Alcohol and Drug Abuse Research Program grants to BMW according to the State of Washington Initiative Measure No. 171, grants from the Swedish Council for Working Life and Social Research (FAS), AFA Forsäkring and the Swedish Science Research Council to GB and R01 DA023924 and the State of Florida to JPM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health, the States of Florida, Michigan and Washington or the country of Sweden.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Amalric M, Cline EJ, Martinez JL, Jr, Bloom FE, Koob GF. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology (Berl) 1987;91:14–19. doi: 10.1007/BF00690919. [DOI] [PubMed] [Google Scholar]
- Bakalkin G, Watanabe H, Jezierska J, Depoorter C, Verschuuren-Bemelmans C, Bazov I, et al. Prodynorphin mutations cause the neurodegenerative disorder spinocerebellar ataxia type 23. Am J Hum Genet. 2010;87:593–603. doi: 10.1016/j.ajhg.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, Kreek MJ. Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacology. 2005;30:2254–2262. doi: 10.1038/sj.npp.1300811. [DOI] [PubMed] [Google Scholar]
- Bazov I, Watanabe H, Kononenko O, Kuntic V, Sarkisyan D, Taqi M, et al. The endogenous opioid system in human alcoholics: molecular adaptations in brain areas involved in cognitive control of addiction. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00366.x. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Vogel Z, Ignatova E, Avidor-Reiss T, Zippel R, Levy R, et al. Opioid modulation of extracellular signal-regulated protein kinase activity is ras-dependent and involves Gbetagamma subunits. J Neurochem. 1998;70:635–645. doi: 10.1046/j.1471-4159.1998.70020635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif B, Wand GS, McCaul ME, Kim YK, Ilgin N, Dannals RF, et al. Mu-opioid receptor binding measured by [11C]carfentanil positron emission tomography is related to craving and mood in alcohol dependence. Biol Psychiatry. 2004;55:255–262. doi: 10.1016/j.biopsych.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Harris RA. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol. 2006;40:73–86. doi: 10.1016/j.alcohol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Kelley EA, Mitchell JM, D’Esposito M, Fields HL. Now or Later? An fMRI study of the effects of endogenous opioid blockade on a decision-making network. Pharmacol Biochem Behav. 2009;93:291–299. doi: 10.1016/j.pbb.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- Brown SA, Schuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49:412–417. doi: 10.15288/jsa.1988.49.412. [DOI] [PubMed] [Google Scholar]
- Brownstein MJ. Opioid peptides: search for the precursors. Nature. 1980;287:678–679. doi: 10.1038/287678a0. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘natural’ reinforcement. Physiol Behav. 2002;76:379–387. doi: 10.1016/s0031-9384(02)00760-6. [DOI] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Lyons AM, Shay CF, Dunton O, McLaughlin JP. Endogenous kappa opioid activation mediates stress-induced deficits in learning and memory. J Neurosci. 2009;29:4293–4300. doi: 10.1523/JNEUROSCI.6146-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Carr G, Fibiger H, Phillips S. Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ, editors. The Neuropharmacological basis of reward. Oxford: Clarendon Press; 1989. pp. 264–319. [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in wistar kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MR, Rodd ZA, Murphy JM, Simon JR. Chronic ethanol consumption increases dopamine uptake in the nucleus accumbens of high alcohol drinking rats. Alcohol. 2006;40:103–109. doi: 10.1016/j.alcohol.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle RM, Mannes AJ. Dynorphin: friend or foe? Pain. 2000;87:235–239. doi: 10.1016/S0304-3959(00)00360-2. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, et al. Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious kappa-opioid receptor agonist: structural and functional considerations. J Pharmacol Exp Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- Chen F, Lawrence AJ. Effect of chronic ethanol and withdrawal on the mu-opioid receptor- and 5-Hydroxytryptamine(1A) receptor-stimulated binding of [(35)S]Guanosine-5′-O-(3-thio)triphosphate in the fawn-hooded rat brain: A quantitative autoradiography study. J Pharmacol Exp Ther. 2000;293:159–165. [PubMed] [Google Scholar]
- Collins SL, Gerdes RM, D’Addario C, Izenwasser S. Kappa opioid agonists alter dopamine markers and cocaine-stimulated locomotor activity. Behav Pharmacol. 2001;12:237–245. doi: 10.1097/00008877-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Coonfield DL, Hill KG, Kaczmarek HJ, Ferraro FM, III, Kiefer SW. Low doses of naltrexone reduce palatability and consumption of ethanol in outbred rats. Alcohol. 2002;26:43–47. doi: 10.1016/s0741-8329(01)00180-x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- D’Addario C, Di Benedetto M, Izenwasser S, Candeletti S, Romualdi P. Differential time course of effects of kappa-opioid agonist treatment on dynorphin A levels and kappa-opioid receptor density. J Mol Neurosci. 2004;24:307–314. doi: 10.1385/JMN:24:2:307. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Differences in the peripheral levels of beta-endorphin in response to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Alcohol Clin Exp Res. 2005;29:1965–1975. doi: 10.1097/01.alc.0000187599.17786.4a. [DOI] [PubMed] [Google Scholar]
- Dalman FC, O’Malley KL. kappa-Opioid tolerance and dependence in cultures of dopaminergic midbrain neurons. J Neurosci. 1999;19:5750–5757. doi: 10.1523/JNEUROSCI.19-14-05750.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. Kappa-opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. doi: 10.1016/0166-2236(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Wang J, Tian H, Pochareddy S, Xuei X, Wetherill L, et al. A regulatory variation in OPRK1, the gene encoding the kappa-opioid receptor, is associated with alcohol dependence. Hum Mol Genet. 2008;17:1783–1789. doi: 10.1093/hmg/ddn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden AI, Jacobs TP. Dynorphin induces partially reversible paraplegia in the rat. Eur J Pharmacol. 1983;91:321–324. doi: 10.1016/0014-2999(83)90487-9. [DOI] [PubMed] [Google Scholar]
- Femenia T, Manzanares J. Increased ethanol intake in prodynorphin knockout mice is associated to changes in opioid receptor function and dopamine transmission. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00378.x. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Fuentealba JA, Gysling K, Magendzo K, Andres ME. Repeated administration of the selective kappa-opioid receptor agonist U-69593 increases stimulated dopamine extracellular levels in the rat nucleus accumbens. J Neurosci Res. 2006;84:450–459. doi: 10.1002/jnr.20890. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary beta-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry. 1996;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Gieryk A, Ziolkowska B, Solecki W, Kubik J, Przewlocki R. Forebrain PENK and PDYN gene expression levels in three inbred strains of mice and their relationship to genotype-dependent morphine reward sensitivity. Psychopharmacology (Berl) 2010;208:291–300. doi: 10.1007/s00213-009-1730-1. [DOI] [PubMed] [Google Scholar]
- Gilligan SB, Reich T, Cloninger CR. Etiologic heterogeneity in alcoholism. Genet Epidemiol. 1987;4:395–414. doi: 10.1002/gepi.1370040602. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Gabriel KI, Cunningham CL. Topiramate does not affect the acquisition or expression of ethanol conditioned place preference in DBA/2J or C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:783–790. doi: 10.1111/j.1530-0277.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- Grisel JE, Mogil JS, Grahame NJ, Rubinstein M, Belknap JK, Crabbe JC, et al. Ethanol oral self-administration is increased in mutant mice with decreased beta-endorphin expression. Brain Res. 1999;835:62–67. doi: 10.1016/s0006-8993(99)01384-0. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Holloway RL. Brain shrinkage in chronic alcoholics: a pathological study. Br Med J (Clin Res Ed) 1985;290:501–504. doi: 10.1136/bmj.290.6467.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood HJ, Fountain D, Livermore G. Economic costs of alcohol abuse and alcoholism. Recent Dev Alcohol. 1998;14:307–330. doi: 10.1007/0-306-47148-5_14. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. Major depression in 6050 former drinkers: association with past alcohol dependence. Arch Gen Psychiatry. 2002;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Aldrich JV, Anderson KJ, Bakalkin G, Christie MJ, Hall ED, et al. Pathobiology of dynorphins in trauma and disease. Front Biosci. 2005;10:216–235. doi: 10.2741/1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey JC, Winder DG, Kash TL. Chronic ethanol exposure leads to divergent control of dopaminergic synapses in distinct target regions. Alcohol. 2008;42:179–190. doi: 10.1016/j.alcohol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Schenk S, Partridge B, Shippenberg TS. Increased responsiveness of mesolimbic and mesostriatal dopamine neurons to cocaine following repeated administration of a selective kappa-opioid receptor agonist. Synapse. 1998;30:255–262. doi: 10.1002/(SICI)1098-2396(199811)30:3<255::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Holter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9:41–48. [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, et al. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105:105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Ignatova EG, Belcheva MM, Bohn LM, Neuman MC, Coscia CJ. Requirement of receptor internalization for opioid stimulation of mitogen-activated protein kinase: biochemical and immunofluorescence confocal microscopic evidence. J Neurosci. 1999;19:56–63. doi: 10.1523/JNEUROSCI.19-01-00056.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami A, Duvauchelle CL. Dopamine mechanisms and cocaine reward. Int Rev Neurobiol. 2004;62:45–94. doi: 10.1016/S0074-7742(04)62002-2. [DOI] [PubMed] [Google Scholar]
- Jamensky NT, Gianoulakis C. Content of dynorphins and kappa-opioid receptors in distinct brain regions of C57BL/6 and DBA/2 mice. Alcohol Clin Exp Res. 1997;21:1455–1464. [PubMed] [Google Scholar]
- Jarjour S, Bai L, Gianoulakis C. Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcohol Clin Exp Res. 2009;33:1033–1043. doi: 10.1111/j.1530-0277.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Jiang HK, Owyang VV, Hong JS, Gallagher M. Elevated dynorphin in the hippocampal formation of aged rats: relation to cognitive impairment on a spatial learning task. Proc Natl Acad Sci U S A. 1989;86:2948–2951. doi: 10.1073/pnas.86.8.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Cvejic S, Devi LA. Kappa opioid receptor endocytosis by dynorphin peptides. DNA Cell Biol. 2000;19:19–27. doi: 10.1089/104454900314672. [DOI] [PubMed] [Google Scholar]
- June HL, Cummings R, Eiler WJ, Foster KL, McKay PF, Seyoum R, et al. Central opioid receptors differentially regulate the nalmefene-induced suppression of ethanol- and saccharin-reinforced behaviors in alcohol-preferring (P) rats. Neuropsychopharmacology. 2004;29:285–299. doi: 10.1038/sj.npp.1300338. [DOI] [PubMed] [Google Scholar]
- June HL, Grey C, Warren-Reese C, Durr LF, Ricks-Cord A, Johnson A, et al. The opioid receptor antagonist nalmefene reduces responding maintained by ethanol presentation: preclinical studies in ethanol-preferring and outbred Wistar rats. Alcohol Clin Exp Res. 1998;22:2174–2185. [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. Self-regulation and self-medication factors in alcoholism and the addictions. Similarities and differences. Recent Dev Alcohol. 1990;8:255–271. [PubMed] [Google Scholar]
- Koob GF. Neurobiology of addiction. Toward the development of new therapies. Ann N Y Acad Sci. 2000;909:170–185. doi: 10.1111/j.1749-6632.2000.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KM, Szakall I, O’Brien D, Wang R, Vinod KY, Saito M, et al. Decreased oral self-administration of alcohol in kappa-opioid receptor knock-out mice. Alcohol Clin Exp Res. 2005;29:730–738. doi: 10.1097/01.alc.0000164361.62346.d6. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Lai J, Luo MC, Chen Q, Ma S, Gardell LR, Ossipov MH, et al. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat Neurosci. 2006;9:1534–1540. doi: 10.1038/nn1804. [DOI] [PubMed] [Google Scholar]
- Lai J, Ossipov MH, Vanderah TW, Malan TP, Jr, Porreca F. Neuropathic pain: the paradox of dynorphin. Mol Interv. 2001;1:160–167. [PubMed] [Google Scholar]
- Lam MP, Marinelli PW, Bai L, Gianoulakis C. Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology (Berl) 2008;201:261–271. doi: 10.1007/s00213-008-1267-8. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Li JG, Luo LY, Krupnick JG, Benovic JL, Liu-Chen LY. U50,488H-induced internalization of the human kappa opioid receptor involves a beta-arrestin-and dynamin-dependent mechanism. Kappa receptor internalization is not required for mitogen-activated protein kinase activation. J Biol Chem. 1999;274:12087–12094. doi: 10.1074/jbc.274.17.12087. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22:165–171. doi: 10.1016/s0741-8329(00)00118-x. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Rosin A, Dahlin I, Georgieva J, Franck J. Ethanol alters the effect of kappa receptor ligands on dopamine release in the nucleus accumbens. Physiol Behav. 2007;92:167–171. doi: 10.1016/j.physbeh.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brene S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120:137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loacker S, Sayyah M, Wittmann W, Herzog H, Schwarzer C. Endogenous dynorphin in epileptogenesis and epilepsy: anticonvulsant net effect via kappa opioid receptors. Brain. 2007;130:1017–1028. doi: 10.1093/brain/awl384. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Blockade of ethanol reward by the kappa opioid receptor agonist U50,488H. Alcohol. 2009;43:359–365. doi: 10.1016/j.alcohol.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM, Stohler CS, Zubieta JK. Positron emission tomography measures of endogenous opioid neurotransmission and impulsiveness traits in humans. Arch Gen Psychiatry. 2009;66:1124–1134. doi: 10.1001/archgenpsychiatry.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maldonado R. The neurobiology of addiction. J Neural Transm. 2003;(Suppl):1–14. doi: 10.1007/978-3-7091-0541-2_1. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2005;29:1821–1828. doi: 10.1097/01.alc.0000183008.62955.2e. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2006;30:982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 2003;169:60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience. 2004;127:777–784. doi: 10.1016/j.neuroscience.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Ritvo EC, Morgan RO, Salvato FR, Goldberg G, Welch B, et al. A double-blind, placebo-controlled pilot study to evaluate the efficacy and safety of oral nalmefene HCl for alcohol dependence. Alcohol Clin Exp Res. 1994;18:1162–1167. doi: 10.1111/j.1530-0277.1994.tb00098.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merg F, Filliol D, Usynin I, Bazov I, Bark N, Hurd YL, et al. Big dynorphin as a putative endogenous ligand for the kappa-opioid receptor. J Neurochem. 2006;97:292–301. doi: 10.1111/j.1471-4159.2006.03732.x. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Bandy AL. Naltrexone effects on ethanol consumption and response to ethanol conditioned cues in C57BL/6 mice. Psychopharmacology (Berl) 2000;151:321–327. doi: 10.1007/s002130000479. [DOI] [PubMed] [Google Scholar]
- Miner LL. Cocaine reward and locomotor activity in C57BL/6J and 129/SvJ inbred mice and their F1 cross. Pharmacol Biochem Behav. 1997;58:25–30. doi: 10.1016/s0091-3057(96)00465-0. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182:384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Homayoun H. Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology. 2008;33:42–55. doi: 10.1038/sj.npp.1301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology. 2011;61:35–42. doi: 10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nguyen XV, Masse J, Kumar A, Vijitruth R, Kulik C, Liu M, et al. Prodynorphin knockout mice demonstrate diminished age-associated impairment in spatial water maze performance. Behav Brain Res. 2005;161:254–262. doi: 10.1016/j.bbr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Middaugh LD, Tavernetti M. Ethanol consumption and placepreference conditioning in the alcohol-preferring C57BL/6 mouse: relationship with motor activity patterns. Alcohol Clin Exp Res. 1999;23:683–692. [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology (Berl) 2005;181:327–336. doi: 10.1007/s00213-005-2259-6. [DOI] [PubMed] [Google Scholar]
- Pellow S. Anxiolytic and anxiogenic drug effects in a novel test of anxiety: are exploratory models of anxiety in rodents valid? Methods Find. Exp Clin Pharmacol. 1986;8:557–565. [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Picker MJ, Mathewson C, Allen RM. Opioids and rate of positively reinforced behavior: III. Antagonism by the long-lasting kappa antagonist norbinaltorphimine. Behav Pharmacol. 1996;7:495–504. [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, et al. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewlocka B, Turchan J, Lason W, Przewlocki R. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett. 1997;238:13–16. doi: 10.1016/s0304-3940(97)00829-x. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol. 2003;64:445–449. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, et al. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- Rosin A, Lindholm S, Franck J, Georgieva J. Downregulation of kappa opioid receptor mRNA levels by chronic ethanol and repetitive cocaine in rat ventral tegmentum and nucleus accumbens. Neurosci Lett. 1999;275:1–4. doi: 10.1016/s0304-3940(99)00675-8. [DOI] [PubMed] [Google Scholar]
- Roy A, DeJong J, Lamparski D, George T, Linnoila M. Depression among alcoholics. Relationship to clinical and cerebrospinal fluid variables. Arch Gen Psychiatry. 1991;48:428–432. doi: 10.1001/archpsyc.1991.01810290040007. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacology. 2008;33:877–891. doi: 10.1038/sj.npp.1301459. [DOI] [PubMed] [Google Scholar]
- Saland LC, Abeyta A, Frausto S, Raymond-Stintz M, Hastings CM, Carta M, et al. Chronic ethanol consumption reduces delta-and mu-opioid receptor-stimulated G-protein coupling in rat brain. Alcohol Clin Exp Res. 2004;28:98–104. doi: 10.1097/01.ALC.0000108658.00243.BF. [DOI] [PubMed] [Google Scholar]
- Sandin J, Nylander I, Georgieva J, Schott PA, Ogren SO, Terenius L. Hippocampal dynorphin B injections impair spatial learning in rats: a kappa-opioid receptor-mediated effect. Neuroscience. 1998a;85:375–382. doi: 10.1016/s0306-4522(97)00605-2. [DOI] [PubMed] [Google Scholar]
- Sandin J, Nylander I, Silberring J. Metabolism of beta-endorphin in plasma studied by liquid chromatography-electrospray ionization mass spectrometry. Regul Pept. 1998b;73:67–72. doi: 10.1016/s0167-0115(97)01065-3. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am J Psychiatry. 1997a;154:948–957. doi: 10.1176/ajp.154.7.948. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bucholz KK, Nurnberger JI, Jr, Hesselbrock VM, Crowe RR, et al. The life-time rates of three major mood disorders and four major anxiety disorders in alcoholics and controls. Addiction. 1997b;92:1289–1304. [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunk E, Aigner C, Stefanova N, Wenning G, Herzog H, Schwarzer C. Kappa opioid receptor activation blocks progressive neurodegeneration after kainic acid injection. Hippocampus. 2010 doi: 10.1002/hipo.20813. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De WH, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Sheedy D, Garrick T, Dedova I, Hunt C, Miller R, Sundqvist N, et al. An Australian Brain Bank: a critical investment with a high return! Cell Tissue Bank. 2008;9:205–216. doi: 10.1007/s10561-008-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, et al. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. Motivational properties of opioids: evidence that an activation of delta-receptors mediates reinforcement processes. Brain Res. 1987;436:234–239. doi: 10.1016/0006-8993(87)91667-2. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–566. [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker WJ, Vavrousek-Jakuba E, Arons CD, Kwok FC. The acquisition and maintenance of voluntary ethanol drinking in the rat: effects of dopaminergic lesions and naloxone. Behav Brain Res. 2002;137:139–148. doi: 10.1016/s0166-4328(02)00290-5. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Goebel SM, Martin KM, Knapp PE, Marinova Z, et al. Dynorphin A (1–17) induces apoptosis in striatal neurons in vitro through alpha-amino- 3-hydroxy-5-methylisoxazole-4-propionate/kainite receptor-mediated cytochrome c release and caspase-3 activation. Neuroscience. 2003;122:1013–1023. doi: 10.1016/j.neuroscience.2003.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Spanagel R, Stohr T, Shippenberg TS. Strain differences in the rewarding and dopamine-releasing effects of morphine in rats. Psychopharmacology (Berl) 1995;117:240–247. doi: 10.1007/BF02245193. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I Temporal dynamics of affect Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanolconditioned place preference and self-administration. Psychopharmacology (Berl) 2010;210:199–209. doi: 10.1007/s00213-010-1844-5. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O’Brien CP. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15:281–289. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Mackler SA, Volpicelli JR, O’Brien CP. Effect of acamprosate and naltrexone, alone or in combination, on ethanol consumption. Alcohol. 2001;23:109–116. doi: 10.1016/s0741-8329(00)00137-3. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Tan-No K, Cebers G, Yakovleva T, Hoon GB, Gileva I, Reznikov K, et al. Cytotoxic effects of dynorphins through non-opioid intracellular mechanisms. Exp Cell Res. 2001;269:54–63. doi: 10.1006/excr.2001.5309. [DOI] [PubMed] [Google Scholar]
- Tan-No K, Takahashi H, Nakagawasai O, Niijima F, Sato T, Satoh S, et al. Pronociceptive role of dynorphins in uninjured animals: N-ethylmaleimide-induced nociceptive behavior mediated through inhibition of dynorphin degradation. Pain. 2005;113:301–309. doi: 10.1016/j.pain.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Taqi MM, Bazov I, Watanabe H, Nyberg F, Yakovleva T, Bakalkin G. Prodynorphin promoter SNP associated with alcohol dependence forms noncanonical AP-1 binding site that may influence gene expression in human brain. Brain Res. 2011a;1385:18–25. doi: 10.1016/j.brainres.2011.02.042. [DOI] [PubMed] [Google Scholar]
- Taqi MM, Bazov I, Watanabe H, Sheedy D, Harper C, Alkass K, et al. Prodynorphin CpG-SNPs associated with alcohol dependence: elevated methylation in the brain of human alcoholics. Addict Biol. 2011b;16:499–509. doi: 10.1111/j.1369-1600.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Torrens M, Fonseca F, Mateu G, Farre M. Efficacy of antidepressants in substance use disorders with and without comorbid depression. A systematic review and meta-analysis. Drug Alcohol Depend. 2005;78:1–22. doi: 10.1016/j.drugalcdep.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Turchan J, Przewlocka B, Toth G, Lason W, Borsodi A, Przewlocki R. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience. 1999;91:971–977. doi: 10.1016/s0306-4522(98)00637-x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von ZM, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Verbeek DS, Van de Warrenburg BP, Wesseling P, Pearson PL, Kremer HP, Sinke RJ. Mapping of the SCA23 locus involved in autosomal dominant cerebellar ataxia to chromosome region 20p13-12.3. Brain. 2004;127:2551–2557. doi: 10.1093/brain/awh276. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behav Neurosci. 2009;123:926–935. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ettenberg A. Intracerebroventricular ethanol-induced conditioned place preferences are prevented by fluphenazine infusions into the nucleus accumbens of rats. Behav Neurosci. 2007;121:401–410. doi: 10.1037/0735-7044.121.2.401. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2008a;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Regarding “Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake”. FASEB J. 2008b;22:2113–2114. doi: 10.1096/fj.08-0702LTR. [DOI] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2010 doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2011;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, et al. Comparison of pharmacological activities of three distinct kappa ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- Williams KL, Woods JH. Oral ethanol-reinforced responding in rhesus monkeys: effects of opioid antagonists selective for the mu-, kappa-, or delta-receptor. Alcohol Clin Exp Res. 1998;22:1634–1639. doi: 10.1111/j.1530-0277.1998.tb03960.x. [DOI] [PubMed] [Google Scholar]
- Xuei X, Dick D, Flury-Wetherill L, Tian HJ, Agrawal A, Bierut L, et al. Association of the kappa-opioid system with alcohol dependence. Mol Psychiatry. 2006;11:1016–1024. doi: 10.1038/sj.mp.4001882. [DOI] [PubMed] [Google Scholar]
- Yakovleva T, Marinova Z, Kuzmin A, Seidah NG, Haroutunian V, Terenius L, et al. Dysregulation of dynorphins in Alzheimer disease. Neurobiol Aging. 2007;28:1700–1708. doi: 10.1016/j.neurobiolaging.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, et al. Subtype-selective corticotropin-releasing factor receptor agonists exert contrasting, but not opposite, effects on anxiety-related behavior in rats. J Pharmacol Exp Ther. 2007;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]