Abstract
In most eutherian mammals, sexually dimorphic masculinization is established by androgen-producing fetal Leydig cells in the embryonic testis. Fetal Leydig cells, which lack expression of the testis-determining gene SRY, arise after the appearance of SRY-expressing Sertoli cells. Therefore, the appearance and differentiation of fetal Leydig cells are probably regulated by factors derived from Sertoli cells. Results from mouse genetic models have revealed that maintenance and differentiation of fetal Leydig cell population depends upon a balance between differentiation-promoting and differentiation-suppressing mechanisms. Although paracrine signaling via Sertoli cell–derived Hedgehog ligands is necessary and sufficient for fetal Leydig cell formation, cell-cell interaction via Notch signaling and intracellular transcription factors such as POD1 are implicated as suppressors of fetal Leydig cell differentiation. This review provides a model that summarizes the recent findings in fetal Leydig cell development.
Keywords: Reproductive genetics, testis, Hedgehog, Notch, steroidogenic factor 1
The SRY gene (sex-determining region of the Y chromosome) determines the sex of the gonad in most mammalian species; however, masculinization of the embryo is controlled by hormones and can be accomplished independent of genetic components (Jost, 1947, 1953). Products of fetal Leydig cells, a testis-specific cell type, are responsible for masculinization of the embryo. Through the action of Leydig cell–produced androgens and insulin-like factor 3 (INSL3), the male reproductive tract (or Wolffian duct) is maintained, male secondary sexual characteristics are established, and testicular descent is induced (Haider, 2004; Barsoum and Yao, 2006; Park et al, 2007). Without functional fetal Leydig cells, the male embryos develop the appearance of a female despite the presence of testes. To understand where fetal Leydig cells arise and how they differentiate, researchers have developed various transgenic and knockout mouse models that shed lights on these processes. Before the formation of fetal mouse testis (embryonic day [E]11.5–E12.5), gonadal primordium is composed of a mixture of immigrating primordial germ cells and undefined somatic progenitor cells (Swain and Lovell-Badge, 1999; McLaren, 2000; Brennan and Capel, 2004). These somatic progenitor cells in the gonads express various transcription factors, including steroidogenic factor 1 (Sf1, also known as nuclear receptor 5A1), Wilms tumor 1 (Wt1), GATA transcription factor 4 (Gata4), and Lim homeobox gene 9 (Lhx9) (Luo et al, 1994; Hatano et al, 1996; Birk et al, 2000; Mazaud et al, 2002). These progenitor cells (referred to as SF1-positive cells hereafter) are the sources of at least 2 somatic cell lineages in the testis: the supporting-cell lineage (Sertoli cells) that nourishes the germ cells and the steroidogenic lineage fetal Leydig cells.
At E10.5, a subpopulation of the SF1-positive somatic cells starts to express Sry and differentiate into Sertoli cells (Gubbay et al, 1990; Koopman et al, 1990; Lovell-Badge and Robertson, 1990; Hacker et al, 1995; Albrecht and Eicher, 2001). Via interaction with SF1, SRY triggers expression of SRY-box-containing gene 9 (Sox9), which itself is sufficient to induce testis formation (Sekido et al, 2004; Bullejos and Koopman, 2005; Kanai et al, 2005; Sekido and Lovell-Badge, 2008). The SOX9-positive Sertoli cell population then expands via the action of fibroblast factor 9 signaling and prostaglandin D2 (Schmahl et al, 2000; Wilhelm et al, 2005, 2007). By inducing endothelial cell migration and peritubular myoid cell differentiation, Sertoli cells orchestrate the formation of the testis cords (Wilhelm et al, 2007; Cool et al, 2008; Combes et al, 2009), the physical structures that separate germ cells and Sertoli cells from the testis interstitium.
Steroidogenically active fetal Leydig cells appear in the testis interstitium about 24 hours after Sertoli cell differentiation (Habert et al, 2001; Yao et al, 2002; Barsoum and Yao, 2006). Numbers of fetal Leydig cells increase dramatically from E12.5 to E15.5; however, fetal Leydig cells are mitotically inactive during this period (Orth, 1982; Byskov, 1986; Migrenne et al, 2001). Expansion of fetal Leydig cell population could result from transformation of SF1-positive progenitor cells and/or from addition of cells from sources such as the neighboring mesonephros (Merchant-Larios and Moreno-Mendoza, 1998), neural crest (Mayerhofer et al, 1996), and coelomic epithelium (Karl and Capel, 1998; Schmahl et al, 2000). Regardless of their origins, fetal Leydig cells lack expression of Sry or Sox9, indicating that their differentiation is dependent upon cues from the Sry/Sox9-expressing Sertoli cells. Desert hedgehog (Dhh) and platelet-derived growth factor A (Pdgfa), 2 Sertoli cell–derived signaling molecules, have been implicated in differentiation of fetal Leydig cells (Clark et al, 2000; Pierucci-Alves et al, 2001; Yao and Capel, 2002; Yao et al, 2002; Walterhouse et al, 2003; Brennan and Capel, 2004; Ross and Capel, 2005; Barsoum and Yao, 2006). In mice, Dhh mRNA is expressed in differentiating Sertoli cells at E11.5 and its receptor Ptch1 is localized to the testis interstitium (Yao et al, 2002). Testes of Dhh knockout embryos develop fewer fetal Leydig cells and abnormal testis cord organization. In the prepubertal and adult stages, Dhh knockout mice have spermatogenesis defects and lack adult Leydig cells (Bitgood et al, 1996; Clark et al, 2000; Pierucci-Alves et al, 2001; Yao et al, 2002; Yao and Capel, 2002). Pdgfa, on the other hand, is also expressed in Sertoli cells, whereas one of its receptors, PDGF receptor α (Pdgfrα), is present in the testis interstitium (Brennan et al, 2003). In Pdgfrα knockout male embryos, Sertoli cell proliferation, mesonephric cell migration, and fetal Leydig cell differentiation are all reduced (Brennan et al, 2003). Consequently, the Leydig cell defects in Pdgfrα knockout testes are likely secondary to defects in Sertoli cell differentiation and progenitor cell migration/proliferation. Sphingosine phosphate lyase 1 (Spgl1) and pleckstrin homology domain–containing family A1 (Plekha1), 2 putative downstream targets of PDGF signaling, are recently identified as important for male fertility (Schmahl et al, 2008). However, in both Sgpl1−/− and Plekha1−/− mice, the testicular morphology and subsequent male development appear normal before postnatal day 20 (Schmahl et al, 2008). This indicates that these 2 genes are unlikely to be involved in fetal Leydig cell development.
We recently developed a transgenic model in which the Hedgehog (Hh) pathway was ectopically activated in the SF1-positive progenitor cells of the fetal ovary (Barsoum et al, 2009). Under normal circumstances, the Hh pathway is inert in the fetal ovary because of lack of Hh ligands (Bitgood et al, 1996; Clark et al, 2000; Pierucci-Alves et al, 2001; Yao et al, 2002; Yao and Capel, 2002). We used the Cre/loxP system to activate the Hh pathway in the SF1-positive somatic cells of the fetal ovary by targeting Smoothened (SMO), a transmembrane protein responsible for transducing the intracellular signaling pathway induced by Hh ligands. When the SF1-cre transgenic line was crossed to the Smo/YFP (SmoYFP) line, Cre recombinase under the control of the Sf1 promoter removed the STOP sequence upstream of the SmoYFP transgene. Removal of the STOP sequence allowed the transcription of a constitutively active form of mutated Drosophila Smoothened (smo) fused with a yellow fluorescent protein gene (YFP) (Jeong et al, 2004). The SmoYFP transgene thus activated the Hh pathway regardless of the presence or absence of the Hh ligands. Ectopic activation of the Hh pathway in fetal ovaries transformed SF1-positive somatic cells into functional fetal Leydig cells. These ectopic fetal Leydig cells produced androgens and INSL3 that caused virilization of female embryos and descent of the ovaries. Sertoli cells and other testicular components were not found in the affected ovaries, indicating that the appearance of fetal Leydig cells was a direct consequence of Hh activation. Along with the findings in Dhh knockout models, these results demonstrate that the Hh pathway is necessary and sufficient for the induction of fetal Leydig cell differentiation (Barsoum et al, 2009). In addition, the ability of SF1-positive progenitor cells in the fetal ovary to differentiate into fetal Leydig cells supports that the SF1-positive somatic cells are bona fide progenitor cells for fetal Leydig cells.
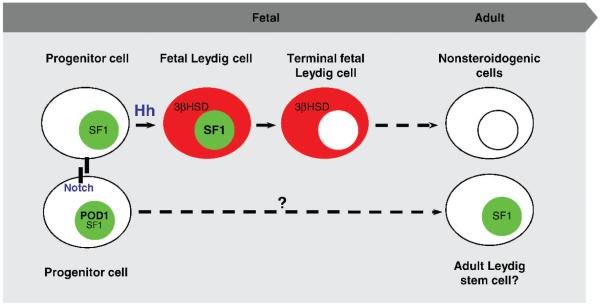
In contrast to the Hh pathway, which promotes fetal Leydig cell differentiation, the Notch pathway suppresses fetal Leydig cell differentiation. When the Notch pathway was inhibited in the fetal testis either by treatment with a chemical inhibitor or by inactivation of Hes1 (hairy and enhancer of split 1), a key intracellular component downstream of Notch receptor, numbers of fetal Leydig cells were significantly increased (Tang et al, 2008). Conversely, constitutive activation of the Notch pathway in the SF1-positive progenitor cells inhibited Leydig cell differentiation. The Notch pathway controls cell fate determination via interaction between membrane-bound ligands and Notch receptor in adjacent cells. We therefore speculate that a subpopulation of SF1-positive progenitor cells in the testis interstitium is set aside as the pool of undifferentiated stem cells. These putative stem cells are prevented from entering the differentiation mode via Notch receptor and its downstream signaling (see model in Figure).
Figure.
Model for maintenance and differentiation of fetal Leydig cells in mice: SF1+/3βHSD− progenitor cells are transformed into fetal Leydig cells (SF+/3βHSD+) in response to Sertoli cell–derived Hedgehog ligands (Hh). The fetal Leydig cells eventually lose SF1 expression in fetal life and then steroidogenic ability in adulthood. A subpopulation of the progenitor cells is prevented from entering differentiation model via the Notch pathway. The progenitor cell status is putatively maintained as a result of POD1, which down-regulates SF1 expression. Color figure available online at www.andrologyjournal.org.
Increasing numbers of fetal Leydig cells were also reported in embryos lacking Pod1 (Tcf21/capsulin/ epicardin), a basic helix-loop-helix transcription factor (Cui et al, 2004). Similar to our Hh activation model, Pod1 knockout ovary had ectopic appearance of steroidogenic cells (presumably fetal Leydig cells). Interestingly, the Hh pathway was not activated in the Pod1 knockout ovaries. However in the absence of Pod1, Sf1 expression was elevated, implying that POD1 may suppress fetal Leydig cell differentiation by counteracting Sf1 transcription in the progenitor cells (Figure). SF1, a transcription factor that controls expression of steroidogenic enzymes, is critical for establishment of both Sertoli and Leydig cell lineages (Luo et al, 1994). Sf1 is present in the gonadal primordium of both sexes but later becomes testis-specific after the onset of sex determination (Luo et al, 1994). In the fetal testis, Sf1 expression was down-regulated in Sertoli cells, whereas it was up-regulated in fetal Leydig cells (Ikeda et al, 1994; Parker and Schimmer, 1997; Parker et al, 2002; Yao et al, 2002). This increased Sf1 expression in fetal Leydig cells was partially linked to DHH derived from Sertoli cells (Yao et al, 2002; Park et al, 2007). In addition, when the Hh pathway was ectopically activated in the ovary, Sf1 expression was up-regulated, followed by appearance of fetal Leydig cells (Barsoum et al, 2009). It has been show that without Sf1, Leydig cell differentiation does not occur (Morohashi et al, 1992; Leers-Sucheta et al, 1997; Reinhart et al, 1999; Koskimies et al, 2002; Val et al, 2003). In gonad-specific Sf1 knockout mice, expression of 2 fetal Leydig cell markers, cytochrome P450 side chain cleavage (Scc) and the steroidogenic acute regulatory protein (StAR), were decreased (Jeyasuria et al, 2004). Also, in Sf1 heterozygous embryos, fetal Leydig cell markers such as cytochrome P450, subfamily XVII (Cyp17) and Scc were reduced at E13.5 (Park et al, 2005). Decreases in steroidogenic enzyme expression in the fetal testis were also reported in mouse embryos lacking the transcriptional factor X-linked aristaless-related homeobox gene (Arx; Kitamura et al, 2002). Arx is strongly expressed in peritubular myoid cells, endothelium, and fibroblasts, but not in Leydig cells. This suggests that Arx could play a role in either establishment of fetal Leydig cell population or regulation of steroidogenesis.
The SF1-positive cells represent an undifferentiated progenitor cell pool for both adrenals and gonads that originate from a common adrenogonadal primordium (Ikeda et al, 1994; Hatano et al, 1996). When adrenals and gonads separate, a fetal adrenal enhancer controls Sf1 expression in SF1-positive cells of adrenal gland (Zubair et al, 2006). At E11.5, the gonadal pool of SF1-positive cells acquires their own enhancer activity within Sf1 gene (from −589 to +85) that contains WT1 and LHX9 binding sites essential for Sf1 gene transcription (Wilhelm and Englert, 2002). Under the effect of SRY/ SOX9 and other transcriptional factors (Barsoum and Yao, 2006), some gonadal SF1-positive cells become Sertoli cells. The remaining SF1-positive cells in the interstitium begin their path to steroidogenesis in response to activation of the Hh pathway (Figure). Using double immunohistochemistry for SF1 and steroidogenic enzyme 3βHSD, we characterized the transition of SF1-positive progenitor cells into steroidogenic fetal Leydig cells. Before E13.0, fetal testes contained only SF1-positive/ 3βHSD-negative (SF1+/3βHSD−) progenitor cells in the interstitium. Twelve hours later (E13.5), SF1+/3βHSD+ (fetal Leydig cells) started to appear, intermingling with the SF1+/3βHSD− progenitor cells. By E16.5, some of the SF1+/3βHSD+ cells turned off SF1 expression and became only 3βHSD+ (SF1−/3βHSD+; terminally differentiated fetal Leydig cells). The proportion of these 3 populations (SF1+/3βHSD− progenitor, SF1+/3βHSD+ fetal Leydig cells, and SF1−/3βHSD+ terminal differentiated cells) was changed dramatically in response to ectopic activation of the Hh pathway. Ectopic activation of the Hh pathway in the SF1-positive cells resulted in a decrease in the progenitor population and an increase in fetal Leydig cell population compared to the control (unpublished data). This observation further confirms that the SF1-positive progenitor cells are transformed into fetal Leydig cells in response to Hh stimulation.
In this review, we present the hypothesis that establishment of fetal Leydig cell population is balanced by differentiation-promoting factors such as Hh and differentiation-inhibiting factors such as Notch and POD1. It remains to be determined how Hh and Notch pathways interact to maintain a progenitor cell population. If the progenitor cells are indeed present in fetal life, what will they become in the adult testis? We are currently using inducible transgenic approaches and lineage-tracing models to address these questions.
Acknowledgments
We thank Drs Buck Hales and Ken-Ichirou Morohashi for supplying antibodies. We also appreciate the technical help of Lou Ann Miller and Denise Archambeault for proofreading the manuscript at University of Illinois at Urbana-Champaign.
Supported by US National Institutes of Health grant NIH-HD046861 and HD059961 (H.H.Y.), the March of Dimes Birth Defects Foundation (H.H.Y.), and UIUC College of Veterinary Medicine’s Billie Field Graduate Fellowship (I.B.B.).
References
- Albrecht KH, Eicher EM. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240(1):92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Barsoum I, Yao HH. The road to maleness: from testis to Wolffian duct. Trends Endocrinol Metab. 2006;17(6):223–228. doi: 10.1016/j.tem.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HH. Activation of the hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudoher-maphroditism. Dev Biol. 2009;329:96–103. doi: 10.1016/j.ydbio.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg JA, Parker KL, Porter FD, Westphal H. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature. 2000;403(6772):909–913. doi: 10.1038/35002622. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6(3):298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5(7):509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17(6):800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Delayed Sry and Sox9 expression in developing mouse gonads underlies B6-Y(DOM) sex reversal. Dev Biol. 2005;278(2):473–481. doi: 10.1016/j.ydbio.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol Rev. 1986;66(1):71–117. doi: 10.1152/physrev.1986.66.1.71. [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63(6):1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- Combes AN, Wilhelm D, Davidson T, Dejana E, Harley V, Sinclair A, Koopman P. Endothelial cell migration directs testis cord formation. Dev Biol. 2009;326(1):112–120. doi: 10.1016/j.ydbio.2008.10.040. [DOI] [PubMed] [Google Scholar]
- Cool J, Carmona FD, Szucsik JC, Capel B. Peritubular myoid cells are not the migrating population required for testis cord formation in the XY gonad. Sex Dev. 2008;2(3):128–133. doi: 10.1159/000143430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Ross A, Stallings N, Parker KL, Capel B, Quaggin SE. Disrupted gonadogenesis and male-to-female sex reversal in Pod1 knockout mice. Development. 2004;131(16):4095–4105. doi: 10.1242/dev.01266. [DOI] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346(6281):245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol. 2001;179(1–2):47–74. doi: 10.1016/s0303-7207(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121(6):1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol. 2004;233:181–241. doi: 10.1016/S0074-7696(04)33005-6. [DOI] [PubMed] [Google Scholar]
- Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1(7):663–671. doi: 10.1046/j.1365-2443.1996.00254.x. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994;8(5):654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18(8):937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, de Rooij DG, Themmen APN, Behringer RR, Parker KL. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004;18(7):1610–1619. doi: 10.1210/me.2003-0404. [DOI] [PubMed] [Google Scholar]
- Jost A. Recherches sur la différentiation sexuelle de lembryonde de lapin. Arch Anat Microsc Morph Exp. 1947;36:271–315. [Google Scholar]
- Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog Horm Res. 1953;8:379–418. [Google Scholar]
- Kanai Y, Hiramatsu R, Matoba S, Kidokoro T. From SRY to SOX9: mammalian testis differentiation. J Biochem. 2005;138(1):13–19. doi: 10.1093/jb/mvi098. [DOI] [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203(2):323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, Matsuo M, Kamijo S, Kasahara M, Yoshioka H, Ogata T, Fukuda T, Kondo I, Kato M, Dobyns WB, Yokoyama M, Morohashi K. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–369. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348(6300):450–452. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- Koskimies P, Levallet J, Sipila P, Huhtaniemi I, Poutanen M. Murine relaxin-like factor promoter: functional characterization and regulation by transcription factors steroidogenic factor 1 and DAX-1. Endocrinology. 2002;143(3):909–919. doi: 10.1210/endo.143.3.8683. [DOI] [PubMed] [Google Scholar]
- Leers-Sucheta S, Morohashi K, Mason JI, Melner MH. Synergistic activation of the human type ii 3beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase promoter by the transcription factor steroidogenic factor-1/adrenal 4-binding protein and phorbol ester. J Biol Chem. 1997;272(12):7960–7967. doi: 10.1074/jbc.272.12.7960. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development. 1990;109(3):635–646. doi: 10.1242/dev.109.3.635. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77(4):481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Mayerhofer A, Lahr G, Seidl K, Eusterschulte B, Christoph A, Gratzl M. The neural cell adhesion molecule (NCAM) provides clues to the development of testicular Leydig cells. J Androl. 1996;17(3):223–230. [PubMed] [Google Scholar]
- Mazaud S, Oreal E, Guigon CJ, Carre-Eusebe D, Magre S. Lhx9 expression during gonadal morphogenesis as related to the state of cell differentiation. Gene Expression Patterns. 2002;2(3–4):373–377. doi: 10.1016/s1567-133x(02)00050-9. [DOI] [PubMed] [Google Scholar]
- McLaren A. Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol. 2000;163(1–2):3–9. doi: 10.1016/s0303-7207(99)00234-8. [DOI] [PubMed] [Google Scholar]
- Merchant-Larios H, Moreno-Mendoza N. Mesonephric stromal cells differentiate into Leydig cells in the mouse fetal testis. Exp Cell Res. 1998;244(1):230–238. doi: 10.1006/excr.1998.4215. [DOI] [PubMed] [Google Scholar]
- Migrenne S, Pairault C, Racine C, Livera G, Géloso A, Habert R. Luteinizing hormone-dependent activity and luteinizing hormone-independent differentiation of rat fetal Leydig cells. Mol Cell Endocrinol. 2001;172(1–2):193. doi: 10.1016/s0303-7207(00)00339-7. [DOI] [PubMed] [Google Scholar]
- Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992;267(25):17913–17919. [PubMed] [Google Scholar]
- Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203(4):485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- Park SY, Meeks JJ, Raverot G, Pfaff LE, Weiss J, Hammer GD, Jameson JL. Nuclear receptors Sf1 and Dax1 function cooperatively to mediate somatic cell differentiation during testis development. Development. 2005;132(10):2415–2423. doi: 10.1242/dev.01826. [DOI] [PubMed] [Google Scholar]
- Park SY, Tong M, Jameson JL. Distinct roles for steroidogenic factor 1 and Desert hedgehog pathways in fetal and Adult Leydig cell development. Endocrinology. 2007;148(8):3704–3710. doi: 10.1210/en.2006-1731. [DOI] [PubMed] [Google Scholar]
- Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res. 2002;57(1):19–36. doi: 10.1210/rp.57.1.19. [DOI] [PubMed] [Google Scholar]
- Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18(3):361–377. doi: 10.1210/edrv.18.3.0301. [DOI] [PubMed] [Google Scholar]
- Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod. 2001;65(5):1392–1402. doi: 10.1095/biolreprod65.5.1392. [DOI] [PubMed] [Google Scholar]
- Reinhart AJ, Williams SC, Clark BJ, Stocco DM. SF-1 (steroidogenic factor-1) and C/EBP{beta} (CCAAT/enhancer binding protein-{beta}) cooperate to regulate the murine StAR (steroidogenic acute regulatory) promoter. Mol Endocrinol. 1999;13(5):729–741. doi: 10.1210/mend.13.5.0279. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Capel B. Signaling at the crossroads of gonad development. Trends Endocrinol Metab. 2005;16(1):19–25. doi: 10.1016/j.tem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127(1):65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Rizzolo K, Soriano P. The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev. 2008;22(23):3255–3267. doi: 10.1101/gad.1723908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol. 2004;274(2):271–279. doi: 10.1016/j.ydbio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453(7197):930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Swain A, Lovell-Badge R. Mammalian sex determination: a molecular drama. Genes Dev. 1999;13(7):755–767. doi: 10.1101/gad.13.7.755. [DOI] [PubMed] [Google Scholar]
- Tang H, Brennan J, Karl J, Hamada Y, Raetzman L, Capel B. Notch signaling maintains Leydig progenitor cells in the mouse testis. Development. 2008;135(22):3745–3753. doi: 10.1242/dev.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Val P, Lefrancois-Martin AM, Veyssiere G, Martinez A. SF-1 a key player in the development and differentiation of steroidogenic tissues. Nucl Recept. 2003;1(1):8. doi: 10.1186/1478-1336-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterhouse DO, Lamm MLG, Villavicencio E, Iannaccone PM. Emerging roles for hedgehog-patched-Gli signal transduction in reproduction. Biol Reprod. 2003;69(1):8–14. doi: 10.1095/biolreprod.103.015941. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002;16(14):1839–1851. doi: 10.1101/gad.220102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Hiramatsu R, Mizusaki H, Widjaja L, Combes AN, Kanai Y, Koopman P. SOX9 regulates prostaglandin D synthase gene transcription in vivo to ensure testis development. J Biol Chem. 2007;282(14):10553–10560. doi: 10.1074/jbc.M609578200. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol. 2005;287(1):111–124. doi: 10.1016/j.ydbio.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Yao HH-C, Whoriskey W, Capel B. Desert hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16(11):1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HH, Capel B. Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev Biol. 2002;246(2):356–365. doi: 10.1006/dbio.2002.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26(11):4111–4121. doi: 10.1128/MCB.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]