Abstract
Lactobacillus rhamnosus strain GG (LGG) has been studied extensively as a probiotic in humans. However, the ability of an organism to survive passage through the intestinal tract and exert beneficial effects cannot be directly extrapolated between species. This study evaluated the ability of LGG to survive gastrointestinal transit in dogs and assessed whether oral administration of LGG is safe, in order to determine whether studies evaluating the efficacy of LGG in the treatment of canine disease are indicated. Dogs were divided into 5 groups receiving doses of 0 (control group, n = 4), 1 × 109 (group 1, n = 8), 1 × 1010 (group 2, n = 8), 5 × 1010 (group 3, n = 8) and 5 × 1011 (group 4, n = 4) colony forming units per day, orally, for 5 days. Lactobacillus rhamnosus GG was detected in the feces of 4/8 dogs in groups 1 and 2, 5/8 dogs in group 3, 4/4 dogs in group 4, and 0/4 dogs in the control group. Fecal colonization was significantly greater in group 4 than in any other group (P < 0.001). Differences between groups 1, 2, and 3 were not significant. No adverse effects were noted. Fecal colonization of LGG in dogs is somewhat variable; however, clinical studies are indicated to evaluate this organism in the treatment and prevention of canine disease.
Introduction
Probiotics have been defined as “living microorganisms, which upon ingestion in certain numbers, exert health effects beyond inherent basic nutrition” (1). The concept of probiotics was first reported by Elie Metchnikoff in 1907 (2). He postulated that consumption of fermented milk products was responsible for longevity of certain ethnic groups and suggested that these products manipulated the intestinal microflora to maintain the normal balance between pathogenic and nonpathogenic bacteria (2). A variety of microorganisms, typically lactic acid bacteria, such as lactobacilli, bifidobacteria, and enterococci, have been evaluated as potential probiotics (3). A small number of yeast have also been evaluated (4,5). Probiotic therapy is being used increasingly in human and veterinary medicine. Appealing properties of probiotics include the ability to reduce antibiotic use, the apparently high index of safety, and the public's positive perception about “natural” or “alternative” therapies. Probiotics are classified, and generally regarded as safe, as opposed to antibiotics, which have a number of recognized adverse effects (6).
Commercial probiotic preparations are available for human and animal use; however, little or no objective research has been done on many. Based on the definition of probiotics stated above, it is clear that adequate numbers of viable organisms must reach the intestinal tract. For this to happen, probiotic organisms must be able to survive transit through the acidic environment of the stomach and resist digestion by bile. Organisms that survive acid and bile must possess a variety of other properties, including the ability to adhere to intestinal epithelial cells, colonize the intestinal tract, produce an antimicrobial factor, and inhibit enteric pathogens (7,8,9,10,11). Other properties, such as immunomodulation, modulation of metabolic activities, and inactivation of procarcinogens, are also desirable (8,12). An organism can only be considered to be a probiotic after these properties have been identified and a positive health effect has been documented.
One of the best-studied probiotics in human medicine is Lactobacillus rhamnosus strain GG (LGG). Lactobacillus rhamnosus GG has been shown to survive acid and bile digestion and colonize the gastrointestinal tracts of humans (13,14,15,16). It also possesses powerful adhesive properties, suppresses bacterial enzyme activity, can displace or eliminate certain components of the normal intestinal flora, and produces an antimicrobial substance active against a variety of bacteria, including Escherichia coli Salmonella spp., Clostridium spp., Streptococcus spp., and Bacteroides spp. (11). In humans, LGG has been shown to be effective in the treatment of several forms of diarrhea, including rotaviral diarrhea in children, acute nonrotaviral diarrhea in children, antibiotic associated diarrhea in children and adults, “travellers'” diarrhea, and relapsing C. difficile diarrhea in placebo-controlled studies (11,17,18,19,20,21,22,23,24). Recent studies using animal models have suggested that LGG may be beneficial in the treatment of inflammatory bowel disease, pouchitis, and ulcerative colitis in humans (25). These results suggest that probiotics, particularly LGG, might be of value in the treatment of canine gastrointestinal disease.
Some authors believe that probiotic organisms should be naturally occurring in their target species to be effective (9). However, cross-species efficacy has been demonstrated for some probiotic strains, including LGG (26). Therefore, despite being of human origin, LGG may possess probiotic properties in dogs. However, prior to evaluating the efficacy of any probiotic, it should be demonstrated that the organism has the ability to survive transit through the gastrointestinal tract of the intended host. This does not indicate that an organism will have probiotic properties in the given species; however, intestinal survival and fecal colonization are a prerequisite for efficacy. This study was designed to determine the ability of LGG to adequately survive intestinal transit in dogs, to evaluate whether intestinal survival is dose dependent, and to determine whether any adverse effects occur following LGG administration.
Materials and methods
Thirty-two clinically healthy adult beagles were enrolled in the study. Animals were housed according to University of Guelph Animal Care Committee guidelines. Diet and management were not altered. Dogs were housed in close proximity, but comingling was not allowed. Dogs were divided into 5 groups. Lactobacillus rhamnosus GG was administered, PO, at doses of 1 × 109 colony-forming units (cfu) (group 1, n = 8), 1 × 1010 cfu (group 2, n = 8), 5 × 1010 cfu (group 3, n = 8), 5 × 1011 cfu (group 4, n = 4), and 0 cfu (control group, n = 4), once daily for 5 d (days 0 through 4). The LGG was administered by opening capsules containing freeze-dried LGG and mixing the contents with a small portion of canned food. Dogs were monitored daily for changes in clinical condition, vital parameters, appetite, and fecal consistency. Freshly passed fecal samples were collected on days 0, 1, 3, 5, 6, 7, 9, and 11. Fecal samples were refrigerated and processed within 4 h or stored at -80°C until being processed.
One gram of feces was serially diluted in phosphate buffered saline (pH 7.2). Aliquots of the serial dilutions were inoculated onto deMan, Rogosa, Sharpe (MRS) agar, a culture medium for the isolation of lactic acid bacteria, and incubated in an anaerobic chamber at 37°C for 72 h. Colonies were identified as LGG based on colonial morphology (large, white, creamy colonies), gram stain appearance (gram positive uniform rods), and the inability to ferment lactose (27). Randomly selected isolates were confirmed as LGG by using a biochemical identification assay (API 50 CHL; BioMerieux, St. Laurent, Quebec). Overall growth on MRS agar on day 0 was also recorded.
A general linear model procedure with contrasts of the overall mean LGG level was used to compare the area under the curve for LGG over days between groups. Univariate analysis on the residuals of the log10 LGG level was run. Linear regression was used to evaluate the association between day 0 MRS growth and LGG colonization on each sampling day. A statistical software package (SAS; SAS Institute Inc., Cary, North Carolina, USA) was used and a P-value of < 0.05 was considered significant for all comparisons.
Results
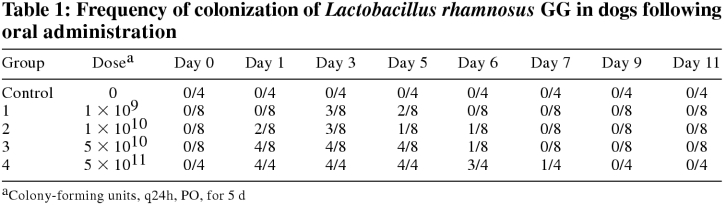
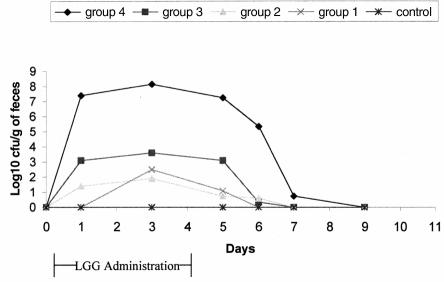
Lactobacillus rhamnosus strain GG was not detected in the feces of any dog prior to administration. All dogs in groups 1–3 readily consumed the food containing the probiotic. One dog in group 4 was slow to consume the food containing the probiotic, but all was consumed eventually. No adverse effects were noted. Lactobacillus rhamnosus strain GG was not present in the feces of control dogs at any point during the study. Detectable levels of LGG were present in the feces of 4/8 dogs in group 1, 4/8 in group 2, 5/8 in group 3, and 4/4 in group 4 (Table 1). The mean number of positive samples per dog was 0.63 in group 1 (range 0–2), 0.9 in group 2 (range 0–3), 1.6 in group 3 (range 0–4), and 4 in group 4 (range 3–5). The LGG was detected in feces 24 h after cessation of administration in 2/8 dogs in group 1, 1/8 in group 2, 4/8 dogs in group 3, and 4/4 dogs in group 4. Forty-eight hours after cessation of administration, LGG was still present in the feces of 1/8 dogs in each of groups 2 and 3, and 3/4 of dogs in group 4. After 72 h, LGG was present in the feces of only 1 dog in group 4.
Table 1.
Fecal levels of LGG in group 4 were significantly higher than in groups 1, 2, and 3 (P < 0.001, 0.001, and 0.004, respectively). Differences between groups 1, 2, and 3 were not statistically significant (P > 0.09).
The mean growth on MRS agar at day 0 was log10 7.5 ± 1.2 (mean ± standard deviation) with a range of log10 4.8 to log10 9.1. There were no significant intergroup differences in day-0 MRS growth (mean log10 7.2 -7.7). There was no association between the levels of MRS growth on day 0 and fecal LGG levels for any day of the study (P 0.51–0.95).
Discussion
This study has demonstrated that LGG can survive gastrointestinal transit in dogs and do so without causing any clinically evident adverse effects. Fecal colonization of LGG in dogs appears to be less efficient than in humans. Mean fecal levels of 105 to 107 cfu/g were reported following PO administration to humans at a dose of 1 × 1010 CFU/d (16,27) This level was achieved only in group 4, which received a higher oral dose (5 × 1011 CFU/d) of LGG. The significant difference in fecal LGG level between group 4 and the other groups cannot be attributed simply to a higher oral dose moving passively through the intestinal tract. The difference in dose between group 1 and group 4 was only 2.7 log10, while the difference between mean fecal levels during the administration period was 5.6–7.3 log10. This suggests that intestinal adhesion and colonization was responsible for the difference. Differentiation of delayed gastrointestinal transit from true intestinal colonization can be difficult, and intestinal biopsies would be required for confirmation that intestinal colonization had actually occurred. The reason that LGG was detected in relatively high levels in the feces of some dogs, while it was infrequently or never detected in other dogs administered the same dose is unclear. Differences in the gastrointestinal microflora between dogs could play a role in the variation that was seen in the study. Dogs with high, preexisting colonization by lactic acid bacteria may be more resistant to colonization with “foreign” lactobacilli. Bacterial species may be able to limit colonization of similar organisms through stable occupation of certain environmental or nutritional niches, or through the production of specific antibacterial products. Many lactobacilli can produce bacteriocins, bactericidal substances that are only effective against lactobacilli or closely related species (28). In this study, however, there was no association between day-0 MRS growth and colonization. Specific identification of resident lactic acid bacteria was not performed, so it is possible that colonization by LGG was inhibited by specific, unidentified components of the bacterial microflora in some dogs. Our understanding of the interactions between components of the intestinal microflora is poor, so critical assessment is difficult. It is possible that LGG, being of human origin, is better adapted to colonize the human gastrointestinal tract at a lower dose than is required in dogs. This may relate to inherent differences in the bacterial microflora among species, or it may be due to a variable ability to adhere to intestinal epithelial cells of different species.
Persistence of LGG in dogs is shorter than that reported in humans. Goldin et al (13) reported that 87% of humans excreted LGG in feces for 4 d following cessation of administration, while 33% shed LGG after 7 d. While LGG persists better in some humans than in others, it is accepted that daily administration of high doses is required to maintain high fecal levels. Clinically, persistence should be less important than colonization during administration.
Fecal levels of LGG were used as an indicator of the ability to colonize in this trial. While fecal colonization is widely used to assess probiotic colonization (13,15,27), fecal samples may underestimate colonization when compared with mucosal biopsy samples (14). Mucosal biopsies were not performed, as it was desired to assess intestinal levels of LGG over time. Mucosal biopsies would have required anesthesia, which could have affected intestinal motility and, potentially, colonization.
Determining the optimum dosing regimen prior to commencing clinical trials is important. This may be particularly true for probiotic organisms that are not being administered to their natural host, as is the case with administration of LGG to dogs. In humans, doses of 6 × 109 to 1 × 1011 CFU/d have been used in clinical trials (17,20,22,23,24,29). The optimum dosing of LGG for future studies is unclear. Colonization was best in group 4 with a dose of 5 × 1011 CFU/dog/d. This required 50 capsules of probiotic per day. The commercial form of LGG (Culturelle; CAG Functional Foods, Omaha, Nebraska, 63103-0820) contains at least 2 × 1010 cfu/capsule, however, 25 capsules would still be required daily. This level of administration would be difficult and expensive. There was not a statistically significant difference in colonization between groups 1, 2, and 3. Despite the lack of significance between groups 1, 2, and 3, it seems logical that a higher dose would be preferable. Since the group 4 dose may not be practical, a dose of approximately 5 × 1010 CFU/d could be considered; however, further studies are required to determine the optimum therapeutic dose.
Lactobacillus rhamnosus strain GG cannot be termed a canine probiotic at this point; however, this study provides the basis for future research involving this organism in canine disease. Because this study demonstrated that LGG could be safely administered to dogs and that it can survive gastrointestinal transit, it would seem logical to pursue further studies regarding this organism. Efficacy studies are indicated to determine whether LGG has a role in the prevention or treatment of canine disease. It is also possible that LGG would colonize better in dogs with diarrhea, because of disruption of the normal, protective intestinal microflora. This should be examined further. CVJ
Figure 1. Fecal recovery of Lactobacillus rhamnosus strain GG (LGG) in dogs following oral administration.
Footnotes
Acknowledgment
The authors thank Gabrielle Monteith for assistance with the statistical analysis.
This study was approved by the University of Guelph Animal Care Committee. This study was supported by the Ontario Veterinary College Pet Trust. The Lactobacillus rhamnosus strain GG preparation that was used in this study was supplied by CAG Functional Foods, Omaha, Nebraska, 63103-0820.
Address correspondence and reprint requests to Dr. J.S. Weese.
References
- 1.Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol 1998;39:237–238. [DOI] [PubMed]
- 2.Metchnikoff E. The Prolongation of Life, London: William Heinemann, 1907.
- 3.Fuller R. Probiotics in human medicine. Gut 1991;32:439–442. [DOI] [PMC free article] [PubMed]
- 4.Filho-Lima JVM, Vieira EC, Nicoli JR. Antagonistic effect of Lactobacillus acidophilus, Saccharomyces boulardii and Escherichia coli combinations against experimental infections with Shigella flexneri and Salmonella enteritidis subsp. typhimurium in gnotobiotic mice. J Appl Microbiol 2000;88:365–370. [DOI] [PubMed]
- 5.McFarland LV, Surawicz CM, Greenburg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994;271:1913–1918. [PubMed]
- 6.Reid G. In defense of probiotics. Am Soc Microbiol News 2000;66:261.
- 7.Dunne C, O'Mahoney L, Murphy L, et al. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr 2001;73:386S–392S. [DOI] [PubMed]
- 8.Gibson GR, Fuller R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J Nutr 2000;130:391S–395S. [DOI] [PubMed]
- 9.Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol 2000;78:80–88. [DOI] [PubMed]
- 10.Ouwehand AC, Niemi P, Salminen SJ. The normal faecal microflora does not affect the adhesion of probiotic bacteria in vitro. FEMS Microbiol Lett 1999;177:35–38. [DOI] [PubMed]
- 11.Gorbach SL. Probiotics and gastrointestinal health. Am J Gastroenterol 2000;95 Suppl:S1–S4. [DOI] [PubMed]
- 12.Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J Biotech 2000;84:197–215. [DOI] [PubMed]
- 13.Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S. Survival of Lactobacillus species (Strain GG) in human gastrointestinal tract. Dig Dis Sci 1992;37:121–128. [DOI] [PubMed]
- 14.Alander M, Satokari R, Korpela R, et al. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol 1999;65:351–354. [DOI] [PMC free article] [PubMed]
- 15.Saxelin M, Ahokas M, Salminen S. Dose response on the faecal colonisation of Lactobacillus strain GG administered in two different formulations. Microbiol Ecol Health Dis 1993;6:119–122.
- 16.Saxelin M, Elo S, Salminen S, Vapaatalo H. Dose response colonisation of faeces after oral administration of Lactobacillus casei strain GG. Microbiol Ecol Health Dis 1991;4:209–214.
- 17.Armuzzi A, Cremonin F, Ojetti V, et al. Effect of Lactobacillus GG supplementation on antibiotic-associated gastrointestinal side effects during Helicobacter pylori eradication therapy: a pilot study. Digestion 2001;63:1–7. [DOI] [PubMed]
- 18.Oberhelman RA, Gilman RH, Sheen P, et al. A placebo-controlled trial of Lactobacillus GG to prevent diarrhea in undernourished Peruvian children. J Pediatr 1999;134:15–20. [DOI] [PubMed]
- 19.Saavedra J. Probiotics and infectious diarrhea. Am J Gastroenterol 2000;95 Suppl:S16–S18. [DOI] [PubMed]
- 20.Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr 1999;135:564–568. [DOI] [PubMed]
- 21.Oksanen PJ, Salminen S, Saxelin M, et al. Prevention of travellers' diarrhoea by Lactobacillus GG. Ann Med 1990;22:53–56. [DOI] [PubMed]
- 22.Guandalini S, Pensabene L, Zikri MA, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter Euopean trial. J Pediatr Gastroenterol Nutr 2000;30:54–60. [DOI] [PubMed]
- 23.Raza S, Graham SM, Allen SJ, Sultana S, Cuevas L, Hart CA. Lactobacillus GG promotes recovery from acute nonbloody diarrhea in Pakistan. Pediatr Infect Dis J 1995;14:107–111. [DOI] [PubMed]
- 24.Isolauri E, Juntunen M, Pautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics 1991;88:90–97. [PubMed]
- 25.Zhou JS, Shu Q, Rutherfurd KJ, Prasad J, Gopal PK, Gill HS. Acute oral toxicity and bacterial translocation studies on potentially probiotic strains of lactic acid bacteria. Food Chem Toxicol 2000;38:153–161. [DOI] [PubMed]
- 26.Lee DJ, Drongowski RA, Coran AG, Harmon CM. Evaluation of probiotic treatment in a neonatal animal model. Pediatr Surg Int 2000;16:237–242. [DOI] [PubMed]
- 27.Saxelin M, Pessi T, Salminen S. Fecal recovery following oral administration of Lactobacillus Strain GG (ATCC 53103) in gelatine capsules to healthy volunteers. Int J Food Microbiol 1995;25:199–203. [DOI] [PubMed]
- 28.Kalenhammer TR. Bacteriocins of lactic acid bacteria. Biochimie 1988;70:337–349. [DOI] [PubMed]
- 29.Gupta P, Andrew H, Kirschner BS, Guandalini S. Is Lactobacillus GG helpful in children in Crohn's disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr 2000;31: 453–457. [DOI] [PubMed]