Abstract
Diffuse peritoneal malignant mesothelioma is a rare, progressive, and ultimately fatal disease and it can present as primary peritoneal carcinoma or ovarian cancer. Differential diagnosis is important to establish appropriate management. In this article the clinical presentation, immunuhistochemical and histopathological features of 8 diffuse peritoneal malignant mesothelioma cases presented as peritoneal carcinoma or ovarian cancer are evaluated. According to findings of all reported cases, we concluded that clinical distinction of malignant mesothelioma from ovarian cancer or peritoneal adenocarcinoma is very difficult. Differential diagnosis is reliably achieved by immune profile of the tumors with a systematic approach of both positive and negative mesothelioma markers.
Keywords: Peritoneal mesotheliomas, peritoneal adenocarcinoma, ovarian cancer
Introduction
Because of the differences in the treatment modalities and prognostic implications, diffuse peritoneal malignant mesothelioma (DPMM) should be distinguished from both ovarian serous cancer (OSC) and primary peritoneal serous carcinoma (PPSC). Due to only about 30 reported cases of DPMM mimicking OSC or PPSC may preclude determining approaches in differential diagnosis and management of these cases [1-5]. In the present study, 8 DMPM cases evaluated preoperatively as ovarian cancer or PPSC were discussed under the light of clinical, histopathological and immunohistochemical (IHC) findings.
Cases
Patient files of cases treated at our clinic with the diagnosis of ovarian cancer or peritoneal adenocarcinoma between the years 2000 and 2011 were evaluated. During this analysis, it was revealed that in 8 patients out of 783 (0.1%), the postoperative diagnosis had changed into DPMM. Complaints of these patients at presentation, administered treatments, survival results, and histopathological and IHC evaluation results are analyzed.
The mean age at the time of diagnosis was 56.5 (range: 40 - 72) years. Clinical characteristics of the patients were summarized in Table 1. All patients complained of abdominal distention and pain. Five cases were presented with ovarian mass and 3 with clinical appearance of peritoneal carcinomatosis without ovarian mass. All cases had massive ascites. Ca125 levels were in the range of 13-1299 IU/mL and were higher than 20 IU/mL in 5 patients. Preoperative ascites cytology was evaluated in 2 cases and both were reported as adenocarcinoma.
Table 1.
Clinical features and outcomes of the patients.
| Case | Age (years) | Preoperative apperarence | Ca 125 (IU/mL) | Treatment | Overall Survival (months) | Outcome |
|---|---|---|---|---|---|---|
| 1 | 42 | Peritoneal carcinomatosis | 244 | Neoadjuvant chemotherapy followed by TAH, BSO, omentectomy, appendectomy BPPLND | 12 | DOD |
| 2 | 68 | Bilateral ovarian mass | 750 | TAH, BSO, omentectomy, appendectomy, BPPLND and adjuvant chemotherapy | 33 | ANED |
| 3 | 40 | Ovarian mass and rectosigmoid colon involvement | 19 | TAH, BSO, Rectosigmoid resection, BPPLND and adjuvant chemotherapy | 9 | AWD |
| 4 | 51 | Ovarian mass | 20 | TAH, BSO peritoneal biopsies and chemotherapy | 7 | ANED |
| 5 | 55 | Ovarian mass and rectosigmoid colon involvement | 67 | TAH, BSO, Subtotal colectomy, splenectomy and adjuvant chemotherapy | 48 | ANED |
| 6 | 71 | Ovarian mass peritoneal carcinomatosis | 1299 | Unilateral salpingo-oophorectomy, omentectomy and adjuvant chemotherapy | 24 | DOD |
| 7 | 53 | Peritoneal carcinomatosis | 160 | Peritoneal biopsy followed by chemotherapy | 9 | DOD |
| 8 | 72 | Ovarian mass and rectosigmoid colon involvement | 13 | TAH, BSO, rectosigmoid colectomy, appendectomy, cholecystectomy, BPPLND | 1 | DOD |
Note: Massive ascites was presence in all cases. Lymph nodes were positive in 3 of 4 patients who underwent lymph node dissection. TAH: total abdominal hysterectomy. BSO: bilateral salpingooophorectomy. BPPLND: bilateral pelvic and paraaortic lymp node dissection. DOD: death of disease. ANED: Alive, no evidence of disease. AWD: Alive with disease.
In pathologic examination 7 of the cases were proved to have ovarian involvement; bilateral in 6 (case number 1-5, 8) and unilateral in 1 case (case number 6), together with peritoneal involvement. Only peritoneal biopsy material was obtainable in our pathology department for the remaining one case (case number 7). Ovarian involvement was nodular and infiltrative pattern was prominent in case 1, 2, and 3, while case 4 and 6 revealed superficial tumor deposits with focal ovarian stromal infiltration. Lymphovascular invasion was prominent in case 2, 3 and 8. Tumors exhibited different histologic patterns with a common feature of tubulopapillary structures and epithelioid appearance. Tumors were all cytologically atypical. Each tumor had been examined by IHC markers varying according to the potential differential diagnosis. Table 2 summarizes the IHC panel and positivity rates for each case. Positivity rates for each marker were summarized as 8/8 (positive cases / analyzed cases) for CK7; 0/8 for CK20; 6/8 for calretinin; 7/7 for CK5/6; 2/2 for WT1; 2/2 for D2-40; 6/6 for mesothelin; 0/4 for Ber-EP4; 3/3 for CA125; 0/4 for CEA (m); 0/2 for TAG72; 0/5 for MOC31; 0/3 for CD15; 2/2 for EMA; 0/6 for ER; 0/5 for PR; and 1/1 for Vimentin.
Table 2.
Immunohistochemical characteristics of the cases.
| Case number | CK7/CK20 profile | Expression characteristics of immunohistochemical markers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Calretinin | CK5/6 | WT1 | D2-40 | Mesothelin | Ber-EP4 | CA125 | CEAm | TAG72 | MOC-31 | CD15 | EMA | ER/PR | ||
| 1 | +/- | +(nuc/cyt) | +(cyt) | N.A. | N.A. | +(mem) | N.A. | +(cyt) | N.A. | N.A. | N.A. | N.A. | N.A. | -/- |
| 2 | +/- | +(nuc/cyt) | +(cyt) | +(nuc) | N.A. | +(mem) | N.A. | N.A. | N.A. | N.A. | - | N.A. | N.A. | -/- |
| 3 | +/- | +(nuc/cyt) | +(cyt) | N.A. | +(mem) | +(mem) | - | N.A. | N.A. | - | - | N.A. | N.A. | -/N.A. |
| 4 | +/- | +(nuc/cyt) | +(cyt) | N.A. | +(mem) | N.A. | - | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | -/- |
| 5 | +/- | +(nuc/cyt) | +(cyt) | +(nuc) | N.A. | +(mem) | - | +(cyt) | - | N.A. | N.A. | -/- | +(mem) | - |
| 6 | +/- | +(nuc/cyt) | N.A. | N.A. | N.A. | +(mem) | N.A. | N.A. | - | - | - | N.A. | N.A. | N.A. |
| 7 | +/- | -(cyt) | +(cyt) | N.A. | N.A. | N.A. | - | +(cyt) | - | N.A. | - | - | N.A. | N.A. |
| 8* | +/- | -(cyt) | +(cyt) | N.A. | N.A. | +(mem) | N.A. | N.A. | - | N.A. | - | - | +(mem) | -/- |
Note: NA: not applied, +: positive, -: negative, nuc: nuclear, mem: membranous, cyt: cytoplasmic.
Case 8 showed also Vimentin positivity not shown here.
Discussion and review of the literature
Ovarian masses, grow out of metastatic lesion and primarily originate from ovaries, may display similar gross appearance, and thus, may unhinge the surgeon whether the tumor is primary or metastatic, at first impression. This dilemma becomes more important in distinguishing a primary peritoneal tumor involving ovaries from a primary ovarian tumor widely spreading peritoneum. PPSC and DPMM are two distinct entities which may mimic primary OSC in several ways in terms of clinical presentation, serologic markers, and surgeon impression during surgery and morphologic features in pathologic examination.
Presentation
Secondary ovarian involvement of DPMM was reported in 24 cases and primary ovarian mesothelioma in 7 patients [1-5]. The presence of diffuse peritoneal involvement in all of our patients suggested that these cases were primary DPMM rather than being of primary ovarian origin.
Cytology
Most patients have ascites, while the cells obtained from this ascites rarely reveal results in terms of positive findings. In one study, parasynthesis was performed preoperatively on 4 out of 15 DPMM cases diagnosed by laparotomy or laparoscopy and all of these cytology results were reported as adenocarcinoma [6]. Similarly in our series, the cytology results of 2 patients were reported as adenocarcinoma. However, nowadays cytologic cell block preparation practice uncovers the facility of immunohistochemistry application, which will be discussed later, and provides more reliable results for the differential diagnosis.
Histopathology
Mesotheliomas may reveal a wide range of histologic pattern; particularly epithelioid mesotheliomas with prominent tubulopapillary pattern may closely mimic serous carcinomas of ovaries or peritoneum. Ovarian tumor exhibiting bilaterality, nodular involvement, stromal invasion with infiltrative pattern, superficial microscopic tumor deposits, lymphovasculary invasion and/or single cell infiltration favors a metastatic lesion rather than a primary origin [7]. However, these morphologic features are not specific and can not reliably differentiate between malignant mesothelioma and primary ovarian or peritoneal serous carcinoma. All of our cases revealed extensive disease with at least both peritoneal and ovarian involvement. Moreover for each case the pathologic examination was compatible with a tumor comprising tubulopapillary pattern which may be encountered in numerous malignancies whether they are primary or metastatic.
Immunohistochemistry
The definite diagnosis could be achieved by ancillary tests, in which immunohistochemistry plays the principle role, and is absolutely essential in the differential diagnosis. In this context, a wide range of immune panel may be applied for ovarian tumors to differentiate them from not only malignant mesothelioma or PPSC, but also other metastatic tumors which may potentially involve ovary and/or peritoneum such as pancreatic, biliary, colorectal, gastric, breast, pulmonary and other female genital tract adenocarcinomas. Because the critical issue is differentiating malignant mesothelioma from others, and there is no absolute specific marker for mesothelioma, a combined use of positive and negative markers of mesothelioma is performed, and the overall immune phenotype modifies the final diagnosis [8,9].
In this context, podoplanin, calretinin, CK5/6, WT1, thrombomodulin, mesothelin, and D2-40 antibodies are proposed as positive markers of mesothelioma that are commonly expressed in mesotheliomas, but not in carcinomas, while Ber-EP4, MOC-31, TAG72 (B72.3), CA19-9, CD15 (Leu-M1), monoclonal CEA (CEAm) and BG-8 antibodies are negative markers for mesothelioma that are commonly expressed in adenocarcinomas. Furthermore, the panel may be extended by some miscellaneous markers; Ttf-1, EMA, CA125, ER, CK7, and CK20 antibodies which may also help in the differential diagnosis [8,9].
Differential role of CK7/CK20 immune profile is valuable for malignant mesotheliomas with high percentage of CK7 positivity and definite CK20 negativity [10]. In the present study, CK7+/CK20- immune profile was constant for each case. Nuclear calretinin positivity and Ber-EP4 negativity together is a strong indicator for malignant mesothelioma. Furthermore CK5/6 is also very useful in discriminating malignant mesothelioma from adenocarcinoma with high rate of positivity in the former, and an inconsiderable positivity in the latter. Most of our cases revealed diffuse and strong nuclear calretinin positivity with three exceptions, case 3, 7 and 8. Nevertheless, the diagnosis was supported by D2-40 positivity in case 3, which showed focal nuclear calretinin positivity, and CK5/6 positivity in case 7 and 8. D2-40 is a sensitive marker for mesothelial cells; and its characteristic membranous staining pattern is considered to have a high specifity for particularly epithelioid mesotheliomas while differentiating them from adenocarcinomas [11]. Mesothelin is highly sensitive for malignant mesothelioma, but its specificity is relatively low that some other tumors including ovarian cancer may exhibite mesothelin positivity. Nevertheless diffuse and strong membranous mesothelin expression serves as a strong indicator of particularly epithelioid mesothelioma rather than an ovarian carcinoma [9,12]. Malign mesotheliomas are also characterized by strong and diffuse membranous EMA positivity (expression on the luminal aspects of the tumor cells), but this staining pattern does not discriminate from adenocarcinoma. ER positivity in malignant mesothelioma is a very rare phenomenon, and its positivity most probably indicates a serous carcinoma rather than a mesothelioma. Compatible with these findings, none of our cases revealed ER positivity [13]. Although WT-1 protein is highly sensitive for epithelioid mesotheliomas, it has no benefit in discriminating from serous carcinomas [14]. Besides the expression of positive mesothelioma markers, none of the cases revealed adenocarcinoma markers such as; Ber-EP4, CEAm, MOC-31, and Tag72, and supported the diagnosis on the other hand. ER and PR negativity also served as significant indicators of malignant mesothelioma. Thus, not only the positivity, but also the negativity of certain markers is valuable in the differential diagnosis. Figures were shown to represent some of the cases with common tubulopapillary morphology, ovarian involvement and immune profile of positive and negative markers (Figure 1, 2, 3, 4, and 5; corresponding to Case 1, 2, 3, 4 and 7, respectively).
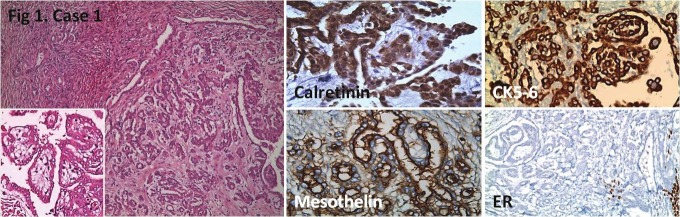
Figure 1.
Nodular tumor involvement in ovarian stroma. Positive calretinin, mesothelin and CK5/6 staining, and negative ER staining is seen. Hematoxylin Eosin (HE); x100, x200 (inlet), IHC; x400, x400, x200, x200, respectively.
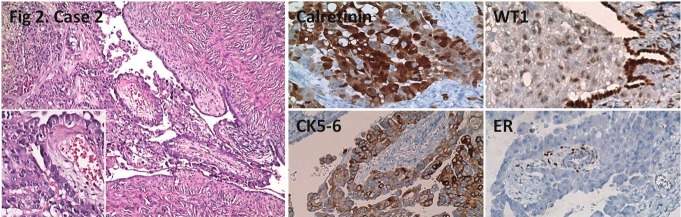
Figure 2.
Tubulopapillary tumor next to ovary. Positive calretinin, CK5/6, WT1 staining, and negative ER staining withpositive control in residue ovarian stroma is seen. HE; x100, x200 (inlet), IHC; x200.
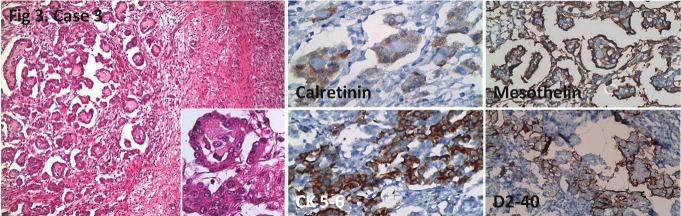
Figure 3.
Nodular tumor involvement in ovary. Focal nuclear calretinin positivity, positive CK5/6, mesothelin and D2-40 staining is seen. HE; x100, x400 (inlet), IHC; x400, x200, x200, x200, respectively.
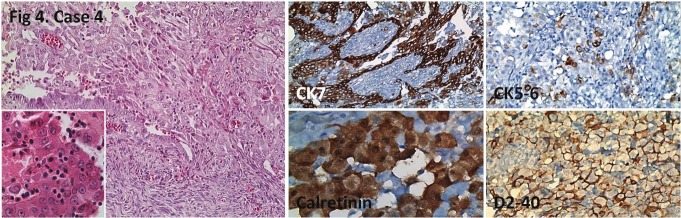
Figure 4.
Tubulopapillary tumor infiltrating ovary from the surface. Positive CK7, calretinin, CK5/6 (focal), and D2-40staining. HE; x200, x400 (inlet), IHC; x200, x400, x200, x200, respectively.
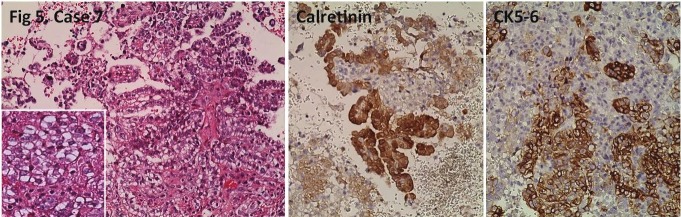
Figure 5.
Tubulopapillary tumor in the peritoneum. Nonspecific cytoplasmic calretinin positivity, and diffuse CK5/6positivity is seen. HE, IHC; x200.
Treatment and results
In a series evaluating ovarian involvement of mesothelioma cases, biopsy or adnecsectomy or intraperitoneal chemotherapy following total abdominal hysterectomy and bilateral salpingo-oophorectomy were performed [1]. Half of these patients died in the first year of follow-up [1]. In patients with extensive peritoneal involvement, the survival was worse as expected. In other series, varying treatments including chemoradiation were administered following conservative, palliative, or surgical treatments [2-5].
Among 8 patients we evaluated, we thought that optimal cytoreduction could be achieved with primary surgery, especially in patients presented with ovarian mass, thus primary surgery was performed. In other patients, surgery was considered following neoadjuvant chemotherapy because of peritoneal carcinomatosis signs. The chemotherapies administered were paclitaxel and carboplatin based. Mean survival time was 17.87 months (range: 1-48 months). Four cases were alive while the other four cases died (Table 1).
These practices can of course be questionable. However, as DPMM is rare, its management, especially in cases with ovarian metastases and presenting as ovarian cancers is yet unclear along with the benefits of cytoreductive surgery. Recently a multimodal approach using radiotherapy in combination with surgery and intraperitoneal chemotherapy has been evaluated for the treatment of patients with DPMM [15]. Chemotherapy agents have been used alone or in combination and chemotherapy protocols are variable. The treatment currently considered as standard for selected patients with this disease is surgical cytoreduction with intraperitoneal chemotherapy [15].
Conclusion
Malignant mesotheliomas can apply to or be referred to gynecologic oncology clinics with primary ovarian masses or peritoneal carcinomatosis. This rate was calculated as 0.1% in our cases. Clinical distinction of malignant mesothelioma from ovarian cancer or peritoneal adenocarcinoma is very difficult. Differential diagnosis is reliably achieved by immune profile of the tumors with a systematic approach of both positive and negative mesothelioma markers. It is not yet clear whether or not the treatment approach in patients presented with ovarian mass is different from mesothelioma patients with peritoneal involvement. More series are required to determine the optimal management.
References
- 1.Goldblum J, Hart WR. Localized and diffuse mesotheliomas of the genital tract and peritoneum in women. A clinicopathological study of nineteen true mesothelial neoplasms, other than adenomatoid tumors, multicystic mesotheliomas and localized fibrous tumors. Am J Surg Pathol. 1995;19:1124–1137. doi: 10.1097/00000478-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Clement PB, Young RH, Scully RE. Malignant mesotheliomas presenting as ovarian masses. A report of nine cases, including two primary ovarian mesotheliomas. Am J Surg Pathol. 1996;20:1067–1080. doi: 10.1097/00000478-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Attanoos RL, Gibbs AR. Primary malignant gonadal mesotheliomas and asbestos. Histopathology. 2000;37:150–159. doi: 10.1046/j.1365-2559.2000.00942.x. [DOI] [PubMed] [Google Scholar]
- 4.Mani H, Merino MJ. Mesothelial neoplasms presenting as, and mimicking, ovarian cancer. Int J Gynecol Pathol. 2010;29:523–528. doi: 10.1097/PGP.0b013e3181e6a3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addis BJ, Fox H. Papillary mesothelioma of ovary. Histopathology. 1983;7:287–298. doi: 10.1111/j.1365-2559.1983.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 6.Eltabbakh GH, Piver MS, Hempling RE, Recio FO, Intengen ME. Clinical picture, response to therapy, and survival of women with diffuse malignant peritoneal mesothelioma. J Surg Oncol. 1999;70:6–12. doi: 10.1002/(sici)1096-9098(199901)70:1<6::aid-jso2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.McCluggage WG, Wilkinson N. Metastatic neoplasms involving the ovary: a review with an emphasis on morphological and immunohistochemical features. Histopathology. 2005;47:231–247. doi: 10.1111/j.1365-2559.2005.02194.x. [DOI] [PubMed] [Google Scholar]
- 8.Davidson B. New diagnostic and molecular characteristics of malignant mesothelioma. Ultrastructural Pathology. 2008;32:227–240. doi: 10.1080/01913120802454298. [DOI] [PubMed] [Google Scholar]
- 9.Ordonez NG. What are the current best immunohistochemical markers for the diagnosis of epithelioid mesothelioma? A review and update. Hum Pathol. 2007;38:1–16. doi: 10.1016/j.humpath.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Chu P, Wu E, Weiss LM. Cytokeratin 7 and Cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962–972. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]
- 11.Chu AY, Litzky LA, Pasha TL, Acs G, Zhang PJ. Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol. 2005;18:105–110. doi: 10.1038/modpathol.3800259. [DOI] [PubMed] [Google Scholar]
- 12.Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–197. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 13.Ordonez NG. Value of estrogen and progesterone receptor immunostaining in distinguishing between peritoneal mesotheliomas and serous carcinomas. Hum Pathol. 2005;36:1163–1167. doi: 10.1016/j.humpath.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Hashi A, Yuminamochi T, Murate S-I, Iwamoto H, Honda T, Hoshi K. Wilms tumor gene immunoreactivity in primary serous carcinomas of the fallopian tube, ovary, endometrium, and peritoneum. Int J Gynecol Pathol. 2003;22:374–377. doi: 10.1097/01.pgp.0000092130.10100.88. [DOI] [PubMed] [Google Scholar]
- 15.Munkholm-Larsen S, Cao CQ, Yan TD. Malignant peritoneal mesothelioma. World J Gastrointest Surg. 2009;1:38–48. doi: 10.4240/wjgs.v1.i1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]