Summary
The Silk stent (Balt, Montmorency, France) is a retractable device designed to achieve curative reconstruction of the parent artery associated with an intracranial aneurysm. We present our initial experience with the Silk flow-diverting stent in the management and follow-up of 25 patients presenting with intracranial aneurysms.
Twenty-five patients (age range, 34-81 years; 24 female) were treated with the Silk flow-diverting device. Aneurysms ranged in size from small (5), large (10) and giant (10) and included wide-necked aneurysms, multiple, nonsaccular, and recurrent intracranial aneurysms. Nine aneurysms were treated for headache, 14 for mass effect. None presented with haemorrhage. All patients were pretreated with dual antiplatelet medications for at least 72 hours before surgery and continued taking both agents for at least three months after treatment. A total of 25 Silk stents were used. Control MR angiography and/or CT angiography was typically performed prior to discharge and at one, three, six and 12 months post treatment. A follow-up digital subtraction angiogram was performed between six and 19 months post treatment.
Complete angiographic occlusion or subtotal occlusion was achieved in 15 patients in a time frame from three days to 12 months. Three deaths and one major complication were encountered during the study period. Two patients, all with cavernous giant aneurysms, experienced transient exacerbations of preexisting cranial neuropathies and headache after the Silk treatment. Both were treated with corticosteroids, and symptoms resolved completely within a month.
In our experience the Silk stent has proven to be a valuable tool in the endovascular treatment of intracranial giant partially thrombosed aneurysms and aneurysms of the internal carotid artery cavernous segment presenting with mass effect. The time of complete occlusion of the aneurysms and the risk of the bleeding is currently not predictable.
Key words: brain aneurysm, endovascular therapy, flow-diverting stent
Introduction
Over the past 30 years major advances have been made in the endovascular treatment (EVT) of intracranial aneurysms and the technique has become widely accepted. The randomized Subarachnoid International Aneurysm Trial reported an absolute risk reduction of 7% at one year for patients undergoing EVT versus surgical clipping 1-3. However, when the follow-up was extended to seven years late retreatment (more than three months after the first EVT) was around sevenfold more common than in patients treated surgically 4. Nevertheless, there was no statistically significant difference in the risk of death from aneurysm bleeding at five years between EVT and surgery patients 5.
A number of techniques have been developed in recent years and included new materials designed to obtain a more stable outcome thereby reducing the percentage of recurrences among EVT-treated aneurysms. Some studies have demonstrated similar mortality, morbidity and initial occlusion rates for all types of materials 6. In addition, there is evidence that the long-term stability of EVT is enhanced when balloon remodelling techniques are used, while the medium-term outcomes of stent-assisted coiling to treat complex aneurysms are encouraging 6,7. Better results are expected with the use of stents in EVT for cerebral aneurysms because stents:
– support coils within the aneurysm in wide-necked aneurysms;
– allow greater packing density within the aneurysmal sac;
– lead to flow changes within the vessel;
– serve as a scaffold for endothelial growth;
– strengthen vessel structure.
In our experience, the Leo stent (Balt, Montmorency, France), when used to protect the aneurysm neck in two patients led to marked slowing of intra-aneurysmal flow disclosed by angiographic control at the end of the EVT procedure. Subsequent follow-up documented complete thrombosis of the sac without coil deployment. Similar experiences have been reported by other centres 8,9.
Many in vitro and in vivo studies have argued that stents can divert flow within the artery and decrease the blood flow within the aneurysm. These studies demonstrated that placement of a high porosity stent (i.e. a device with a high number of pores × mm2) will result in spontaneous thrombosis of the aneurysm without occluding the vessels crossed by the device. As a result of these studies two micropore flow-diverting stents were designed:
– Silk by Balt, Montmorency, France
– Pipeline embolization device (PED) by ev3, Irvine, California, USA.
Flow-diverting stents were designed to obtain a greater change in vessel haemodynamics resulting in a diversion of flow within the device thanks to its highly porous dense mesh structure. Experimental tests on the Pipeline embolization device (PED) in rabbits demonstrated a directly proportional correlation between stent porosity, flow changes and thrombogenicity 10, 11.
The Silk and PED stents were marketed to promote flow diversion away from the aneurysms and, at the same time, to preserve the patency of branch vessels and perforating arteries (100 micron - 1 mm) crossed by the device.
Release of an endovascular metallic prosthesis triggers an immune response with platelet adhesion and subsequent activation of the coagulation cascade. Compared to traditional intracranial stents, flow-diverting devices have a larger metallic surface accounting for 30-35% of the total area. We present a single centre experience in the treatment of 25 intracranial aneurysms using Silk stents, and their long-term follow-up (12 months).
Materials and Methods
Patients
Since December 2007 our Neuroradiology Service has treated 30 patients with a Silk flow-diverting device, of whom 25 were treated prior to August 2009 and were followed with CT and MR controls for 12 months. This group consisted of 24 women and one male, aged between 34 and 81 years. Treated lesions comprised 23 aneurysms of the carotid siphon (nine giant, nine large and five with a maximum diameter <10 mm), one large aneurysm arising at the origin of the PICA and one giant fusiform aneurysm of the basilar artery in the only male patient (Table 1).
Table 1.
| Patients | Sex | Site | Size |
|---|---|---|---|
| FC | F | Ophthalmic tract of carotid artery |
>10 mm |
| AG | F | Ophthalmic tract of carotid artery |
>10 mm |
| MS | F | Supraclinoid tract of carotid artery |
<10 mm |
| JO | F | Supraclinoid tract of carotid artery |
>10 mm |
| TM | F | Ophthalmic tract of carotid artery |
<10 mm |
| AM | F | Ophthalmic tract of carotid artery |
<10 mm |
| AMG | F | Cavernous tract of carotid artery |
>25 mm |
| VM | F | Ophthalmic tract of carotid artery |
<10 mm |
| SM | F | Ophthalmic tract of carotid artery |
<10 mm |
| AG | F | Ophthalmic tract of carotid artery |
>25 mm |
| FM | F | Posterior communicating cerebral artery |
>10 mm |
| MPT | F | Cavernous tract of carotid artery |
>25 mm |
| LT | F | Ophthalmic tract of carotid artery |
>10 mm |
| CU | F | Ophthalmic tract of carotid artery |
>10 mm |
| EG | F | Posterior communicating cerebral artery |
>10 mm |
| AI | F | Cavernous tract of carotid artery |
>25 mm |
| MCS | F | Cavernous tract of carotid artery |
>25 mm |
| LM | F | Cavernous tract of carotid artery |
>25 mm |
| MS | F | Ophthalmic tract of carotid artery |
>10 mm |
| GMF | F | Cavernous tract of carotid artery |
>25 mm |
| AR | F | Ophthalmic tract of carotid artery |
>10 mm |
| VU | F | Cavernous tract of carotid artery |
>25 mm |
| FF | M | Basilar artery | >25 mm |
| AS | F | Posterior inferior cerebellar artery |
>10 mm |
| BM | F | Cavernous tract of carotid artery |
>25 mm |
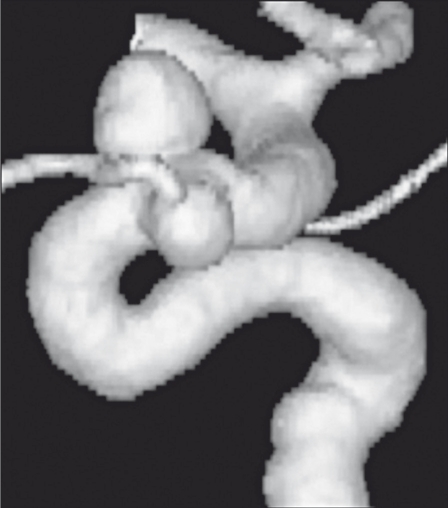
The selection of aneurysms to be treated was limited to the carotid siphon and included only large or giant lesions 12,13. The five selected small aneurysms with diameters between 4 and 9 mm had very wide necks and often an irregular morphology with multiple partially confluent lesions (Figure 1).
Figure 1.
Volume rendering reconstruction of 3D angiographic examination. In this case we show two carotid-ophthalmic saccular aneurysms with wide necks and different spatial orientation: note that the ophthalmic artery originates from the aneurysm sac making these lesions difficult to treat using endovascular coils.
On the basis of our preliminary experience, in August 2009 we decided to treat one giant aneurysm of the basilar artery and a partially thrombosed aneurysm in the PICA. Nine aneurysms were treated for headache, 14 for mass effect, one was a residual lesion after clipping surgery and one a reperfusion after endovascular coiling in a patient with multiple aneurysms. No patients presented with hemorrhage.
Stent Data and Angiographic Technique
We used the Silk stent in all our patients. The Silk stent is made of 48 nitinol and two platinum braided strands (compared to only 16 nitinol wires in the Leo stent) giving the device a good radio-opacity. The higher number of braided strands narrows the spaces between the struts (0.12-0.25 mm), while the higher metal surface area coverage has an inversely proportional effect on stent flexibility and navigability. Because of its low radial force, the Silk stent has difficulty opening fully within the vessel lumen. To ensure stability, the device must be positioned at a length at least one and a half times the vessel diameter above and below the aneurysm neck. The Silk stent is a retractable device attached to a high friction delivery system with a 200 cm steel plunger mounted on a microcatheter (Vasco 21 or 25) with a variable external diameter (Vasco 21: proximal 3.1F and distal 2.4F; Vasco 25: proximal 3.3F and distal 3F) and a constant internal lumen measuring 0.023 inches. The plunger has a distal portion in nitinol with the same length as the stent and a 15 mm long radio-opaque floppy portion that extends beyond the stent with a 45° tip. As the stent is released the plunger advances into the vessel for a length equal to that of the stent. Silk sizes currently available are from 15 to 40 mm long and between 2 and 5.5 mm in diameter. In addition, stents can be tailor-made to order and new variable diameter Silk devices were recently produced.
Angiography was performed on a biplane system (GE Healthcare, Milwaukee, Wisconsin, USA) and selective catheterization of the internal carotid and vertebral arteries performed with a 4F diagnostic catheter (Cordis). The examination was carried out using a 3D rotational technique with VR reconstructions and measurements of the vessel calibre and aneurysm neck.
>Stents were deployed using a 90 cm long 4F introducer (Balt). After stent release standard control views were taken (PA and LL). Only one stent was positioned in all patients and stent placement was never preceded by coil release into the aneurysmal sac.
Drug Management and Follow-up
Use of the flow-diverting stent must be associated with a high-dose antiplatelet medication and anticoagulant therapy before, during and after treatment. All patients received dual antiplatelet therapy (ticlopidine and aspirin) for four days before treatment.
EVT was carried out under general anaesthesia and antiplatelet (3000 IU heparin at the beginning of the procedure followed by 1000 IU hourly) and antiplatelet therapy (1 g aspirin on stent release).
The therapeutic protocol after treatment comprised: heparin for 48 hours after the procedure followed by dual antiplatelet therapy for three months (ticlopidine and aspirin) and aspirin alone thereafter for at least a month.
Follow-up was carried out by CT angiography and/or MR angiography 14 on discharge and one, three, six and 12 months after treatment.
Results
Technical Findings
Time for procedure completion varied from 15 to 50 min. Stent placement was successful in all patients. We changed stent size in one case to enhance the stability of the device. The diameters of the stents deployed varied from 3 to 5.5 mm (one 3 mm, two 3.5 mm, 15 4 mm, six 4.5 mm, one 5.5 mm). A 5.5 mm wide stent 60 mm long was deployed to cover the entire length of the basilar artery aneurysm.
Distal stent migration was detected at angiographic control after correct placement of the device to protect the neck of a left carotid siphon aneurysm. CT angiography and MR angiography follow-up disclosed imperfect opening of the Silk stent not adhering well to the vessel walls.
Angiographic Findings
Outcome was assessed according to the classification system devised by Oxford Neurovascular and Neuroradiology Research Unit (ONNRU) 14 and was also compared to the O'Kelly classification 15. Nineteen patients underwent long-term angiographic follow-up (range between 6 and 19 months) to disclose the development of any intrastent intimal hyperplasia.
Results were deemed positive (Table 2) if aneurysms were completely thrombosed in a time frame from three days to 12 months or if they showed an almost complete shrinkage of the sac portion patent to blood flow (grade 3-4 of the ONNRU classification).
Table 2.
| GRADE | MAXIMUM DIAMETER | ||
|---|---|---|---|
| <10 mm | 10-25 mm | >25 mm | |
| Grade 0 | 2 | ||
| Grade 1 | 1 | ||
| Grade 2 | 2 | 2 | |
| Grade 3 | 1 | 3 | |
| Grade 4 | 1 | 6 | >4 |
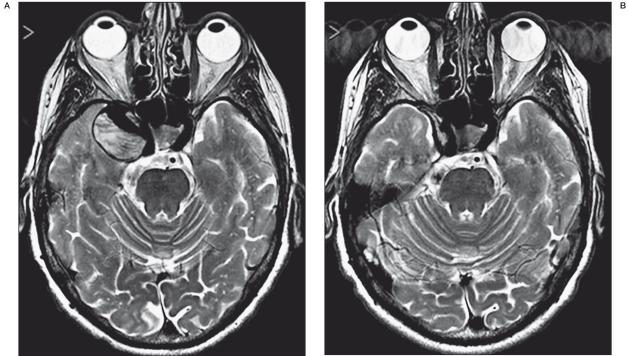
Thrombosis was faster in giant aneurysms that in partially thrombosed lesions even when the neck exceeded 4 mm. Over time, thrombosis may lead to a complete resorption as observed in five of our patients two of whom had a complete resolution of symptoms caused by cavernous sinus syndrome (Figure 2A,B).
Figure 2.
A,B) Pre-treatment T2-w MR images reveal a partially trombosed intracavernous giant aneurysm (A). The 3-month follow-up image reveals a complete resorption of the lesion with a resolution of nerve compression symptoms.
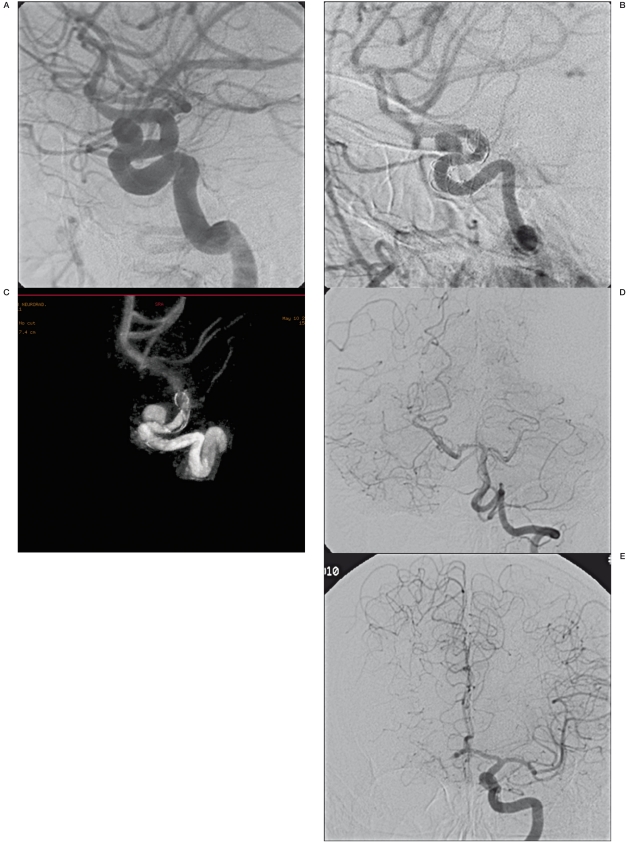
Aneurysm closure was not achieved in three patients at more than 12 months after EVT, but two of these patients showed a reduced calibre of the treated carotid artery at six-month follow-up. Subsequent angiographic examination disclosed suboptimal opening of the stent and intimal hyperplasia with vessel stenosis but well-compensated haemodynamics through the circle of Willis without clinical symptoms (Figure 3A-C).
Figure 3.
A-E) Carotid-ophthalmic saccular aneurysm of the left carotid artery (A). Images “b” and “c” show neointimal hypertrophy (arrow) and incorrect stent opening (ring). This condition could be the basis of blood flow changes which prevent aneurysm occlusion. 13 months later, the patient developed a good haemodynamic compensation without clinical symptoms.
The last angiographic follow-up in two patients showed slow flow within and below the stent which had worsened during the course of follow-up. Therefore the protocol was applied administering an intravenous bolus, pump and intra-arterial infusion of antiplatelet agents (GpIIbIIIa inhibitors - ReoPro) together with an intra-arterial fibrinolytic drug (Urokinase) leading to complete vessel recanalization. Neither of these patients had clinical sequelae or morphological changes at MR follow-up.
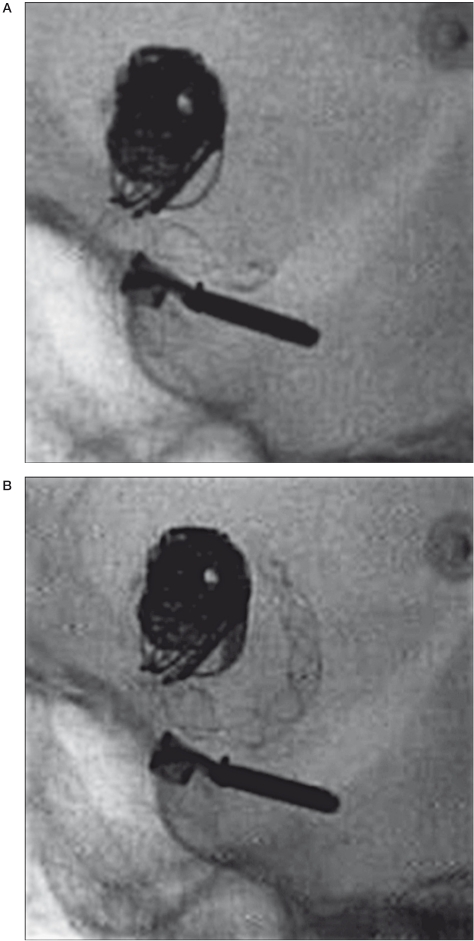
Thrombosis in one patient developed after stent displacement in the initial portion of the M1 (Figure 4) during injection of contrast medium for angiographic control. The stent measured 4×35 mm and had seemed the right size for the vessel to be treated.
Figure 4.
A,B) Note a clip and coils of previous treatments and the correct position and a good opening of the stent before angiographic control (A). After angiographic control we observed stent migration into M1 segment (B).
We observed three haemorrhagic events. Periprocedural bleeding occurred in one patient after stent release in the left vertebral artery to cover a partially thrombosed aneurysm at the origin of the postero-inferior cerebellar artery. Final angiographic control in this patient displayed leakage of contrast medium into the interpeduncular cistern due to iatrogenic rupture of the right artery of Percheron as the stent plunger moved forward during stent release. The bleeding was stopped by occlusion of this artery by insertion of two coils. Another patient presented post-procedural bleeding with haemorrhage located in a lenticular-capsular region possibly caused by small lacerations of the vessel wall related to microwire manipulation. We believe the antiplatelet medication and anticoagulant therapy were responsible for the fatal outcome. A spontaneous bleed was observed in another aneurysm and proved fatal as it occurred during antiplatelet and anticoagulant therapy after the patient was discharged from hospital seven days following stent placement. Spontaneous aneurysm rupture was demonstrated at autopsy. Lastly, we observed one case of acute obstructive third ventricular hydrocephalus one week after placement of a Silk stent (5.5×60 mm) in the basilar artery to cover the neck of a giant aneurysm. The patient subsequently underwent external ventricular drainage placement leading to acute fatal bleeding. There were no cases of thrombosis of the stent or terminal branches covered by the device (ophthalmic artery, anterior choroidal artery, postero-inferior cerebellar artery).
Discussion
Endovascular insertion of metal coils represents a proven technique for the treatment of intracranial aneurysms, including acute lesions. However, the treatment does have the drawbacks of coil compaction due to repeated blood flow pulsations and sac regrowth caused by residual flow proximal to the aneurysm neck 1-5. Ongoing efforts to develop new materials and techniques to enhance results have not had a significant impact on patient outcomes to date 6,7. In the last three years flow-diverting stents have been devised to restore the treated vessel wall and stimulate spontaneous thrombosis of the aneurysm sac and vessel wall neoendothelialization 10-13,16-19. In our experience, we observed closure or marked shrinkage of the patent portion of an aneurysmal sac after intervals ranging from three days to ten months. The reason for this variable time interval remains unclear and could depend on factors such as:
– Morphological features of the aneurysm and vessel of origin.
We found that giant partially thrombosed aneurysms have more acute thrombosis: complete occlusion was observed after just three days in three of our patients. This led us to believe that the slower and more turbulent the flow within an aneurysm, as is often the case in giant lesions, the greater the effect created by a flow-diverting stent. In addition, partial thrombosis within an aneurysm will facilitate enzymatic progression in the formation of new thrombus. As the thrombus has a higher osmolarity than blood this will draw fluids into the aneurysmal sac increasing the size of the lesion. So in the patients described, this rapid sac occlusion resulted in an initial worsening of symptoms caused by compression of adjacent nerves. By contrast, we found small aneurysms more difficult to occlude as they have faster and less turbulent flow.
– The following observations can be made with respect to stent placement (adhesion to vessel walls; position in relation to the aneurysm neck).
The closer the stent adheres to the vessel walls, the better its flow-diversion effect will be. Correct opening of the stent and good wall adhesion are only ensured by an “assisted” stent release with a continuous push and pull mechanism by the microcatheter-stent system to support the poor radial force of the device.
During follow-up we found that patients with persistent patent aneurysms more than 12 months after treatment did not have perfect stent adhesion to the vessel walls. Poor stent-vessel wall alignment may give rise to excessive neointimal development and/or stent migration. If the stent is not well-opposed to the vessel wall, the intervening space can be filled by neotima resulting in vessel narrowing. We encountered narrowing of the internal carotid artery following treatment in two of our patients and correlated this finding with the fibromuscular dysplasia (Figure 5).
Figure 5.
A,B) Angiographic examination of a carotid-ophthlamic aneurysm of the right carotid artery. The lateral images revealed the irregular appearance of the internal carotid artery (A). One month follow-up angiographic control showed a reduction of vessel diameter and blood flow in middle cerebral artery (B).
Generally speaking, the release of any intravascular prosthesis or wall stress caused by angioplasty will trigger new endothelial growth. Neointimal structure changes continuously with an “early” (proliferative) and a late (stable) neointima formation 20. A 5.8% incidence of intracranial intra-stent stenosis has been described, but fortunately the incidence of symptomatic stenosis is very low (1.3%) 21.
Downward stent migration is an unforeseable event that occurred in one of our patients despite careful measurement of vessel calibre and good stent opening. One explanation for this could be the development of an intra-stent thrombus: the sudden cessation of flow and the increased pressure during injection of contrast medium may have mobilised the device.
– The following observations can be made with respect to sensitivity to antiaggregant therapy and aneurysmal sac thrombosis.
The enhanced thrombogenicity of the Silk stent requires that means that EVT must be performed with prolonged antiplatelet and anticoagulant therapy to avoid stent thrombosis. Even though this therapy does not increase the risk of aneurysm rupture it will make any bleed more severe or even fatal. Fatal aneurysm rupture after deployment of a flow-diverting stent has also been described in other cohorts 22. Possible causes of this dramatic event recently proposed include increased pressure in the aneurysmal sac 23 and rapid sac thrombosis. Some of these authors 24 recommend combining flow-diverting stents with the insertion of metal coils to compartmentalize and stabilize sac thrombosis. We never placed coils in the aneurysmal sac in combination with Silk stent deployment. This resulted in complete obliteration of the aneurysm in five patients, two of whom had a resolution of nerve compression symptoms (cavernous sinus syndrome). We question the rationale and/or scientific basis underlying the proposal to deploy “some coils” with flow-diverting stents 24.
A limitation of the stent system is the small size of the space between the stent struts which prevents microcatheter access. Therefore once the Silk stent has been positioned no coils can be inserted into the aneurysm. Should the aneurysm remain patent after stent placement the only endovascular therapeutic alternative is to insert a second stent in the same position (“stent-in-stent technique) to enhance the flow-diverting effect of the device.
Conclusions
In our experience the Silk stent has an excellent potential for the endovascular treatment of intracranial aneurysms, in particular unruptured giant partially thrombosed aneurysms as well as aneurysms of the internal carotid artery cavernous segment and otherwise untreatable giant aneurysms presenting with mass effect.
The indication for the deployment of Silk stents aneurysms smaller than 10 mm remains to be proven.
Technically-speaking, Silk stent deployment is relatively quick and straightforward thanks to the good navigability of the device. Stent release, however, is arduous due to the device's low radial force and must be assisted by the system's push and pull mechanism. The risk of bleeding during anticoagulant and antiplatelet therapy at the time or after Silk stent deployment is a concern as it occurred in three of our patients.
The results with the use of the Silk flow-diverting stents will likely improve with further definition of their indications, the in-depth assessment of intra-aneurysmal flow haemodynamics before and after stent placement and an improved understanding of antiplatelet therapy administered.
References
- 1.International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274 . doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 2.Molyneux AJ, Kerr RSC, Yu LM, et al. for the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups and aneurysm occlusion. Lancet. 2005;366:809–817 . doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 3.van der Schaaf I, Algra A, Wermer M, et al. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage (Review) The Cochrane Library. 2009;37:4. doi: 10.1002/14651858.CD003085.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Campi A, Ramzi N, Molyneux AJ, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT) Stroke. 2007;38:1538–1544 . doi: 10.1161/STROKEAHA.106.466987. [DOI] [PubMed] [Google Scholar]
- 5.Molyneux AJ, Kerra RSC, Birks J, et al. for the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427–433 . doi: 10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurre W, Berkefeld J. Materials and techniques for coiling of cerebral aneurysms: how much scientific evidence do we have. Neuroradiology. 2008;50:909–927 . doi: 10.1007/s00234-008-0446-y. [DOI] [PubMed] [Google Scholar]
- 7.Guglielmi G. The beginning and the evolution of the endovascular treatment of intracranial aneurysms: from the first catheterization of brain arteries to the new stents. J NeuroInterv Surg. 2009;1:53–55 . doi: 10.1136/jnis.2009.000422. [DOI] [PubMed] [Google Scholar]
- 8.Vanninen R, Manninen H, Ronkainen A, et al. Broad-based intracranial aneurysms. Thrombosis induced by stent placement. Am J Neuroradiol. 2003;24:263–266 . [PMC free article] [PubMed] [Google Scholar]
- 9.Pumar JM, Castiñeira JA, Vazquez F, et al. Exclusion of a cavernous aneurysm by Leo stent. Interventional Neuroradiology. 2006;12:57–60 . doi: 10.1177/159101990601200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadasivan C, Cesar L, Seong J, et al. An original flow diversion device for the treatment of intracranial aneurysms. Evaluation in the rabbit elastase-induced model. Stroke. 2009;40:952–958 . doi: 10.1161/STROKEAHA.108.533760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38:2346–2352 . doi: 10.1161/STROKEAHA.106.479576. [DOI] [PubMed] [Google Scholar]
- 12.Leonardi M, Dall’Olio M, Princiotta C, et al. Treatment of carotid siphon aneurysms with a microcell stent. A case report. Interventional Neuroradiology. 2008;14:429–434 . doi: 10.1177/159101990801400408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirillo L, Dall’Olio M, Princiotta C, et al. The use of flow-diverting stents in the treatment of giant cerebral aneurysms: preliminary results. Neuroradiology J. 2010;23:220–224 . doi: 10.1177/197140091002300212. [DOI] [PubMed] [Google Scholar]
- 14.Toni F, Marliani AF, Cirillo L, et al. 3T MRI in the evaluation of brain aneurysms treated with flow-diverting stents: preliminary experience. Neuroradiology J. 2009;22:588–599 . doi: 10.1177/197140090902200512. [DOI] [PubMed] [Google Scholar]
- 15.O’Kelly CJ, Krings T, Fiorella D, et al. A novel grading scale for the angiograophic assessment of intracranial aneurysms treated using flow diversion stents. Interventional Neuroradiology. 2010;16:133–137 . doi: 10.1177/159101991001600204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1115–1121 . doi: 10.1227/01.neu.0000325873.44881.6e. [DOI] [PubMed] [Google Scholar]
- 17.Lylyk P, Mirando C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64:632–642 . doi: 10.1227/01.NEU.0000339109.98070.65. [DOI] [PubMed] [Google Scholar]
- 18.Byrne JV, Beltechi R, Yarnold JA, et al. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PLoS One. 2009;5:e12492. doi: 10.1371/journal.pone.0012492. doi:10.1371/journal.pone.0012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: The Budapest experience with the Pipeline embolization device. Am J Neuroradiol. 2010;31:1139. doi: 10.3174/ajnr.A2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim WH, Hong KM, Virmani R, et al. Histopathologic analysis of in-stent neointimal regression in a porcine coronary model. Coron Artery Dis. 2000;11:273–277 . doi: 10.1097/00019501-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Fiorella D, Albuquerque FC, Woo H, et al. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006;59:34–42 . doi: 10.1227/01.NEU.0000219853.56553.71. [DOI] [PubMed] [Google Scholar]
- 22.Turowski B, Macht S, Kulcsàr Z, et al. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (Silk-Stent): Do we need to rethink our concepts? Neuroradiology. 2011;53:37–41 . doi: 10.1007/s00234-010-0676-7. [DOI] [PubMed] [Google Scholar]
- 23.Cerebral JR, Mut F, Raschi M, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. Am J Neuroradiol. 2011;32:27–33 . doi: 10.3174/ajnr.A2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulcsar Z, Houdart E, Bonafé A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. Am J Neuroradiol. 2011;32:20–25 . doi: 10.3174/ajnr.A2370. [DOI] [PMC free article] [PubMed] [Google Scholar]