Summary
A blood blister-like (BBL) or dissecting aneurysm should be carefully considered if located at a non-branching site of the supra-clinoid internal carotid artery (ICA). Several surgical and endovascular treatment methods have been proposed but they all carry a relatively high risk of morbidity and mortality.
This study evaluated the effectiveness of a novel Silk flow-diverting device (SFD) placed in the early acute stage.
Three patients presenting with acute subarachnoid haemorrhage caused by small blister-like aneurysms of the carotid siphon were treated within 48 hours after admission by placement of SFDs. More than one device was placed to cover the lesion. None of the patients were premedicated and started anti-platelet therapy during the procedure. All aneurysms were successfully occluded.
A good outcome was observed in two out of three treated patients. No thromboembolic or haemorrhagic event occurred during or after the procedures, or during follow-up (6-14 months). SFD prevented rebleeding and the use of these devices could be proposed as an option to treat fragile uncoilable BBL aneurysms, even in the early acute phase without anti-platelet premedication. Larger studies and long-terms results are necessary.
Key words: brain, cerebral, anterior communicating, aneurysm
Introduction
When a ruptured aneurysm is detected angiographically at a non-branching site of the supra-clinoid internal carotid artery (ICA) located in the dorsal or anterior wall of the ICA, a blood blister-like (BBL) or dissecting aneurysm should be carefully considered. BBL are rare lesions comprising 0.3-1% of all intracranial aneurysms and 0.9-6.6% of ICA aneurysms 1-3. BBL aneurysms are fairly small, sometimes appearing as just a protrusion of the vessel wall and are usually associated with diffuse subarachnoid haemorrhage and a severe clinical condition. Their behaviour is extremely variable with a life-threatening evolution. BBL aneurysms are not easily detected at the first angiography due to their small dimensions and unusual site, but because of their rapid growth they appear more evident a few days later and tend to re-rupture 1,2. The early recognition of these lesions is crucial to adequate treatment planning and patient survival.
Several surgical and endovascular treatment methods have been proposed to date but they all carry a relatively high risk of morbidity and mortality. The new Silk devices are self-expandable stents with a very dense NiTinol mesh designed to produce flow diversion and reconstruct laminar flow in the parent artery and exclude the aneurysm 4,5. Although data on the long-term outcomes of these devices are not available there is increasing experience of their successful use for otherwise untreatable aneurysms or lesions with a high risk of recurrence after standard endovascular procedures.
We describe our experience of three patients with diffuse subarachnoid haemorrhage due to small aneurysms at non-branching sites of the left ICA, successfully treated with Silk Flow-Diverting (SFD) devices placed in the acute stage.
Material and Methods
Two women and one man (aged from 42 to 56 years) presented with acute subarachnoid haemorrhage caused by small blister-like aneurysms of the carotid siphon. All patients were treated in the early acute stage, within 48 hours after admission. Carotid occlusion test was performed to evaluate collateral circulation through the anterior communicating artery. None of the patients were pre-loaded with anti-platelet drugs. Aspirin was started during the procedures and clopidogrel loading was started in the intensive care unit at the end of endovascular treatment. Silk Flow-Diverters (Balt Extrusion, Montmorency, France) were implanted with the stent-within-a-stent technique, one inside the other to achieve a more dense mesh in front of the aneurysm, but in a telescopic fashion positioning the next device with the proximal edge below the end of the previous one. This technique served mainly to avoid possible inflow between overlapping stents and to better stabilize the SFDs keeping the proximal end in contact with the arterial wall. All SFDs were implanted in the carotid syphon below the A1-M1 bifurcation in two patients (cases 1 and 2), whereas the first SFD was implanted from M1 to the carotid syphon in one patient (case 3). Coil placement was attempted without success in two patients during the same treatment session.
Case 1
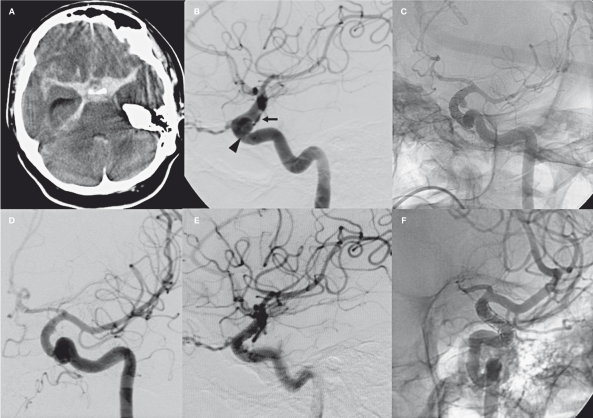
A 56-year-old woman was admitted to the Emergency Department with a GCS 8. CT scan on admission showed a Fisher 3 subarachnoid haemorrhage with blood concentrated in the left supraclinoid cistern. Selective angiography showed an 8 mm intracavernous aneurysm and a 3 mm BBL aneurysm in the lateral aspect of the supraclinoid ICA (Figure 1). An external shunt was placed due to increasing ventricular dilatation. On the basis of the angiographic findings, after case discussion with neurosurgeons we opted for endovascular treatment. Simple and balloon-assisted coiling was attempted unsuccessfully due to the aneurysm site and its wide-neck morphology. Carotid occlusion was well-tolerated through the anterior communicating artery. Three SFDs were implanted one inside the other covering the entire carotid segment. The three 4 mm diameter Silk devices were positioned below the A1-M1 bifurcation. We first inserted the shortest one (20 mm) followed by 25 mm and 30 mm devices to deploy the proximal edge of the SFD a few millimeters below the end of the previous one. A bolus of 5000 IU of heparin and 500 mg of aspirin was administered intravenously (i.v.) just before first stent deployment. After insertion of the second stent we obtained a complete disappearance of the intracavernous aneurysm and persistent flow in the supraclinoid BBL lesion. A third SFD device was positioned. The final result was a flow reduction throughout the carotid artery and middle cerebral artery (MCA) with the left anterior cerebral artery (ACA) supported by the contralateral carotid artery. We even observed flow reduction in the BBL aneurysm. The patient received 300 mg clopidogrel through a naso-gastric tube one hour later in the intensive care unit. She was kept intubated with i.v. administration of 10 ml/h of nimodipine and 250 mg ASA, oral 75 mg clopidogrel and subcutaneous 5000 IU of low molecular weight heparin (LMWH). The control angiogram performed 24 hours later showed a complete restoration of carotid artery and MCA flow and initial dye stagnation in the BBL aneurysm. A seven-day control angiogram (Figure 1C) showed partial exclusion of the BBL aneurysm. We stopped ASA to facilitate aneurysm occlusion, whereas clopidogrel and 5000 UI of LMWH were maintained to prevent artery thrombosis and thromboembolic events. The control angiogram performed six days later (Figure 1D) demonstrated complete occlusion of the BBL aneurysm with normal flow through the carotid siphon. This was confirmed at 30 day follow-up CT angiography (CTA). The patient was discharged two months later in good clinical condition medicated with 75mg of clopidogrel. At six month follow-up she had completely recovered without neurological deficit. Clopidogrel was then suspended. The seven month control angiogram confirmed complete healing of the BBL aneurysm and patency of the carotid siphon and its branches (Figure 1E). A 20% in-stent stenosis was found and 100 mg of aspirin was then started (Figure 1F). Clinical condition and angiographic (CTA) pattern remained stable at 16 month follow-up.
Figure 1.
Case 1. A) Acute CT scan showing diffuse subarachnoid hemorrhage. B) Lateral view of the left carotid artery showing the 8 mm intracavernous aneurysm (arrowhead) and the 3 mm BBL aneurysm of the supraclinoid siphon (black arrow). C) The 7 day control angiogram shows a complete disappearance of the intracavernous aneurysm and persistence of the BBL aneurysm. At that time we stopped ASA maintaining clopidogrel and LMWH. D) Control angiogram performed six days later shows a small remnant of the BBL (white arrow). E-F) 7 month control angiogram shows complete healing of the carotid siphon and in-stent restenosis.
Case 2
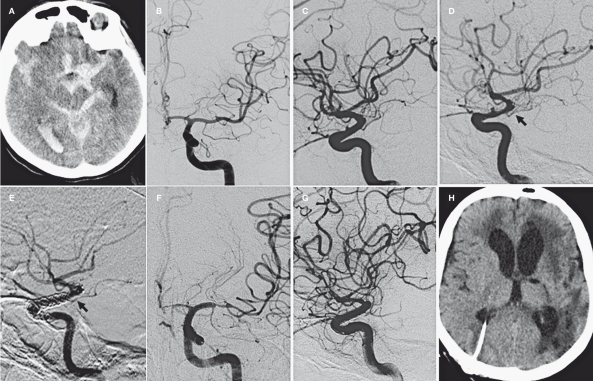
A comatose (GCS 8) 50-year-old woman was admitted. Emergent CT scan showed a Fisher 4 subarachnoid haemorrhage with blood concentrated in the left supraclinoid cistern and sylvian fissure. Selective angiography showed a < 2 mm BBL aneurysm in the dorsal aspect of the left supraclinoid ICA. A ventricular shunt was placed. After interdisciplinary consultation we opted for endovascular treatment. The procedure was performed 48 hours after admission (Figure 2). The preliminary angiogram showed initial vasospasm with narrowing of the left M1 and ACA and modification of aneurysm shape. Microcatheter positioning for simple coiling was attempted unsuccessfully due to the aneurysm site and small dimensions. Angiographic carotid occlusion test showed compensatory circulation through the anterior communicating artery despite initial vasospasm. Two SFDs were implanted one inside the other covering the entire carotid segment. The two 4 mm SFD were positioned below the A1-M1 bifurcation. We first positioned a 20 mm SFD followed by a 25 mm device that was deployed with the proximal edge a few millimeters below the end of first one. A bolus of 5000 UI of heparin and 500 mg of aspirin was administered intravenously just before the first stent deployment. After insertion of the second Silk we observed flow reduction in the carotid siphon and tight narrowing of the MCA due to increased vasospasm. Through the Vasco21 microcatheter still positioned in M1 we placed a Solitaire stent (EV3, Irvine, CA, USA) obtaining prompt reopening of the MCA and restoration of carotid flow. One milligram of nimodipine was slowly injected selectively into the carotid artery. Because of the pre-existing vasospasm and the short-lasting effect of nimodipine injection, we decided to deploy the Solitaire stent. The BBL aneurysm was partially excluded with dye stagnation. The patient received 300 mg clopidogrel through a naso-gastric tube one hour later in the intensive care unit. The patient was kept intubated with i.v. administration of 10 ml/h nimodipine and 250 mg aspirin, oral 75 mg clopidogrel and subcutaneous 5000 IU of LMWH. The control angiogram performed 24 hours later (Figure 2E) showed a complete restoration of flow in the carotid siphon and MCA. Severe vasospasm was still present in ACA and MCA territory. The vasospasm spared the entire stented arterial segment. The seven-day angiogram showed complete occlusion of the BBL aneurysm (Figure 2F) and severe persistent vasospasm in distal M1 and ACA. The anterior choroidal artery disappeared with a correlated ischemic lesion in its distal territory. CT scans showed infarct areas even in the left M1 and ACA territories. Double anti-platelet therapy was maintained for three months then clopidogrel was stopped. The patient was sent to a rehabilitation center in poor clinical condition with aphasia and right hemiplegia. A two month control angiogram (Figure 2G) confirmed healing of the BBL aneurysm and patency of the carotid siphon. Her neurological status did not improve significantly in the next eight months.
Figure 2.
Case 2. A) CT scan on admission showing subarachnoid and intraventricular bleeding. B-C) Acute AP and lateral view angiogram of the left carotid artery. D) 48 hour pre-treatment angiogram shows a modification of the BBL aneurysm and vasospasm of the carotid siphon and its branches. E) 24 hour control angiogram. Double telescopic Silk stents in the carotid siphon and Solitaire stent in M1 segment. Complete disappearance of the aneurysm. The AChA is still patent (black arrow). F) 7 day control angiogram. Severe vasospasm of ACA and MCA that spared the entire stented arterial segment. G-H) 2 month control angiogram and CT scan. Absence of the AChA and stable occlusion of the aneurysm. Infarct areas in AChA and MCA territories.
Case 3
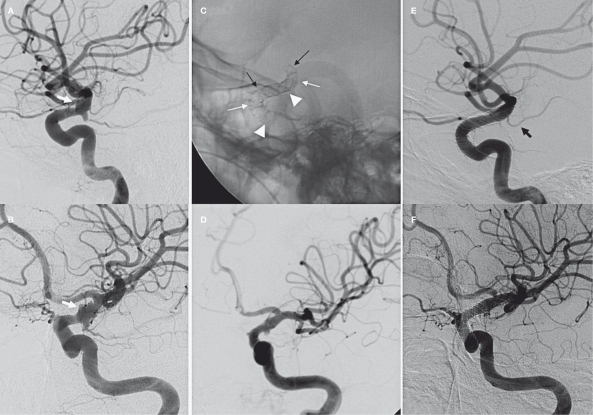
A 42-year-old man with GCS 14 was transferred to our Institution with an angiographically diagnosed BBL aneurysm of the left carotid siphon. The patient had bled the first time five days before emergency admission complaining of worsening headache with vomiting and confusion. Emergent CT scan showed a Fisher 3 subarachnoid haemorrhage. Selective angiography disclosed a 5 mm irregular shaped BBL aneurysm in the dorsal aspect of the left supraclinoid ICA extending to the A1-M1 bifurcation and an infundibular dilatation of the posterior communicating artery (Figure 3). The endovascular procedure was performed 24 hours after emergency admission. Coiling was not attempted due to aneurysm morphology. Angiographic carotid occlusion test showed good collateral circulation through the anterior communicating artery. Three SFDs were implanted from M1 to the siphon one inside the other covering the entire carotid segment. The first 4 mm Silk was positioned from M1 to the C5-C6 segment of the carotid siphon, the second device was inserted below the A1-M1 bifurcation and the third one just below the anterior choroidal artery. We first positioned a 20 mm SFD followed by 25 mm and 30 mm devices (Figure 3C). A bolus of 5000 UI of heparin and 500 mg of aspirin was administered intravenously just before the first stent deployment. One milligram of nimodipine was injected selectively into the carotid artery before and after the first SFD placement. After the third SFD we obtained a significant reduction of aneurysm filling and a good reconstruction of the arterial wall (Figure 3D). The patient received 300 mg clopidogrel through a naso-gastric tube one hour later in the intensive care unit. The patient was awakened 12 hours later without neurological deficits. He was treated with i.v. administration of 10 ml/h nimodipine, oral 300 mg aspirin and 75 mg clopidogrel and subcutaneous 5000 IU LMWH. The control angiogram performed five days later showed a complete restoration of the carotid siphon with normal flow in the ACA and MCA territories. This was confirmed at 30 day CTA control. The patient was discharged without neurological deficit. Double anti-platelet therapy was maintained for three months, then clopidogrel was stopped. The eight month control angiogram (Figure 3E) showed a complete healing of the artery and patency of the AChA. The posterior communicating artery is supported by posterior circulation. The patient is neurologically intact and 100 mg of aspirin is continued.
Figure 3.
Case 3. A-B) Different projections of the left carotid siphon showing aneurysm of the C6 segments (white arrows). C) Triple telescopic Silk stent in the carotid siphon. The first stent is placed from M1 to the ophthalmic segment (black arrows), the second one just before the A1-M1 bifurcation (white arrows) and the third one just below the AChA origin (arrowheads). D) Angiographic result at the end of the procedure. E-F) 8 month control angiogram showing a complete disappearance of the BBL aneurysm. Patency of the AChA (black arrow) and narrowing of A1 origin.
Discussion
Blood blister-like (BBL) aneurysms have some unique anatomo-pathological and clinical characteristics distinctive from the more frequent berry aneurysms. The histological characteristics of BBL aneurysms include focal wall defects covered with clots and fibrous tissue. The focal wall defects may be the result of laceration of the ICA wall caused by ulceration and penetration into the internal elastica lamina resulting from arteriosclerosis. The walls of BBL aneurysms are composed of only normal adventitia, in an abrupt transformation from the sclerotic ICA wall. By contrast, berry aneurysms are formed by thickened intima and/or adventitia with rich collagen and inflammatory cells continuous with the three-layered arterial wall 1-3. Dissection of the ICA is often observed in patients harbouring BBL aneurysms. Hypertension and hemodynamic stresses may also play a role 1,3. Bleeding is usually severe despite the small dimensions of the lesions. Because of their atypical location and small size BBL aneurysms are frequently not visible at CTA or at the first angiogram but grow rapidly becoming evident at a second or third examination and tending to re-rupture 1,2.
The evolution of a BBL into a saccular-like aneurysm on a subsequent angiogram is just an illusion. This new saccular aneurysm does not have the thick wall of a true berry aneurysm and the morphological change is probably related to lysis of the intra-aneurysmal clot 3.
The clinical course is usually extremely life-threatening and the therapeutic options are not obvious. The options include direct surgery with clipping, wrapping or encircling clipping, the use of extracranial-intracranial bypass or endovascular techniques.
The main surgical problem is the extremely fragile aneurysm wall and the complete absence of a definite neck 2. The arterial defect may be more extensive than as depicted by CTA or selective angiography. On the basis of surgical literature, intraoperative or early postoperative re-ruptures of BBL aneurysms occurred in 47% of directly clipped patients, and 20% of patients treated with clipping on wrapping material 2,6-8.
Endovascular options include carotid artery occlusion and treatment to preserve arterial patency 9. Carotid occlusion could be performed proximal to the lesion or with trapping of the diseased segment. Proximal occlusion does not eliminate the risk of retrograde flow and lesion progression with consequent rebleeding 4-5, whereas precise coil placement for the trapping technique may not be easy or safe because of the irregular shape and unclear lesion borders. Moreover, due to the extreme fragility of the wall, coils should not be densely packed to avoid aneurysm re-rupture. In both these cases the inflow reduction may entail a considerable risk of hemispheric ischaemia due to inadequate perfusion in case of cerebral vasospasm, despite good collaterals and a well-tolerated occlusion test 6,7.
More recently, the stent-assisted technique has been used alone or in combination with coils 10-12. The main problem with this technique is the need for aggressive anti-platelet therapy 9,13. This therapeutic regimen obliges the physician to restore the arterial wall and to occlude the wall laceration to avoid possible catastrophic re-bleeding. Moreover, this therapy exposes the patient to possible bleeding in case of ventricular shunt placement. Because of these considerations some authors suggest a multi-step treatment or to stabilize the patient and wait for the cerebral vasospasm to end before any endovascular approach 14.
Nevertheless, endovascular treatment is associated with frequent lesion regrowth 8 exposing patients to an unpredictable evolution. Because of the small size, unfavourable position and wide-necked morphology of BBL aneurysms, microcatheter positioning and safe coil detachment seems unlikely due to the risk of further aneurysm rupture or frequent aneurysm regrowth and coil migration. On the other hand, wide cell stent placement alone, even with the stent-within-a-stent technique, does not guarantee effective haemodynamic changes and reduction of flow in the aneurysm promoting thrombosis and healing 15-17. Alternatively, covered stents represent a possible therapeutic option. The main advantage of using stent-grafts is the ability to seal the aneurysm at the time of stent deployment, making coiling unnecessary. This option is potentially hazardous due to the stiffness of the stent itself and of the delivery system that could lead to vessel injury, rupture and acute carotid occlusion 12. On the other hand, the occlusion of side branches should be seriously considered in case of successful placement of the stent.
In our patients we opted for the novel Silk flow-diverting (SFD) stents to obtain a rapid occlusion of the aneurysm with preservation of carotid flow. This treatment also makes aneurysm catheterization and coil deployment unnecessary. All patients were treated in the very acute phase and were not premedicated with antiplatelet drugs. For this reason collateral circulation was tested in all cases to ascertain the possible effect of carotid occlusion due to an acute stent thrombosis. Because reconstruction of the affected carotid siphon may be of critical importance in the treatment of BBL aneurysms 18, and to reduce inflow within the aneurysm, the use of stents that mimic a covered stent but have the flexibility of a bare stent seems to be a good option. This method could further alter the inflow within the aneurysm, promoting stasis and fast thrombosis. In our patients we decided to overlap more than one SFD as in the stent-in-stent technique to achieve immediate aneurysm exclusion and to use conventional anti-platelet therapy more safely. The aim was to place stents one within the other until aneurysm filling was significantly reduced or even excluded from the flow. Our intention was partially modified during treatments due to carotid flow reduction caused, in our opinion, by a combination of increased thickness of the metal mesh and arterial spasm. These side-effects occurred after the third stent in case 1 and after the second stent in case 2. In the third case we decided to inject nimodipine selectively before and after the first stent to prevent vasospasm. We believe that vasospasm could be related to multiple passages of the delivery system in such a very acute phase. This complication was never observed in our elective patients. The worst vasospasm reaction occurred in our second case with tight narrowing of M1 and flow stagnation in the carotid siphon. We decided to restore the M1 flow positioning a self-expandable Solitaire stent through the Vasco 21 microcatheter already in place, followed by further selective nimodipine injection. This unconventional procedure was designed to restore the flow rapidly and avoid in-stent thrombosis in the acutely implanted stent.
In all cases we started with the shortest stent and then placed the next device 5 mm longer to detach the lower extremity of the stent below the previous one in contact with arterial wall in a telescopic fashion. This was done to avoid possible inflow between overlapping stents and to better stabilize the SFDs keeping the proximal end in contact with the arterial wall. This choice was based on the characteristic low radial force of SFDs compared to all other stents and the unpredictable evolution of a SFD in contact with a high density metal mesh. To our knowledge, there are no data describing the behaviour of SFDs positioned one inside the other.
An important issue is the effect of highly dense devices in front of important branches such as the A1 origin and the anterior choroidal artery (AChA). In our experience SFDs were positioned in front of the aneurysms possibly sparing the anterior cerebral artery and AChA. We carefully positioned the SFDs below the A1-M1 bifurcation in cases 1 and 2, whereas in case 3 we had to place the first Silk from the M1 segment because of the aneurysm site. We observed flow reduction of the AChA only in our second case just after the second stent deployment and complete occlusion at follow-up, with consequent ischemic infarction. In case 1 the distal edge of the SFDs did not cause occlusion of the artery (Figure 1) or ischemic events in the AChA territory. In case 3 we covered the ostium of the AChA with the first two SFDs and we carefully positioned the third device just below the artery origin (Figure 3). At eight month control angiogram the AChA was patent. Every effort should be taken to minimize the metal mesh density in front of side branches.
In our small series no thromboembolic event occurred during or after the procedures even though patients were not premedicated. We discontinued double anti-platelet therapy seven days after SFD deployment in case 1, maintaining only clopidogrel and LMWH. This decision was taken to promote aneurysm thrombosis that was presumably delayed by the double anti-platelet regimen. The use of anti-platelet agents in the setting of ruptured aneurysms is still controversial due to the risk of haemorrhagic complications or to acute occlusion of the parent artery 19. When needed (cases 1 and 2) a ventricular shunt was positioned before endovascular treatment.
Many debatable explanations could account for the positive evolution of our cases, such as the different behaviour of SFD devices in small aneurysms versus conventional larger lesions, the haemostatic mechanism of small aneurysms and the modification of coagulative and fibrinolytic cascade in patients with acute subarachnoid haemorrhage treated in this time window 20-22. This small and early experience indicates that SFD placement could be a good choice in the cases described here, possibly better than wide cell stents or stent-assisted coiling. However, given the narrow cell design of SFDs the overlapping stent technique may not be necessary and we may have overtreated our patients. Other authors 23,24 have described successful single-stent flow diversion to prevent rebleeding of sub-acutely ruptured aneurysms obtaining stable disappearance of small “uncoilable” lesions.
Conclusion
Given that the best treatment for BBL aneurysms has yet to be established, our cases show that endovascular treatment with the new flow-diverting devices prevented rebleeding and occluded the aneurysms. This procedure was performed in fragile ruptured uncoilable BBL lesions in the early acute phase without anti-platelet premedication. The primary risk of using multiple devices remains side branch occlusion. Larger studies and long-terms results are necessary.
References
- 1.Ishikawa T, Nakamura N, Houkin K, et al. Pathological consideration of a “blister-like” aneurysm at the superior wall of the internal carotid artery: case report. Neurosurgery. 1997;40:403–406. doi: 10.1097/0006123-199702000-00038. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa A, Suzuki M, Ogasawara K. Aneurysms at nonbranching sites in the supraclinoid portion of the internal carotid artery: internal carotid artery trunk aneurysms. Neurosurgery. 2000;47:578–586. doi: 10.1097/00006123-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Kwon TH, Kim JH, et al. Internal carotid artery dorsal wall aneurysm with configurational change: Are they all false aneurysms? Surg Neurol. 2006;66:441–443. doi: 10.1016/j.surneu.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Leonardi M, Dall’olio M, Princiotta C, et al. Treatment of carotid siphon aneurysms with a microcell stent. A case report. Interv Neuroradiol. 2008;29(14):429–434. doi: 10.1177/159101990801400408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szikora I, Nelson PK, Berentei Z, et al. The potential of flow modification in the treatment of intracranial aneurysms. Interv Neuroradiol. 2008;14(Suppl 1):77–80. doi: 10.1177/15910199080140S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sim SY, Shin YS, Cho KG, et al. Blood blister-like aneurysms at nonbranching sites of the internal carotid artery. J Neurosurg. 2006;105:400–405. doi: 10.3171/jns.2006.105.3.400. [DOI] [PubMed] [Google Scholar]
- 7.Baskaya MK, Ahmed AS, Ates O, et al. Surgical treatment of blood blister-like aneurysms of the supraclinoid internal carotid artery with extracranial-intracranial bypass and trapping. Neurosurg Focus. 2008;24:E13. doi: 10.3171/FOC/2008/24/2/E13. [DOI] [PubMed] [Google Scholar]
- 8.Meling TR, Sorteberg A, Bakke SJ, et al. Blood blister-like aneurysms of the internal carotid artery trunk causing subarachnoid hemorrhage: treatment and outcome. J Neurosurg. 2008;108:662–671. doi: 10.3171/JNS/2008/108/4/0662. [DOI] [PubMed] [Google Scholar]
- 9.Krings T, Choi IS. The many faces of intracranial arterial dissections. Interv Neuroradiol. 2010;16:151–160. doi: 10.1177/159101991001600206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanoue S, Kyosue H, Matsumoto S, et al. Ruptured “blisterlike” aneurysm with a pseudoaneurysm formation requiring delayed intervention with endovascular coil embolization. J Neurosurg. 2004;101:159–162. doi: 10.3171/jns.2004.101.1.0159. [DOI] [PubMed] [Google Scholar]
- 11.Park JH, Park IS, Han DH, et al. Endovascular treatment of blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2007;106:812–819. doi: 10.3171/jns.2007.106.5.812. [DOI] [PubMed] [Google Scholar]
- 12.Lee BH, Kim BM, Park MS, et al. Reconstructive endovascular treatment of ruptured blood blister-like aneurysms of the internal carotid artery. J Neurosurg. 2009;110:431–436. doi: 10.3171/2008.7.JNS08257. [DOI] [PubMed] [Google Scholar]
- 13.Korja M, Rautio R, Valtonen S, et al. Primary treatment of ruptured blood blister-like aneurysms with stent-assisted coil embolization: report of two cases. Acta Radiol. 2008;49:180–183. doi: 10.1080/02841850701675735. [DOI] [PubMed] [Google Scholar]
- 14.Gaughen JR Jr, Hasan D, Dumont AS, et al. The efficacy of endovascular stenting in the treatment of supraclinoid internal carotid artery blister aneurysms using a stent-in-stent technique. Am J Neuroradiol. 2010;31:1132–1138. doi: 10.3174/ajnr.A2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim BM, Chung EC, Park SI, et al. Treatment of blood blister-like aneurysm of the internal carotid artery with stent-assisted coil embolization followed by stent-within-a-stent technique. Case report. J Neurosurg. 2007;107(6):1211–1213. doi: 10.3171/JNS-07/12/1211. [DOI] [PubMed] [Google Scholar]
- 16.Otani N, Takasato Y, Masaoka H, et al. Clinical and radiological findings and surgical management of ruptured aneurysms at the non-branching sites of the internal carotid artery. J Clin Neurosci. 2009;16:1018–1023. doi: 10.1016/j.jocn.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Ahn JY, Cho JH, Jung JY, et al. Blister-like aneurysms of the supraclinoid internal carotid artery: challenging endovascular treatment with stent-assisted coiling. J Clin Neurosci. 2008;15:1058–1061. doi: 10.1016/j.jocn.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneuryms. Neurosurgery. 2006;59:291–300. doi: 10.1227/01.NEU.0000223650.11954.6C. [DOI] [PubMed] [Google Scholar]
- 19.Tähtinen OI, Vanninen RL, Manninen HI, et al. Wide-necked intracranial aneurysms: treatment with stent-assisted coil embolization during acute (<72 hours) subarachnoid hemorrhage--experience in 61 consecutive patients. Radiology. 2009;253:199–208. doi: 10.1148/radiol.2531081923. [DOI] [PubMed] [Google Scholar]
- 20.Morga R, Czepko R, Dembinska-Kieć A, et al. Assessment of the haemostatic system in patients surgically treated for ruptured cerebral aneurysm. Neurol Neurochir Pol. 2007;41:296–305. [PubMed] [Google Scholar]
- 21.Antovic J, Bakic M, Zivkovic M, et al. Blood coagulation and fibrinolysis in acute ischaemic and haemorrhagic (intracerebral and subarachnoid haemorrhage) stroke: does decreased plasmin inhibitor indicate increased fibrinolysis in subarachnoid haemorrhage compared to other types of stroke? Scand J Clin Lab Invest. 2002;62:195–199. doi: 10.1080/003655102317475452. [DOI] [PubMed] [Google Scholar]
- 22.Ebihara T, Kinoshita K, Utagawa A, et al. Changes in coagulative and fibrinolytic activities in patients with intracranial hemorrhage. Acta Neurochir Suppl. 2006;96:69–73. doi: 10.1007/3-211-30714-1_17. [DOI] [PubMed] [Google Scholar]
- 23.Kulcsár Z, Wetzel SG, Augsburger L, et al. Effect of flow diversion treatment on very small ruptured aneurysms. Neurosurgery. 2010;67:1–5. doi: 10.1227/01.NEU.0000372920.39101.55. [DOI] [PubMed] [Google Scholar]
- 24.Rasskazoff S, Selvaggio J, Brouwer PA, et al. Endovascular treatment of a ruptured blood blister-like aneurysm with a flow-diverting stent. Interv Neuroradiol. 2010;16:255–258. doi: 10.1177/159101991001600304. [DOI] [PMC free article] [PubMed] [Google Scholar]