Summary
Blood blister-like aneurysms (BBLA) are rare lesions sometimes difficult to recognize and in most cases associated with diffuse subarachnoid haemorrhage and severe clinical conditions. BBLA are life-threatening because they tend to enlarge rapidly and to rebleed, and no consensus has so far been reached on the best management strategy. We describe a patient with a BBLA in the right ICA treated successful by a two-stage embolization procedure first with coils and an open cell stent (Neuroform 3) and later by further coil placement and insertion of a flow-diverting stent (Silk).
Key words: blood blister-like aneurysm, endovascular treatment, coils, micropore stent, flow-diverting stent
Introduction
Blood blister-like aneurysms (BBLA) are rare lesions sometimes difficult to recognize and in most cases associated with diffuse subarachnoid haemorrhage and severe clinical conditions. BBLA are life-threatening because they tend to enlarge rapidly and to rebleed, and no consensus has so far been reached on the best management strategy.
We describe a patient with a broken BBLA in the right ICA treated successful by a two-stage embolization procedure, with coils and an open-cell stent (Neuroform3, Boston Scientific, Fremont, CA, USA) during sub-acute phase of subarachnoid haemorrhage and later with further coil placement and insertion of a flow-diverting stent (Silk, Balt Extrusion, Montmorency, France).
Case report
A 43-year-old man suffered a diffuse subarachnoid haemorrhage (Fisher grade 3) and recovered in a peripheral hospital. After nine days pending a decision if and where to transfer the patient, he was transferred in a stable clinical condition (HH grade 1) to our hospital where angiography disclosed an aneurysm in the supraclinoid segment of the right ICA (Figure 1). The lesion was enlarged and deformed with respect to the examination done on the same day of bleeding on the first hospital admission, thereby confirming the aneurysm as a BBLA.
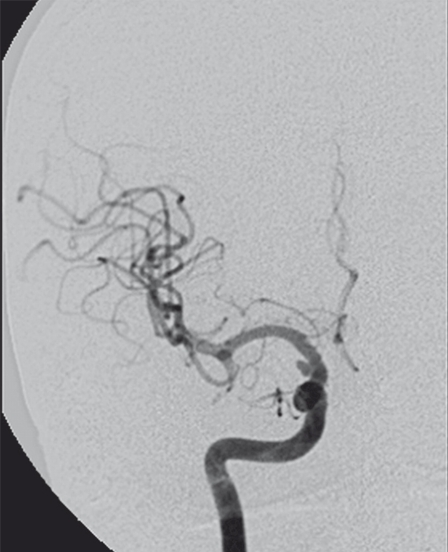
Figure 1.
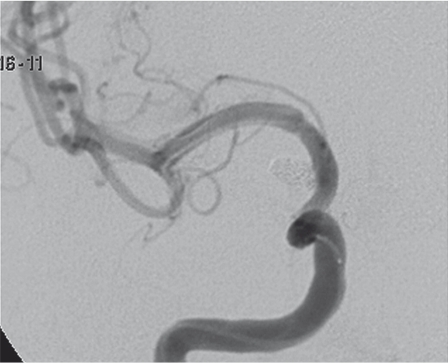
A-P right ICA angiogram on day 9 after SAH shows a small BBLA on the superolateral surface of the supraclinoidal ICA. The aneurysm is enlarged and deformed with respect to the angiogram done elsewhere on day 1. The right A1 is severely narrowed and ipsilateral carotid occlusion test was not tolerated.
As often occurs during the subacute phase of subarachnoid haemorrhage, the angiogram disclosed a significant vasospasm with right A1 branch severely narrowed and carotid occlusion test was poorly tolerated by the patient. Under these conditions occlusion or trapping of the carotid artery is contraindicated and the ischemic risk related to endovascular treatments with stent deployment is also increased.
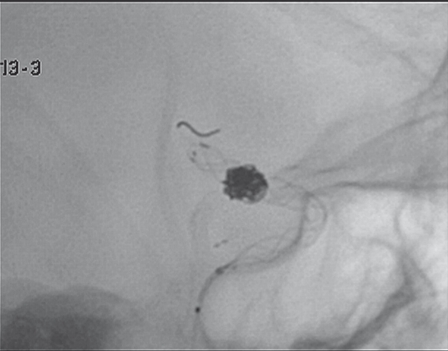
Taking into account the peculiar fragility of the wall of BBLA, after i.v. administration of 1 g aspirin and 5000 IU heparin, a balloon microcatheter (Hyperform, ev3 Endovascular, Plymouth, MN, USA) was left in position uninflated in the petrous portion of the ICA, to be inflated at the level of the aneurysmal neck in case of aneurysm rupture. The balloon was kept proximal to reduce the higher risk of thrombogenic action if located in a distal thinner part of the vessel. Then we chose an open-cell stent (Neuroform3 4×30, Boston Scientific, Fremont, CA, USA) that in our experience has proved poorly thrombogenic even without the usual preparatory dual antiplatelet treatment and the device was deployed to cover the neck of the aneurysm. The tip of the microcatheter was then positioned in the aneurysmal sac through the stent struts and two coils (DeltaPlush, Micrus Endovascular, San Jose, CA, USA) were deployed obtaining sub-total aneurysm occlusion (Figure 2).
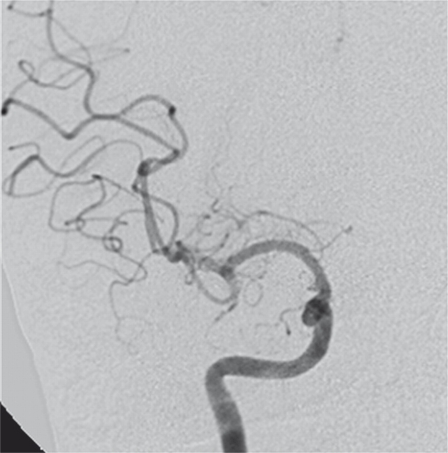
Figure 2.
A-P right ICA angiogram after stent-assisted coiling shows sub-total aneurysm occlusion.
Notwithstanding our prudence and treatment, during final angiographic control, acute embolic occlusion of right M2 branches was noted (Figure 3A). The balloon was promptly withdrawn as potentially the source of embolism, and an i.v. bolus of abciximab (full dose related to body weight) was delivered followed by i.v. infusion for 12 hours. Fifteen minutes after i.v. bolus, the MCA remained occluded and then the microcatheter tip was advanced into the vessel and was used for i.a. delivery of abciximab close to the thrombus. This attempt also proved unsuccessful, and then urokinase (300,000 IU) was slowly injected through the microcatheter, obtaining reperfusion of the posterior branches of the MCA. At the final control, angiography confirmed sub-total aneurysm obliteration with regular stent patency while a CT scan excluded any sign of rebleeding and displayed mild signs of acute right fronto-insular infarction in the territory supplied by the corresponding occluded MCA branch.
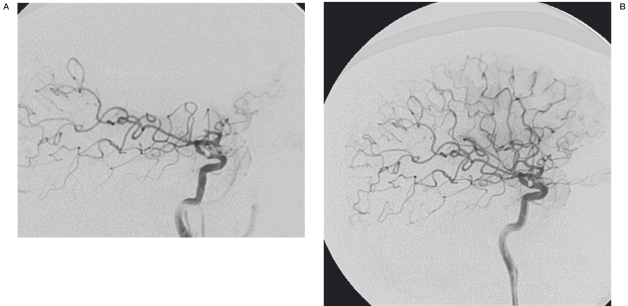
Figure 3.
A) L-L right ICA final control angiogram after stent-assisted coiling shows acute embolic occlusion of right M2 branches. B) L-L right ICA angiogram on day 1 after embolization shows resolution of the distal vessel occlusion, stent patency and aneurysm occlusion.
The patient was kept on assisted ventilation under strict clinical monitoring and i.v. infusion of abciximab was maintained for 12 hours. On the following day he underwent follow-up angiography showing resolution of the distal vessel occlusion, stent patency and aneurysm occlusion (Figure 3B). CT scan confirmed right fronto-insular acute ischaemic infarct. Dual antiplatelet therapy was instituted with aspirin (300 mg/day) and ticlopidine (500 mg/day), according to our usual protocol for intracranial stenting, and the patient was kept in intensive care for the first week after treatment.
MR angiography follow-up was done two weeks and eight weeks after embolization, with the last scan showing refilling and growing of the embolized aneurysmal sac (as frequently occurs with BBLA).
In agreement with the patient a second endovascular treatment was planned and angiography, done ten weeks after bleeding, confirmed major refilling with regrowth of the aneurysmal sac and resolution of vasospasm.
The aneurysm was embolized again (Figure 4) using coils (Axium2, ev3 Endovascular, Plymouth, MN, USA), obtaining complete obliteration. At this point, to stabilize occlusion and enhance vessel wall repair, a flow-diverting stent (Silk 4×30, Balt Extrusion, Montmorency, France) was deployed, using the telescopic technique, within the previously positioned Neuroform 3 stent (Figure 5), taking advantage of the dual antiplatelet treatment that had been prolonged since the first embolization procedure.
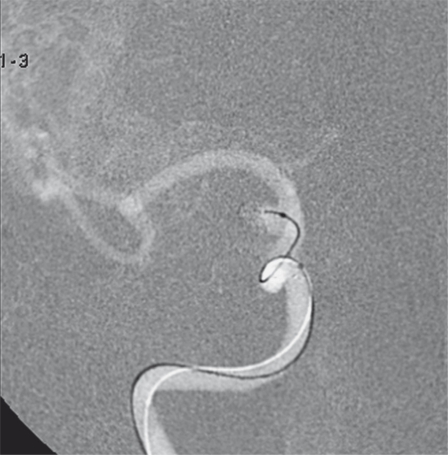
Figure 4.
A-P right ICA angiogram on week 9 after coiling confirms major refilling with regrowth of the aneurysmal sac and resolution of right A1 vasospasm. The tip of the microcatheter is positioned in the aneurysmal sac through the stent struts to complete coil embolization.
Figure 5.
L-L right ICA intra-operative angiogram shows correct positioning and full expansion of the Silk flow-diverting stent deployed using the telescopic technique within the previously positioned Neuroform 3 stent.
Just after the deployment of the Silk stent dye opacification disappeared within the coil mesh and around the aneurysmal neck. A marked reduction of contrast flow velocity was demonstrated in the right ICA, probably related to the haemodynamic effect of the overlying stent struts and to vasospasm at stents extremities (Figure 6). The patient was cautiously administered i.a. and i.v. nimodipine infusion and volumetric CT examination confirmed correct positioning and full expansion of the Silk stent.
Figure 6.
Post-operative A-P right ICA control angiogram after re-coiling of the aneurysmal sac and telescopic stent-in-stent insertion of a Silk flow-diverter stent shows that the aneurysm is completely obliterated. Vasospasm is recognizable at the upper end of the stents, treated with i.a. and i.v. nimodipine infusion.
Subsequent MR angiography follow-up at discharge, one and four months after treatment showed stable findings with aneurysm occlusion and patency of the carotid artery. The patient has had an almost complete recovery of his slight motor deficit and has resumed his normal activities. He was kept on antiplatelet therapy with aspirin (300 mg/day) and ticlopidine (500 mg/day) for two months after insertion of the Silk stent and thereafter on aspirin alone (300 mg/day for the first month, then 100 mg/day forever). Follow-up MR angiography and angiography were performed at six months showing stable unmodified findings.
Discussion
Blood blister-like (BBLA) aneurysms are rare lesions (0.5-2% of ruptured intracranial aneurysms) arising at non-branching sites of the supraclinoidal internal carotid artery 1,2 or more rarely at other locations 3,4. Unlike the more common saccular aneurysms, BBLA are characterized by a thin fragile and unstable wall made only of the tunica adventitia and fibrinous material so that their size and shape tend to change rapidly until they rupture; these features hamper both the choice and implementation of appropriate treatment 2,3.
Both surgical and endovascular management of BBLA carry a high risk of peri and post-procedural complications with high mortality and morbidity rates, especially when the lesions are treated in the acute and sub-acute phases following subarachnoid haemorrhage 5-7 because the frequently associated vasospasm makes less effective the hemodynamic compensation provided by the circle of Willis and by pial vessels, with increased risk for ischemic stroke following parent artery occlusion, done as a treatment of choice (even when EC-IC bypass 8 is associated) or as an emergency procedure after intra-operative aneurysm rupture.
For this reason, a number of staged therapeutic strategies have been advocated, in which an initial treatment is undertaken in the acute phase to protect the aneurysm sac during the time required to resolve vasospasm, and a later treatment is used in the chronic phase to achieve permanent exclusion from the arterial circulation of the aneurysm alone or together with corresponding carotid section, by surgical or endovascular technique 9,10.
Coiling of BBLA carries a significant risk of rupturing the fragile aneurysm wall, especially when the coil mesh is densely compacted. In addition, it is usually difficult to achieve stable coil placement because of the small size and typical shallow dome shape and wide poorly defined neck of these aneurysms. Coils can readily migrate into the ICA even a long time after treatment as the aneurysm sac and neck enlarge, leading to possible thrombo-embolic complications. To overcome these difficulties a stent can be positioned to cover the aneurysm neck, but this entails antiplatelet treatment that will worsen the patient's prognosis in case of rebleeding 5,11,12.
Literature reports emphasize that blister aneurysm embolization with coils will not offer long-term protection from rebleeding, probably because dissecting disease affects both the wall of the aneurysm than the wall of the close portion of carotid, and therefore when treating only the sac it is difficult to achieve stable occlusion 5,7. However, even when BBLA enlarge after coiling, especially when stent-assisted, they appear to be protected against early rebleeding, at least in the first weeks, probably due to reduced haemodynamic stress on the fragile aneurysmal walls 7,12,13.
The positive effect of stent in these aneurysms can derive from the reduction of intra-aneurysmal flow, redirected by the struts and also by the healing effect on the diseased artery wall done by the stent that create a mesh in front of the aneurismal neck to be covered by endothelial cells 14,15.
Recent reports have described interesting outcomes after deployment of two or three stents at the BBLA neck using the telescopic technique to reduce the size of stent cells and thereby enhance the flow-diverting haemodynamic effect 16,19. A similar or even superior hemodynamic effect can be obtained using one or more of the new flow-diverting stents 20-23 characterized by very narrow cells, and this positive effect was recently reported also for BBLA 24.
On the other hand, using only stents without coils aneurysm occlusion is delayed for days or weeks and probably during this period of time the aneurysm is not completely protected from rupture. Furthermore stent deployment entails dual antiplatelet treatment that will worsen the patient's prognosis in case of rebleeding 25,26.
For these reasons, when it is feasible, we prefer to do stent-assisted coiling of these ruptured aneurysms followed by flow-diverter stent-in-stent deployment. We would limit the use of flow-diverter stents without coils to cases with intact BBLA or to cases of broken BBLA with unfavorable morphology in which coiling is not viable.
Conclusions
When a blister-like aneurysm is disclosed in a patient presenting with subarachnoid haemorrhage, choosing the right endovascular treatment is difficult because no consensus has so far been reached on the best management strategy.
In the reported case we treated a broken BBLA in the sub-acute phase of subarachnoid hemorrhage with associated vasospasm, using a two-stage endovascular strategy.
In accordance with this strategy, when the morphology of the ruptured aneurysm is appropriate and adverse conditions to parent artery occlusion or aneurysmal trapping are associated (vasospasm, inadequate collateral circles, unfavorable anatomical site) coiling, preferably stent-assisted and avoiding dense coil compaction, can be undertaken in the acute and sub-acute phase of subarachnoid hemorrhage to reduce the fatal risk of early rebleeding. Several weeks later after strict clinical and angiographic monitoring, treatment can be completed, if necessary, by inserting more coils into the aneurysmal sac and deploying a flow-diverting stent to cover the aneurysmal neck. This device will serve the dual purpose of making aneurysm occlusion permanent and providing the necessary scaffolding to facilitate healing of the vessel wall 14,15,27.
When the morphology of the ruptured aneurysm is unfavorable for both coiling and parent artery occlusion, or in the rare cases of incidental finding of intact BBLA, we believe that treatment with telescopic deployment of flow-diverter stents is the most promising therapeutic option.
In both the staged employment after coiling and in direct telescopic deployment without coils, the new flow-diverting stents offer interesting prospects in the treatment of these rare and difficult aneurysms, but management is neither straightforward nor univocal.
A series of technical and methodological factors have yet to be addressed, namely the treatment schedule (direct versus staged strategy) and the type of antiplatelet and anticoagulant therapy to be instituted before, during and after treatment to manage a delicate balance between hemorrhagic and thrombo-embolic risks.
References
- 1.Ogawa A, Suzuki M, Ogasawara K. Aneurysms at nonbranching sites in the supraclinoid portion of the internal carotid artery: internal carotid artery trunk aneurysms. Neurosurgery. 2000;47:578–586. doi: 10.1097/00006123-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa T, Nakamura N, Houkin K, et al. Pathological consideration of a “blister-like” aneurysm at the superior wall of the internal carotid artery: case report. Neurosurgery. 1997;40:403–406. doi: 10.1097/0006123-199702000-00038. [DOI] [PubMed] [Google Scholar]
- 3.Meckel S, Singh TP, Undrén P, et al. Endovascular treatment using predominantly stent-assisted coil embolization and antiplatelet and anticoagulation management of ruptured blood blister-like aneurysms. Am J Neuroradiol. 2011;32:764–771. doi: 10.3174/ajnr.A2392. Epub 2011 Mar 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris TC, Brophy BP. Blister-like aneurysm of the anterior communicating artery. J Clin Neurosci. 2009;16:1098–1100. doi: 10.1016/j.jocn.2008.10.025. Epub 2009 May 20. [DOI] [PubMed] [Google Scholar]
- 5.Meling TR, Sorteberg A, Bakke SJ, et al. Blood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J Neurosurg. 2008;108:662–671. doi: 10.3171/JNS/2008/108/4/0662. [DOI] [PubMed] [Google Scholar]
- 6.Chung JH, Shin YS, Lim YC, et al. Ideal internal carotid artery trapping technique without bypass in a patient with insufficient collateral flow. J Korean Neurosurg Soc. 2009;45:260–263. doi: 10.3340/jkns.2009.45.4.260. Epub 2009 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee BH, Kim BM, Park MS, et al. Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2009;110:431–436. doi: 10.3171/2008.7.JNS08257. [DOI] [PubMed] [Google Scholar]
- 8.Park EK, Ahn JS, Kwon DH, et al. Result of extracranial-intracranial bypass surgery in the treatment of complex intracranial aneurysms: outcomes in 15 cases. J Korean Neurosurg Soc. 2008;44:228–33. doi: 10.3340/jkns.2008.44.4.228. Epub 2008 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanoue S, Kiyosue H, Matsumoto S, et al. Ruptured “blisterlike” aneurysm with a pseudoaneurysm formation requiring delayed intervention with endovascular coil embolization. Case report. J Neurosurg. 2004;101:159–162. doi: 10.3171/jns.2004.101.1.0159. [DOI] [PubMed] [Google Scholar]
- 10.Islam MS, Manabe H, Hasegawa S, et al. Successful staged treatment for ruptured blister-like dissecting aneurysm of the intracranial internal carotid artery: acute GDC embolization for the blister-like aneurysm followed by proximal occlusion with extracranial-intracranial bypass in the chronic stage. Minim Invasive Neurosurg. 2004;47:165–168. doi: 10.1055/s-2004-818490. [DOI] [PubMed] [Google Scholar]
- 11.Tähtinen OI, Vanninen RL, Manninen HI, et al. Wide-necked intracranial aneurysms: treatment with stent-assisted coil embolization during acute (< 72 hours) subarachnoid hemorrhage--experience in 61 consecutive patients. Radiology. 2009;253:199–208. doi: 10.1148/radiol.2531081923. [DOI] [PubMed] [Google Scholar]
- 12.Korja M, Rautio R, Valtonen S, et al. Primary treatment of ruptured blood blister-like aneurysms with stent-assisted coil embolization: report of two cases. Acta Radiol. 2008;49:180–183. doi: 10.1080/02841850701675735. [DOI] [PubMed] [Google Scholar]
- 13.Ahn JY, Cho JH, Jung JY, et al. Blister-like aneurysms of the supraclinoid internal carotid artery: challenging endovascular treatment with stent-assisted coiling. J Clin Neurosci. 2008;15:1058–1061. doi: 10.1016/j.jocn.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Piotin M, Blanc R, Spelle L, et al. Stent-assisted coiling of intracranial aneurysms. clinical and angiographic results in 216 consecutive aneurysms. Stroke. 2010;41:110–115. doi: 10.1161/STROKEAHA.109.558114. Epub 2009 Dec 3. [DOI] [PubMed] [Google Scholar]
- 15.Lawson MF, Newman WC, Chi YY, et al. Stent-associated flow remodeling causes further occlusion of incompletely coiled aneurysms. Neurosurgery. 2011 Mar 23; doi: 10.1227/NEU.0b013e3182181c2b. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Kim BM, Chung EC, Park SI, et al. Treatment of blood blister-like aneurysm of the internal carotid artery with stent-assisted coil embolization followed by stent-within-a-stent technique. Case report. . J Neurosurg. 2007;107:1211–1213. doi: 10.3171/JNS-07/12/1211. [DOI] [PubMed] [Google Scholar]
- 17.Gaughen JR Jr, Hasan D, Dumont AS, et al. The efficacy of endovascular stenting in the treatment of supraclinoid internal carotid artery blister aneurysms using a stent-in-stent technique. Am J Neuroradiol. 2010;31:1132–1138. doi: 10.3174/ajnr.A2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery. 2006;59:291–300. doi: 10.1227/01.NEU.0000223650.11954.6C. [DOI] [PubMed] [Google Scholar]
- 19.Kim YW, Park IS, Baik MW, et al. Endovascular treatment of blood blister-like aneurysms using multiple self-expanding stents. J Korean Neurosurg Soc. 2011;49:116–119. doi: 10.3340/jkns.2011.49.2.116. Epub 2011 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne JV, Beltechi R, Yarnold JA, et al. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PloS One. 2010;5(9) doi: 10.1371/journal.pone.0012492. pii: e12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonardi M, Dall’Olio M, Princiotta C, et al. Treatment of carotid siphon aneurysms with a microcell stent. A case report. Interventional Neuroradiology. 2008;14:429–434. doi: 10.1177/159101990801400408. Epub 2009 Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1115–1120. doi: 10.1227/01.neu.0000325873.44881.6e. [DOI] [PubMed] [Google Scholar]
- 23.Kulcsár Z, Wetzel SG, Augsburger L, et al. Effect of flow diversion treatment on very small ruptured aneurysms. Neurosurgery. 2010;67:789–793. doi: 10.1227/01.NEU.0000372920.39101.55. [DOI] [PubMed] [Google Scholar]
- 24.Rasskazoff S, Silvaggio J, Brouwer P.A, et al. Endovascular treatment of a ruptured blood blister-like aneurysm with a flow-diverting stent. Interventional Neuroradiology. 2010;16:255–258. doi: 10.1177/159101991001600304. Epub 2010 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turowski B, Macht S, Kulcsár Z, et al. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent): do we need to rethink our concepts? Neuroradiology. 2011;53:37–41. doi: 10.1007/s00234-010-0676-7. Epub 2010 Mar 26. [DOI] [PubMed] [Google Scholar]
- 26.Cebral JR, Mut F, Raschi M, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. Am J Neuroradiol. 2011;32:27–33. doi: 10.3174/ajnr.A2398. Epub 2010 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64:632–642. doi: 10.1227/01.NEU.0000339109.98070.65. [DOI] [PubMed] [Google Scholar]