Abstract
Objective
Green tea has been found to possess anti-inflammatory, anti-oxidative and anti-carcinogenic properties. The present study examines the association between green tea drinking and hepatocellular carcinoma (HCC) and its interactions with other risk or protective factors and single nucleotide polymorphisms (SNP) of inflammation and oxidative stress related genes.
Methods
A population-based case-control study with 204 primary HCC cases and 415 healthy controls was conducted in Taixing, China. Epidemiological data were collected using a standard questionnaire. SNPs of genes of the inflammation and metabolic pathways were genotyped at the UCLA Molecular Epidemiology Laboratory. Logistic regression was performed to estimate adjusted odds ratios and 95% confidence intervals.
Results
Longer duration and larger quantities of green tea consumption were inversely associated with primary HCC. Individuals who drank green tea longer than 30 years were at lowest risk (adjusted OR = 0.44, 95% CI: 0.19-0.96) compared with non-drinkers. A strong interaction was observed between green tea drinking and alcohol consumption (adjusted OR for interaction = 3.40, 95% CI: 1.26-9.16). Green tea drinking was also observed to have a potential effect modification on HBV/HCV infection, smoking and polymorphisms of inflammation related cytokines, especially for IL-10.
Conclusion
Green tea consumption may protect against development of primary HCC. Potential effect modifications of green tea on associations between primary HCC and alcohol drinking, HBV/HCV infection, and inflammation-related SNPs were suggested.
Keywords: Green tea, Primary hepatocellular carcinoma, Inflammation, Alcohol
1. Introduction
Primary liver cancer (PLC) is the sixth most common cancer and the third most common cancer-related death worldwide. PLC has a wide geographic variation, with approximately 82% of PLC cases and deaths occurring in the developing countries [1]. According to Globocan 2002, an estimated 345,844 new PLC cases and 321,851 deaths occurred in China each year, accounting for more than half of PLC cases and deaths in the world. Hepatocellular carcinoma (HCC) comprises 85-90% of PLC, and the two terms are often used interchangeably [2].
Chronic infections by hepatitis B (HBV) and hepatitis C (HCV), aflatoxin B1 (AFB1) and excessive alcohol consumption have been associated with increased risk for HCC [3-7]. Tobacco smoking may increase HCC risk, but the results are conflicting [8,9]. Among these known or suspected risk factors, inflammation may play an important role in infectious and non-infectious pathways leading to HCC. Chronic infections with HBV/HCV can induce chronic inflammation in liver tissue, which creates a procarcinogenic environment for HCC development. Chronic alcohol consumption and tobacco smoking are known non-infectious causes of chronic inflammation and may play a role in liver cell injury [10,11]. Chronic inflammation is characterized, in part, by altered cytokine levels, which may contribute to the pathogenesis of HCC [12]. In addition, liver tissue inflammation can increase oxidative stress, which is thought to be important in the initiation and promotion of HCC [13,14]. Single nucleotide polymorphisms (SNPs) of cytokines may alter gene expression level and are functionally associated with liver disease and HCC [12,15].
Green tea contains many polyphenols. In vitro and in vivo studies have suggested that polyphenols, particularly epigallo-cathechin-3 gallate (EGCG), have anti-inflammatory and antioxidant properties [16-19]. Epidemiological studies suggest that, though not conclusive, green tea consumption is associated with reduced risk of gastrointestinal cancers, including stomach cancer [20-22], esophageal cancer [23-25] and colorectal cancer[26,27]. Increased green tea consumption is inversely associated with breast cancer in case-control studies [28,29], but not in cohort studies [30,31]. In addition, green tea drinking is associated with breast cancer recurrence [32,33]. Very few studies have been conducted to examine the effect of green tea drinking on the development of HCC, and the published results are inconclusive [34-38]. There have been no published epidemiological studies that investigate interactions between polymorphisms of inflammation-related cytokines and green tea consumption on HCC risk. We hypothesize that the antiinflammatory effects of green tea may protect against HCC and SNPs of inflammation pathways may modify the effect of green tea drinking on HCC.
2. Materials and methods
2.1. Study population
The current study includes 204 newly diagnosed HCC cases and 415 controls from a population-based case-control study conducted in Taixing city, China. The original study population has been described in detail previously [20,39]. Briefly, the original study included three cancer sites (esophagus, stomach and liver) and one common control group. The ratio of the combined three cancer cases to the common control is 3:2. The current study only includes newly diagnosed HCC patients and all population controls. Therefore, the HCC case-to-control ratio is 1:2 in this study. Eligible cases were identified using the Taixing Tumor Registry between January 1 and June 30, 2000. Healthy controls were randomly selected from the residential areas where cancer cases originated and were frequency-matched to the combined case group (esophagus, stomach and liver) on residential area, gender and age. Cases and controls were at least 20 years old and living in Taixing at least 10 years. If a selected control did not fit the criteria, or refused to be interviewed, we recorded their basic demographic data and used the same selection process to choose another control. Recruitment rates were 57% for eligible cases and 89% for controls. Since controls were not individual-matched to cases, but were instead frequency-matched to the combined case group, the age and gender distribution of controls corresponds to all three cancer sites and does not exactly match the HCC cases.
2.2. Data collection
All cases and controls completed a standardized interviewer-based questionnaire to collect information on demographic factors, smoking history, alcohol drinking habits, tea drinking habits, drinking water, personal, family cancer history, etc. An 8-milliliter blood sample was collected from 194 cases (95%) and 397 controls (96%). Genomic DNA was isolated from the specimens using a modified phenol-chloroform protocol.
2.3. Laboratory assays
2.3.1. PCR-analysis of gene polymorphisms
Genotyping was performed in the Molecular Epidemiology Laboratory at Department of Epidemiology, School of Public Health at UCLA. Genotypes for GSTM1, GSTT1 and ALDH2 rs671 polymorphisms were determined using the PCR-RFLP method as previously described [20,40]. The other SNPs (ALDH2 rs886205, IL-1α rs17561, IL-10 rs1800871, IL-10 rs 1800872, IL-10 rs 1800896, IL-13 rs20541, TNF-α rs1800629, and TNF-β rs909253) were genotyped using the SNPlex assay (Applied Biosystems [ABI], Foster City, CA) with call rates of >85% and reproducibility of 0.978 (randomly regenotyping 3% of samples). Two SNPs (ALDH2 rs886205 and IL-10 rs1800871) were also genotyped using the ABI’s Taqman assay with call rates of >97% and reproducibility of 0.989 (randomly regenotyping 5% of samples). The concordance between SNPlex and Taqman is 94.3% for IL-10 rs1800871 and 98.8% for ALDH2 rs886205. Detailed description of SNPlex and Taqman methods were published elsewhere [41-43].
2.3.2. HBsAg, anti-HCV and plasma aflatoxin B1-albumin adduct detection
The presence of HBsAg in serum was measured by enzyme-linked immunosorbant assay (ELISA) using kits from the Reagent Company of the Shanghai Hospital for Infectious Diseases. Anti-HCV IgG antibody was measured by ELISA using kits from Shanghai Huamei Biological Company. Aflatoxin B1 (AFB1)-albumin adduct levels were measured from plasma as previously described [44].
2.4. Definition of green tea and alcohol drinking
Green tea ever-drinkers were defined as individuals who drank at least one cup of green tea per day for more than half a year. Tea concentration was categorized into three levels: low (tea leaves were <25% of the volume of the cup), moderate (tea leaves were between 25% and 50% of the volume of the cup) and high (volume of tea leaves was >50% of cup volume). One standard drink of alcohol was defined as any alcoholic beverage containing 14 g of pure alcohol (National Institute on Alcohol Abuse and Alcoholism, the United States).
2.5. Statistical analysis
All analyses were performed using SAS 9.2 software. We used unconditional logistic regression models to obtain odds ratios (ORs) and 95% confidence intervals (CIs). We adjusted each independent variable for potential confounders, including age (continuous), gender (male or female), education (ordinal, five categories), income (continuous), body mass index (BMI, continuous), family history of primary liver cancer (yes or no), pack-years of smoking (continuous), alcohol drinking (never/occasionally vs. often/everyday) and HBSAg status (positive vs. negative). Associations were considered statistically significant if the P-value <0.05 at two-sided test. Unconditional logistic regression was used to evaluate multiplicative interactions between green tea and other risk factors. Departures from multiplicative effects were assessed by including main effect variables and their product terms in the logistic regression model.
3. Results
The distributions of demographic characters among cases and controls are shown in Table 1. We observed a higher proportion of males and younger individuals among cases, which is consistent with characteristics of HCC in high-risk areas. Education levels and BMI were higher among controls (P < 0.05). There is no obvious difference in the income class between cases and controls (P = 0.063).
Table 1.
Distribution of demographic characters among cases and controls.
| Cases |
Controls |
p-Value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gender | |||||
| Male | 159 | 77.9 | 287 | 69.2 | 0.0221 |
| Female | 45 | 22.1 | 128 | 30.8 | |
| Age | |||||
| <40 | 31 | 15.2 | 31 | 7.5 | 0.0003 |
| 40-50 | 54 | 26.5 | 69 | 16.6 | |
| 50-60 | 54 | 26.5 | 136 | 32.8 | |
| 60-70 | 42 | 20.6 | 116 | 28.0 | |
| ≥70 | 23 | 11.3 | 63 | 15.2 | |
| Education | |||||
| Illiteracy | 44 | 21.6 | 73 | 17.6 | 0.0018 |
| Primary school | 77 | 37.8 | 142 | 34.2 | |
| Middle school | 70 | 34.3 | 124 | 29.9 | |
| High school | 13 | 6.4 | 66 | 15.9 | |
| College and above | 0 | 0.0 | 10 | 2.4 | |
| Monthly income per capita (Yuan) | |||||
| <60 | 63 | 30.9 | 88 | 21.2 | 0.0643 |
| 60-100 | 35 | 17.2 | 74 | 17.8 | |
| 100-160 | 58 | 28.4 | 135 | 32.5 | |
| ≥160 | 48 | 23.5 | 118 | 28.4 | |
| BMI | |||||
| ≤22 | 106 | 52.0 | 180 | 43.4 | 0.0440 |
| >22 | 98 | 48.0 | 235 | 56.6 | |
Compared with green tea non-drinkers, subjects who drank more than 250 g of green tea per month (about ≥2 cups had per day), a crude OR of 0.58 (95% CI: 0.34–1.00) and an adjusted OR of 0.55 (95% CI: 0.28–1.09), while subjects who drank green tea longer than 30 years had a crude OR of 0.38 (95% CI: 0.20–0.74) and an adjusted OR of 0.44 (95% CI: 0.19-0.96). No obvious association was observed between green tea concentration, green tea drinking age onset, green tea temperature and HCC. After adjusting for potential confounding factors, no obvious associations were observed between alcohol drinking, tobacco smoking and HCC risk. Chronic HBV and HCV infection markers, HBsAg and anti-HCV, were much more prevalent among cases than controls, with adjusted ORs of 5.07 (95% CI: 3.38–7.60) and 4.44 (95% CI: 1.82–10.85), respectively. Ingestion of moldy foods was moderately associated with HCC, with an adjusted OR of 2.39 (95% CI: 1.44–3.98). Using a refrigerator was protective, with an adjusted OR of 0.36 (95% CI: 0.19-0.69). Raw water drinking was associated with HCC, while tap water drinking was inversely related to HCC (Ptrend < 0.05). A family history of HCC was associated with HCC, with an adjusted OR of 3.06 (95% CI: 1.80-5.19) (Table 2).
Table 2.
Tea drinking and potential risk factors among cases and controls.
| Cases N |
Controls N |
cOR and 95% CI | aOR and 95% Cla | |
|---|---|---|---|---|
| Years of green tea drinking | ||||
| Never | 111 | 216 | Ref | Ref |
| <20 | 32 | 60 | 1.04 (0.64–1.69) | 0.69 (0.36–1.32) |
| 20–30 | 27 | 60 | 0.88 (0.53–1.46) | 1.05 (0.55–1.99) |
| ≥30 | 12 | 61 | 0.38 (0.20–0.74) | 0.44 (0.19–0.96) |
| P trend | 0.0131 | 0.1297 | ||
| Monthly consumption of green tea (g/month) | ||||
| Never | 111 | 216 | Ref | Ref |
| <125 | 23 | 42 | 1.07 (0.61–1.86) | 1.21 (0.62–2.36) |
| 125–250 | 24 | 50 | 0.93 (0.55–1.60) | 0.76 (0.38–1.51) |
| ≥250 | 21 | 70 | 0.58 (0.34–1.00) | 0.55 (0.28–1.09) |
| P trend | 0.0849 | 0.0806 | ||
| Green tea concentration | ||||
| Never | 111 | 216 | Ref | Ref |
| Low | 10 | 20 | 0.97 (0.44–2.15) | 0.84 (0.33–2.14) |
| Moderate | 42 | 125 | 0.65 (0.43–0.99) | 0.60 (0.34–1.05) |
| High | 21 | 36 | 1.14 (0.63–2.04) | 1.12 (0.54–2.31) |
| P trend | 0.3190 | 0.4685 | ||
| Green tea temperature | ||||
| Never | 111 | 216 | Ref | Ref |
| Normal temperature | 50 | 125 | 0.78 (0.52–1.16) | 0.76 (0.45–1.31) |
| High temperature | 14 | 47 | 0.58 (0.31–1.10) | 0.50 (0.23–1.07) |
| P trend | 0.0571 | 0.0688 | ||
| Age (years) of green tea drinking onset | ||||
| Never | 111 | 216 | Ref | Ref |
| >28 | 29 | 89 | 0.63 (0.39–1.02) | 0.56 (0.30–1.02) |
| ≤28 | 41 | 92 | 0.87 (0.56–1.34) | 0.89 (0.49–1.60) |
| P trend | 0.3159 | 0.5411 | ||
| Alcohol drinking | ||||
| Never/occasionally | 116 | 279 | Ref | Ref |
| Often/everyday | 76 | 133 | 1.37 (0.96–1.96) | 1.29 (0.79–2.09) |
| Drinks per day (1960–1999) | ||||
| Never | 123 | 248 | Ref | Ref |
| 0–2 | 29 | 66 | 0.89 (0.54–1.44) | 0.90 (0.49–1.65) |
| >2 | 52 | 101 | 1.04 (0.70–1.55) | 1.30 (0.75–2.25) |
| P trend | 0.9322 | 0.3719 | ||
| Pack–year of smoking | ||||
| Never | 85 | 217 | Ref | Ref |
| <20 | 53 | 85 | 1.59 (1.04–2.44) | 1.12 (0.62–2.01) |
| 20–40 | 42 | 86 | 1.25 (0.80–1.95) | 0.88 (0.48–1.64) |
| ≥40 | 12 | 26 | 1.18 (0.57–2.44) | 0.92 (0.35–2.37) |
| P trend | 0.2768 | 0.6904 | ||
| HBV infection | ||||
| HBsAg− | 72 | 312 | Ref | Ref |
| HBsAg+ | 132 | 102 | 5.61 (3.90–8.07) | 5.07 (3.38–7.60) |
| HCV infection | ||||
| Anti-HCV− | 183 | 403 | Ref | Ref |
| Anti-HCV+ | 18 | 12 | 3.03 (1.56–7.00) | 4.44 (1.82–10.85) |
| Moldy food intake | ||||
| No | 143 | 339 | Ref | Ref |
| Yes | 48 | 66 | 1.72 (1.13–2.62) | 2.39 (1.44–3.98) |
| Using refrigerator | ||||
| No | 173 | 305 | Ref | Ref |
| Yes | 18 | 86 | 0.37 (0.22–0.63) | 0.36 (0.19–0.69) |
| Raw water drinking | ||||
| Never | 95 | 248 | Ref | Ref |
| Few/ever | 27 | 44 | 1.60 (0.94–2.73) | 1.84 (0.96–3.53) |
| Sometimes | 52 | 62 | 2.19 (1.41–3.39) | 2.18 (1.30–3.67) |
| Often | 11 | 5 | 5.74 (1.94–16.97) | 3.78 (0.96–14.96) |
| P trend | <0001 | 0.0006 | ||
| Tap water drinking | ||||
| Never use tap water | 151 | 263 | Ref | Ref |
| Use tap water <3yrs | 23 | 67 | 0.60 (0.36–1.00) | 0.56 (0.30–1.02) |
| Use tap water >3yrs | 30 | 85 | 0.61 (0.39–0.98) | 0.63 (0.36–1.10) |
| P trend | 0.0154 | 0.0416 | ||
| Family history of liver cancer | ||||
| No | 150 | 375 | Ref | Ref |
| Yes | 53 | 39 | 3.40 (2.16–5.35) | 3.06 (1.80–5.19) |
Adjusted for age, gender, education, income, BMI, family history, pack-year, alcohol drinking and HBSAg.
Table 3 shows the possible interactions between environmental risk factors and green tea drinking. A more than multiplicative interaction between green tea and alcohol drinking was observed (adjusted OR for interaction 3.40, 95% CI: 1.26–9.16). The adjusted ORs for interactions between green tea drinking and smoking, and green tea drinking and HBV or HCV infection were 1.98 (95% CI: 0.78-5.03) and 1.87 (95% CI: 0.81-4.31), respectively. Green tea drinking does not modify the effect of AFB1 on HCC.
Table 3.
Interactions between green tea and other risk factors.
| Cases N |
Controls N |
cOR and 95% CI | aOR and 95% CIa | ||
|---|---|---|---|---|---|
| Green tea drinking | Alcohol drks/day | ||||
| Ever | <2 | 46 | 105 | Ref | Ref |
| Ever | ≥2 | 27 | 76 | 0.81 (0.46–1.42) | 0.85 (0.44–1.64) |
| Never | ≤2 | 86 | 191 | 1.03 (0.67–1.58) | 0.93 (0.52–1.66) |
| Never | >2 | 25 | 25 | 2.28 (1.19–4.39) | 2.68 (1.24–5.82) |
| OR for interaction | 2.74 (1.20–6.27) | 3.40 (1.26–9.16) | |||
| Green tea drinking | Smoking | ||||
| Ever | Never | 22 | 60 | Ref | Ref |
| Ever | Ever | 51 | 121 | 1.15 (0.64–2.07) | 0.76 (0.38–1.55) |
| Never | Never | 61 | 153 | 1.09 (0.61–1.93) | 0.89 (0.41–1.90) |
| Never | Ever | 50 | 63 | 2.17 (1.17–4.00) | 1.34 (0.63–2.85) |
| OR for interaction | 1.73 (0.81–3.69) | 1.98 (0.78–5.03) | |||
| Green tea drinking | HBV/HCV infection | ||||
| Ever | No | 26 | 126 | Ref | Ref |
| Ever | Yes | 45 | 55 | 3.97 (2.23–7.06) | 3.88 (2.10–7.19) |
| Never | No | 31 | 163 | 0.92 (0.52–1.63) | 1.01 (0.52–1.94) |
| Never | Yes | 79 | 52 | 7.36 (4.26–12.74) | 7.29 (3.84–13.82) |
| OR for interaction | 2.02 (0.93–4.38) | 1.87 (0.81–4.31) | |||
| Green tea drinking | AFB1 | ||||
| Ever | Low | 35 | 117 | Ref | Ref |
| Ever | High | 32 | 44 | 2.43 (1.35–4.39) | 2.17 (1.14–4.15) |
| Never | Low | 57 | 127 | 1.50 (0.92–2.45) | 1.55 (0.86–2.81) |
| Never | High | 40 | 73 | 1.83 (1.07–3.14) | 1.90 (1.00–3.61) |
| OR for interaction | 0.50 (0.23–1.09) | 0.56 (0.24–1.32) |
Adjusted for age, gender, education, income, BMI, family history, pack-year, alcohol drinking and HBSAg.
Table 4 shows the possible interactions between gene polymorphisms and green tea drinking. More than multiplicative interactions were observed between green tea drinking and polymorphisms of IL-10/-819 (rs 1800871) and IL-10/-592 (rs 1800872). Among green tea non-drinkers, an increased risk for HCC was associated with the presence of C/C genotype in IL-10/-819 and IL-10/-592, while for green tea drinkers, a decreased risk for HCC was found with the presence of C/C genotype in IL-10/-819 and IL-10/-592. When evaluating the interactions for green tea and gene polymorphisms, we assumed green tea drinkers with major alleles to be at lowest risk and usually used this category as reference. For IL-10/-819 and IL-10/-592, we used the green tea drinkers with any variant alleles as reference instead. IL-10 is an anti-inflammatory cytokine and its major alleles may be associated with HCC risk [45-47]. Therefore, we assume green tea drinkers with any variant alleles of IL-10 might be at lower risk. For IL-10/-1082 (rs 1800896), green tea drinkers with homozygous variant alleles (A/A) were used as reference, due to the very few subjects with homozygous major alleles (G/G). No obvious interactions were found between polymorphisms of GSTM1, GSTT1, IL-1α (rs17561), IL-13 (rs20541), TNF-α (rs1800629), TNF-β (rs909253) and green tea consumption on HCC risk (data are not shown).
Table 4.
Interactions between green tea and gene polymorphism.
| Cases N |
Controls N |
cOR &95%CI | aOR &95%CIa | ||
|---|---|---|---|---|---|
| Green tea drinking | ALDH2 rs886205 | ||||
| Ever | C/C | 49 | 135 | Ref | Ref |
| Ever | C/T or T/T | 17 | 35 | 1.34 (0.69–2.60) | 1.10 (0.50–2.40) |
| Never | C/C | 84 | 169 | 1.37 (0.90–2.08) | 1.39 (0.79–2.43) |
| Never | C/T or T/T | 22 | 36 | 1.68 (0.90–3.14) | 2.28 (1.03–5.03) |
| OR for interaction | 0.92 (0.38–2.24) | 1.50 (0.52–4.37) | |||
| Green tea drinking | IL-10 rs1800871 | ||||
| Ever | T/T or C/T | 62 | 140 | Ref | Ref |
| Ever | C/C | 3 | 24 | 0.28 (0.08–0.97) | 0.21 (0.05–0.83) |
| Never | T/T or C/T | 82 | 183 | 1.01 (0.68–1.50) | 1.17 (0.68–2.00) |
| Never | C/C | 19 | 15 | 2.86 (1.37–5.99) | 3.17 (1.29–7.79) |
| OR for interaction | 10.01 (2.39–41.97) | 13.14 (2.60–66.52) | |||
| Green tea drinking | IL-10 rs1800872 | ||||
| Ever | A/A or A/C | 57 | 136 | Ref | Ref |
| Ever | C/C | 2 | 21 | 0.23 (0.05–1.00) | 0.18 (0.04–0.95) |
| Never | A/A or A/C | 77 | 177 | 1.04 (0.69–1.56) | 1.18 (0.68–2.043) |
| Never | C/C | 14 | 13 | 2.57 (1.14–5.81) | 2.81 (1.08–7.28) |
| OR for interaction | 10.89 (2.02–58.77) | 13.13 (1.98–86.94) | |||
| Green tea drinking | IL-10 rs1800896 | ||||
| Ever | A/A | 53 | 125 | Ref | Ref |
| Ever | A/G or G/G | 7 | 40 | 0.41 (0.17–0.98) | 0.35 (0.13–0.92) |
| Never | A/A | 79 | 153 | 1.22 (0.80–1.86) | 1.40 (0.80–2.48) |
| Never | A/G or G/G | 19 | 37 | 1.21 (0.64–2.30) | 1.20 (0.54–2.67) |
| OR for interaction | 2.41 (0.83–6.97) | 2.46 (0.72–8.43) |
Adjusted for age, gender, education, income, BMI, family history, pack-year, alcohol drinking and HBsAg.
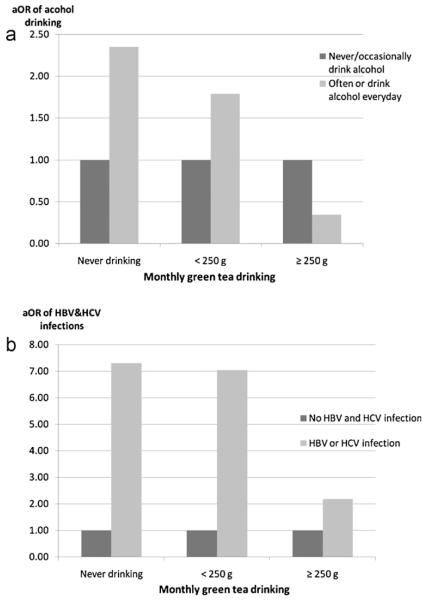
Fig. 1 shows the study participants stratified into three groups based on their green tea drinking status: non-drinkers, low-level drinkers (≥250 g/month) and high-level drinkers (<250 g/month). The stratified analyses showed the greatest protection from green tea drinking (>250 mg) on individuals who drank alcohol and with HBV or HCV infection.
Fig. 1.
Alcohol drinking, smoking and HBV&HCV infections among different green tea drinkers. (a) Alcohol drinking, green tea drinking and HCC. Study subjects were stratified into three groups based on their green tea drinking amounts: never drinking, less than 250 g/month and more than 250 g/month. In each green tea drinking categories, the adjusted ORs of alcohol drinking were presented, using subjects who never or occasionally drank alcohol as reference. (b) HBV/HCV infection, green tea drinking and HCC. Study subjects were stratified into three groups based on their green tea drinking amounts: never drinking, less than 250 g/ month and more than 250 g/month. In each green tea drinking categories, the adjusted ORs of HBV/HCV infection were presented, using subjects who have no HBV and HCV infection as reference.
4. Discussion
Of the three major types of tea, green tea (non-fermented), oolong tea (half-fermented) and black tea (fermented), green tea has the highest quantity of tea polyphenols [48]. Green tea has been used as herbal medicine and healthy beverage in China since ancient times. Green tea drinking is thought to provide protection against tumor initiation and development in multiple organs [48-50]. The present study found a protective role of green tea drinking against HCC. In the crude analysis, dose–response relationships were suggested, and longer years and increased consumption of green tea were associated with decreased odds of developing HCC. However, the associations were no longer apparent after adjusting for potential confounding factors.
The association between green tea drinking and HCC are consistent with published studies in China [51]. A prospective cohort study found an inverse association between tea drinking and HCC mortality rates in males [34]. One phase II chemoprevention trial suggested that green tea polyphenols are effective in diminishing oxidative DNA damage in individuals at high-risk of HCC [52]. Conflicting results were observed in populations other than the Chinese population. In Japan, one prospective cohort study found green tea was inversely associated with liver cancer incidence, while no association was observed in another study[37,38]. A case-control study in Italy reported no association between tea and HCC [35].
The conflicting results may arise for the following reasons. First, most previous analyses were simply based on frequency of tea consumption, with no detailed information on type of tea, consumption amount and duration of tea drinking. Other limitations include the study heterogeneity due to inclusion of a variety of liver diseases as study endpoints, and not adjusting for the effects of viral hepatitis infections in some studies. In the present study, green tea drinking habits were assessed in more detail. Our analyses were also limited to green tea drinking and HCC cases, which may preclude the possible influence from different concentrations of polyphenols in various types of tea as well as the heterogeneous causes of different liver diseases.
This study found a possible interaction between green tea drinking and infections of HBV and/or HCV (HBV/HCV). Among individuals infected with HBV/HCV, the risk of HCC in green tea non-drinkers is almost twice that of drinkers. The results implicated that green tea consumption might have a potential HCC prevention benefits among individuals with chronic hepatitis infection. Intervention trials are needed in the future to provide more convincing evidence. Persistent infections with HBV/HCV induce chronic inflammation which may also be associated with oxidative stress. Both chronic inflammation and oxidative stress may contribute to the development of HCC [53]. The anti-inflammatory and anti-oxidative properties of tea polyphenols may explain the positive interactions between green tea and HBV/ HCV infections.
Results from the present study also suggest possible interactions between green tea drinking and SNPs of inflammation related cytokines. Cytokines are generally grouped into two categories, pro-inflammatory cytokines (e.g., IL-1α, IL-1β, IL-2, TNF-α and IFN-γ) and anti-inflammatory cytokines (e.g., IL-4, IL-5, IL-8, IL-10 and IL-13) [12]. Some studies have shown that high IL-10-producing alleles (major allele of IL-10/-819 and IL-10/-592) were associated with increased HCC risk [45-47]. In the current study, IL-10 major alleles were associated with increased risk among green tea non-drinkers but decreased risk among green tea drinkers. Green tea might reverse the harmful effect of the high IL-10-producing alleles. The relatively small number of subjects in certain categories may limit our ability to detect the true association. Large-scaled studies are needed to further explore the interactions between green tea and SNPs of inflammation related cytokines.
Alcohol consumption is known to cause HCC in developed countries [4]. In the present study, no obvious association was observed between alcohol drinking and HCC without considering tea drinking status. However we observed a strong interaction between green tea and alcohol drinking, and the highest odds of HCC was found among individuals who drank alcohol without concurrent green tea drinking. Possible explanations for this interaction may be due to the anti-oxidative effects of tea polyphenols. Ethanol is eliminated from the body by its oxidation. Acetaldehyde, the first oxidation product of ethanol, is thought to be mainly responsible for the carcinogenic effect of alcohol [54]. Previous evidence showed that green tea may protect against alcohol-related oxidative modification [55-57]. Aldehyde dehydrogenase (ALDH) is the key enzyme in eliminating acetaldehyde, and the null-allele variant of ALDH2 may lead to accumulation of acetaldehyde [58]. Our study found that green tea non-drinkers with variant ALDH2 (rs886205) alleles were at higher risk than other groups. However, no significant interaction between ALDH2 and green tea drinking was found. This result is consistent with one animal experiment [59].
Several methodological issues need to be discussed. Selection bias may occur due to the relatively low participation rate (57%) of HCC cases. High fatality rate of HCC led to a great proportion of newly diagnosed cases dying before our interviewers could reach them. Thus it is possible the enrolled cases only represent less severe patients among all the qualified cases. In retrospective case-control studies, information bias, especially recall bias and corresponding differential misclassification, are important issues. Since the association between green tea and liver cancer had not been established yet, participants in this study were not aware of the possible benefits from green tea drinking. Besides that, several green tea drinking related variables were collected. Thus, the possibility of differential recall bias would be minimal. There is a possibility of reverse causality if cases of liver cancer started to drink green tea after diagnosis. However, this will lead to an underestimation of the observed protective association. Standard protocols and blinding were utilized in the assays so as to reduce other information bias and possible misclassification. Since we used common controls for all three cancer sites, potential residual confounding effects of age and gender might exist, even though these two factors were adjusted for in the multivariate analysis. The relatively small sample size also limited our ability to detect moderate interactions and compromised the precision of measurements. However, the current report has provided preliminary evidence on the association of green tea drinking with HCC and its potential modifying effect on other factors. Large-scale epidemiological studies are needed in the future to further study the complex role of green tea drinking in the development of HCC.
In conclusion, our results support the hypothesis that long years and high amount of green tea drinking may be a protective factor against primary HCC. Green tea drinking modified the effect of alcohol drinking on the development of primary HCC. Potential effect modifications of green tea drinking on associations between primary HCC and hepatitis virus infections and inflammation related SNPs, especially for IL-10, were suggested.
Acknowledgments
The authors thank Dr. Regina Santella and her laboratory for their training and assistance in the aflatoxin B1-albumin adduct detection.
Role of the funding source
This work is supported in part by the International Union against Cancer (UICC) Technology Transfer fellowship (ICRETT) awarded to Dr. Li-Na Mu, and by the Foundation for the Author of National Excellent Doctoral Dissertation of P.R. China, No. 200157, awarded to Dr. Lin Cai. The study was also partially supported by the NIH National Institute of Environmental Health Sciences, National Cancer Institute, Department of Health and Human Services, Grants CA09142, ES 011667 as well as the Alper Research Program for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center.
Abbreviations
- PLC
primary liver cancer
- HCC
hepatocellular carcinoma
- HBV
hepatitis B
- HCV
hepatitis C
- HBsAg
hepatitis B virus surface antigen
- SNPs
single nucleotide polymorphisms
- AFB1
aflatoxin B1
- OR
odds ratio
- 95% CI
95% confidence interval
- ALDH
aldehyde dehydrogenase
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- [1].Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- [2].McGlynn KA, Tsao L, Hsing AW, Devesa SS, Fraumeni JF., Jr International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290–6. doi: 10.1002/ijc.1456. [DOI] [PubMed] [Google Scholar]
- [3].Yu MC, Yuan J-M. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127:S72–78. doi: 10.1016/j.gastro.2004.09.018. [DOI] [PubMed] [Google Scholar]
- [4].IARC Consumption of Alcoholic Beverages and Ethyl Carbamate (Urethane) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2007;96 [PMC free article] [PubMed] [Google Scholar]
- [5].Qian GS, Ross RK, Yu MC, Yuan JM, Gao YT, Henderson BE, et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3:3–10. [PubMed] [Google Scholar]
- [6].Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, et al. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943–6. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- [7].Wang LY, Hatch M, Chen CJ, Levin B, You SL, Lu SN, et al. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. Int J Cancer. 1996;67:620–5. doi: 10.1002/(SICI)1097-0215(19960904)67:5<620::AID-IJC5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- [8].Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, et al. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498–502. [PubMed] [Google Scholar]
- [9].Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–24. doi: 10.1016/j.jhep.2004.10.005. see comment. [DOI] [PubMed] [Google Scholar]
- [10].Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–21. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- [11].Moszczynski P, Zabinski Z, Moszczynski P, Jr, Rutowski J, Slowinski S, Tabarowski Z. Immunological findings in cigarette smokers. Toxicol Lett. 2001;118:121–7. doi: 10.1016/s0378-4274(00)00270-8. [DOI] [PubMed] [Google Scholar]
- [12].Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80:1197–213. doi: 10.1189/jlb.0506297. [DOI] [PubMed] [Google Scholar]
- [13].Mena S, Ortega A, Estrela JM. Oxidative stress in environmental-induced carcinogenesis. Mutat Res. 2009;674:36–44. doi: 10.1016/j.mrgentox.2008.09.017. [DOI] [PubMed] [Google Scholar]
- [14].Sasaki Y. Does oxidative stress participate in the development of hepatocellular carcinoma? J Gastroenterol. 2006;41:1135–48. doi: 10.1007/s00535-006-1982-z. [DOI] [PubMed] [Google Scholar]
- [15].Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, et al. Cytokine gene polymorphism in human disease: on-line databases. Genes Immun. 2001;2(Suppl. 1):61–70. doi: 10.1038/sj.gene.6363733. see comment. [DOI] [PubMed] [Google Scholar]
- [16].Tipoe GL, Leung T-M, Hung M-W, Fung M-L. Green tea polyphenols as an antioxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007;7:135–44. doi: 10.2174/187152907780830905. [DOI] [PubMed] [Google Scholar]
- [17].Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1 beta-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. J Nutr. 2004;134:1039–44. doi: 10.1093/jn/134.5.1039. [DOI] [PubMed] [Google Scholar]
- [18].Yang F, de Villiers WJ, McClain CJ, Varilek GW. Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutr. 1998;128:2334–40. doi: 10.1093/jn/128.12.2334. [DOI] [PubMed] [Google Scholar]
- [19].Cabrera C, Gimenez R, Lopez MC. Determination of tea components with antioxidant activity. J Agric Food Chem. 2003;51:4427–35. doi: 10.1021/jf0300801. [DOI] [PubMed] [Google Scholar]
- [20].Mu L-N, Lu Q-Y, Yu S-Z, Jiang Q-W, Cao W, You N-C, et al. Green tea drinking and multigenetic index on the risk of stomach cancer in a Chinese population. Int J Cancer. 2005;116:972–83. doi: 10.1002/ijc.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Myung SK, Bae WK, Oh SM, Kim Y, Ju W, Sung J, et al. Green tea consumption and risk of stomach cancer: a meta-analysis of epidemiologic studies. Int J Cancer. 2009;124:670–7. doi: 10.1002/ijc.23880. [DOI] [PubMed] [Google Scholar]
- [22].Hoshiyama Y, Kawaguchi T, Miura Y, Mizoue T, Tokui N, Yatsuya H, et al. Green tea and stomach cancer - a short review of prospective studies. J Epidemiol. 2005;2(15 Suppl.):S109–12. doi: 10.2188/jea.15.S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF., Jr Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994;86:855–8. doi: 10.1093/jnci/86.11.855. [DOI] [PubMed] [Google Scholar]
- [24].Wang JM, Xu B, Rao JY, Shen HB, Xue HC, Jiang QW. Diet habits alcohol drinking, tobacco smoking, green tea drinking, and the risk of esophageal squamous cell carcinoma in the Chinese population. Eur J Gastroenterol Hepatol. 2007;19:171–6. doi: 10.1097/MEG.0b013e32800ff77a. [DOI] [PubMed] [Google Scholar]
- [25].Wu M, Liu A-M, Kampman E, Zhang Z-F, Van’t Veer P, Wu D-L, et al. Green tea drinking, high tea temperature and esophageal cancer in high- and low-risk areas of Jiangsu Province, China: a population-based case-control study. Int J Cancer. 2009;124:1907–13. doi: 10.1002/ijc.24142. [DOI] [PubMed] [Google Scholar]
- [26].Kumar N, Shibata D, Helm J, Coppola D, Malafa M. Green tea polyphenols in the prevention of colon cancer. Front Biosci. 2007;12:2309–15. doi: 10.2741/2233. [DOI] [PubMed] [Google Scholar]
- [27].Arab L, Il’yasova D. The epidemiology of tea consumption and colorectal cancer incidence. J Nutr. 2003;133:3310S–8. doi: 10.1093/jn/133.10.3310S. [DOI] [PubMed] [Google Scholar]
- [28].Wu AH, Yu MC, Tseng CC, Hankin J, Pike MC. Green tea and risk of breast cancer in Asian Americans. Int J Cancer. 2003;106:574–9. doi: 10.1002/ijc.11259. [DOI] [PubMed] [Google Scholar]
- [29].Zhang M, Holman CD, Huang JP, Xie X. Green tea and the prevention of breast cancer: a case-control study in Southeast China. Carcinogenesis. 2007;28:1074–8. doi: 10.1093/carcin/bgl252. [DOI] [PubMed] [Google Scholar]
- [30].Key TJ, Sharp GB, Appleby PN, Beral V, Goodman MT, Soda M, et al. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81:1248–56. doi: 10.1038/sj.bjc.6690837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Suzuki Y, Tsubono Y, Nakaya N, Koizumi Y, Tsuji I. Green tea and the risk of breast cancer: pooled analysis of two prospective studies in Japan. Br J Cancer. 2004;90:1361–3. doi: 10.1038/sj.bjc.6601652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Inoue M, Tajima K, Mizutani M, Iwata H, Iwase T, Miura S, et al. Regular consumption of green tea and the risk of breast cancer recurrence: follow-up study from the Hospital-based Epidemiologic Research Program at Aichi Cancer Center (HERPACC), Japan. Cancer Lett. 2001;167:175–82. doi: 10.1016/s0304-3835(01)00486-4. [DOI] [PubMed] [Google Scholar]
- [33].Nakachi K, Suemasu K, Suga K, Takeo T, Imai K, Higashi Y. Influence of drinking green tea on breast cancer malignancy among Japanese patients. Jpn J Cancer Res. 1998;89:254–61. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Evans AA, Chen G, Ross EA, Shen F-M, Lin W-Y, London WT. Eight-year follow-up of the 90000-person Haimen City cohort: I. Hepatocellular carcinoma mortality, risk factors, and gender differences. Cancer Epidemiol Biomarkers Prev. 2002;11:369–76. [PubMed] [Google Scholar]
- [35].Montella M, Polesel J, La Vecchia C, Dal Maso L, Crispo A, Crovatto M, et al. Coffee and tea consumption and risk of hepatocellular carcinoma in Italy. Int J Cancer. 2007;120:1555–9. doi: 10.1002/ijc.22509. [DOI] [PubMed] [Google Scholar]
- [36].Wang N, Zheng Y, Jiang Q, Yu X, Chen Y. Tea and reduced liver cancer mortality. Epidemiology. 2008;19:761. doi: 10.1097/EDE.0b013e3181811603. [DOI] [PubMed] [Google Scholar]
- [37].Ui A, Kuriyama S, Kakizaki M, Sone T, Nakaya N, Ohmori-Matsuda K, et al. Green tea consumption and the risk of liver cancer in Japan: the Ohsaki Cohort study. Cancer Causes Control. 2009;20:1939–45. doi: 10.1007/s10552-009-9388-x. [DOI] [PubMed] [Google Scholar]
- [38].Inoue M, Kurahashi N, Iwasaki M, Shimazu T, Tanaka Y, Mizokami M, et al. Effect of coffee and green tea consumption on the risk of liver cancer: cohort analysis by hepatitis virus infection status. Cancer Epidemiol Biomarkers Prev. 2009;18:1746–53. doi: 10.1158/1055-9965.EPI-08-0923. [DOI] [PubMed] [Google Scholar]
- [39].Lu H, Cai L, Mu L-N, Lu Q-Y, Zhao J, Cui Y, et al. Dietary mineral and trace element intake and squamous cell carcinoma of the esophagus in a Chinese population. Nutr Cancer. 2006;55:63–70. doi: 10.1207/s15327914nc5501_8. [DOI] [PubMed] [Google Scholar]
- [40].Harada S, Zhang S. New strategy for detection of ALDH2 mutant. Alcohol Alcohol Suppl. 1993;1A:11–3. doi: 10.1093/alcalc/28.supplement_1a.11. [DOI] [PubMed] [Google Scholar]
- [41].Hussain SK, Mu LN, Cai L, Chang SC, Park SL, Oh SS, et al. Genetic variation in immune regulation and DNA repair pathways and stomach cancer in China. Cancer Epidemiol Biomarkers Prev. 2009;18:2304–9. doi: 10.1158/1055-9965.EPI-09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Oh SS, Chang SC, Cai L, Cordon-Cardo C, Ding BG, Greenland S, et al. Single nucleotide polymorphisms of 8 inflammation-related genes and their associations with smoking-related cancers. Int J Cancer. 2010 doi: 10.1002/ijc.25214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Park SL, Chang SC, Cai L, Cordon-Cardo C, Ding BG, Greenland S, et al. Associations between variants of the 8q24 chromosome and nine smoking-related cancer sites. Cancer Epidemiol Biomarkers Prev. 2008;17:3193–202. doi: 10.1158/1055-9965.EPI-08-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen SY, Chen CJ, Tsai WY, Ahsan H, Liu TY, Lin JT, et al. Associations of plasma aflatoxin B1-albumin adduct level with plasma selenium level and genetic polymorphisms of glutathione S-transferase M1 and T1. Nutr Cancer. 2000;38:179–85. doi: 10.1207/S15327914NC382_6. [DOI] [PubMed] [Google Scholar]
- [45].Tseng LH, Lin MT, Shau WY, Lin WC, Chang FY, Chien KL, et al. Correlation of interleukin-10 gene haplotype with hepatocellular carcinoma in Taiwan. Tissue Antigens. 2006;67:127–33. doi: 10.1111/j.1399-0039.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- [46].Miyazoe S, Hamasaki K, Nakata K, Kajiya Y, Kitajima K, Nakao K, et al. Influence of interleukin-10 gene promoter polymorphisms on disease progression in patients chronically infected with hepatitis B virus. Am J Gastroenterol. 2002;97:2086–92. doi: 10.1111/j.1572-0241.2002.05926.x. [DOI] [PubMed] [Google Scholar]
- [47].Shin HD, Park BL, Kim LH, Jung JH, Kim JY, Yoon JH, et al. Interleukin 10 haplotype associated with increased risk of hepatocellular carcinoma. Hum Mol Genet. 2003;12:901–6. doi: 10.1093/hmg/ddg104. [DOI] [PubMed] [Google Scholar]
- [48].Koo MWL, Cho CH. Pharmacological effects of green tea on the gastrointestinal system. Eur J Pharmacol. 2004;500:177–85. doi: 10.1016/j.ejphar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- [49].Ju J, Lu G, Lambert JD, Yang CS. Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol. 2007;17:395–402. doi: 10.1016/j.semcancer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pastore RL, Fratellone P. Potential health benefits of green tea (Camellia sinensis): a narrative review. Explore (NY) 2006;2:531–9. doi: 10.1016/j.explore.2006.08.008. [DOI] [PubMed] [Google Scholar]
- [51].Jin X, Zheng RH, Li YM. Green tea consumption and liver disease: a systematic review. Liver Int. 2008;28:990–6. doi: 10.1111/j.1478-3231.2008.01776.x. [DOI] [PubMed] [Google Scholar]
- [52].Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, et al. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–8. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
- [53].Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–83. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- [54].Poschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–65. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- [55].Dobrzynska I, Sniecinska A, Skrzydlewska E, Figaszewski Z. Green tea modulation of the biochemical and electric properties of rat liver cells that were affected by ethanol and aging. Cell Mol Biol Lett. 2004;9:709–21. [PubMed] [Google Scholar]
- [56].Skrzydlewska E, Ostrowska J, Stankiewicz A, Farbiszewski R. Green tea as a potent antioxidant in alcohol intoxication. Addict Biol. 2002;7:307–14. doi: 10.1080/13556210220139523. [DOI] [PubMed] [Google Scholar]
- [57].Augustyniak A, Waszkiewicz E, Skrzydlewska E. Preventive action of green tea from changes in the liver antioxidant abilities of different aged rats intoxicated with ethanol. Nutrition. 2005;21:925–32. doi: 10.1016/j.nut.2005.01.006. [DOI] [PubMed] [Google Scholar]
- [58].Jelski W, Szmitkowski M. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the cancer diseases. Clin Chim Acta. 2008;395:1–5. doi: 10.1016/j.cca.2008.05.001. [DOI] [PubMed] [Google Scholar]
- [59].Chrostek L, Tomaszewski W, Szmitkowski M. The effect of green tea on the activity of aldehyde dehydrogenase (ALDH) in the liver of rats during chronic ethanol consumption. Rocz Akad Med Bialymst. 2005;50:220–3. [PubMed] [Google Scholar]