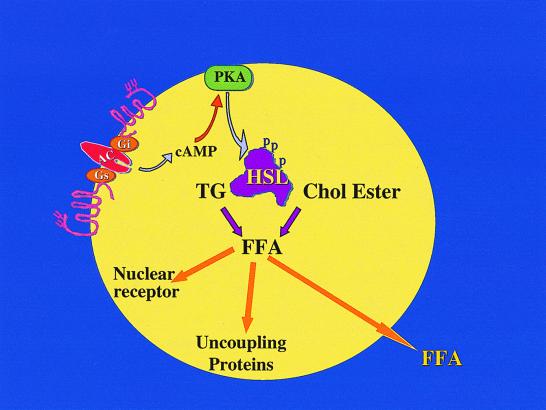
The mobilization of fatty acid from triglycerides and cholesterol esters provides the primary source of energy in mammals. Hormone-sensitive lipase (HSL) is a multifunctional tissue lipase that plays a critical role in this process. The enzyme has broad specificity, catalyzing the hydrolysis of tri-, di-, and monoacylglycerols, as well as cholesterol esters. HSL has been studied most extensively in adipose tissue, where it is thought to catalyze the major rate-limiting step in lipolysis. The lipase is acutely activated by cAMP-dependent phosphorylation, which also leads to its redistribution from the cytoplasm to the lipid droplet (1, 2) (Fig. 1). Regulation of adipocyte HSL is the primary means by which lipolytic agents, such as catecholamines, stimulate the release of free fatty acids (FFAs) and thus control circulating levels. Plasma FFAs profoundly influence carbohydrate and lipid utilization, storage, and synthesis, both in liver and muscle. Moreover, fatty acids produced in fat cells can uncouple ATP synthesis, resulting in adaptive thermogenesis (3). Products of fatty acid metabolism are also thought to bind directly to nuclear receptors, thus regulating transcription of genes involved in lipid synthesis and breakdown (4). These observations predict HSL to be an important player in controlling the balance of substrate utilization and storage.
Figure 1.
The regulation of hormone-sensitive lipase. Lipolytic hormones such as catecholamines and ACTH (corticotropin) stimulate the activity of adenylyl cyclase, generating cAMP, which in turn activates protein kinase A. Protein kinase A catalyzes the phosphorylation of HSL, resulting in increased activity (1, 2) and access to substrate (17). The enzyme can hydrolyze both triglycerides and cholesterol esters, producing FFAs and glycerol or free cholesterol. Free fatty acids may have several biological roles, activating uncoupling proteins in adipocytes and nuclear receptors in a variety of cells, and are also released into the circulation to indirectly regulate metabolism in other tissues.
In addition to adipocytes, HSL is found in skeletal muscle, heart, brain, pancreatic beta cells, adrenal gland, ovaries, testes, and macrophages. Although triglyceride hydrolysis is probably also important in muscle and pancreas, cholesterol ester hydrolysis appears to play a separate biological role. In macrophages, HSL has been suggested to regulate foam cell formation (5). The presence of the enzyme in adrenal and reproductive tissues suggests a potential role in hormone-regulated steroidogenesis and possibly in sperm development, by regulating the availability of free cholesterol (6).
Hormone-Sensitive Lipase Plays a Critical Role in Spermatogenesis.
In this issue of PNAS, Osuga et al. report the targeted disruption of the HSL gene in mice (7). Although mice homozygous for the mutant HSL allele (HSL−/−) appeared normal, the males were sterile because of oligospermia. Those few sperm found in the epididymis from the knockout mice were not motile, and the epithelial cells in seminiferous tubules were extensively vacuolated, presumably with lipid. Although there was no reduction in epididymal triglyceride lipase activity, there was a complete absence of cholesterol esterase activity in testes of HSL−/− mice, with a significant rise in cholesterol ester levels. Interestingly, other steroidogenic tissues were not greatly effected because circulating steroid hormone levels were normal in the HSL−/− mice. This indicates that the effects of disruption of the gene on spermatogenesis were direct, and not secondary to hypogonadism resulting from hormone insufficiency. Interestingly, female mice were unaffected by disruption of the HSL allele, suggesting that similar processes are not critical for oogenesis.
These findings implicate an important role for fatty acids in the regulation of spermatogenesis. There is a novel, testes-specific splice variant of HSL found in Sertoli cells that encodes a longer form of the enzyme with one additional exon (8), although the impact of the additional sequences on the activity or regulation of the enzyme remains unknown. Studies have documented changes in both cholesterol and triglyceride content during epidydimal maturation (9). Moreover, pathological states of fatty acid deficiency have been identified that result in testicular degeneration and sterility (10). Thus, a suggested role for cholesterol ester hydrolysis in spermatogenesis is now conclusively demonstrated with the HSL knockout.
There remains much to learn about the precise pathways required for normal sperm development that are impacted by loss of the HSL allele. First, the enzymology of the testes-specific isoform of the lipase deserves further scrutiny. Does this enzyme show a substrate specificity different from that expressed in other tissues? The lack of changes in testicular TG content in the face of a significant reduction in cholesterol ester hints at a distinct substrate preference for the larger testicular isoform. The authors also point out the interesting possibility that retinyl ester hydrolysis might contribute significantly to this phenotype. Male sterility is a defining feature of retinoic acid X receptor-deficient mice (11), suggesting that the unavailability of ligands for these receptors might have profound effects on testicular development. Besides questions concerning the substrates of testicular HSL, there are also issues regarding the relative roles of the potential products of the enzyme. In addition to retinoic acid derivatives, it is likely that the flux of free intracellular cholesterol might impact significantly on steroid synthesis in Sertoli cells. Other steroidogenic tissues were apparently not influenced in a major way because circulating hormone levels were not appreciably altered. However, it may be that the testes are more sensitive to smaller changes in local concentrations of steroid hormones. This possibility will require more investigation. It is equally possible that the fatty acids themselves have a major role in sperm development. Indeed, fatty acid derivatives can serve as ligands for a number of nuclear receptor transcription factors, including the PPARs, HNF4, and others (4). Importantly, many of these receptors exist in an obligate heterodimer with retinoic acid X receptor. It will be of interest to determine whether these receptors play an important role in the developing sperm and, if so, how their state of activation is changed during spermatogenesis.
HSL Is Not Absolutely Required for Lipolysis in Adipose Tissue.
The lipolytic process in fat cells has been targeted in attempts to combat both obesity and insulin resistance. Several β3 adrenergic receptor agonists have been designed to activate adaptive thermogenesis. These drugs can activate adenylyl cyclase in responsive tissues, resulting in the uncoupling of energy expenditure, in the process producing heat. As a primary target of cAMP-dependent kinase, HSL was thought to be the central player in this pathway, leading to the generation of intracellular fatty acids that can activate uncoupling proteins (3). Thus, it is quite startling that the disruption of the HSL gene has a relatively small effect on metabolism in the fat cell and no apparent impact on weight or cold sensitivity, β3 agonist treatment, or oxygen consumption.
Detailed studies of the HSL−/− mice focused on brown and white adipose tissue. Surprisingly, triglyceride lipase activity was unaffected by deletion of the HSL gene in brown adipocytes, although cholesterol esterase activity was completely eliminated. Every other physiological measurement indicated that the thermogenic response in these animals was completely preserved. The only other detectable change in these cells was a marked hypertrophy characterized by a significant increase in the size and vacuolization of individual fat cells. Thus, the most likely explanation for these findings is the presence of a second, catecholamine-sensitive lipase responsible for UCP activation whereas HSL is primarily responsible for cholesterol ester hydrolysis, perhaps regulating lipid accumulation.
Although triglyceride lipase activity is unaffected in brown fat cells from HSL−/− mice, it is partially attenuated in white fat. Moreover, the stimulation of lipolysis in vitro and in vivo is also blunted in the knockout mice. White adipocytes are larger in the HSL−/− mice because of increased lipid content. However, as seen in brown adipose tissue, cholesterol esterase activity was completely eliminated, and there was a significant residual triglyceride lipase activity in white fat cells.
Although the data are preliminary, these studies suggest a fresh look at the enzymatic activity of hormone-sensitive lipase. The enzyme has been widely studied as a classical target of hormone action in the adipocyte for over 30 years. Its activation by protein kinase A is a classic example of the regulation of intermediary metabolism by phosphorylation, thus making it an attractive explanation for the effects of catecholamines and insulin on lipid metabolism in the adipocyte (1, 2). However, the primary phenotype of disruption of the HSL gene seems to be expressed as an abatement of cholesterol esterase activity. This may be the primary function of the enzyme, indicating that we need to look carefully for another hormone-regulated lipase in fat, and perhaps other tissues.
Does Hormone-Sensitive Lipase Control Insulin Action or Secretion?
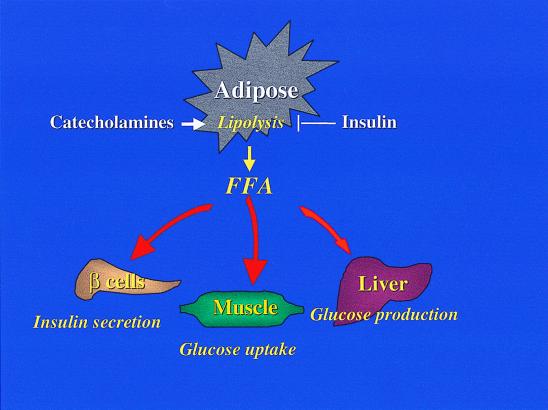
Although the presumed existence of another adipose triglyceride lipase makes it difficult to draw further conclusions on the role of lipolytic enzymes in adaptive thermogenesis and obesity, additional studies on the HSL−/− mice can contribute significantly to our knowledge about lipolysis in the regulation of metabolism (Fig. 2). A number of obvious questions surface regarding the metabolic status of the mice. First, there is a major inhibition of catecholamine-stimulated lipolysis in the knockout mice, along with a 50% reduction in circulating FFAs and glycerol in the fasted state. One wonders about the sensitivity of the affected fat cells to insulin and whether glucose transport and utilization are impaired in brown and white adipose tissue. Previous studies have shown that smaller fat cells are more sensitive to insulin (12), implying that HSL−/− mice might exhibit selective insulin resistance in adipocytes. Changes in adipocyte size and number might also profoundly impact the regulation of leptin or tumor necrosis factor α synthesis.
Figure 2.
Free fatty acids control glucose and lipid metabolism. Upon activation of HSL, fat cells release FFAs into the circulation, where they have pleiotropic effects on different tissues involved in glucose homeostasis. Excessive FFAs can reduce insulin-stimulated glucose uptake in muscle. In liver, FFAs can directly increase glucose output via increased gluconeogenesis. Together, these effects can increase plasma glucose. In the pancreatic beta cell, FFAs are required to maintain normal glucose-stimulated insulin secretion. However, an excess of FFAs can block insulin secretion.
On the other hand, it will also be interesting to examine lipolysis in the postprandial state and to assess the impact of lowered levels of FFAs on liver and muscle (13). Is there a reduction in hepatic glucose production? Is there an increase in glucose uptake and utilization in muscle? Despite the fact that bigger fat cells are associated with insulin resistance, lower levels of circulating FFAs might increase insulin sensitivity in muscle and liver. Although there was no effect of the knockout on weight, the relative sensitivity of these animals to insulin will be of interest, as assessed by glucose and insulin tolerance tests and hyperinsulinemic euglycemic clamp experiments. Such studies will also require investigation of the sensitivity of these animals to a high fat diet, among other stresses.
In addition to indirect effects on peripheral tissues, it will be of great interest to evaluate the role of HSL on insulin secretory capacity. Numerous studies (14) have suggested that fatty acids can profoundly influence insulin secretion in the pancreatic beta cell. Although exogenous fatty acids are required for glucose-stimulated insulin secretion in the isolated islet, an excess of fatty acids can be detrimental to the beta cell, resulting in “lipotoxicity.” Although the precise mechanism of this effect remains uncertain, high levels of FFAs correlate with beta cell dysfunction in models of type 2 diabetes (15). Further-more, HSL is expressed in the beta cell (16), and endogenous lipolysis has been proposed to play an important role in the regulation of insulin secretion by cAMP-elevating hormones such as GLP. Thus, it will be quite interesting to extend the analysis of the HSL−/− mice to the dynamics of insulin secretion and action.
Acknowledgments
I thank Drs. R Mackenzie, J. Granneman, and C. Burant for helpful comments.
Footnotes
See companion article on page 787.
References
- 1.Stralfors P, Olsson H, Belfrage P. In: The Enzymes. Boyer P D, Krebs E G, editors. London: Academic; 1987. pp. 147–177. [Google Scholar]
- 2.McKnight G S, Cummings D E, Amieux P S, Sikorski M A, Brandon E P, Planas J V, Motamed K, Idzerda R L. Recent Prog Horm Res. 1998;53:139–159. [PubMed] [Google Scholar]
- 3.Himms-Hagen J. Prog Lipid Res. 1989;28:67–115. doi: 10.1016/0163-7827(89)90009-x. [DOI] [PubMed] [Google Scholar]
- 4.Tontonoz P, Hu E, Spiegelman B M. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 5.Escary J-L, Choy H A, Reue K, Schotz M C. Arterioscler Thromb Vasc Biol. 1998;18:991–998. doi: 10.1161/01.atv.18.6.991. [DOI] [PubMed] [Google Scholar]
- 6.Yeaman S J. Biochim Biophys Acta. 1990;1052:128–132. doi: 10.1016/0167-4889(90)90067-n. [DOI] [PubMed] [Google Scholar]
- 7.Osuga J-i, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer F B, Tsutsumi O, Yamada N. Proc Natl Acad Sci USA. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holst L S, Langin D, Mulder H, Laurell H, Grober J, Bergh A, Mohrenweiser H W, Edgren G, Holm C. Genomics. 1996;35:441–447. doi: 10.1006/geno.1996.0383. [DOI] [PubMed] [Google Scholar]
- 9.Rana A P, Majumder G C, Misra S, Ghosh A. Biochim Biophys Acta. 1991;1061:185–196. doi: 10.1016/0005-2736(91)90284-f. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald M L, Rogers Q R, Morris J G, Cupps P T. J Nutr. 1984;114:719–726. doi: 10.1093/jn/114.4.719. [DOI] [PubMed] [Google Scholar]
- 11.Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub M P, LeMeur M, Chambon P. Proc Natl Acad Sci USA. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, et al. J Clin Invest. 1998;101:1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boden G. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 14.Milburn J L, Jr, Hirose H, Lee Y H, Nagasawa Y, Ogawa A, Ohneda M, Beltran del Rio H, Newgard C B, Johnson J H, Unger R H. J Biol Chem. 1995;270:1295–1299. doi: 10.1074/jbc.270.3.1295. [DOI] [PubMed] [Google Scholar]
- 15.Shimabukuro M, Higa M, Zhou Y-T, Wang M-Y, Newgard C B, Unger R H. J Biol Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 16.Mulder H, Holst L S, Svensson H, Degerman E, Sundler F, Ahrén B, Rorsman P, Holm C. Diabetes. 1999;48:228–232. doi: 10.2337/diabetes.48.1.228. [DOI] [PubMed] [Google Scholar]
- 17.Syu L-J, Saltiel A R. Mol Cell. 1999;4:109–115. doi: 10.1016/s1097-2765(00)80192-6. [DOI] [PubMed] [Google Scholar]