Abstract
Ependymomas constitute the most common type of primary spinal cord tumors, and are subclassified as myxopapillary ependymoma, classic ependymoma, and anaplastic ependymoma. Ependymomas can be further subclassified based on morphologic phenotype: cellular, papillary, tanycytic, clear cell, pigmented and epithelioid. Giant cell ependymoma (GCE), a rare variant, has recently been described. Reported cases have exhibited a wide anatomic distribution, including spinal cord, cerebrum and cerebellum. We report here three cases of GCE, arising from cerebrum in a 5-year-old girl, spinal cord in a 34-year-old female and cerebellum in an 86-year-old female respectively. Histologically those cases showed prominent pleomorphic giant cells with focal perivascular pseudorosettes in all cases. Tumor cells were immunopositive for GFAP and EMA. Only the first case was qualified for anaplastic ependymoma. No recurrence was noted in these three cases after 57, 46 and 6 months of follow-up respectively. By reviewing the literature, GCEs arising from spinal cord and cerebellum tended to have low-grade morphology while supratentorially located GCEs tended to have anaplastic features. GCEs were preferentially located in extraventricular regions. Anaplastic GCEs in adult population seemed to pursue a more aggressive behavior. Gross total resection should still be the main treatment for GCEs.
Introduction
Ependymomas are slow growing tumors of children and young adults, which account for 3-9% of all neuroepithelial tumors. Ependymomas are the most common primary intramedullary spinal cord neoplasms, accounting for 50 to 60% of spinal cord gliomas [1]. They consist of myxopapillary ependymoma (WHO grade I), classic ependymoma (WHO grade II), and anaplastic ependymoma (WHO grade III). WHO grade II ependymomas have four variants: cellular, papillary, tanycytic and clear cell [1]. Since the extent of tumor removal is the most significant prognostic factor for long-term survival, the gross total resection should be the primary treatment goal. In 1996, the first two cases of giant cell ependymoma of the filum terminale were described by Zec et al [2]. In recent years, another 17 cases of giant cell ependymoma have been reported, which were located in spinal cord, cerebrum and cerebellum [3-17]. We report three cases of giant cell ependymoma (GCE) arising from cerebrum, spinal cord and cerebellum respectively. To our knowledge, this represents the largest case serials of giant cell ependymomas.
Case report
Case 1
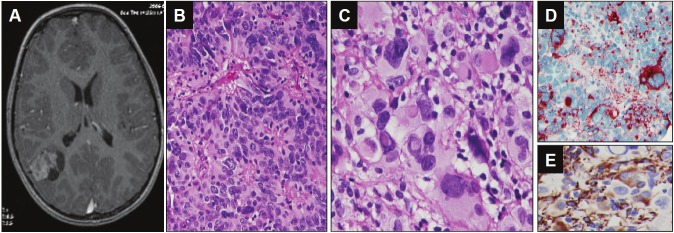
A 5-year-old girl with a history of headache and left side weakness. The Magnetic resonance imaging (MRI) showed an extraventricular heterogeneously enhanced solid and cystic mass in the right parietal lobe (Figure 1). The gross total resection was achieved. The histologic diagnosis was anaplastic ependymoma, WHO grade III (Figure 1). No recurrence was noted after 57 months of follow-up.
Figure 1.
Neuroimaging and histological findings of the tumor from case 1. A. Axial gadolinium-enhanced T1-weighted MRI image demonstrated a heterogeneously enhanced solid and cystic mass in right parietal lobe. B. Perivascular pseudorosette (Hematoxylin and eosin 200X). C. The giant cell with eosinophilic cytoplasm, eccentrically located single or multiple nuclei with prominent nucleoli, and intranuclear cytoplasmic inclusions (Hematoxylin and eosin, 400X). D. EMA immunohistochemical stain showed perinuclear dot-like or ring pattern. E. GFAP immunohistochemical stain showed positivity of tumor cells.
Case 2
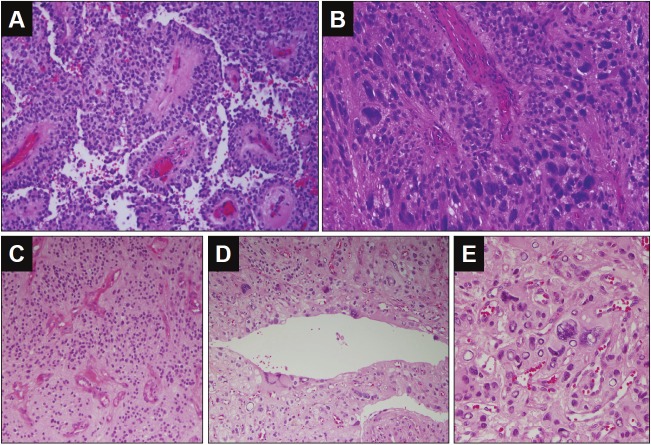
A 34-year-old female with a history of tingling and numbness in the right side of body and with recent progressive weakness in the right side of body. The MRI showed well-defined, slightly T1-hypointense, intramedullary enhancing lesion of the cervical spinal cord. T2-weighted imaging revealed a cystic caudal region with both rostral and caudal parenchyma edema. The gross total resection of the tumor was performed. The histologic diagnosis was ependymoma, WHO grade II (Figure 2). There was no recurrence after 46 months of follow-up.
Figure 2.
Histological findings of the tumor from case 2 and case 3. A-B for case 2: A. Low power view of perivascular pseudorosette and pseudo-papillary structure (Hematoxylin and eosin 200X). B. Perivascular pseudorosette formed with pleomorphic tumor cells (Hematoxylin and eosin, 400X). C-E for case 3: C. Low power view of perivascular pseudorosette in well-differentiated area of this tumor (Hematoxylin and eosin 100X). D. Ependymal epithelial surfaces formed with pleomorphic tumor cells (Hematoxylin and eosin, 200X). E. Pleomorphic giant tumor cells. (Hematoxylin and eosin, 400X).
Case 3
An 86-year-old female with a history of vertigo and abnormal gait. The MRI showed a heterogeneously enhancing solid and cystic lesion in the right cerebellum hemisphere. The tumor was grossly totally resected. The histologic diagnosis was ependymoma, WHO grade II (Figure 2). No recurrence was seen after 6 months of follow-up.
All cases showed prominent pleomorphic giant cells with abundant eosinophilic cytoplasm and distinct cell borders (Figure 1 and 2). Typical perivascular pseudorosettes were seen focally in all cases. Focal ependymal epithelial surfaces were identified in the case 3. There were strong cytoplasmic positivity for GFAP and perinuclear dot-like immunopositivity for EMA in tumor cells for all three cases (Figure 1). The case 1 exhibited hypercellularity, increased mitotic activity, including the presence of atypical bizarre mitotic figures, and florid microvascular proliferation, consistent with anaplastic ependymoma (WHO grade III).
Discussion
In present report, we described three ependymal tumors with abundant giant cells with focal perivascular pseudopapillary pattern. Giant cell glioblastoma, pleomorphic xanthoastrocytoma (PXA), subependymal giant cell astrocytoma (SEGA), and atypical teratoid/rhabdoid tumor (AT/RT) should be considered in the differential diagnosis on the H&E sections. Giant cell glioblastoma, the major differential diagnosis, is characterized by prominent micovascular proliferation and pseudopalisading necrosis [18]. Those features did not present in all our cases. PXA is composed of pleomorphic astrocytic cells and occasional lipdized cells with perivascular lymphocytic infiltration and prominent eosinophilic granular bodies [18]. PXA usually occurs in the superficial location of cerebrum with frequent meningeal involvement. SEGA is almost exclusively associated with tuberous sclerosis and also located around the lateral ventricle. Well-formed perivascular pseudorosette usually does not present in SEGA [19]. In the third case, there were focal rhabdoid cells, raising the possibility of AT/RT. The immunohistochemical stain for INI1 was performed and demonstrated diffuse immmunoreactivity of tumor cells to INI1 antibody. Typically AT/RT tumor cells are negative for INI1 [20,21]. In our three cases, tumor cells were strongly positive for GFAP, and prominent EMA positivity was present in perinuclear dot-like and ring patterns, diagnostic of ependymoma.
Besides our present three cases, 18 cases of giant cell ependymoma have been described in the literature [2-17]. Five out of 21 (23.8%) cases occurred in children and 16 out of 21 (76.2 %) occurred in adult. The median age of these 21 patients was 34 years ranging in age from 5 to 89 years. The male to female ratio was 1.1:1 (Table 1) [2-17]. The clinical presentations were non-specific and depended on the location of tumor. Ten out of 21 cases (47.6 %) were located in spinal cord. Six cases occurred at cervical spinal cord, two at thoracic spinal cord and two at filum teminale. Seven out of 21 cases (33.3%) were supratentorially located. Two cases occurred in temporal lobe, and one in frontal lobe, parietal lobe, occipital lobe, temporo-parietal lobe and suprasellar region respectively (Table 1). All supratentorial giant cell ependymomas were originated at extraventicular regions. Only one case occurred in the frontal lobe and extended to the lateral ventricle. Four out of 21 cases (19.1%) occurred in cerebellum (Three were extraventricular while one was in the 4th ventricle) (Table 1) [2-16]. Fifteen out of 21 cases (71.4%) had low grade (WHO grade II) histology, among which eight were arising from spinal cord; four from cerebellum and one from fourth ventricle [2,6,8-13,16,17]. Six cases out of 21 cases (28.6%) had anaplastic features (WHO grade III), among which five were arising from the cerebrum and one from cerebellum [3-5,7,15]. Five out of seven supratentorial giant cell ependymomas were high grade tumors [3-5,15].
Table 1.
Clinical and pathologic features of giant cell ependymomas
| Age | 5-89 years (median =34 years) |
| Sex | 11 male : 10 female (ratio = 1.1:1) |
| Location | Spinal cord : 10 cases (6 at cervical spinal cord, 2 at filum teminale, and 2 at thoracic spinal cord) |
| Supratentorial: 7 cases (2 in temporal , 1 in frontal, 1 in parietal, 1 in occipital, 1 in temporo-parietal and 1 in suprasellar region) (All are extraventricular and 1 extends to the lateral ventricle) | |
| Cerebellum: 4 cases (3 are extraventricular and 1 in 4th ventricle) | |
| WHO grade | WHO grade II: 15 cases |
| WHO Grade III: 6 cases | |
| Treatment | Gross total resection in 17 cases and subtotal resection in one case |
| Radiation therapy for 3 cases after surgery | |
| Chemotherapy and radiation therapy for 3 cases | |
| Follow-up time and prognosis | 1.5-35 months (median = 7 months) |
| 5 patients had recurrence (4 WHO grade III and 1 WHO grade II) | |
| 1 patient died of anaplastic ependymoma (WHO grade III) | |
| 1 patient with WHO grade II ependymoma died of surgical complication |
Besides one autopsy case, seventeen patients were underwent gross total resection and one case underwent subtotal resection [2-16]. Three anaplastic ependymoma cases were treated with radiation therapy after gross total resection [3,4,15]. Two anaplastic ependymoma cases and one supratentorial ependymoma case were treated with the combination of chemotherapy and radiation therapy after surgery [5,7,10]. Eighteen cases have been followed-up ranging from 1.5 to 57 months with the median of 15.5 months [2-10,12-16]. Five patients with anaplastic ependymoma and one patient with WHO grade II ependymoma developed recurrent tumor [3,5-7,15]. The patient with anaplastic ependymoma in the temporal lobe died of disease after 20 months of follow-up even though she was treated with gross total resection, and the combination of radiation and chemotherapy [5]. One of two patients with ependymoma (WHO grade II) in the thoracic spinal cord died of complications after surgery [13].
Giant cell ependymomas arising from spinal cord (all cases 100%) and cerebellum (3 out of four cases, 75%) tended to have low-grade morphology [2,6-9,11,13,14,16,17]. GCEs arising from supratentorial locations (5 out of 7 cases, 71.4 %) tended to have anaplastic features [3-5,10,12,15]. Anaplastic GCEs in general had a higher chance to recur compared to WHO grade II GCEs. However, two suparatentiorial anaplastic GCEs in children had no recurrence after 46 and 57 months of follow-up respectively. Although the majority of patients with anaplastic ependymoma were treated with radiation therapy with/without chemotherapy after surgery, gross total resection should still be the treatment of choice for GCEs.
In conclusion, we report here three cases of GCE, which occurred in cerebrum, spinal cord and cerebellum respectively. These tumors have prominent pleomorphic giant cells as well as focal ependymal differentiation. Their ependymal origin is confirmed by immunohistochemistry. By reviewing all published cases, extraventricular regions of cerebrum and cerebellum are the preferential location for GCEs. GCEs in spinal cord and cerebellum tend to be WHO grade II morphology while supratentorially located GCEs tend to have anaplastic features. Anaplastic GCEs in adult population most likely have a more aggressive behavior. Gross total resection still remains as the first line treatment for GCEs.
References
- 1.Wiestler O, Schiffer D, Coons S. Ependymal tumor. 2000:71–79. [Google Scholar]
- 2.Zec N, De GU, Schofield DE, Scott RM, Anthony DC. Giant cell ependymoma of the filum terminale. A report of two cases. Am J Surg Pathol. 1996;20:1091–1101. doi: 10.1097/00000478-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Brown DF, Chason DP, Schwartz LF, Coimbra CP, Rushing EJ. Supratentorial giant cell ependymoma: a case report. Mod Pathol. 1998;11:398–403. [PubMed] [Google Scholar]
- 4.Pimentel J, Kepes JJ, Moura Nunes JF, Bentes C, Miguens J, Antunes JL. Supratentorial giant cell ependymoma. Clin Neuropathol. 2001;20:31–37. [PubMed] [Google Scholar]
- 5.Moritani S, Kushima R, Bamba M, Kobayashi TK, Oka H, Fujimoto M, Hattori T, Okabe H. Highly anaplastic extraventricular ependymoma arising in an adult, mimicking metastatic adenocarcinoma with heavy stromal inflammation and emperiporesis. Pathol Int. 2003;53:539–546. doi: 10.1046/j.1440-1827.2003.01517.x. [DOI] [PubMed] [Google Scholar]
- 6.Fourney DR, Siadati A, Bruner JM, Gokaslan ZL, Rhines LD. Giant cell ependymoma of the spinal cord. Case report and review of the literature. J Neurosurg. 2004;100:75–79. doi: 10.3171/spi.2004.100.1.0075. [DOI] [PubMed] [Google Scholar]
- 7.Jeon YK, Jung HW, Park SH. Infratentorial giant cell ependymoma: a rare variant of ependymoma. Pathol Res Pract. 2004;200:717–725. doi: 10.1016/j.prp.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Pal P, Fernandes H, Ellison DW. Woman aged 24 years with fourth ventricular mass. Brain Pathol. 2005;15:367–368, 373. doi: 10.1111/j.1750-3639.2005.tb00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper PB, Katus M, Moores L, Geyer D, Smirniotopoulos JG, Sandberg GD, Rushing EJ. Rare giant cell ependymoma in an octogenarian. Case report and review of the literature. J Neurosurg. 2006;105:908–911. doi: 10.3171/jns.2006.105.6.908. [DOI] [PubMed] [Google Scholar]
- 10.Adamek D, Dec M, Sobol G, Urbanowicz B, Jaworski M. Giant cell ependymoma: a case report. Clin Neurol Neurosurg. 2008;110:176–181. doi: 10.1016/j.clineuro.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Szpak GM, Lewandowska E, Schmidt-Sidor B, Pasennik E, Modzelewska J, Stepien T, Zdaniuk G, Kulczycki J, Wierzba-Bobrowicz T. Giant cell ependymoma of the spinal cord and fourth ventricle coexisting with syringomyelia. Folia Neuropathol. 2008;46:220–231. [PubMed] [Google Scholar]
- 12.Sangoi AR, Lim M, Dulai M, Vogel H, Chang S. Suprasellar giant cell ependymoma: a rare neoplasm in a unique location. Hum Pathol. 2008;39:1396–1401. doi: 10.1016/j.humpath.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Shamji MF, Benoit BG, Perry A, Jansen GH. Giant cell ependymoma of the thoracic spine: pathology case report. Neurosurgery. 2009;64:E566–E567. doi: 10.1227/01.NEU.0000338428.01654.A4. [DOI] [PubMed] [Google Scholar]
- 14.Barbagallo GM, Caltabiano R, Parisi G, Albanese V, Lanzafame S. Giant cell ependymoma of the cervical spinal cord: case report and review of the literature. Eur Spine J. 2009;18:186–190. doi: 10.1007/s00586-008-0789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahlback HS, Brandal P, Krossnes BK, Fric R, Meling TR, Meza-Zepeda LA, Danielsen HE, Heim S. Multiple chromosomal monosomies are characteristic of giant cell ependymoma. Hum Pathol. 2011;42:2042–2046. doi: 10.1016/j.humpath.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi P, Gupta A, Pasricha S, Patel D. Giant cell ependymoma of a cervical spinal cord. Indian J Pathol Microbiol. 2011;54:201–203. doi: 10.4103/0377-4929.77406. [DOI] [PubMed] [Google Scholar]
- 17.Gessi M, Kuchelmeister K, Lauriola L, Pietsch T. Rare hitological variants in ependymomas: Histopathological analysis of 13 cases. Vichows Arch. 2011;459:423–429. doi: 10.1007/s00428-011-1126-6. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Diaz H, Kleinschmidt-DeMasters BK, Powell SZ, Yachnis AT. Giant cell glioblastoma and pleomorphic xanthoastrocytoma show different immunohistochemical profiles for neuronal antigens and p53 but share reactivity for class III beta-tubulin. Arch Pathol Lab Med. 2003;127:1187–1191. doi: 10.5858/2003-127-1187-GCGAPX. [DOI] [PubMed] [Google Scholar]
- 19.Sharma MC, Ralte AM, Gaekwad S, Santosh V, Shankar SK, Sarkar C. Subependymal giant cell astrocytoma--a clinicopathological study of 23 cases with special emphasis on histogenesis. Pathol Oncol Res. 2004;10:219–224. doi: 10.1007/BF03033764. [DOI] [PubMed] [Google Scholar]
- 20.Biegel JA, Fogelgren B, Zhou JY, James CD, Janss AJ, Allen JC, Zagzag D, Raffel C, Rorke LB. Mutations of the INI1 rhabdoid tumor suppressor gene in medulloblastomas and primitive neuroectodermal tumors of the central nervous system. Clin Cancer Res. 2000;6:2759–2763. [PubMed] [Google Scholar]
- 21.Judkins AR, Mauger J, Ht A, Rorke LB, Biegel JA. Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol. 2004;28:644–650. doi: 10.1097/00000478-200405000-00013. [DOI] [PubMed] [Google Scholar]