Summary
Arteriovenous malformation (AVM) patients who initially present with intracerebral haemorrhage may have an identifiable source of bleeding on angiogram, which can be a treatment target. Previous work suggests that the re-bleed rate may be lowered if a weak area is eliminated.
A retrospective cohort study was conducted on patients who presented over a six-year period with a bled AVM. Cases were reviewed looking for the source of the hemorrhage by correlating haematoma location on CT or MRI and any angio-architectural weakness seen on digital subtraction angiography (DSA). Neuroendovascular notes were reviewed to identify the treatment targets.
One hundred patients presented with a brain AVM with a 1.7:1 male: female ratio, 41 patients had an initial presentation of hemorrhage. The source of hemorrhage was identified in 18 subjects with 11 intranidal false aneurysms, five flow-related aneurysms, two associated aneurysms and one venous pouch.
The location of haemorrhage on the presenting scan significantly correlated with the identified bleeding source using Chi-square analysis (P-value 0.039). Partial targeted embolization was used successfully in 90% with a 9% related technical complication rate not resulting in long-term morbidity or mortality. The mean follow-up period was 34 months with an annual hemorrhage rate of 0.7%.
In just under half the patients with AVM bleeding a source of haemorrhage can be identified on DSA and in most cases this will be an intranidal false aneurysm. Flow-related and associated aneurysms in patients with brain AVM can cause haemorrhage and these patients are more likely to have SAH than intracerebral haemorrhage.These weak points are a good target for partial endovascular treatment, are usually accessible and may reduce the higher haemorrhage rate expected over the next two years.
Key words: arteriovenous malformation, intranidal aneurysm, flow-related aneurysm, haemorrhage, targeted embolization
Introduction
A brain arteriovenous malformation (AVMs) is an abnormal connection between arteries and veins with a characteristic nidus. They can present with hemorrhage (50%), epilepsy (40%), and focal neurological deficits or may be asymptomatic. In patients who present with a haemorrhage the incidence of AVM re-bleeding is increased from 2-4% / year to 6-18% / year for at least a year following the initial haemorrhage 1-6.
The ideal treatment of an AVM is complete obliteration of the lesion which removes the risk of further haemorrhage and reduces the seizure risk 15. This is however not always possible at an acceptable risk.
Important factors in deciding whether an AVM is treated include clinical presentation, location, size and features such as venous drainage, which determine the risk of complications. Treatment options include microsurgical excision, endovascular neuro-intervention, radiosurgery or observation.
Attempts at cure, particularly with larger lesions, have been associated with a high rate of complications 9-14. However an alternative approach to cure is one where endovascular intervention is directed at specific angiographic target areas in an attempt to improve symptoms and reduce the associated morbidity 3,15,16. This study sought to find a clear correlation between presenting with a haemorrhage and having an identifiable angiographic bleeding source. Although we may be finding something that is a consequence rather than a cause of bleeding it allows more certain correlation between bleed and lesion. As the AVM haemorrhage rate is significantly increased after a bleed, treatment of any weakness responsible for haemorrhage also offers an opportunity to lower this bleed rate to baseline or below.
Subjects and Methods
The Neuro-Endovascular Database is an ongoing prospective project collecting clinical and neurovascular notes of patients admitted to Groote Schuur and UCT Private Academic Hospitals. All new AVM patients are captured as well as any subsequent symptomatic events that patients may present with. Subjects were selected from the database if they were presenting for the first time and had an arteriovenous malformation presenting with haemorrhage. A retrospective folder review was conducted to find the demographic and clinical data of the subjects. All patients had undergone a CT brain scan that identified the hemorrhage. A consensus review of all the scans was conducted by the authors to identify the location of the hemorrhage (intracerebral, subarachnoid or intraventricular) and associated findings such as, cerebral oedema, mass effect and hydrocephalus. The haemorrhage site was then compared to the AVM angioarchitecture as seen on DSA in order to identify the source of the bleeding.
The bleeding point was identified by looking for the presence of an aneurysm or a venous pouch. A flow-related aneurysm was defined as a downstream saccular aneurysm in a vessel supplying the nidus and an intranidal false aneurysm was identified as a defect with a smooth wall that was larger than the nidus channels with contrast often lingering within the defect. Nidus irregularities that did not demonstrate all these features were ignored as the identification of a nidal aneurysm other than a false aneurysm is subjective, relying on DSA quality, timing and observer opinion. An associated aneurysm was defined as a saccular aneurysm located away from the vessels supplying the nidus. A venous pouch was a localized eccentric dilation of a vein draining the nidus.
The angiograms were also assessed for location and size of the nidus, fistulous and choroidal supply, the number of draining veins and deep venous drainage. The angiograms and angiogram notes were used to assess the neuro-intervention for technical success and complication occurrence.
Follow-up data was collected from folders. A telephonic interview was also conducted to assess the clinical status of the subjects with particular interest on the: current level of function, presence of headaches, seizures, residual focal deficits and history of rebleeding.
Results
In the review period 100 new subjects presented with a cerebral AVM. The group that presented with hemorrhage was comprised of 41 subjects who collectively had 44 episodes of hemorrhage. The demographic profile of the patients is shown in Table 1. Subjects presented with a sudden severe headache in 61% of cases and ten subjects (22%) presented with a generalized or focal seizure as a result of their haemorrhage. There were 21 (47%) patients that presented with focal neurological deficits ranging from dysphasia, memory loss, and long tract signs. The average age of the subjects was 36.2 years with a distribution of 11 to 62 years. The median Glasgow Coma Score at presentation was 13 and there were no patients that died on arrival or during admission.
Table 1.
Gender Distribution.
| AVM | Male | Female |
|---|---|---|
| Total | 63 | 37 |
| Bled | 31 | 10 |
| Identified bleeding source |
16 | 3 |
The location of haemorrhage on the presenting scans, categorized as either intracerebral haemorrhage, intraventricular hemorrhage or subarachnoid hemorrhage, significantly correlated with the identified bleeding source using Chi-square analysis (Table 2). Nine patients presented with SAH and of these five had bleeding from an aneurysm distant from the nidus (Figures 1-3), only two patients with nidal rupture developed significant SAH. Twenty-eight patients had a parenchymal haemorrhage associated with the nidus and in 17 of these patients no bleeding point was visualized, however of the 11 where a weakness was found, eight had an intranidal false aneurysm (Figures 4-6).
Table 2.
Location of the haematoma vs. source of bleeding for the 44 bleeding events.
| Haemorrhage | Flow-related aneurysm |
Intranidal aneurysm |
Associated aneurysm |
Venous pouch |
Source not identified |
|---|---|---|---|---|---|
| SAH | 4 | 2 | 1 | 0 | 2 |
| IVH | 0 | 1 | 0 | 0 | 6 |
| ICH | 1 | 8 | 1 | 1 | 17 |
| Chi-square 16.25; Correlation 0.52; P-value 0.039 | |||||
Figure 1.
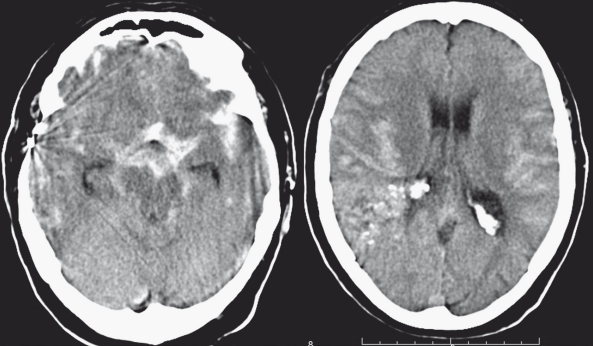
Patient 1. A 60-year-old patient presenting with SAH 20 years after failed surgery for a large right parietal AVM. Haemorrhage is mostly in the supra-sellar cisterns and peri-mesencephalic cisterns. There is hemispheral cistern blood but no parenchymal blood that would indicate nidus haemorrhage.
Figure 2.
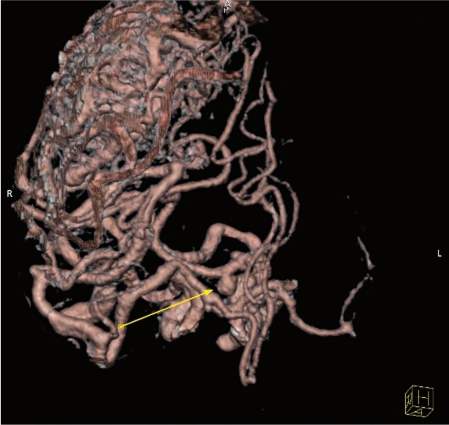
Patient 1. A CT angiogram revealed a right P1 proximal flow related aneurysm corresponding to the SAH localization.
Figure 3.
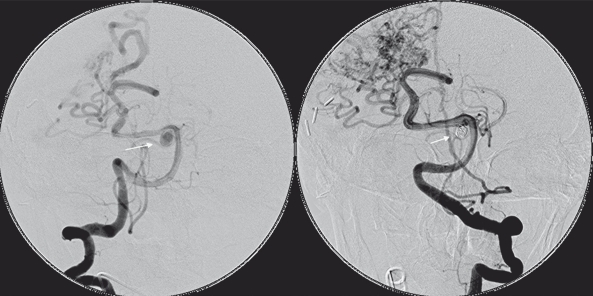
Patient 1. Vertebral angiography demonstrating the P1 aneurysm before and after coiling.
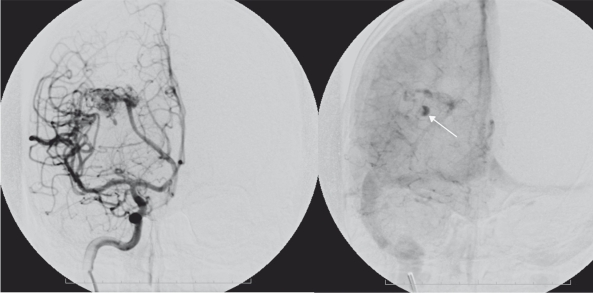
Figure 4.
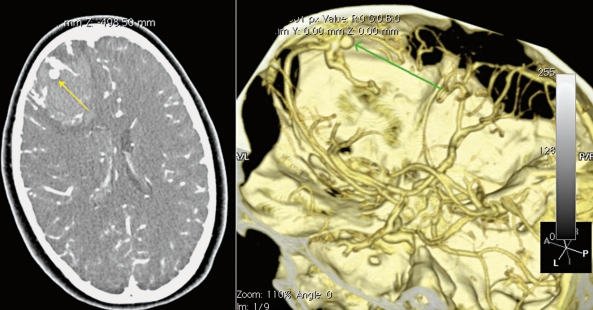
Patient 2. A 14 year old female presenting with sudden onset of headache. A CT angiogram demonstrates a right frontal parenchymal haemorrhage with an associated intranidal false aneurysm.
Figure 5.
Patient 2. DSA demonstrates the typical features of an intranidal false aneurysm.
The source of bleeding (Table 3) was identified in 18 subjects with 19 episodes of hemorrhage. Eleven of the patients had a clear intranidal rupture with a false aneurysm visible on DSA done shortly after presentation. Eight patients did not bleed from the nidus, five bled from a flow-related aneurysm, two from an associated aneurysm and one from a venous pouch. The patient that presented with a repeat bleed, had a right parietal AVM that initially presented with SAH and a ruptured flow related aneurysm was identified on the anterior communicating artery, the aneurysm was wide-necked and it was not amenable to endovascular treatment, he re-presented a month later with intracerebral hemorrhage and no source of the bleeding could be found, so he was managed conservatively, he presented after four years with another intracerebral hematoma, an intranidal aneurysm was identified and treated, he has not bled since.
Table 3.
Bleeding source vs. partial targeted neuro-endovascular treatment.
| Source of bleeding | Number of patients (%) | Bleeding point treated | Failed |
|---|---|---|---|
| Flow-related aneurysm | 5 (11.3) | 4 | 1 |
| Intranidal false aneurysm | 11 (25) | 11 | 0 |
| Associated aneurysm | 2 (4.5) | 1 | 1 |
| Venous pouch | 1 (2,2) | 1 | 0 |
All the subjects with an identifiable source of hemorrhage were managed with targeted endovascular treatment and this was successful in 90% of the cases. There was a 9% procedure related complication rate, one patient had glue reflux with no clinical consequences and the other had thrombi-embolic occlusion of a distal left MCA branch with transient dysphasia, there was no long-term morbidity related to the procedure. The procedure had to repeated on some patients in order to achieve the targeted result. There was no mortality related to the procedure.
Surgical intervention to relieve mass effect from the AVM bleed was required in only three patients. Of the 41 patients who presented with haemorrhage 11 (27%) were considered cured during the follow-up period. Six were endovascularly cured with NBCA embolization, two patients had surgical excision of the nidus at the time of haematoma removal. A further three had radiosurgery with cure on follow-up angiography. The remaining patients had only partial embolization as a treatment strategy.
Long-term follow-up data were only available for 30 patients as some patients were referred from far away and were not contactable. 1074 months of patient follow-up were completed for this cohort (mean of 34 months). Only one patient had rebleeding during this period and bled on two separate occasions giving an annual haemorrhage rate of 0.7%.
Discussion
Arteriovenous malformations present with haemorrhage in 50% of cases, this accounts for 2% of all patients presenting with stroke 17,18. There is an equal gender distribution of all patients that have an AVM, but there are slightly more male patients than females who present with a haematoma 19. Multiple factors have been found to be associated with an increased risk of hemorrhage such as a single draining vein, deep seated AVM location, small size AVM, deep venous drainage, advanced age and aneurysms associated with an AVM 20,28. The annual risk of hemorrhage in a patient with an unruptured AVM is 2-5% / year with annual morbidity and mortality rate of 1% and 2.7% respectively 1-3,19. The ruptured AVM rebleeding risk is 6%-18% within the first year and it drops to 2% per year 29 for the next 20 years. This is most likely because the rupture point remains weak and susceptible to re-haemorrhage until vascular repair is complete.
For AVMs presenting with haemorrhage the primary treatment objective remains exclusion and cure of the AVM and associated vascular weaknesses. This is however not possible in all cases as sometimes the AVM is too large or the lesion is inaccessible. Even in patients where cure is possible by surgery or radiosurgery, exclusion of the recent bleed site is advantageous. In the case of surgery it makes haematoma drainage possible without the need to urgently exclude the AVM, which can be operated on electively. In patients who require radiosurgery it could reduce the high bleed risk that the patient faces in the next year or two to a safer level.
Partial targeted embolization is a safe alternative to complete excision or occlusion of an AVM and a useful adjunct even when these treatments are planed. In patients that present with haemorrhage, a localised area of weakness representing the rupture point can be identified in some patients 9. Le Feuvre et al. had a cohort of nine patients that had intranidal aneurysms, which were a treatment target 15. The one-year follow-up of these patients showed a lower rebleeding rate than expected. The present study sought to analyze in more detail the group of AVM patients presenting with haemorrhage.
We had one hundred patients that presented with a brain AVM over a six-year period and only 41 patients initially presented with hemorrhage. These numbers are small but the distribution of the patients that presented with hemorrhage is consistent with the natural history so this sample is likely representative of a larger AVM population.
It has been previously shown that the location of hemorrhage could not be used to predict the presence of an aneurysm 29. In our cohort, patients that presented with a subarachnoid hemorrhage were more likely to have bled from a flow-related or associated aneurysm and patients presenting with intracerebral bleeding more likely to have a nidal false aneurysm. Based on this finding AVM patients who present with SAH should be carefully investigated, to look for an aneurysmal source of bleeding.
We were unable to conclusively identify the source of haemorrhage in 25 out of 44 bleeding episodes (57%) which is dissapointing. We did however apply strict criteria when reviewing the presenting scans and angiograms. There had to be a good correlation between the haemorrhage site and the angiographic weakness and we only identified intranidal false anerysms as a nidal bleeding source. Although other nidal irregularities were observed, and some were even called nidal aneurysms in procedure notes, we ignored these lesions because they could not definetely be identified as the bleeding site. Nidal aneurysms have been associated with a higher haemorrhage risk however there is currently no clear evidence that it is the nidal aneurysm that ruptures when this patient group presents with haemorrhage 6,7,25.
Other authors have identified flow-related and associated AVM aneurysms as a sign of vessel fragility, as this group of patients has a higher haemorrhage rate, although the source of bleeding was not felt to be aneurysmal 5,6,15. In our series, five episodes of bleeding out of 44 were identified as aneurysmal in origin. Given that only seven patients in this study were identified as having flow/associated aneurysms our findings offer a different perspective and perhaps AVM-related aneurysms should be considered a bleeding source.
We use neuroendovascular embolization as our first line of treatment for patients that have an identified angioarchitectural target. All patients presenting with hemorrhage were embolized with NBCA and one patient was treated with Onyx. NBCA has been shown to effectively occlude AVM feeders and the AVM nidus. Only three patients had a craniotomy 7,30,33 to evacuate the hemorrhage because of mass effect confirming that AVM-related bleeds are often well tolerated and that emergency surgery is not required unless patients have deteriorating symptoms.
Partial targeted embolization was our primary treatment for all those patients that had an intranidal false aneurysm. All of the patients with an intranidal aneurysm could have a catheter passed up to or into the nidus and the false aneurysm was occluded successfully. The flow-related aneurysms were all easily accessible on the AVM feeding artery and all patients were treated using bare platinum coils except for one patient who had a wide-necked anterior communicating artery aneurysm that could not be embolized. This aneurysm was thought to be the source of bleeding in this patient and was the only patient where the target could not be treated. This patient was the only one to rebleed in the series. Associated aneurysms have been thought to signal a widespread vasculopathy but we found these to be uncommon.
Although follow-up data were not complete for all patients the rebleed rate of 0.7% was significantly lower than would be expected for patients presenting with haemorrhage.
Conclusion
In just under a half of patients with AVM bleeding a source of haemorrhage can be identified on DSA and in most cases this will be an intranidal false aneurysm. Flow-related and associated aneurysms in patients with brain AVM can cause haemorrhage. These patients are more likely to have SAH than intracerebral haemorrhage. These weak points are a good target for partial endovascular treatment, are usually accessible and may reduce the higher haemorrhage rate expected over the next two years.
References
- 1.Brown RD Jr, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352–357. doi: 10.3171/jns.1988.68.3.0352. [DOI] [PubMed] [Google Scholar]
- 2.Ondra SL, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387–391. doi: 10.3171/jns.1990.73.3.0387. [DOI] [PubMed] [Google Scholar]
- 3.Krings T, Hans FJ, Geibprasert S, et al. Partial “targeted” embolisation of brain arteriovenous malformations. Eur Radiol. 2010;20:2723–2731. doi: 10.1007/s00330-010-1834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernesniemi JA, Dashti R, Juvela S, et al. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008;63:823–829. doi: 10.1227/01.NEU.0000330401.82582.5E. discussion 829-831. [DOI] [PubMed] [Google Scholar]
- 5.da Costa L, Wallace MC, Ter Brugge KG, et al. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke. 2009;40:100–105. doi: 10.1161/STROKEAHA.108.524678. [DOI] [PubMed] [Google Scholar]
- 6.Redekop G, TerBrugge K, Montanera W, et al. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539–546. doi: 10.3171/jns.1998.89.4.0539. [DOI] [PubMed] [Google Scholar]
- 7.Meisel HJ, Mansmann U, Alvarez H, et al. Effect of partial targeted N-butyl-cyano-acrylate embolization in brain AVM. Acta Neurochir (Wien) 2002;144:879–887. doi: 10.1007/s00701-002-0978-6. discussion 888. [DOI] [PubMed] [Google Scholar]
- 8.Achrol AS, Guzman R, Varga M, et al. Pathogenesis and radiobiology of brain arteriovenous malformations: implications for risk stratification in natural history and posttreatment course. Neurosurg Focus. 2009;26:E9. doi: 10.3171/2009.2.FOCUS0926. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann A, Mast H, Mohr JP, et al. Determinants of staged endovascular and surgical treatment outcome of brain arteriovenous malformations. Stroke. 2005;36:2431–2435. doi: 10.1161/01.STR.0000185723.98111.75. [DOI] [PubMed] [Google Scholar]
- 10.Speizler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476–483. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 11.Spetzler RF. A 3-tier classification of cerebral arteriovenous malformations. J Neurosurg. 2011;114:842–849. doi: 10.3171/2010.8.JNS10663. [DOI] [PubMed] [Google Scholar]
- 12.Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003;98:3–7. doi: 10.3171/jns.2003.98.1.0003. [DOI] [PubMed] [Google Scholar]
- 13.Schaller C, Schramm J, Haun D. Significance of factors contributing to surgical complications and to late outcome after elective surgery of cerebral arteriovenous malformations. J Neurol Neurosurg Psychiatry. 1998;65:547–554. doi: 10.1136/jnnp.65.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Feuvre D, Taylor A. Target embolization of AVMs: identification of sites and results of treatment. Interventional Neuroradiology. 2007;13:389–394. doi: 10.1177/159101990701300411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Yu T, Wang S, et al. Surgical treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2010;67:1359–1370. doi: 10.1227/NEU.0b013e3181eda216. discussion 1370. [DOI] [PubMed] [Google Scholar]
- 16.Alexander MJ, Tolbert ME. Targeting cerebral arteriovenous malformations for minimally invasive therapy. Neurosurgery. 2006;59 Suppl 3:S178–S183. doi: 10.1227/01.NEU.0000238530.44912.01. discussion S3. [DOI] [PubMed] [Google Scholar]
- 17.Stapf C, Labovitz DL, Sciacca RR, et al. Incidence of adult brain arteriovenous malformation hemorrhage in a prospective population based stroke survey. Cerebrovasc Dis. 2002;13:43–46. doi: 10.1159/000047745. [DOI] [PubMed] [Google Scholar]
- 18.A.-Shahi R, Warlow C. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain. 2001;124:1900–1926. doi: 10.1093/brain/124.10.1900. [DOI] [PubMed] [Google Scholar]
- 19.Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331–337. doi: 10.3171/jns.1983.58.3.0331. [DOI] [PubMed] [Google Scholar]
- 20.Hofmeister C, Stapf C, Hartmann A, et al. Demographic, morphological, and clinical characteristics of 1289 patients with brain arteriovenous malformation. Stroke. 2000;31:1307–1310. doi: 10.1161/01.str.31.6.1307. [DOI] [PubMed] [Google Scholar]
- 21.Stefani MA, Porter PJ, terBrugge KG, et al. Angioarchitectural factors present in brain arteriovenous malformations associated with hemorrhagic presentation. Stroke. 2002;33:920–924. doi: 10.1161/01.str.0000014582.03429.f7. [DOI] [PubMed] [Google Scholar]
- 22.Stefani MA, Porter PJ, terBrugge KG, et al. Large and deep brain arteriovenous malformations are associated with risk of future hemorrhage. Stroke. 2002;33:1220–1224. doi: 10.1161/01.str.0000013738.53113.33. [DOI] [PubMed] [Google Scholar]
- 23.Dashe JF. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2007;68:535. doi: 10.1212/01.wnl.0000256978.74872.60. author reply 535. [DOI] [PubMed] [Google Scholar]
- 24.Khaw AV, Mohr JP, Sciacca RR, et al. Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke. 2004;35:660–663. doi: 10.1161/01.STR.0000117093.59726.F9. [DOI] [PubMed] [Google Scholar]
- 25.Mansmann U, Meisel J, Brock M, et al. Factors associated with intracranial hemorrhage in cases of cerebral arteriovenous malformation. Neurosurgery. 2000;46:272–279. doi: 10.1097/00006123-200002000-00004. discussion 279-281. [DOI] [PubMed] [Google Scholar]
- 26.Miyasaka Y, Yada K, Ohwada T, et al. An analysis of the venous drainage system as a factor in hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:239–243. doi: 10.3171/jns.1992.76.2.0239. [DOI] [PubMed] [Google Scholar]
- 27.Spetzler RF, Hargraves RW, McCormick PW, et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:918–923. doi: 10.3171/jns.1992.76.6.0918. [DOI] [PubMed] [Google Scholar]
- 28.Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350–1355. doi: 10.1212/01.wnl.0000210524.68507.87. [DOI] [PubMed] [Google Scholar]
- 29.Mast H, Young WL, Koennecke HC, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997;350:1065–1068. doi: 10.1016/s0140-6736(97)05390-7. [DOI] [PubMed] [Google Scholar]
- 30.Li TL, Fang B, He XY, et al. Complication analysis of 469 brain arteriovenous malformations treated with N-butyl cyanoacrylate. Interventional Neuroradiology. 2005;11:141–148. doi: 10.1177/159101990501100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jafar JJ, Davis AJ, Berenstein A, et al. The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78:60–69. doi: 10.3171/jns.1993.78.1.0060. [DOI] [PubMed] [Google Scholar]
- 32.Tamatani S, Ito Y, Koike T, et al. Efficacy of diluted NBCA mixture for embolization of arteriovenous malformations. Interventional Neuroradiology. 1999;5(Suppl 1):161–165. doi: 10.1177/15910199990050S129. [DOI] [PubMed] [Google Scholar]
- 33.Yu SC, Chan MS, Lam JM, et al. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. Am J Neuroradiol. 2004;25:1139–1143. [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford PM, West CR, Chadwick DW, et al. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1–10. doi: 10.1136/jnnp.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]