Abstract
Background
Cancer causes significant symptom burden and diminished quality of life. Despite the expansion of supportive and palliative care services (SPCS), little is known about rates of utilization and barriers to access to these services among oncology outpatients.
Methods
We performed a cross-sectional survey in three outpatient medical oncology clinics. Patients with a diagnosis of breast, lung, or gastrointestinal (GI) cancer and a Karnofsky score of ≥60 were included. Patients reported their use of SPCS and any perceived barriers. Multivariable logistic regression was used to identify factors associated with SPCS use.
Results
Among 313 participants, (50.5%) had not used SPCS since cancer diagnosis. The most common services used were nutrition (26.5%), psychiatric/psychological counseling (29.7%), and physical therapy (15.1%). Pain/palliative care and cancer rehabilitation consultations were used by 8.5% and 4.1% of participants, respectively. In multivariate analysis, graduate education was associated with greater SPCS use (adjusted odds ratio [AOR] 2.14, 95% confidence interval [CI] 1.08-4.26) compared with those with high school or less, whereas having lung cancer was associated with less SPCS use (AOR 0.48, 95% CI 0.24-0.96) when compared with those having breast cancer. The biggest reported barriers to using SPCS were a lack of awareness (22.4%) and lack of physician referral (23%).
Conclusions
Approximately half of these patients had not accessed SPCS since cancer diagnosis and cite lack of awareness and physician nonreferral as barriers. Further research is needed to understand patients' needs and beliefs regarding SPCS, and how to integrate SPCS into conventional treatments to improve cancer care.
Introduction
Cancer is the one of the leading causes of death in individuals living in the United States, with an estimated 569,490 deaths from cancer occurring in 2010.1 Cancer, especially in its advanced form and despite treatment, is often accompanied by significant symptom burden, psychosocial distress, and poor quality of life.2–7 As treatments advance and cancer is increasingly considered a chronic illness, the number of cancer survivors has exceeded 11 million and continues to grow.8 Emerging data suggest that survivors continue to experience considerable symptom distress that may impact quality of life.9,10
In responding to needs experienced by patients, several national and international organizations have supported expansion and integration of supportive and palliative care services (SPCS) into standard cancer care.11–14 The National Comprehensive Cancer Network guidelines for palliative care define supportive and palliative care as that given to improve the quality of life of patients who have a serious or life-threatening disease, with the goal of early prevention or treatment of the symptoms and side effects caused by a disease and its treatment.12 Despite the increasing emphasis on SPCS, there are limited data on the extent to which these services are available in cancer centers in the United States. Most recently, a 2009 survey of cancer centers showed that although most reported having palliative care programs (98% versus 78% for National Cancer Institute-designated and non-NCI cancer centers, respectively), the breadth of services and level of integration varied widely among centers.15
There is a growing body of literature on unmet needs in cancer patients and survivors,16–24 which indicates a gap between the need for SPCS and the availability and usage of these services. To date, little is known about the determinants of use and barriers to access for supportive and palliative cancer care in the outpatient oncology setting. The goals of this study were to: 1) measure the rate of utilization of SPCS at an urban, academic medical cancer center; 2) identify factors related to use of SPCS; and 3) identify and describe patient-reported barriers to access to SPCS.
Methods
Study design and patients
We conducted a cross-sectional survey study in three outpatient oncology clinics at one academic cancer center that mainly treat breast, lung, and gastrointestinal cancers. Eligible participants were patients aged 18 or older who had a primary diagnosis of cancer, a Karnofsky score of 60 or greater, and were seen between June and August of 2010. Additional inclusion criteria stipulated the approval of the patient's oncologist and the patient's ability to understand and provide informed consent in English. Patients were excluded if they were new patients (defined as patients who were being seen for the first time in the outpatient clinic), or if they were unable to understand the requirements of the study. Trained research assistants approached potential study subjects in the waiting area of the oncology clinics. After a written informed consent process, each participant was given a self-report survey. The study protocol was approved by the institutional review board of the Hospital of the University of Pennsylvania.
Outcome measurement
Use of any SPCS was our primary outcome variable. We asked participants, “Since your cancer diagnosis, have you received the following supportive care services to deal with your physical and psychological symptoms?” The services included were: psychiatric consultation (seeing a medical doctor who specializes in mental health), individual psychosocial counseling, cancer support group, nutritional counseling, cancer rehabilitation (seeing a medical doctor who specializes in rehabilitation), physical therapy, and palliative care consultation (seeing a medical doctor who specializes in pain and symptom management), all currently available to patients seen in our comprehensive cancer center. These are also services that appeared to be largely available in other major U.S. cancer centers.15 Participants who responded as having used any one (or more) of the services versus those who responded that they had never used any of these services were dichotomized into two groups: SPCS users and SPCS nonusers, respectively.
We queried SPCS users about satisfaction with the services they used by asking, “How satisfied are you with these supportive care services in helping you deal with the impact of your cancer or treatment?” Satisfaction was measured on a 5-point Likert scale with answers ranging from “not at all satisfied” to “very much satisfied.”
We also queried participants on perceived barriers to access to SPCS by asking, “What are some of the difficulties you experience in using one of more of the above services?” The barriers included in the survey were: expense, time limitations, difficulty in obtaining transportation, lack of knowledge of SPCS, and lack of referral by physicians. These patient-reported barriers were identified from the literature25–27 as well as from cognitive interviews with 16 patients.
Participants reported sociodemographic variables included gender, age, race/ethnicity, education level, employment status, and marital status. We used medical records to abstract cancer type (breast, lung, gastrointestinal [GI], other) and cancer stage (localized versus metastatic), prior cancer treatments (i.e., surgery, chemotherapy, and radiation therapy), and treatment status (in-treatment, or post-treatment).
Statistical analysis
We performed statistical analyses using STATA software (Windows version 11.0, StataCorpLP, College Station, TX). Descriptive statistics were used to examine the distribution of the outcomes and covariates. Next, we used χ2 tests to identify factors that are associated with SPCS use. Multivariate logistic regression analyses were then conducted to identify independent predictors of SPCS use, using only variables that were significant at the p=0.10 level in the χ2 analyses. Because all breast cancer patients were females, we separately fitted models that include gender or cancer type. All analyses were two-sided at significance level 0.05. We also performed exploratory analyses to determine if specific barriers differ by key sociodemographic groups (age, gender, race/ethnicity, and educational attainment).
Results
Of the 382 consecutive patients screened for eligibility based on the initial criteria, 339 (88.7%) agreed to participate. Of the 43 (11.0%) who refused, 6 (1.6%) cited lack of time to complete the survey, and 37 (9.7%) did not want to participate in research. Additionally, 9 subjects withdrew consent and 17 subjects did not provide complete data that could be used for the current analyses, resulting in the final sample of 313. This population reflected a response rate of 81.9% among eligible subjects.
Among the 313 participants, the mean age was 58.4, standard deviation (SD) of 12.1 and a range of 20-89 years; 240 (76.7%) were Caucasian; 56 (17.9%) were African American; 7 (2.2%) were Asian; 6 (1.9%) were Hispanic; 2 (0.64%) were Native American, and 2 (0.64%) were identified as other. Because the majority of the nonwhite study participants identified themselves as black, we dichotomized the race/ethnicity variable into whites and nonwhites. Whereas 87 (27.8%) reported an education status of high school or less, 146 (46.7%) reported having a college degree, and 78 (24.9%) had some graduate or professional education. Overall, 103 (32.9%) of the participants were diagnosed with lung cancer, 88 (28.1%) with breast cancer, 79 (25.2%) with GI cancer, and 47 (15.0%) with another type of cancer. Table 1 displays the demographic and clinical characteristics for the study participants by SPCS use.
Table 1.
Characteristics of Patient Population by SPCS Use (n=313)
| Characteristic | Total | SPCS non-usersa n (%) | SPCS users n (%) | p-valueb |
|---|---|---|---|---|
| Age, years | 313 | 158 | 155 | |
| ≤55 | 108 | 51 (47.2%) | 57 (52.8%) | |
| 56–65 | 108 | 53 (49.1%) | 55 (50.9%) | 0.452 |
| >65 | 97 | 54 (55.7%) | 43 (44.3%) | |
| Gender | 313 | |||
| Male | 110 | 65 (59.1%) | 45 (40.9%) | 0.025 |
| Female | 203 | 93 (45.8%) | 110 (54.2%) | |
| Race/Ethnicity | 313 | |||
| White | 240 | 121 (50.4%) | 119 (49.6%) | 0.968 |
| non-Whitec | 73 | 37 (50.7%) | 36 (49.3%) | |
| Education | 311* | |||
| High school or less | 87 | 52 (59.8%) | 35 (40.2%) | 0.041 |
| College or higher | 224 | 105 (46.9%) | 119 (53.1%) | |
| Employment | 308* | |||
| No | 173 | 84 (48.5%) | 89 (51.5%) | 0.482 |
| Yes | 135 | 71 (52.6%) | 64 (47.4%) | |
| Marital status | 306* | |||
| Not Married | 115 | 53 (46.1%) | 62 (53.9%) | 0.250 |
| Married/living with partner | 191 | 101 (52.9%) | 90 (47.1%) | |
| Cancer type | 313 | |||
| Breast | 85 | 35 (41.2%) | 50 (58.8%) | 0.014 |
| Lung | 103 | 63 (61.2%) | 40 (38.8%) | |
| GI | 78 | 33 (42.3%) | 45 (57.7%) | |
| Other | 47 | 27 (57.5%) | 20 (42.5%) | |
| Cancer stage | 313 | |||
| Localized disease | 151 | 72 (47.7%) | 79 (52.3%) | 0.339 |
| Metastatic disease | 162 | 86 (53.1%) | 76 (46.9%) | |
| Surgery | 311* | |||
| No | 140 | 84 (60.0%) | 56 (40.0%) | 0.002 |
| Yes | 171 | 73 (42.7%) | 98 (57.3%) | |
| Radiation | 311* | |||
| No | 166 | 92 (55.4%) | 74 (44.6%) | 0.062 |
| Yes | 145 | 65 (44.8%) | 80 (55.2%) | |
| Chemotherapy | 310* | |||
| No | 32 | 20 (62.5%) | 12 (37.5%) | 0.157 |
| Yes | 278 | 137 (49.3%) | 141 (50.7%) | |
| Survivorhsip status | 284* | |||
| In treatment | 193 | 104 (53.9%) | 89 (46.1%) | 0.009 |
| Post treatment | 91 | 34 (37.4%) | 57 (62.6%) |
SPCS, supportive and palliative care services; GI, gastrointestinal.
Not all cells add up to 313 due to missing data.
SPCS non-user: N(%) of patients who did not receive at least one SPCS.
p-value calculated using chi-square analysis.
Non-white: majority (76.7%) were African-American.
Use of SPCS
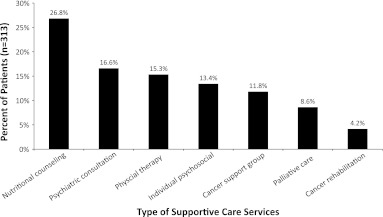
Of 313 participants, 155 (49.5%) reported having used at least one of the services included in our definition of SPCS. The most commonly used SPCS (Fig. 1) were nutritional counseling (26.5%) and psychological counseling/psychiatric consultation (29.6%), followed by physical therapy (15.1%), cancer support group (11.4%), palliative care consultation (8.3%), and cancer rehabilitation consultation (4.0%). Notably, of the 155 patients who used SPCS, 71.6% reported satisfaction with the services they used (either “quite a bit” or “very much” satisfied).
FIG. 1.
Type of supportive care services used expressed as a percent of total patients.
Factors associated with use of SPCS
There were statistically significant differences between SPCS users and SPCS nonusers based on sociodemographic and clinical variables, as represented in Table 1. Women were more likely to use SPCS compared with men (54.2% versus 40.9%, p=0.025). A higher level of education was associated with a higher rate of utilization of SPCS (college or graduate school 53.1% versus high school or less 40.2%, p=0.041). Patients with lung cancer were less likely to use SPCS when compared with breast, GI, and other tumor types (38.8% for lung versus 58.8%, 57.7%, and 42.5% for breast, GI, and other cancers respectively). Patients who had undergone surgery were also significantly more likely to have used SPCS (57.3 versus 40%, p=0.0002), and those who were in the post-treatment phase used SPCS more than those who were undergoing treatment (62.6% versus 46.1%, p=0.009). Age, ethnicity, employment, marital status, and cancer stage (localized versus metastatic) were not associated with SPCS use in the bivariate analysis.
In the multivariate regression analysis (Table 2) using a model that included gender, SPCS use was independently associated with a higher level of education (graduate education OR 2.14, 95% CI 1.98-4.26), p=0.03) and chemotherapy (OR 2.37, 95% CI 1.01-5.56, p=0.047). Patients who were currently undergoing treatment for cancer were less likely to have used SPCS (OR 0.51, 95% CI, 0.29-0.89, p=0.019). In the multivariate regression analysis using a model that included cancer types, SPCS use was independently associated with patients who had undergone radiation (OR 1.81, 95% CI, 1.07-3.05, p=0.026) or chemotherapy (OR 2.97, 95% CI, 1.22-7.22, p=0.016). There was a trend toward a significant association between SPCS use and higher level of education (graduate education OR 2.00, 95% CI, 0.99-4.55, p=0.054). Patients with lung cancer were less likely to use SPCS as compared with those with breast cancer (OR 0.48, 95% CI 0.24-0.96, p=0.037), and patients currently undergoing treatment were also less likely to have used SPCS (OR 0.49, 95% CI, 0.27-0.87, p=0.015).
Table 2.
Factors Associated with SPCS Use (n=313)
| |
|
|
|
Multivariate analyses |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Bivariate analyses |
Model 1a |
|
Model 2b |
||||||
| Characteristics | Odds ratio (OR) | 95% CI | p-value | Odds ratio (OR) | 95% CI | p-value | Odds ratio (OR) | 95% CI | p-value | |
| Gender | ||||||||||
| Male | 1.00 | – | – | 1.00 | – | – | – | – | – | |
| Female | 1.71 | 1.07–2.73 | 0.025 | 1.44 | 0.84–2.46 | 0.180 | – | – | – | |
| Education | ||||||||||
| High school or less | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | |
| College | 1.45 | 0.84–2.47 | 0.179 | 1.66 | 0.93–2.99 | 0.088 | 1.46 | 0.80–2.68 | 0.216 | |
| Graduate | 2.25 | 1.21–4.20 | 0.011 | 2.14 | 1.08–4.26 | 0.030 | 2.00 | 0.99–4.05 | 0.054 | |
| Cancer type | ||||||||||
| Breast | 1.00 | – | – | – | – | – | – | 1.00 | – | – |
| Lung | 0.44 | 0.25–0.80 | 0.007 | – | – | – | – | 0.48 | 0.24–0.96 | 0.037 |
| GI | 0.95 | 0.51–1.78 | 0.884 | – | – | – | – | 1.15 | 0.56–2.35 | 0.711 |
| Other | 0.52 | 0.25–1.07 | 0.075 | – | – | – | – | 0.82 | 0.35–1.89 | 0.636 |
| Surgery* | ||||||||||
| No | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | |
| Yes | 2.01 | 1.28–3.17 | 0.003 | 1.68 | 1.00–2.83 | 0.051 | 1.54 | 0.91–2.62 | 0.110 | |
| Radiation* | ||||||||||
| No | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | |
| Yes | 1.53 | 0.98–2.40 | 0.063 | 1.61 | 0.98–2.67 | 0.062 | 1.81 | 1.07–3.05 | 0.026 | |
| Chemotherapy* | ||||||||||
| No | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | |
| Yes | 1.72 | 0.81–3.64 | 0.160 | 2.37 | 1.01–5.56 | 0.047 | 2.97 | 1.22–7.22 | 0.016 | |
| Survivorhsip status | ||||||||||
| Post treatment | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – | |
| In treatment | 0.51 | 0.31–0.85 | 0.010 | 0.51 | 0.29–0.89 | 0.019 | 0.49 | 0.27–0.87 | 0.015 | |
SPCS, supportive and palliative care services; OR, odds ratio; CI, confidence interval; GI, gastrointestinal.
Services used since cancer diagnosis.
Model 1 includes gender but not cancer type.
Model 2 includes cancer type but not gender.
Patient perceived barriers to using SPCS
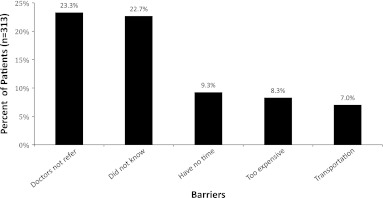
The most common self-reported barriers to use of SPCS (Fig. 2) were lack of knowledge of these services (22.4%) and lack of physician referral (23%). These were followed by lack of time (9.1%), difficulty in transportation (6.9%), and expense (8.2%). In an exploratory analysis of social/demographic factors and reported barriers, nonwhites reported lack of physician referral more frequently than did whites (20.4% versus 32.9% respectively, p=0.03).
FIG. 2.
Patient-reported barriers to access to supportive care services expressed as a percent of total patients.
Discussion
Although cancer remains as a leading cause of death in the United States, a growing population of individuals lives longer following a cancer diagnosis.8 The multitude of physical and psychosocial co-morbidities experienced by cancer patients during and after treatment16–24 demands an integrated model of care that comprehensively addresses the varying needs of these individuals. Whereas several recent studies have consistently demonstrated the benefit of SPCS in improving symptom burden, health-related quality of life, and patient satisfaction,28–33 our study suggests that one in two outpatient cancer patients have not used any of the existing and available SPCS since their diagnosis. Patients with lower levels of education, a diagnosis of lung cancer, or those undergoing active treatment are less likely to receive SPCS. The largest patient-reported barriers to access are lack of physician referral and lack of awareness.
SPCS users in our study were more likely to have a higher level of education, a trend that is consistent with literature correlating higher education levels to improved access to health care and better health outcomes.34–38 Patients with less education and consequently low health literacy may have greater information needs regarding the benefits and availability of SPCS as a part of their cancer care. Although in our study lower educational attainment did not correlate with a perceived lack of awareness of SPCS, a knowledge gap may indeed exist in this subset of patients. More research is needed to identify specific social and technological barriers faced by patients with low education, and there is clearly a need to increase the visibility of SPCS throughout the continuum of cancer care.
Lung cancer patients were half as likely as patients with breast, GI, and other solid malignancies to seek out supportive and palliative care services. This finding is particularly noteworthy in the context of data showing that lung cancer survivors suffer from a higher burden of physical and psychological problems, co-morbid conditions, and lower health-related quality of life and health utility when compared with survivors of breast, colorectal, and prostate cancers.39–45 Although further research is required to understand the specific reasons for this phenomenon, one possible explanation is that lung cancer patients have a shorter median survival after diagnosis compared with patients with breast cancer or colorectal cancers.8 As a result, these patients have less time overall in which to pursue SPCS, and in the limited time available, may choose to pursue anti-cancer therapies over supportive care and symptom management. Interestingly, results of a recent randomized trial showed that introduction of early palliative care in patients with metastatic lung cancer demonstrated improvements in health-related quality of life and mood, less aggressive care at the end of life, and longer survival.28 Thus in this study, the subset of lung cancer patients was identified as an underserved population, and one that may benefit significantly from an integrated framework of care that emphasizes early referral to SPCS in addition to standard therapy.
Patients in this study with a history of chemotherapy and radiation treatment were more likely to use SPCS in the multivariate analysis, likely representing the high symptom burden that often results from these treatments. However, patients undergoing current treatment were less likely to use SPCS, indicating that patients with a greater need for SPCS (i.e., those actively receiving chemoradiation) may not access these services until they have completed treatment. Patients in the post-treatment phase of cancer care, particularly those whose disease is cured or in remission, may also be more hopeful and willing to focus on symptoms and quality of life versus pursuing second-line or salvage therapy. These patients have also likely had a longer exposure to oncology care than newly diagnosed patients, thereby increasing the chances of exposure to SPCS. Seamless integration of supportive care with conventional therapies would help patients receive relief of symptom distress in a timelier manner.
The most common self-reported barriers to use of SPCS were lack of physician referral (23%) and lack of awareness (22.4%) that such services were available at this cancer center. These findings among oncology outpatients are consistent with the current data on barriers to end-of-life care in the hospice population.46–48 Our findings underscore the importance of educating physicians, other health care providers (e.g., nurses, social workers), and patients about the value and availability of SPCS in order to improve the access to these services and ultimately improve patient outcomes.
Within populations of cancer patients, nonwhites (specifically African Americans) and patients of lower economic status have been found to have less exposure to information about palliative care.49,50 Although ethnicity was not an independent predictor of SPCS use in our study, nonwhite participants reported lack of provider referral as a significant barrier. Prior research suggests that nonwhite patients were less likely to engage in shared decision making than whites51–52 and are also less likely to receive effective cancer pain management and palliative care.53–58 Because values and perspective may differ by age,59 gender, and race/ethnicity, so the needs and expectation of different subgroups are likely to be different as well; the design of patient-centered PSCS should not be one-size-fits-all, but should carefully incorporate diverse social cultural perspectives.
This study has several limitations. First, there were no data collected on patient-reported needs, which would give a more accurate estimate of unmet SPCS needs in the oncology patient population. Prior studies have suggested that measures of effectiveness of palliative care interventions are most useful when analyzed against the background of the existing needs of patients,60–62 which highlights the potential need for interviews with patients or a detailed set of questions relating to specific preferences about the extent, type, and duration of services that patients prefer in order to understand whether the services currently offered at most cancer centers fulfill these needs. Second, the study relied on self-report, which is subject to recall bias and therefore is not a direct measure of SPCS use among oncology outpatients. This may have contributed to either underreporting or overreporting of SPCS use by patients. Third, although we reported rates of utilization of SPCS, we did not explore patient or physician attitudes and beliefs toward these services, both of which are likely to affect uptake and utilization. Fourth, we queried only patient-perceived barriers without investigating physician-perceived and system barriers, two potentially important challenges to providing optimal, integrated cancer care. Lastly, the study was conducted at a single, urban, academic comprehensive cancer center, so the findings may not be generalizable to community cancer centers.
To our knowledge, this study is one of the first to characterize the difference between users and nonusers of SPCS in the nonhospice outpatient oncology setting. Although the majority of oncology patients likely experience a significant symptom burden, slightly less than half of the patients surveyed accessed supportive care services. This discrepancy represents an area of unmet need in the cancer care algorithm, and is an area for further study. We also identified common patient-reported barriers to use of SPCS. Further research applying an appropriate conceptual model is needed to elucidate the complex relationships among the unmet needs, determinants of SPCS use, and the barriers to access to these services. In doing so, we will move closer to creating an integrated model of cancer care that better addresses the physical and psychosocial needs that patients face during and beyond cancer treatment.
Acknowledgments
We would like to thank all of the patients who participated in this study. We thank physicians, nurse practitioners, and staff for their support. We would like to thank our hardworking students, Ethan Frankel, Jonathan Burgess, Blake Freidman, Manuel Bramble, Neha Agarwal, and Tiffany Tan for their dedication to the data collection and management process. This study is partially funded by the Penn Institute of Aging Pilot Fund. Dr. Mao is supported by American Cancer Society [CCCDA-08-107-03] and National Institutes of Health [1K23 AT004112-04]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Jemal A. Siegel R. Xu J. Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Cheng KK. Lee DT. Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol. 2010;78:127–137. doi: 10.1016/j.critrevonc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Hopwood P. Stephens RJ. Symptoms at presentation for treatment in patients with lung cancer: Implications for the evaluation of palliative treatment. The Medical Research Council (MRC) Lung Cancer Working Party. Br J Cancer. 1995;71:633–636. doi: 10.1038/bjc.1995.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopwood P. Stephens Depression in patients with lung cancer: Prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18:893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- 5.Lutz S. Norrell R. Bertucio C. Kachnic L. Johnson C. Arthur D. Schwarz M. Palardy G. Symptom frequency and severity in patients with metastatic or locally recurrent lung cancer: A prospective study using the Lung Cancer Symptom Scale in a community hospital. J Palliat Med. 2001;4:157–165. doi: 10.1089/109662101750290191. [DOI] [PubMed] [Google Scholar]
- 6.Paddison JS. Temel JS. Fricchione GL. Pirl WF. Using the differential from complete blood counts as a biomarker of fatigue in advanced non-small-cell lung cancer: An exploratory analysis. Palliat Support Care. 2009;7:213–217. doi: 10.1017/S1478951509000273. [DOI] [PubMed] [Google Scholar]
- 7.So WK. Marsh G. Ling WM. Leung FY. Lo JC. Yeung M. Li GK. Anxiety, depression and quality of life among Chinese breast cancer patients during adjuvant therapy. Eur J Oncol Nurs. 2010;14:17–22. doi: 10.1016/j.ejon.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Altekruse SF, editor; Kosary CL, editor; Krapcho M, editor; Neyman N, editor; Aminou R, editor; Waldron W, editor; Ruhl J, editor; Howlader N, editor; Tatalovich Z, editor; Cho H, editor; Mariotto A, editor; Eisner MP, editor; Lewis DR, editor; Cronin K, editor; Chen HS, editor; Feuer EJ, editor; Stinchcomb DG, editor; Edwards BK, editor. SEER Cancer Statistics Review. 2010. http://seer.cancer.gov/csr/1975_2007/ [Jun 16;2011 ]. pp. 1975–2007.http://seer.cancer.gov/csr/1975_2007/ Based on November 2009 SEER data submission, posted to the SEER web site, 2010.
- 9.Mao JJ. Armstrong K. Bowman MA. Xie SX. Kadakia R. Farrar JT. Symptom burden among cancer survivors: Impact of age and comorbidity. J Am Board Fam Med. 2007;20:434–443. doi: 10.3122/jabfm.2007.05.060225. [DOI] [PubMed] [Google Scholar]
- 10.Yabroff KR. Lawrence WF. Clauser S. Davis WW. Brown ML. Burden of illness in cancer survivors: Findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 11.Foley KM, editor; Gelband H, editor. Improving Palliative Care for Cancer: Summary, Recommendations, 1e. Washington, D.C.: National Cancer Policy Board, National Research Council; 2001. Washington, DC. [PubMed] [Google Scholar]
- 12.Levy MH. Back A. Benedetti C. Billings JA. Block S. Boston B. Bruera E. Dy S. Eberle C. Foley KM. Karver SB. Knight SJ. Misra S. Ritchie CS. Spiegel D. Sutton L. Urba S. Von Roenn JH. Weinstein SM. NCCN clinical practice guidelines in oncology: palliative care. J Natl Compr Canc Netw. 2009;7(4):436–473. doi: 10.6004/jnccn.2009.0031. [DOI] [PubMed] [Google Scholar]
- 13.Pain relief and palliative care. National Cancer Control Programmes: Policies and Managerial Guidelines. 2nd. Geneva: World Health Organization; 2002. pp. 83–91. [Google Scholar]
- 14.Ferris FD. Bruera E. Cherny N. Cummings C. Currow D. Dudgeon D. Janjan N. Strasser F. von Gunten CF. Von Roenn JH. Palliative cancer care a decade later: Accomplishments, the need, next steps—from the American Society of Clinical Oncology. J Clin Oncol. 2009;27:3052–3058. doi: 10.1200/JCO.2008.20.1558. [DOI] [PubMed] [Google Scholar]
- 15.Hui D. Elsayem A. De la Cruz M. Berger A. Zhukovsky DS. Palla S. Evans A. Fadul N. Palmer JL. Bruera E. Availability and integration of palliative care at US cancer centers. JAMA. 2010;303:1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao YC. Liao WY. Shun SC. Yu CJ. Yang PC. Lai YH. Symptoms, psychological distress, and supportive care needs in lung cancer patients. Support Care Cancer. 2011;19:1743–1751. doi: 10.1007/s00520-010-1014-7. [DOI] [PubMed] [Google Scholar]
- 17.Uchida M. Akechi T. Okuyama T. Sagawa R. Nakaguchi T. Endo C. Yamashita H. Toyama T. Furukawa TA. Patients' supportive care needs and psychological distress in advanced breast cancer patients in Japan. Jpn J Clin Oncol. 2011;41:530–536. doi: 10.1093/jjco/hyq230. [DOI] [PubMed] [Google Scholar]
- 18.Thorsen L. Gjerset GM. Loge JH. Kiserud CE. Skovlund E. Fløtten T. Fosså SD. Cancer patients' needs for rehabilitation services. Acta Oncol. 2011;50:212–222. doi: 10.3109/0284186X.2010.531050. [DOI] [PubMed] [Google Scholar]
- 19.Armes J. Crowe M. Colbourne L. Morgan H. Murrells T. Oakley C. Palmer N. Ream E. Young A. Richardson A. Patients' supportive care needs beyond the end of cancer treatment: A prospective, longitudinal survey. J Clin Oncol. 2009;27:6172–6179. doi: 10.1200/JCO.2009.22.5151. [DOI] [PubMed] [Google Scholar]
- 20.Sanders SL. Bantum EO. Owen JE. Thornton AA. Stanton AL. Supportive care needs in patients with lung cancer. Psychooncology. 2010;19:480–489. doi: 10.1002/pon.1577. [DOI] [PubMed] [Google Scholar]
- 21.Harrison JD. Young JM. Price MA. Butow PN. Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009 doi: 10.1007/s00520-009-0615-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Barg FK. Cronholm PF. Straton JB. Keddem S. Knott K. Grater J. Houts P. Palmer SC. Unmet psychosocial needs of Pennsylvanians with cancer: 1986–2005. Cancer. 2007;110:631–639. doi: 10.1002/cncr.22820. [DOI] [PubMed] [Google Scholar]
- 23.Fitch MI. Steele R. Identifying supportive care needs of women with ovarian cancer. Can Oncol Nurs J. 2010;20:66–74. doi: 10.5737/1181912x2026674. [DOI] [PubMed] [Google Scholar]
- 24.Fitch MI. Steele R. Supportive care needs of individuals with lung cancer. Can Oncol Nurs J. 2010;20:15–22. doi: 10.5737/1181912x2011522. [DOI] [PubMed] [Google Scholar]
- 25.Peppercorn JM. Smith TJ. Helft PR. Debono DJ. Berry SR. Wollins DS. Hayes DM. Von Roenn JH. Schnipper LE. American Society of Clinical Oncology: American Society of Clinical Oncology statement: Toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29:755–760. doi: 10.1200/JCO.2010.33.1744. [DOI] [PubMed] [Google Scholar]
- 26.Yabroff KR. Mandelblatt JS. ngham J. The quality of medical care at the end-of-life in the USA: Existing barriers and examples of process and outcome measures. Palliat Med. 2004;18:202–216. doi: 10.1191/0269216304pm880oa. [DOI] [PubMed] [Google Scholar]
- 27.Fiscella K. Franks P. Gold MR. Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA. 2000;283:2579–2584. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- 28.Temel JS. Greer JA. Muzikansky A. Gallagher ER. Admane S. Jackson VA. Dahlin CM. Blinderman CD. Jacobsen J. Pirl WF. Billings JA. Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 29.Hearn J. Higginson IJ. Do specialist palliative care teams improve outcomes for cancer patients? A systematic literature review. Palliat Med. 1998;12:317–332. doi: 10.1191/026921698676226729. [DOI] [PubMed] [Google Scholar]
- 30.Yennurajalingam S. Urbauer DL. Casper KL. Reyes-Gibby CC. Chacko R. Poulter V. Bruera E. Impact of a palliative care consultation team on cancer-related symptoms in advanced cancer patients referred to an outpatient supportive care clinic. J Pain Symptom Manage. 2010 doi: 10.1016/j.jpainsymman.2010.03.017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Higginson IJ. Evans CJ. What is the evidence that palliative care teams improve outcomes for cancer patients and their families? Cancer J. 2010;16:423–345. doi: 10.1097/PPO.0b013e3181f684e5. [DOI] [PubMed] [Google Scholar]
- 32.Casarett D. Pickard A. Bailey FA. Ritchie C. Furman C. Rosenfeld K. Shreve S. Chen Z. Shea JA. Do palliative consultations improve patient outcomes? J Am Geriatr Soc. 2008;56:593–599. doi: 10.1111/j.1532-5415.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 33.Gómez-Batiste X. Porta-Sales J. Espinosa-Rojas J. Pascual-López A. Tuca A. Rodriguez J. Effectiveness of palliative care services in symptom control of patients with advanced terminal cancer: A spanish, multicenter, prospective, quasi-experimental, pre-post study. J Pain Symptom Manage. 2010;40:652–660. doi: 10.1016/j.jpainsymman.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 34.Adler NE. Newman K. Socioeconomic disparities in health: Pathways and policies. Health Aff (Millwood) 2002;21:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 35.Sorlie PD. Backlund E. Keller JB. US mortality by economic, demographic, and social characteristics: The National Longitudinal Mortality Study. Am J Public Health. 1995;85:949–956. doi: 10.2105/ajph.85.7.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Backlund E. Sorlie PD. Johnson NJ. A comparison of the relationships of education and income with mortality: The National Longitudinal Mortality Study. Soc Sci Med. 1999;49:1373–1384. doi: 10.1016/s0277-9536(99)00209-9. [DOI] [PubMed] [Google Scholar]
- 37.Huisman M. Kunst AE. Bopp M. Borgan JK. Borrell C. Costa G. Deboosere P. Gadeyne S. Glickman M. Marinacci C. Minder C. Regidor E. Valkonen T. Mackenbach JP. Educational inequalities in cause-specific mortality in middle-aged and older men and women in eight western European populations. Lancet. 2005;365(9458):493–500. doi: 10.1016/S0140-6736(05)17867-2. [DOI] [PubMed] [Google Scholar]
- 38.Winkleby MA. Jatulis DE. Frank E. Fortmann SP. Socioeconomic status and health: How education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schag C.A. Ganz PA. Wing DS. Sim MS. Lee JJ. Quality of life in adult survivors of lung, colon and prostate cancer. Qual Life Res. 1994;3:127–141. doi: 10.1007/BF00435256. [DOI] [PubMed] [Google Scholar]
- 40.Wilson IB. Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 41.Hewitt M. Rowland JH. Yancik R. Cancer survivors in the United States: Age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 42.Ko CY. Maggard M. Livingston EH. Evaluating health utility in patients with melanoma, breast cancer, colon cancer, and lung cancer: A nationwide, population-based assessment. J Surg Res. 2003;114:1–5. doi: 10.1016/s0022-4804(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 43.Sugimura H. Yang P. Long-term survivorship in lung cancer: A review. Chest. 2006;129:1088–1097. doi: 10.1378/chest.129.4.1088. [DOI] [PubMed] [Google Scholar]
- 44.Piccirillo JF. Tierney RM. Costas I. Grove L. Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 45.Ogle KS. Swanson GM. Woods N. Azzouz F. Cancer and comorbidity: Redefining chronic diseases. Cancer. 2000;88:653–663. doi: 10.1002/(sici)1097-0142(20000201)88:3<653::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 46.Cohen LL. Racial/ethnic disparities in hospice care: A systematic review. J Palliat Med. 2008;11:763–768. doi: 10.1089/jpm.2007.0216. [DOI] [PubMed] [Google Scholar]
- 47.Fadul N. Elsayem A. Palmer JL. Del Fabbro E. Swint K. Li Z. Poulter V. Bruera E. Supportive versus palliative care: What's in a name?: A survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer. 2009;115:2013–2021. doi: 10.1002/cncr.24206. [DOI] [PubMed] [Google Scholar]
- 48.Feeg VD. Elebiary H. Exploratory study on end-of-life issues: Barriers to palliative care and advance directives. Am J Hosp Palliat Care. 2005;22:119–124. doi: 10.1177/104990910502200207. [DOI] [PubMed] [Google Scholar]
- 49.Johnson KS. Kuchibhatla M. Tulsky JA. Racial differences in self-reported exposure to information about hospice care. J Palliat Med. 2009;12:921–927. doi: 10.1089/jpm.2009.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koffman J. Burke G. Dias A. Raval B. Byrne J. Gonzales J. Daniels C. Demographic factors and awareness of palliative care and related services. Palliat Med. 2007;21:145–153. doi: 10.1177/0269216306074639. [DOI] [PubMed] [Google Scholar]
- 51.Cooper-Patrick L. Gallo JJ. Gonzales JJ. Vu HT. Powe NR. Nelson C. Ford DE. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282:583–589. doi: 10.1001/jama.282.6.583. [DOI] [PubMed] [Google Scholar]
- 52.Levinson W. Kao A. Kuby A. Thisted RA. Not all patients want to participate in decision making. A national study of public preferences. J Gen Intern Med. 2005;20:531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cintron A. Morrison RS. Pain and ethnicity in the United States: A systematic review. J Palliat Med. 2006;9:1454–73. doi: 10.1089/jpm.2006.9.1454. [DOI] [PubMed] [Google Scholar]
- 54.Kalauokalani D. Franks P. Oliver JW. Meyers FJ. Kravitz RL. Can patient coaching reduce racial/ethnic disparities in cancer pain control? Secondary analysis of a randomized controlled trial. Pain Med. 2007;8:17–24. doi: 10.1111/j.1526-4637.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- 55.McNeill JA. Reynolds J. Ney ML. Unequal quality of cancer pain management: Disparity in perceived control and proposed solutions. Oncol Nurs Forum. 2007;34:1121–1128. doi: 10.1188/07.ONF.1121-1128. [DOI] [PubMed] [Google Scholar]
- 56.Payne R. Medina E. Hampton JW. Quality of life concerns in patients with breast cancer: Evidence for disparity of outcomes and experiences in pain management and palliative care among African-American women. Cancer. 2003;97(1 Suppl):311–317. doi: 10.1002/cncr.11017. [DOI] [PubMed] [Google Scholar]
- 57.Ward E. Jemal A. Cokkinides V. Singh GK. Cardinez C. Ghafoor A. Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 58.Shavers VL. Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 59.Gott M. Small N. Bernes S. Payne S. Seamark D. Older people's views of a good death in heart failure: Implications for palliative care provision. Soc Sci Med. 2008;67:1113–1121. doi: 10.1016/j.socscimed.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 60.Abarshi E. Onwuteaka-Philipsen B. Donker G. Echteld M. Van den Block L. Deliens L. General practitioner awareness of preferred place of death and correlates of dying in a preferred place: A nationwide mortality follow-back study in the Netherlands. J Pain Symptom Manage. 2009;38:568–577. doi: 10.1016/j.jpainsymman.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Ahmed N. Bestall JC. Ahmedzai SH. Payne SA. Clark D. Noble B. Systematic review of the problems and issues of accessing specialist palliative care by patients, carers and health and social care professionals. Palliat Med. 2004;18:525–542. doi: 10.1191/0269216304pm921oa. [DOI] [PubMed] [Google Scholar]
- 62.Walshe C. Todd C. Caress A. Chew-Graham C. Patterns of access to community palliative care services: A literature review. J Pain Symptom Manage. 2009;37:884–912. doi: 10.1016/j.jpainsymman.2008.05.004. [DOI] [PubMed] [Google Scholar]