Abstract
Recent advances in defining TGF-β signaling pathways have provided a new level of understanding of the role of this pleiotropic growth factor in the development of fibrosis. Here, we review selected topics related to the profibrotic role of TGF-β . We will discuss new insights into the mechanisms of ligand activation and the contribution of Erk1/2 MAPK, PI3K/FAK, and Endoglin/Smad1 signaling pathways to the process of fibrosis. There is growing evidence of the disease-specific alterations of the downstream components of the TGF-β signaling pathway that may be explored for the future therapeutic interventions.
Keywords: TGF-β, fibrosis, Erk1/2/MAPK, PI3K, Alk1, Endoglin, Smad1.
INTRODUCTION
Transforming Growth factor beta proteins (TGF-βs) are a group of three structurally similar proteins, TGF-β1, TGF-β2 and TGF-β3, that are the founding members of a larger family of proteins that comprise of BMPs (bone morphogenetic proteins), GDFs (growth and differentiation factors), activins, inhibins, and MIF (Müllerian inhibitory factor) [1]. TGF-β is an evolutionarily conserved multifunctional cytokine from invertebrates to human, with a diverse spectrum of biological activities ranging from the development of an embryo to the maintenance of tissue homeostasis [2]. Adding to the complexity, the functions of TGF-β are tissue specific and widely vary in a spatio-temporal manner. While a key role of TGF-β signaling in organ fibrosis is supported by ample evidence, specific mechanisms involved in deregulation of this pathway towards fibrotic outcomes vary depending on the affected organ. This review will highlight recent developments in this field focusing on the aspects of TGF-β signaling with the relevance to the process of dermal fibrosis.
ACTIVATION OF LATENT TGF-β
During physiological tissue remodeling expression and activation of TGF-β is tightly controlled, however deregulation of these processes is frequently associated with human fibrotic diseases. While many fibrotic disorders are characterized by the elevated levels of TGF-β, additional mechanisms could also account for the increased TGF-β signaling in fibrosis. High levels of TGF-β were observed in renal fibrosis where it was shown that all three TGF-β isoforms were overexpressed in glomerular and tubulointerstitial compartments of patients with glomerular diseases characterized by excessive extracellular matrix (ECM) deposition [3]. In addition, circulating and urinary levels of TGF-β were also highly elevated in patients with type II diabetes [4]. Elevated levels of TGF-β1 mRNA were found in fibrotic human lungs [5] and in BAL (bronchoalveolar lavage) fluids from patients with scleroderma (SSc, systemic sclerosis) [6]. Likewise, increased circulating levels of TGF-β1 were demonstrated in patients with hypertrophic and restrictive cardiomyopathy [7]. Interestingly, in SSc TGF-β mRNA was only detected in association with inflammatory cells, but not in sclerotic skin, underscoring an important role of elevated TGF-β in initiation, but not in progression of dermal fibrosis in SSc [8]. Furthermore, circulating active TGF-β levels were decreased in SSc and correlated inversely with the degree of skin fibrosis [9].
As TGF-β is secreted in inactive form, factors that contribute to its activation locally in the affected tissues might play a critical role in perpetuation of the fibrotic process during chronic stages of the disease. TGF-β is secreted and deposited into ECM as a large latent complex (LLC) that consists of latent TGF-β binding protein (LTBP) covalently bound to so called small latency complex (SLC). SLC is formed by a homodimer of TGF-β non-covalently bound to an RGD-containing N-terminal latency associated binding peptide (LAP) [10]. Recent studies provided compelling evidence for the integrin-mediated activation of latent TGF-β [reviewed in [11-13]]. LAPs of TGF-β1 and -3, but not TGF-β2, contain RGD motif and can be activated through this mechanism [13]. To date, six integrins, including all five αv integrins, as well as α8β1 have been shown to interact with the RGD-containing LAPs [13]. Integrins αvβ5 and αvβ6 were shown to activate TGF-β via a mechanism that does not require cleavage of latent TGF-β and most likely involves conformational change of the latent complex. This protease-independent mechanism entails contractile cell forces and can be enhanced by agents that stimulate cell contraction such as thrombin, angiotensin-II, endothelin-1 [14], as well as LPA [15]. A protease-dependent mechanism that does not involve cell contraction has been described for integrin αvβ8, which upon binding to SLC induces MT1-MMP (MMP14)-mediated release of TGF-β [16]. Other, non integrin, mediated mechanisms involve proteolytic cleavage of LAP by MMP2, MMP9 and plasmin [rev. in [17]]. Another proposed mechanism involves conformational rearrangement of the LAP-TGF-β complex upon interaction with thrombospondin-1 (TSP-1) [18] or a recently described ADAMTS1 (ADAM metallopeptidase with thrombospondin type motif, 1) [19]. Notably, there is evidence for the excessive activation of TGF-β in human fibrotic diseases. For example, SSc fibroblasts express elevated levels of integrin αvβ5 and αvβ3, which contributes to the activation of the autocrine TGF-β signaling and increased collagen synthesis in these cells [20, 21]. In addition, SSc fibroblasts produce more TSP-1, which may further augment autocrine TGF-β signaling [22, 23]. Importantly, expression of TSP-1 mRNA in the skin in vivo was highly correlated with degree of skin fibrosis in SSc patients [24]. The importance of αvβ6 integrin was demonstrated in animal models of fibrosis, including pulmonary and hepatic fibrosis [25]. Recently, elevated levels of ADAMTS1 were observed in human fibrotic livers. Additional functional studies demonstrated a key role of ADAMTS1 in activation of TGF-β signaling during experimental liver fibrosis [26]. Together, these clinical and experimental data support the view that deregulated activation of latent TGF-β plays an important role in the development of fibrosis.
TGF-β RECEPTORS - OVERVIEW
TGF-β receptors are transmembrane proteins with intrinsic serine/threonine kinases activity and include Type I (TβRI, also termed Activin Like Kinase 5, ALK5) and Type II (TβRII) receptors [27]. Both types of receptor have a short extracellular region, a transmembrane region and a large intracellular cytoplasmic domain. The extracellular domain undergoes glycosylation, and while the TβRII has a high affinity for the ligand, TβRI does not bind to TGF-β. The transmembrane domain of TβRII is constitutively phosphorylated at Ser213 independent of ligand activation and is essential for downstream signaling. In contrast, transmembrane region of TβRI is phosphorylated at Ser165 by TβR-II in a ligand dependent manner. Both TGF-β receptors have the intracellular domain with inherent serine/threonine kinase activity. In TβRI a unique glycine-serine region termed the GS domain is present between kinase and transmembrane domains [28]. Upon ligand binding, TβR-II recruits and activates TβRI by phosphorylating the GS domain. In addition to the major Type I and II receptors, accessory TGF-β receptor such as betaglycan and endoglin are present and collectively termed as Type III receptors. The major function of these co-receptors appears to be to increase the bioavailability of TGF-β to the signaling TGF-β receptors. Interestingly, recent studies have challenged previously held view that TGF-β receptors are present on the cell membrane as preformed homodimers. Zhang et al. have elegantly demonstrated that in the absence of TGF-β receptor isoforms are monomeric and dimerization takes place upon TGF- binding to TβRII [29]. Furthermore, Huang et al. have shown that TGF-β treatment leads to increase of TβRII:TβRI heterodimers rather than heterotetramers [30]. Upon activation, TβRI is relieved of inhibitory effect of the immunophilin protein FKBP12 and actively recruits and phosphorylates the receptor Smads - Smad2 or -3. Smad proteins comprise of three domains, an N-terminal MH1 domain followed by a linked region and a C-terminal MH2 domain. Under resting condition, the MH2 domain and MH1 domain interact with each other. However, receptor-mediated phosphorylation at C-terminal disrupts these interactions exposing N-terminal nuclear localization signal. In addition phosphorylation promotes interaction of MH2 domain with other protein such as Smad4 that as a complex move into nucleus where they regulate large number of genes [31]. In addition to activation of Smad pathway, TGF-β has been shown to elicit non-Smad signaling responses via intermediates such as MAPK, Rho, PI3K-AKT, Src and other signaling molecules (28-31). The remarkable versatility of TGF-β can be attributed to the activation of these non-Smad pathways working in conjunction with Smads to further modulate cellular responses. However, this complexity increases the likelihood of alteration in additional components of this fine-tuned system that ultimately may result in pathological fibrosis.
TGF-β RECEPTOR MEDIATED NON-SMAD SIGNALING
In recent years a significant progress has been made in unraveling the molecular mechanisms involved in the activation of the non-Smad pathways. This topic has been discussed in details in several recent reviews [32-35]. We will discuss selected pathways with a particular relevance to fibrosis.
Erk MAPK Pathway
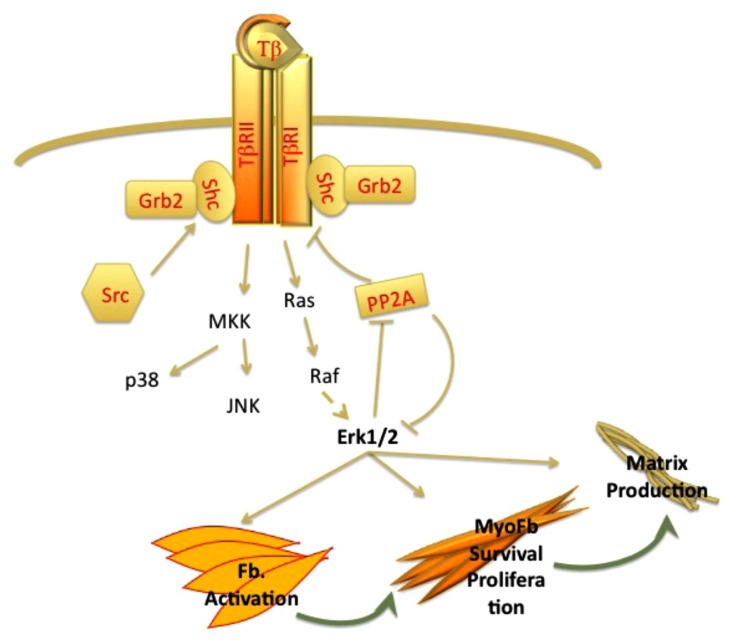
Activation of Erk MAPK plays an important role in fibrosis by regulating myofibroblast transdifferentiation, cell proliferation and survival, as well as matrix synthesis [36-42] (Fig. 1). Recent studies have provided new insights into the molecular mechanisms involved in activation of this pathway by TGF-β. Lawler et al. [43] were first to demonstrate that in addition to serine/threonine phosphorylation, TβRII undergoes autophosphorylation on tyrosine residues. Further studies from Galliher and Schiemann showed that Src-mediated Tyrosine phosphorylation of TβRII, triggers receptor recruitment of Shc and Grb2 resulting in activation of p38 MAPK signaling [44]. Similar to TβRII, TβRI is also phosphorylated on tyrosine residues in response to TGF-β activation leading to the assembly of Shc/Grb2 complex and activation of Erk1/2 MAPK [45] (Fig. 1). While activation of Erk1/2 MAPK by RTKs (receptor tyrosine kinases) is usually rapid and transient, activation of this pathway by TGF-β is characterized by a prolonged kinetics in dermal fibroblasts [46]. Studies by Samuel et al. have shown that this may be due, in part, to the downregulation of the catalytic subunit of PP2A (protein phosphatase 2A), a key cellular phosphatase that targets Erk1/2 MAPK [46]. SSc fibroblasts were also shown to express reduced levels of PP2A as a result of the constitutive activation of TGF-β signaling. As PP2A is also involved in dephosphorylation of TβRI [47], its downregulation may further contribute to the chronic activation of this pathway in scleroderma fibrosis (Fig. 1). Relevant to this finding, Bandyopadhyay and colleagues [48] have demonstrated that varied expression levels of TGF-β receptors might explain tissue-selective activation of Erk1/2 pathway by TGF-β. The authors observed that in contrast to epithelial cells, in which TGF-β inhibited Erk1/2 activation, Erk1/2 was induced by TGF-β in dermal fibroblasts and microvascular endothelial cells. On the other hand, activation of Smad2/Smad3 was comparable in all cell types. Further, investigation into the levels of receptors revealed that unlike the TβRI levels, which were similarly expressed in both cell types, the levels of TβRII were expressed at much higher levels in fibroblasts. Additional depletion experiments indicated that Erk1/2 MAPK activation is mediated by TβRII in a TβRI independent manner in fibroblasts. Given the importantance of Erk1/2 in regulating profibrotic gene expression, these studies may explain failure of the TβRI kinase specific inhibitors to fully normalize activated phenotype of SSc fibroblasts [49, 50].
Fig. (1).
Autocrine regulation of ERK MAPK activation by TGF-β plays a key role in wound healing and fibrosis. TGF-β via Ras-Raf proteins activates Erk pathway. Activated ERK in turn inhibits PP2A, a negative regulator of ERK and TGF-β receptors and stimulates myofibroblast survival and matrix production.
PI3K (Phosphoinositide 3-Kinase)-FAK Pathway
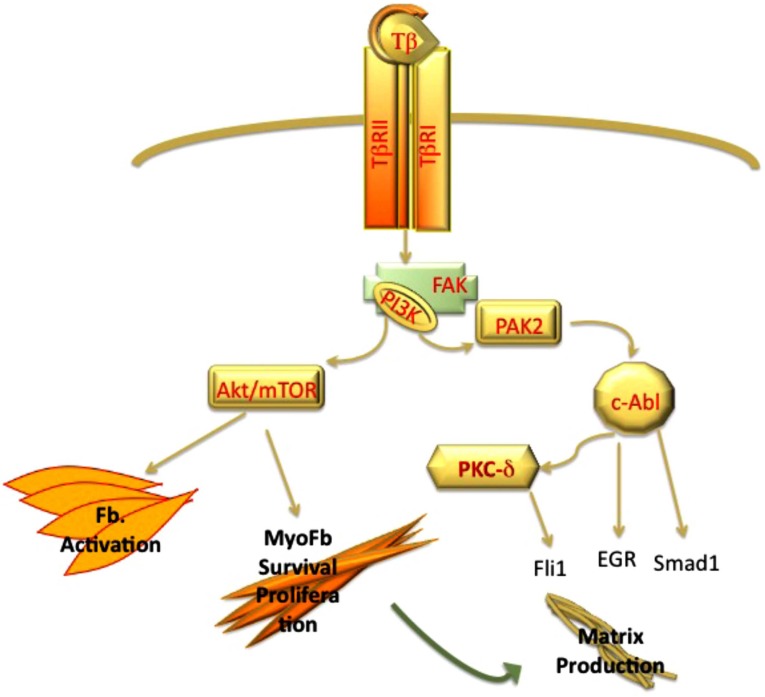
Activation of PI3K pathway and its downstream targets plays a central role in the fibrogenic process induced by TGF-β. In contrast to Erk1/2 MAPK, PI3K activation requires both Type II and Type I receptor [51]. The studies by Leof and his colleagues have provided important insights into activation of the TGF-β-PI3K axis in mesenchymal cells. Collectively, these studies demonstrated that TGF-β stimulation leads to recruitment of the p85 subunit of PI3K to FAK (focal adhesion kinase) that acts as a scaffold to organize this signaling complex. Notably, this function of FAK does not require tyrosine kinase activity and is Src-independent [52]. PI3K is a branch point for the activation of the two important profibrotic pathways: PAK2 (p21 activated Kinase)-Abl (Abelson kinase) and Akt-mTOR1 pathways [53] (Fig. 2). The importance of c-Abl pathway has been well established in experimental models of fibrosis, where it was shown that administration of c-Abl inhibitor, imatinib, reduces organ fibrosis [54]. Downstream targets of c-Abl in fibroblasts include such known profibrotic mediators as Egr [55], Smad1 [56, 57], and PKCδ/Fli1 [57]. Furthermore, recent studies have shown that c-Abl-PKCδ pathway may also contribute to the process of endothelial-mesenchymal transition [58]. The Akt-mTOR branch regulates cell proliferation, cell survival, and metabolism [59] (Fig. 2). The importance of the activation of the PI3K pathway in fibrotic disorders is further underscored by the finding that a key negative regulator of this pathway, PTEN, is underexpressed in several fibrotic disorders, including IPF (Idiopathic Pulmonary Fibrosis) [60], scleroderma [61, 62], and liver fibrosis [63].
Fig. (2).
PI3 kinase pathway contributes to TGF-β induced fibrosis via Akt and PAK2 pathways. Activation of Akt/mTOR pathway plays a key role in activation of fibroblast and myofibroblasts survival. PAK2 via c-Abl induces matrix production via activation of EGR and Smad1 pathway and inhibition Fli1 protein.
The important aspect of the TGF-β-PI3K signaling is its role in the process of EMT (epithelial-mesenchymal transition). While EMT has been shown to be a source of collagen producing cells in experimental models of renal and pulmonary fibrosis [64, 65], whether this process contributes to human disease is debatable. This topic has been a subject of recent reviews and will not be discussed herein [35, 59].
Endoglin-Smad1 Pathway in Fibrosis
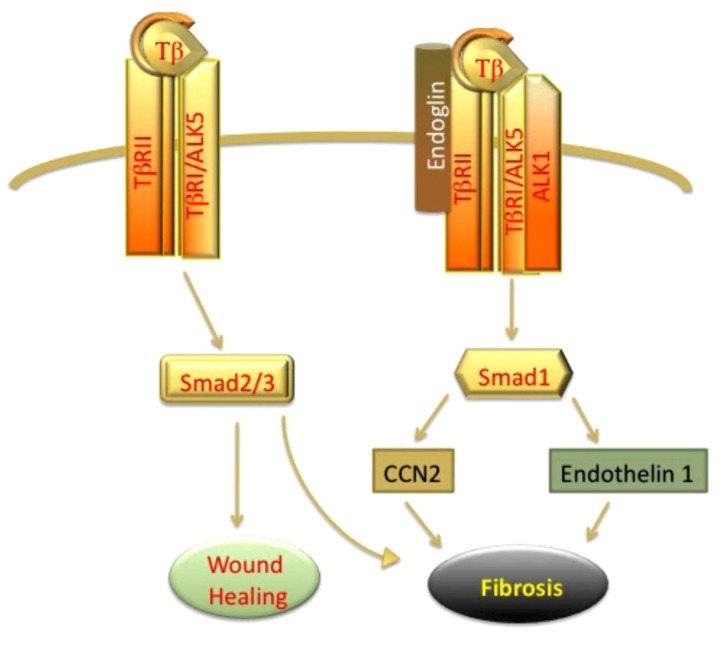
The type III receptors, betaglycan and endoglin, are part of the TGF-β receptor complex. Structural studies revealed that the cytoplasmic regions of both receptors lack any significant signaling domains and that both receptors are similar in their transmembrane domain [66, 67]. However, these accessory receptors differ in their affinity towards Type I and Type II receptors. Betaglycan facilitates binding of TGF-β isoforms to TβRII, and this effect is most pronounced for TGF-β2, which by itself binds receptor with low affinity [rev in [68]]. Endoglin binds TGF-β1 and TGF-β3, but not TGF-β2, and needs the presence of TGFβRII for binding to TGF-β ligands [rev in [67, 69]]. Endoglin is a critical mediator of angiogenesis and aberrant expression of this co-receptor has been associated with several vascular pathologies including HHT (hereditary hemorrhagic talangiectasias), preeclampsia, and tumor angiogenesis [69, 70]. Studies from the laboratory of Peter ten Dijke have provided important insights into the role of endoglin in the TGF-β signaling in endothelial cells. TGF-β has been shown to utilize distinct receptor subtypes to induce different responses in endothelial cells. TGF-β signaling via ALK5 and subsequent phosphorylation of Smad2/3 is associated with inhibition of cell proliferation and migration, as well as other features consistent with induction of quiescent phenotype, while signaling via ALK1 receptor leads to activation of Smad1/5 and promotion of angiogenic response [67]. It has to be noted that ALK5 is also required for signaling through the ALK1 receptor, although the role of ALK5 in this process is not fully defined. Endoglin is upregulated on proliferating endothelial cells and may function as modulator of a balance between ALK1/Smad1 and ALK5/Smad3 by promoting ALK1 signaling and indirectly inhibiting ALK5/Smad3 signaling. Consistent with this concept, high levels of soluble endoglin are present in patients with preeclampsia and contribute to the defective angiogenesis.
There is increasing evidence that elevated expression of endoglin on mesenchymal cells contributes to the profibrotic TGF-β signaling by triggering endothelial-like signaling via activation of ALK1/Smad1. High levels of endoglin have been demonstrated in liver biopsies and patient serum samples in liver fibrosis [71, 72], renal fibrosis [73, 74], as well as dermal fibroblasts and serum of patients with scleroderma [75-80]. The function of endoglin with respect to regulation of the profibrotic effects of TGF-β has been investigated in several experimental systems. While antifibrotic effects of endoglin were described in some studies [78, 81], majority of the studies support a profibrotic role of endoglin. For example, endoglin was shown to promote profibrotic action of Angiotensin II in cardiac fibroblasts [82]. Furthermore, studies of transdifferentiated HSCs (hepatic stellate cells) have linked endoglin to activation of Smad1/5 signaling and subsequent upregulation of α-SMA [72]. In a subset of scleroderma fibroblasts, constitutive activation of ALK1/Smad1 pathway has been shown to contribute to CCN2 and collagen gene expression [41, 56]. Recent studies by Morris et al. have revealed that this subset of SSc fibroblasts is characterized by the elevated levels of endoglin [61]. Furthermore, endoglin was required for activation of Smad1/5 pathway and collagen and CCN2 gene expression in SSc fibroblasts. Notably, endoglin/ALK1 pathway was also shown to mediate expression of another profibrotic mediator, endothelin-1 in SSc and healthy dermal fibroblasts (Fig. 3). Although, less is known about the potential role of betaglycan in fibrotic disorders, recent studies have suggested that deregulation of this co-receptor may also contribute to fibrosis [83, 84].
Fig. (3).
TGF-β activates Smad 2/3 and Smad1 pathway. Activation of classical Smad 2/3 pathway promotes wound healing. Elevated levels of Endoglin in fibroblasts promote Smad1 signaling leading to autocrine upregulation of CCN2 and promoting fibrosis.
CONCLUSIONS
Recent progress in unraveling TGF-β signaling provides a better understanding of the role of this pleiotropic growth factor in fibrosis. While these new information further solidify a central role for TGF-β in development of fibrosis, a critical question regarding suitability of TGF-β as a therapeutic target, remains unanswered. The effective suppression of TGF-β in the affected tissues might be difficult to achieve. Furthermore, serious concerns remain that such a treatment could promote autoimmunity and epithelial hyperplasia [85]. Although anti-TGF-β strategies used to date did not produce toxic effects, this could have been a result of incomplete inhibition of TGF-β, consistent with the absence of beneficial anti-fibrotic effect. The discovery of additional signaling pathways that work in concert with TGF-β in promoting fibrosis may offer new therapeutic options by combining TGF-β suppression with the additional pathway specific inhibition.
ACKNOWLEDGEMENT
The authors were supported by the NIH grants AR42334 and AR44883.
CONFLICT OF INTEREST
Declared none.
REFERENCES
- 1.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 2.Gordon KJ, Blobe GC. Role of transforming growth factor-beta super family signaling pathways in human disease. Biochim Biophys Acta. 2008;1782:197–228. doi: 10.1016/j.bbadis.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T, Noble NA, Cohen AH, et al. Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int. 1996;49:461–9. doi: 10.1038/ki.1996.65. [DOI] [PubMed] [Google Scholar]
- 4.Sharma K, Ziyadeh FN, Alzahabi B, et al. Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes. 1997;46:854–9. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 5.Coker RK, Laurent GJ, Jeffery PK, et al. Localisation of transforming growth factor beta1 and beta3 mRNA transcripts in normal and fibrotic human lung. Thorax. 2001;56:549–56. doi: 10.1136/thorax.56.7.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwicka A, Ohba T, Trojanowska M, et al. Elevated levels of platelet derived growth factor and transforming growth factor-beta 1 in bronchoalveolar lavage fluid from patients with scleroderma. J Rheumatol. 1995;22:1876–83. [PubMed] [Google Scholar]
- 7.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Querfeld C, Eckes B, Huerkamp C, et al. Expression of TGF-beta 1, -beta 2 and -beta 3 in localized and systemic scleroderma. J Dermatol Sci. 1999;21:13–22. doi: 10.1016/s0923-1811(99)00008-0. [DOI] [PubMed] [Google Scholar]
- 9.Dziadzio M, Smith RE, Abraham DJ, et al. Circulating levels of active transforming growth factor beta1 are reduced in diffuse cutaneous systemic sclerosis and correlate inversely with the modified Rodnan skin score. Rheumatology (Oxford) 2005;44:1518–24. doi: 10.1093/rheumatology/kei088. [DOI] [PubMed] [Google Scholar]
- 10.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–69. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 11.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–15. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Worthington JJ, Klementowicz JE, Travis MA. TGF-beta: a sleeping giant awoken by integrins. Trends Biochem Sci. 2011;36:47–54. doi: 10.1016/j.tibs.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu MY, Porte J, Knox AJ, et al. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q) Am J Pathol. 2009; 174:1264–79. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu D, Cambier S, Fjellbirkeland L, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF-beta activation. J Cell Sci. 2003;116:217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 18.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 19.Bourd-Boittin K, Bonnier D, Leyme A, et al. Protease profiling of liver fibrosis reveals the ADAM metallopeptidase with thrombospondin type 1 motif, 1 as a central activator of transforming growth factor beta. Hepatology. 2011;54:2173–84. doi: 10.1002/hep.24598. [DOI] [PubMed] [Google Scholar]
- 20.Asano Y, Ihn H, Jinnin M, et al. Involvement of alphavbeta5 integrin in the establishment of autocrine TGF-beta signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol. 2006;126:1761–9. doi: 10.1038/sj.jid.5700331. [DOI] [PubMed] [Google Scholar]
- 21.Asano Y, Ihn H, Yamane K, et al. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol. 2005;175: 7708–18. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Leask A, Abraham DJ, et al. Thrombospondin 1 is a key mediator of transforming growth factor beta-mediated cell contractility in systemic sclerosis via a mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent mechanism. Fibrogenesis Tissue Repair. 2011;4:9. doi: 10.1186/1755-1536-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mimura Y, Ihn H, Jinnin M, et al. Constitutive thrombospondin-1 overexpression contributes to autocrine transforming growth factor-beta signaling in cultured scleroderma fibroblasts. Am J Pathol . 2005;166:1451–63. doi: 10.1016/s0002-9440(10)62362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farina G, Lafyatis D, Lemaire R, et al. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2010;62:580–8. doi: 10.1002/art.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munger JS, Huang X, Kawakatsu H, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 26.Bourd BK, Bonnier D, Leyme A, et al. Protease profiling of liver fibrosis reveals the adam metallopeptidase with thrombospondin type 1 motif, 1 as a central activator of TGF-beta. Hepatology . 2011;54:2173–84. doi: 10.1002/hep.24598. [DOI] [PubMed] [Google Scholar]
- 27.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 28.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997; 390:465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Jiang Y, Wang Q, et al. Single-molecule imaging reveals transforming growth factor-beta-induced type II receptor dimerization. Proc Natl Acad Sci U S A. 2009;106:15679–83. doi: 10.1073/pnas.0908279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang T, David L, Mendoza V, et al. TGF-beta signalling is mediated by two autonomously functioning TbetaRI: TbetaRII pairs. EMBO J. 2011;30:1263–76. doi: 10.1038/emboj.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–44. [PubMed] [Google Scholar]
- 32.Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol. 2009;19:385–94. doi: 10.1016/j.tcb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Mu Y, Gudey SK, Landstrom M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- 34.Trojanowska M. Noncanonical transforming growth factor beta signaling in scleroderma fibrosis. Curr Opin Rheumatol. 2009;21: 623–9. doi: 10.1097/BOR.0b013e32833038ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res . 2009;19:128–39. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caraci F, Gili E, Calafiore M, et al. TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res. 2008; 57:274–82. doi: 10.1016/j.phrs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Kohan M, Muro AF, White ES, et al. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J. 2010;24:4503–12. doi: 10.1096/fj.10-154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Meyer C, Muller A, et al. IL-13 Induces Connective Tissue Growth Factor in Rat Hepatic Stellate Cells via TGF-{beta}- Independent Smad Signaling. J Immunol. 2011;187:2814–23. doi: 10.4049/jimmunol.1003260. [DOI] [PubMed] [Google Scholar]
- 39.Nagai Y, Miyata K, Sun GP, et al. Aldosterone stimulates collagen gene expression and synthesis via activation of ERK1/2 in rat renal fibroblasts. Hypertension. 2005;46:1039–45. doi: 10.1161/01.HYP.0000174593.88899.68. [DOI] [PubMed] [Google Scholar]
- 40.Nakerakanti SS, Bujor AM, Trojanowska M. CCN2 is required for the TGF-beta induced activation of Smad1-Erk1/2 signaling network. PLoS One. 2011;6:e21911. doi: 10.1371/journal.pone.0021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pannu J, Nakerakanti S, Smith E, et al. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J Biol Chem. 2007;282:10405–13. doi: 10.1074/jbc.M611742200. [DOI] [PubMed] [Google Scholar]
- 42.Ponticos M, Holmes AM, Shi-wen X, et al. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009; 60:2142–55. doi: 10.1002/art.24620. [DOI] [PubMed] [Google Scholar]
- 43.Lawler S, Feng XH, Chen RH, et al. The type II transforming growth factor-beta receptor autophosphorylates not only on serine and threonine but also on tyrosine residues. J Biol Chem. 1997;272: 14850–9. doi: 10.1074/jbc.272.23.14850. [DOI] [PubMed] [Google Scholar]
- 44.Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–8. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 45.Lee MK, Pardoux C, Hall MC, et al. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J . 2007;26:3957–67. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samuel GH, Bujor AM, Nakerakanti SS, et al. Autocrine transforming growth factor beta signaling regulates extracellular signal-regulated kinase 1/2 phosphorylation via modulation of protein phosphatase 2A expression in scleroderma fibroblasts. Fibrogenesis Tissue Repair. 2010;3:25. doi: 10.1186/1755-1536-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griswold PI, Kamibayashi C, Maruoka EM, et al. Physical and functional interactions between type I transforming growth factor beta receptors and Balpha, a WD-40 repeat subunit of phosphatase 2A. Mol Cell Biol. 1998;18:6595–604. doi: 10.1128/mcb.18.11.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bandyopadhyay B, Han A, Dai J, et al. TbetaRI/Alk5-independent TbetaRII signaling to ERK1/2 in human skin cells according to distinct levels of TbetaRII expression. J Cell Sci. 2011;124:19–24. doi: 10.1242/jcs.076505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Shi-wen X, Eastwood M, et al. Contribution of activin receptor-like kinase 5 (transforming growth factor beta receptor type I) signaling to the fibrotic phenotype of scleroderma fibroblasts. Arthritis Rheum. 2006;54:1309–16. doi: 10.1002/art.21725. [DOI] [PubMed] [Google Scholar]
- 50.Ishida W, Mori Y, Lakos G, et al. Intracellular TGF-beta receptor blockade abrogates Smad-dependent fibroblast activation in vitro and in vivo. J Invest Dermatol. 2006;126:1733–44. doi: 10.1038/sj.jid.5700303. [DOI] [PubMed] [Google Scholar]
- 51.Yi JY, Shin I, Arteaga CL. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J Biol Chem. 2005;280:10870–6. doi: 10.1074/jbc.M413223200. [DOI] [PubMed] [Google Scholar]
- 52.Hong M, Wilkes MC, Penheiter SG, et al. Non-Smad transforming growth factor-beta signaling regulated by focal adhesion kinase binding the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem. 2011;286:17841–50. doi: 10.1074/jbc.M111.233676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilkes MC, Leof EB. Transforming growth factor beta activation of c-Abl is independent of receptor internalization and regulated by phosphatidylinositol 3-kinase and PAK2 in mesenchymal cultures. J Biol Chem. 2006;281:27846–54. doi: 10.1074/jbc.M603721200. [DOI] [PubMed] [Google Scholar]
- 54.Gordon J, Spiera R. Imatinib and the treatment of fibrosis: recent trials and tribulations. Curr Rheumatol Rep. 2011;13:51–8. doi: 10.1007/s11926-010-0146-6. [DOI] [PubMed] [Google Scholar]
- 55.Bhattacharyya S, Ishida W, Wu M, et al. A non-Smad mechanism of fibroblast activation by transforming growth factor-beta via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene. 2009;28:1285–97. doi: 10.1038/onc.2008.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pannu J, Asano Y, Nakerakanti S, et al. Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum. 2008;58:2528–37. doi: 10.1002/art.23698. [DOI] [PubMed] [Google Scholar]
- 57.Bujor AM, Asano Y, Haines P, et al. The c-Abl tyrosine kinase controls protein kinase Cdelta-induced Fli-1 phosphorylation in human dermal fibroblasts. Arthritis Rheum. 2011;63:1729–37. doi: 10.1002/art.30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Z, Jimenez SA. Protein kinase Cdelta and c-Abl kinase are required for transforming growth factor beta induction of endothelial-mesenchymal transition in vitro. Arthritis Rheum. 2011; 63:2473–83. doi: 10.1002/art.30317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamouille S, Derynck R. Emergence of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin axis in transforming growth factor-beta-induced epithelial-mesenchymal transition. Cells Tissues Organs. 2011;193:8–22. doi: 10.1159/000320172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White ES, Atrasz RG, Hu B, et al. Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10) Am J Respir Crit Care Med . 2006;173:112–21. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris E, Chrobak I, Bujor A, et al. Endoglin promotes TGF-beta/ Smad1 signaling in scleroderma fibroblasts. J Cell Physiol . 2011;226:3340–8. doi: 10.1002/jcp.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parapuram SK, Shi-Wen X, Elliott C, et al. Loss of PTEN Expression by Dermal Fibroblasts Causes Skin Fibrosis. J Invest Dermatol. 2011;131:1996–2003. doi: 10.1038/jid.2011.156. [DOI] [PubMed] [Google Scholar]
- 63.Peyrou M, Bourgoin L, Foti M. PTEN in liver diseases and cancer. World J Gastroenterol. 2010;16:4627–33. doi: 10.3748/wjg.v16.i37.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coward WR, Saini G, Jenkins G. The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2010;4:367–88. doi: 10.1177/1753465810379801. [DOI] [PubMed] [Google Scholar]
- 65.Fragiadaki M, Mason RM. Epithelial-mesenchymal transition in renal fibrosis - evidence for and against. Int J Exp Pathol. 2011;92: 143–50. doi: 10.1111/j.1365-2613.2011.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gatza CE, Oh SY, Blobe GC. Roles for the type III TGF-beta receptor in human cancer. Cell Signal. 2010;22:1163–74. doi: 10.1016/j.cellsig.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.ten Dijke P, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11:79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 68.Bilandzic M, Stenvers KL. Betaglycan: a multifunctional accessory. Mol Cell Endocrinol. 2011;339:180–9. doi: 10.1016/j.mce.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 69.Perez-Gomez E, Del Castillo G, Juan Francisco S, et al. The role of the TGF-beta coreceptor endoglin in cancer. Scientific World J . 2010;10:2367–84. doi: 10.1100/tsw.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orlova VV, Liu Z, Goumans MJ, et al. Controlling angiogenesis by two unique TGF-beta type I receptor signaling pathways. Histol Histopathol. 2011;26:1219–30. doi: 10.14670/HH-26.1219. [DOI] [PubMed] [Google Scholar]
- 71.Clemente M, Nunez O, Lorente R, et al. Increased intrahepatic and circulating levels of endoglin, a TGF-beta1 co-receptor, in patients with chronic hepatitis C virus infection: relationship to histological and serum markers of hepatic fibrosis. J Viral Hepat. 2006;13:625–32. doi: 10.1111/j.1365-2893.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 72.Meurer SK, Tihaa L, Borkham KE, et al. Expression and functional analysis of endoglin in isolated liver cells and its involvement in fibrogenic Smad signalling. Cell Signal. 2011;23:683–99. doi: 10.1016/j.cellsig.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez PA, Eleno N, Duwell A, et al. Endoglin upregulation during experimental renal interstitial fibrosis in mice. Hypertension . 2002;40:713–20. doi: 10.1161/01.hyp.0000037429.73954.27. [DOI] [PubMed] [Google Scholar]
- 74.Roy CP, Simpson JG, Power DA. Endoglin, a transforming growth factor-beta-binding protein, is upregulated in chronic progressive renal disease. Exp Nephrol. 1997;5:55–60. [PubMed] [Google Scholar]
- 75.Coral APX, Garces MF, Caminos JE, et al. Serum endoglin levels in patients suffering from systemic sclerosis and elevated systolic pulmonary arterial pressure. Int J Rheumatol. 2010. p. ii: 969383. in press. [DOI] [PMC free article] [PubMed]
- 76.Dharmapatni AA, Smith MD, Ahern MJ, et al. The TGF beta receptor endoglin in systemic sclerosis. Asian Pac J Allergy Immunol. 2001;19:275–82. [PubMed] [Google Scholar]
- 77.Fujimoto M, Hasegawa M, Hamaguchi Y, et al. A clue for telangiectasis in systemic sclerosis: elevated serum soluble endoglin levels in patients with the limited cutaneous form of the disease. Dermatology. 2006;213:88–92. doi: 10.1159/000093846. [DOI] [PubMed] [Google Scholar]
- 78.Leask A, Abraham DJ, Finlay DR, et al. Dysregulation of transforming growth factor beta signaling in scleroderma: overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis Rheum. 2002;46:1857–65. doi: 10.1002/art.10333. [DOI] [PubMed] [Google Scholar]
- 79.Morris E, Chrobak I, Bujor A, et al. Endoglin promotes TGF-beta/ Smad1 signaling in scleroderma fibroblasts. J Cell Physiol . 2011;226:3340–8. doi: 10.1002/jcp.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wipff J, Avouac J, Borderie D, et al. Disturbed angiogenesis in systemic sclerosis: high levels of soluble endoglin. Rheumatology (Oxford) 2008;47:972–5. doi: 10.1093/rheumatology/ken100. [DOI] [PubMed] [Google Scholar]
- 81.Velasco S, Alvarez MP, Pericacho M, et al. L- and S-endoglin differentially modulate TGFbeta1 signaling mediated by ALK1 and ALK5 in L6E9 myoblasts. J Cell Sci. 2008;121:913–9. doi: 10.1242/jcs.023283. [DOI] [PubMed] [Google Scholar]
- 82.Chen K, Mehta JL, Li D, et al. Transforming growth factor beta receptor endoglin is expressed in cardiac fibroblasts and modulates profibrogenic actions of angiotensin II. Circ Res. 2004;95:1167–73. doi: 10.1161/01.RES.0000150369.68826.2f. [DOI] [PubMed] [Google Scholar]
- 83.Hermida N, Lopez B, Gonzalez A, et al. A synthetic peptide from transforming growth factor-beta1 type III receptor prevents myocardial fibrosis in spontaneously hypertensive rats. Cardiovasc Res. 2009;81:601–9. doi: 10.1093/cvr/cvn315. [DOI] [PubMed] [Google Scholar]
- 84.Holmes AM, Ponticos M, Shi WX, et al. Elevated CCN2 expression in scleroderma: a putative role for the TGFbeta accessory receptors TGFbetaRIII and endoglin. J Cell Commun Signal. 2011;5:173–7. doi: 10.1007/s12079-011-0140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Varga J, Pasche B. Antitransforming growth factor-beta therapy in fibrosis: recent progress and implications for systemic sclerosis. Curr Opin Rheumatol. 2008;20:720–8. doi: 10.1097/BOR.0b013e32830e48e8. [DOI] [PMC free article] [PubMed] [Google Scholar]