Abstract
Adult mesenchymal stem cells (MSCs) are an attractive cell source for cartilage tissue engineering. In vitro predifferentiation of MSCs has been explored as a means to enhance MSC-based articular cartilage repair. However, there remain challenges to control and prevent the premature progression of MSC-derived chondrocytes to the hypertrophy. This study investigated the temporal effect of transforming growth factor (TGF)-β and β-catenin signaling co-activation during MSC chondrogenic differentiation and evaluated the influence of these predifferentiation conditions to subsequent phenotypic development of the cartilage. MSCs were differentiated in chondrogenic medium that contained either TGFβ alone, TGFβ with transient β-catenin coactivation, or TGFβ with continuous β-catenin coactivation. After in vitro differentiation, the pellets were transplanted into SCID mice. Both coactivation protocols resulted in the enhancement of chondrogenic differentiation of MSCs. Compared with TGFβ activation, transient coactivation of TGFβ-induction with β-catenin activation resulted in heightened hypertrophy and formed highly ossified tissues with marrow-like hematopoietic tissue in vivo. The continuous coactivation of the 2 signaling pathways, however, resulted in inhibition of progression to hypertrophy, marked by the suppression of type X collagen, Runx2, and alkaline phosphatase expression, and did not result in ossified tissue in vivo. Chondrocytes of the continuous co-activation samples secreted significantly more parathyroid hormone-related protein (PTHrP) and expressed cyclin D1. Our results suggest that temporal co-activation of the TGFβ signaling pathway with β-catenin can yield cartilage of different phenotype, represents a potential MSC predifferentiation protocol before clinical implantation, and has potential applications for the engineering of cartilage tissue.
Introduction
Adult mesenchymal stem cells (MSCs) have been considered an attractive cell source for cartilage tissue engineering and regeneration due to their vast proliferative capacity and differentiation potential. However, MSC chondrogenic differentiation has the tendency to progress toward the hypertrophic stage in a process resembling that of endochondral ossification, forming transient, endochondral cartilage instead of stable, articular cartilage-like tissue [1–3]. In vivo ectopic transplantation of MSC-derived cartilage constructs resulted in either chondrocyte dedifferentiation, or vascular invasion and mineralization, depending on the predifferentiation stage of the construct [3–7]. In the case of cartilage repair in vivo, animal studies with implantation of MSCs into cartilage defects indicated differentiation of MSCs into chondrocytic-like cells and resulted in enhanced cartilage repair [8–10]. Steck et al. [10] showed that at this orthotopic site, MSCs differentiated into chondrocytes forming collagen type II positive neocartilage, which was collagen type X-negative, except in areas in close vicinity to the bone. The result indicates that the in vivo niche is able to provide the appropriate signaling molecules and biomechanical cues which shape the fate of the transplanted MSC.
In vitro preconditioning of MSCs has been explored as a means to enhance cartilage repair. Studies using predifferentiated MSCs for cartilage repair have resulted in faster and better cartilage regeneration compared with the use of undifferentiated MSCs, with phenotype stability maintained up to 1 year postimplantation [11,12]. These studies indicate that preconditioned MSCs can be employed to overcome the requirement of an extended regeneration period and to improve the quality of the neocartilage from the often inferior quality derived from undifferentiated MSCs [13,14]. The chondrogenic ability of MSCs can be triggered with various growth factors, including transforming growth factor β (TGFβ), bone morphogenic factors (BMP), fibroblast growth factor, and insulin like growth factor [1]. Canonical β-catenin signaling pathway has been associated with chondrogenesis and cartilage development [15]. Forced expression of the ligands of β-catenin pathways inhibited embryonic mesenchymal cells condensation and transition to cartilage nodules [16–18]. By contrast, activation of the β-catenin induced transcriptional activity was shown to promote chondrocyte differentiation in an Sox9-dependent manner [19]. In addition, β-catenin signaling pathways often crosstalk with other signaling pathways in modulating chondrogenesis; Wnt3A enhances BMP2-mediated chondrogenesis of murine mesenchymal cells [20]; and in adult human marrow stromal cells, β-catenin activation enhanced TGFβ-induced chondrogenic differentiation [21,22]. Further down the endochondral ossification process, β-catenin signaling again plays a crucial role in the hypertrophic maturation of chondrocytes [23–26].
In this study, we investigated the influence of co-activation of TGFβ and β-cateinin signaling pathways to subsequent phenotype development of the cartilage in vitro and in vivo. We showed that with co-activation of the TGFβ and β-catenin signaling pathways, chondrogenic induction can be greatly enhanced. However, further development into hypertrophy and mineralization depended on the temporal period of β-catenin perturbation. Our study suggests that subtle manipulation of β-catenin activation during TGFβ-induced chondrogenesis of MSCs could be employed to derive phenotypically different cartilage for transplantation purposes.
Materials and Methods
Human bone marrow MSCs culture and chondrogenic differentiation
MSCs were generated from bone marrow aspirates of consented human donors after obtaining approval from the hospital Institutional Review Board. The bone marrow aspirate cells were resuspended in DMEM supplemented with 10% FBS and cultured at 37°C in 5% CO2 atmosphere. After 4–5 days, nonadherent cells were removed. When reaching 70%–80% confluency, adherent cells were trypsinized and further expanded. A homogenous MSC population was obtained after 1–2 weeks of culture, and MSC was used between passages 3 and 5.
Chondrogenic differentiation of MSCs was induced through aggregate culture, under conditions previously described [27,28]. Briefly, 2.5×105 cells were centrifuged to form pellets and cultured in a medium containing high glucose DMEM supplemented with 4 mM proline, 50 μg/mL ascorbic acid, 1% ITS-Premix (Becton-Dickinson, San Jose, CA), 1 mM sodium pyruvate, and 0.1 μM dexamethasone (Sigma-Aldrich, St Louis, MO) for up to 35 days in the presence of 5 ng/mL of transforming growth factor-β3 (TGFβ3; R&D Systems, Minneapolis, MN). For the activation of β-catenin signaling pathway, 5 mM lithium chloride (LiCl; Sigma-Aldrich) was included together with TGFβ3 for up to 35 days (continuous co-activation), or the first 7 days in the differentiation period, before changing into the TGFβ3-only chondrogenic media (transient co-activation). Medium was changed every 3 days. All experiments were performed in triplicate.
RNA analysis and real-time polymerase chain reaction
Total RNA was extracted with RNeasy Mini Kit (Qiagen, Chatsworth, CA) following the manufacturer's instructions. cDNA was reverse transcribed by using iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time polymerase chain reaction (PCR) was performed using the Power SYBR Green PCR Master Mix (Applied Biosystem, Foster City, CA) on Applied Biosystems 7500 Real-Time PCR System (Applied Biosystem) at 95°C for 15 min followed by 40 cycles of 15-s denaturation at 94°C, 30-s annealing at 55°C, and 30-s elongation at 72°C. Genes of interest were normalized to the reference gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH). The level of target gene expression was calculated as 2−ΔΔCt. The following forward and reverse primers were used for amplification: for GAPDH, forward 5′-ATGGGGAAGGTGAAGGTCG-3′ and reverse 5′-TAAAAGCAGCCCTGGTGACC-3′; for Sox9, forward 5′-CAGTACCCGCACTTGCACAA-3′ and reverse 5′-CTCGTTCAGAAGTCTCCAGAGCTT-3′; for Aggr, forward 5′-ACTTCCGCTGGTCAGATGGA-3′ and reverse 5′-TCTCGTGCCAGATCATCACC-3′; for Col2, forward 5′-GGCAATAGCAGGTTCACGTACA-3′ and reverse 5′-CGATAACAGTCTTGCCCCACTT-3′; for Col9, forward 5′-CAGGATATCCAGGCCTACCA-3′ and reverse 5′-TCCCTGGTCACCTTCTTCAC-3′; for Col10, forward 5′-CAAGGCACCATCTCCAGGAA-3′ and reverse 5′-AAAGGGTATTTGTGGCAGCATATT-3′; for Runx2, forward 5′-CCCGTGGCCTTCAAGGT-3′ and reverse 5′-TGACAGTAACCACAGTCCCATCTG-3′; for BSP, forward 5′-CGATATTATCTTTACAAGCATGCCTACT-3′ and reverse 5′-GGATGAGTCACTACTGCCCTGAA-3′.
Alkaline phosphatase activity quantification
Supernatants of 4 pellets per treatment from 3 independent donors were collected at various time of chondrogenic differentiation. For detection of alkaline phosphatase activity, the supernatant was incubated with substrate solution containing 10 mg/mL p-nitrophenyl phosphate (Sigma-Aldrich) in 0.1 M glycine, 1 mM MgCl2, 1 mM ZnCl2, pH 9.6, and measured spectrophotometrically in a microplate reader (TECAN Infinite M200) at 405 nm.
Determination of parathyroid hormone-related protein in the culture supernatants
Parathyroid hormone-related protein (PTHrP) level in culture supernatant was determined by enzyme-linked immunoassay (USCN Life Science & Technology Co Ltd, Wuhan, China) according to manufacturer's protocol.
Ectopic transplantation in nude mice
Pellets of MSC subjected to in vitro chondrogenic differentiation conditions for 5 weeks were fixed with fibrin glue to a nonresorbable surgical suture to facilitate harvest and implanted subcutaneously on the backs of nude mice (BALB/c athymic nu/nu). Samples were harvested 8 weeks later. Animal procedures were approved by The NUS Institutional Animal Care and Use Committee.
The pellets harvested were fixed in formalin. Micro-computed tomography (μCT) analysis was performed before the pellets were subjected to de-calcification process. Decalcified pellets were then sectioned and underwent histological and immunohistochemical analysis.
Histology and immunohistochemistry analysis
For tissue processing, formalin-fixed pellets were dehydrated, paraffin embedded, and cut into sections of 5 μm. Tissue sections underwent Alcian blue staining (5%, Sigma-Aldrich) and were counterstained with nuclear fast red (Sigma-Aldrich); Masson's trichrome staining, with sequential incubated in Bouin's solution, Weigert's Haematoxylin solution, and Ponceau Fuchsin solution, and developed with 4% phosphomolybdic acid and Light Green solution (Sigma-Aldrich). Immunohistochemical staining was as published [28] with monoclonal antibodies for collagen type II (Clone 6B3, Chemicon International, Temecula, CA), collagen type X (Clone ×53, Quartett, Berlin, Germany), and cyclin D1 (Clone DCS6, Santa Cruz Biotechnology, Santa Cruz, CA).
Mineralization quantification
μCT analysis was used to quantify mineralization. The pellets were analyzed using the μCT scanner (SMX-100CT X-ray CT Sys, Shimadzu, Japan), based on the following scan settings: X-ray voltage (53 kV), X-ray current (50 μA), detector size (9′′), scaling coefficient (50), and pixel spacing (0.0114 mm/pixel). The pellet was segmented from the 3D volume using a consistent set of threshold gray values (40,549–50,355), which was found to allow a reasonable demarcation of the bone region from the surrounding soft tissues. The segmented bone volume of each pellet was subsequently calculated as a percentage of the total pellet volume.
Statistical analysis
Statistical significance was calculated using the student t-test. Data were presented as the mean±standard deviation, with the level of significance set at P<0.05. All quantitative data reported here were averaged from 3 independent experiments.
Results
Temporal co-activation of TGFβ and β-catenin signaling in MSC chondrogenesis
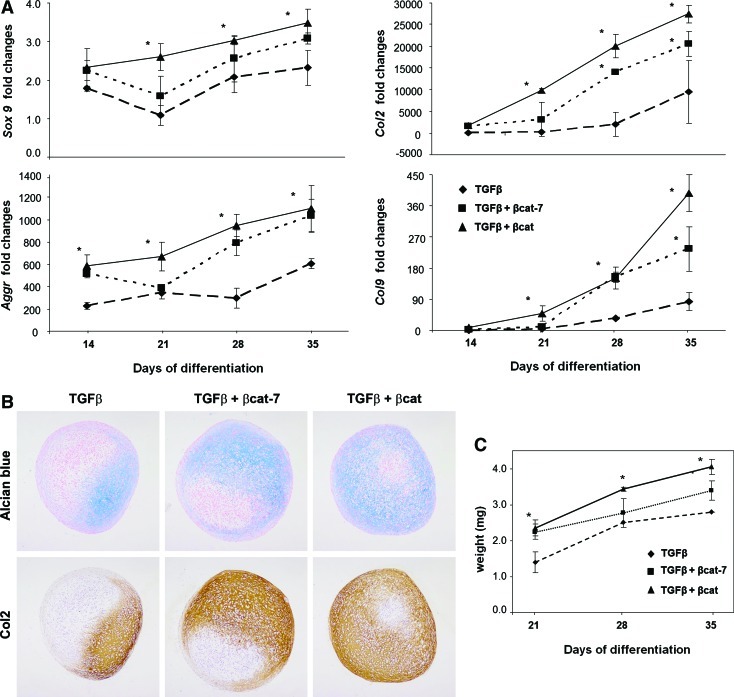
We examined the temporal effect of co-activating TGFβ with β-catenin signaling in MSC chondrogenesis with reference to induction by TGFβ alone. Activation of β-catenin was achieved by using a well-documented GSK3β inhibitor, LiCl, which mimics Wnt signaling by stabilizing cytosolic β-catenin [29,30] and was documented to increase β-catenin nuclear translocation in the presence of TGFβ [30]. MSC pellet culture was treated with TGFβ and LiCl for the entire differentiation period (continuous co-activation, represented by TGFβ3+βcat) or only during the first 7 days before switching to activation by TGFβ3 alone (transient co-activation, represented by TGFβ3+βcat-7). Real-time RT-PCR analysis showed a time-dependent increase of Sox9, Aggr, Col2, and Col9 expression in all samples (Fig. 1A), with a significantly higher level of expression detected in the TGFβ3+βcat samples compared with TGFβ3 alone. Significantly, the levels of these mRNAs in the transient co-activation samples were higher than the TGFβ-activated samples at all time points and were comparable to the continuous co-activation samples. Histological analysis shows an early and enhanced deposition of proteoglycan and type II collagen by both the transient and continuous co-activation samples (Fig. 1B, see also Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd), compared with TGFβ-activation. The increase in ECM deposition was accompanied by a significant increase in pellet weight in both the transient and continuous co-activation groups, with the latter consistently heavier, especially at the later time points (Fig. 1C).
FIG. 1.
MSC pellets were subjected to chondrogenic differentiation in TGFβ, TGFβ with transient or continuous β-catenin co-activation. (A) Expression level of chondrogenic marker genes was quantified by real-time PCR. Expression was normalized to glyceraldehydes-3-phosphate dehydrogenase and presented relative to level in undifferentiated MSC. Results of TGFβ group are represented by dashed lines with diamonds; TGFβ+transient β-catenin co-activation by dotted lines with squares; and TGFβ+continuous β-catenin co-activation by solid lines with triangles. (B) Pellets harvested at day 28 were sectioned and subjected to alcian blue staining and type II collagen immunohistochemistry staining. Representative micrograms from 3 independent experiments of 3 different donors' MSCs are shown. Deposition of proteoglycan and type II collagen was enhanced in the TGFβ+transient β-catenin co-activation pellets, and the continuous β-catenin co-activation pellets. Images were taken at ×100 magnification. (C) The weight of the resulting pellet was determined. The weight of TGFβ+transient β-catenin co-activation pellets increased significantly as much as those of the continuous β-catenin co-activation pellets at day 21 and remained significantly higher than TGFβ pellets at day 35. Data shown are means±SD (n=3, *P<0.05 significant increase compared with TGFβ samples). MSC, mesenchymal stem cell; TGF, transforming growth factor; β-cat, β-catenin; SD, standard deviation. Color images available online at www.liebertonline.com/scd
These results indicate that transient co-activation of both TGFβ3 and β-catenin signaling at the initial period of differentiation is effective in enhancing chondrogenic differentiation of MSC as continuous co-activation, resulting in earlier and enhancement in matrix protein deposition, compared with activation with TGFβ alone.
Hypertrophic development of chondrocytes affected by the extent of β-catenin signaling activation
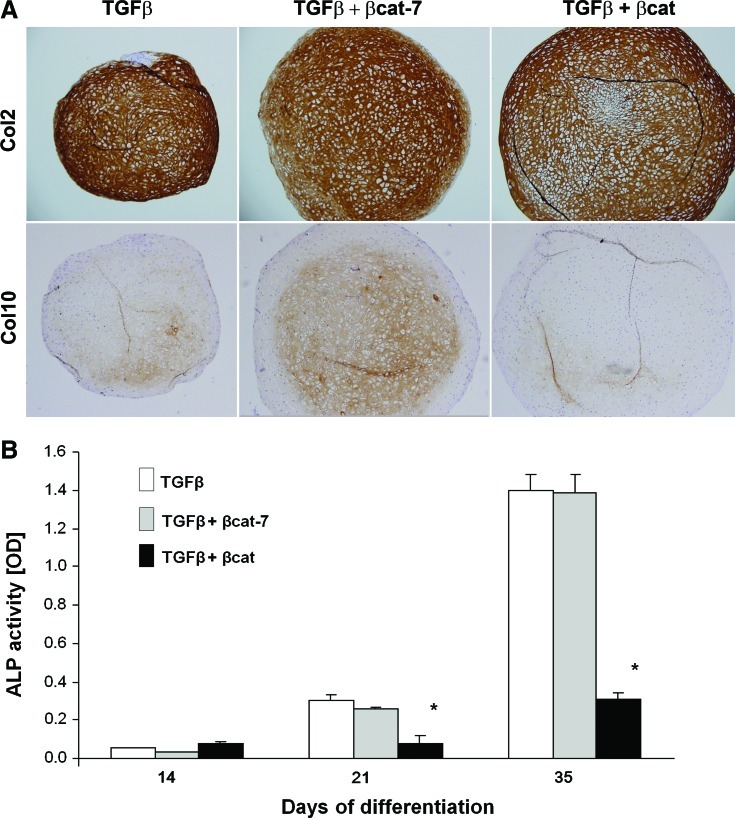
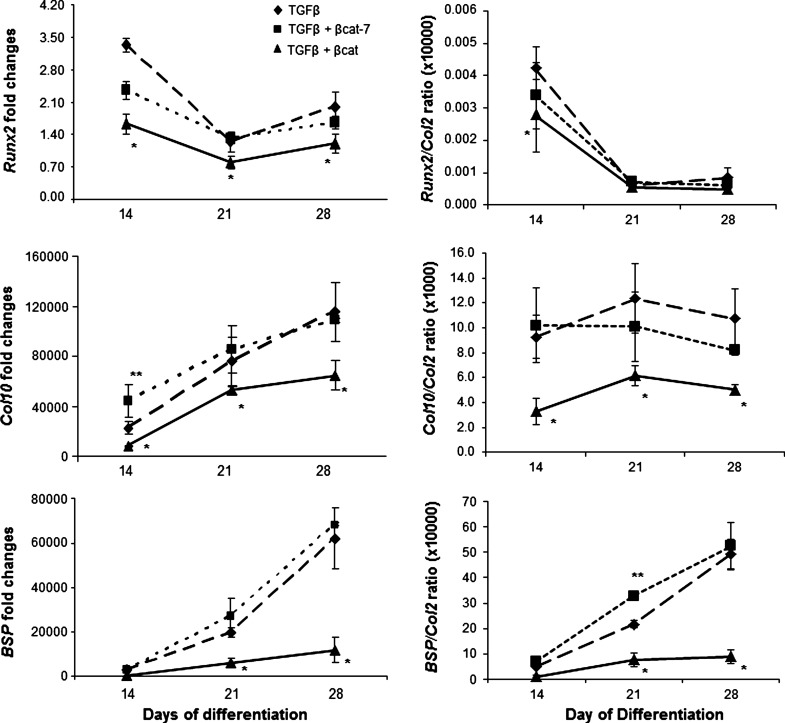
We next examined the development of the MSC-derived chondrocytes to the hypertrophic state in our samples. Immunohistochemical analysis revealed that type X collagen was detectable in the TGFβ-activated sample at day 28, and expression was further increased by day 35 (Fig. 2A). The time-dependant increase in ColX protein was accompanied by the gradual increase in Col10 and BSP mRNA (Fig. 3) indicative of progression toward hypertrophy. Comparatively, expression of ColX protein was enhanced in the transient co-activation samples, which was detectable as early as at day 21, and appeared to be higher at day 35 than the TGFβ-activated samples (Fig. 2A). An early increase in Col10 mRNA expression was detected for the transient co-activation sample at day 14, compared with the control TGFβ-treated sample (Fig. 3). However, no significant difference in Col10 mRNA expression was detected at later time points. The expression of Runx2 and BSP mRNA and alkaline phosphatase activities (Figs. 3 and 2B) in the transient co-activation was comparable to the TGFβ-treated samples. On the other hand, the expression of ColX in the continuous co-activation samples was inhibited at all time points, with the complete lack of ColX except for a trace detected in the day 35 sample (Fig. 2A). Noticeably, the expression of type II collagen was not significantly different at this time point (Fig. 2A, see also Supplementary Fig. S2), indicating that the negative detection of ColX was not due to a lack of chondrogenesis in the continuous co-activation samples. Real-time RT-PCR analysis consistently registered an inhibition of Col10 mRNA in the continuous co-activation samples, which was also accompanied by inhibition in Runx2 and BSP expression at all time points (Fig. 3). The suppressed mRNA expression of Col10 and BSP was maintained after being corrected for its own degree of differentiation presented relative to Col2 expression in the form of Runx2/Col2, Col10/Col2, and BSP/Col2 ratio. The inhibition of hypertrophy development in the continuous co-activation sample was further reflected by the alkaline phosphatase activities, with activities detected only by day 35 and at levels more than 4-fold lower than the other 2 groups (Fig. 2B).
FIG. 2.
Characterization of hypertrophy development of cartilage pellets. MSC pellets were subjected to chondrogenic differentiation in TGFβ, TGFβ with transient or continuous β-catenin co-activation. (A) Pellets harvested at day 35 were immunostained for Type II and Type X collagen. Representative micrograms from 3 independent experiments of 3 different donors' MSCs are shown. (B) Alkaline phosphatase activity in the culture supernatants of MSC pellets derived at various time points during chondrogenesis were analyzed. Results of TGFβ group are represented by white bar; TGFβ+transient β-catenin co-activation by gray bar; and TGFβ+continuous β-catenin co-activation by black bar. Images were taken at ×100 magnification. Data shown are means±SD (n=3, *p<0.05 significant lower compared with TGFβ samples). alkaline phosphatase. Color images available online at www.liebertonline.com/scd
FIG. 3.
Expression level of hypertrophic marker genes was quantified by real-time polymerase chain reaction. Results of TGFβ group are represented by dashed lines with diamonds; TGFβ+transient β-catenin co-activation by dotted lines with squares; and TGFβ+continuous β-catenin co-activation by solid lines with triangles. Expression levels relative to Col2 expression were presented as Runx2/Col2, Col10/Col2, and BSP/Col2 ratio, as means±SD (n=3). **P<0.05 significant increase and *P<0.05 significant decrease, compared with TGFβ samples.
Differential maturation outcome of cartilage after ectopic implantation is affected by temporal β-catenin predifferentiation
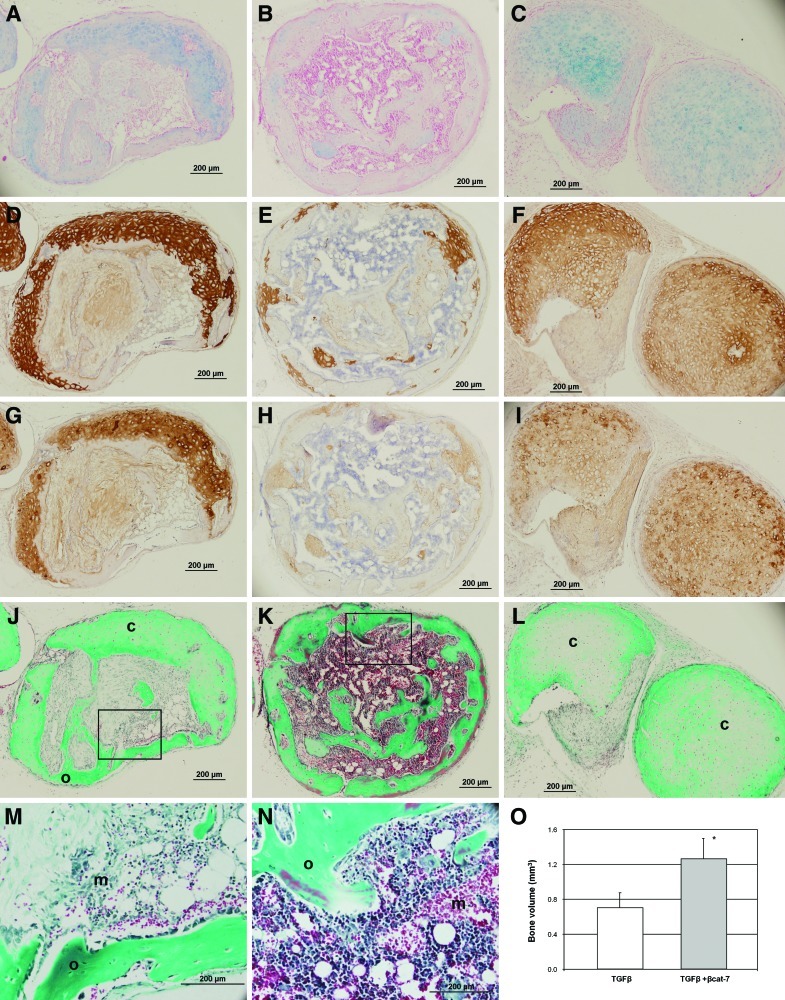
The chondrogenic maturation development of the in vitro MSC-derived cartilage-like tissues was followed up after subcutaneous implantation of the pellets in nude mice. MSC pellets were subjected to 5 weeks of in vitro differentiation. After 8 weeks in vivo, pellets predifferentiated with TGFβ and transient co-activation underwent a spectrum of endochondral ossification development (listed in Table 1); ranging from pellets that maintained their cartilage appearance (with strong positive staining of type II collagen), pellets that underwent remodeling consisting of partial cartilage tissue, and fibrous tissues with vascular invasion (Fig. 4J), to highly ossified tissues encapsulating an inner region of marrow-like hematopoietic tissue with leukocytes, red blood cells, and fat deposits (Fig. 4K). In the TGFβ-treated group (n=12), 2 pellets were unretrievable (presumably degenerated), and 2 pellets had undergone full endochondral ossification, forming matured ossified tissues. Four pellets retained cartilaginous features with positive staining for Alcian blue, type II, and X collagen but little sign of calcification and no vessel formation (image not shown), and 4 pellets underwent the remodeling process consisting of partial cartilage tissue and fibrous tissue with vascular invasion and the appearance of marrow-like hematopoietic tissue next to ossified tissues (Fig. 4J). Comparatively, all pellets in the transient co-activation group (n=11) remained as solid pellets that were easily retrievable. Significantly more pellets (45% compared with 17% in the TGFβ-treated group, Table 1) developed into highly ossified tissues with marrow-like hematopoietic tissue (Fig. 4K) with almost a complete lack of proteoglycan and remnant of type II collagen positive regions. Less pellets retained their cartilage characteristics (18% compared with 33% in the TGFβ-treated group) and a similar number of pellets in the remodeling stage (36% compared with 33% in the TGFβ-treated group). μCT measurement of the bone volume in the pellets after subcutaneous implantation shows a doubling of bone formation in the transient co-activation group compared with the TGFβ-treated group (Fig. 4O).
Table 1.
In Vivo Fate of In Vitro Predifferentiated Cartilage-Like Pellets in Subcutaneous Environment
| In vitro treatment | TGFβ | TGFβ+βcat-7 | TGFβ+βcat |
|---|---|---|---|
| Sample size | 12 | 11 | 7 |
| Degenerated casea | 2 (17%) | 0 (0%) | 4 (57%) |
| Cartilage caseb | 4 (33%) | 2 (18%) | 3 (43%) |
| Remodeling casec | 4 (33%) | 4 (36%) | 0 (0%) |
| Ossification cased | 2 (17%) | 5 (45%) | 0 (0%) |
Un-retrievable pellets after 8 weeks in vivo.
Pellets retaining cartilaginous features with no remodeling.
Pellets undergoing remodeling consisting of cartilage with invading vessels, fibrous tissue, and ossification.
Pellets with mature bone structure surrounding an inner region of marrow-like hematopoietic tissue with leukocytes, red blood cells, and fat tissue. Proportion of each type of pellet tissue within a group was expressed as percentage.
FIG. 4.
Characterization of ectopic cartilage implants. MSC pellets were subjected to 5 weeks differentiation in TGFβ (A, D, G, J, M), TGFβ+transient (B, E, H, K, N) or continuous β-catenin (C, F, I, L) co-activation and implanted subcutaneously in SCID mice for 8 weeks. Serial paraffin sections were stained with alcian blue for proteoglycan (A–C), collagen type II (D–F) and type X (G–I), and Masson's Trichrome (J–N) for ossified tissue with strong blue/green hue and cartilage with tissue whitish green. Representative TGFβ-treated pellet underwent remodeling consisting of partial cartilage tissue and fibrous tissue (A, D, G, J) with vascular invasion and the appearance of marrow-like hematopoietic tissue next to ossified tissues (M, enlarged image of J). Representative of a transient co-activation pellet at the end stage of endochodral ossification with highly ossified tissue surrounding marrow-like hematopoietic inner region (K, N; enlarged image of K). Representative of a continuous co-activation pellet with positive staining for type II collagen (F), which have entered their early hypertrophic phase, staining positive for type X collagen (I), and a low amount of calcification without detectable vessel formation (L). Pellet and insert images were taken at ×100 and ×400 magnification, respectively. c, cartilage; m, marrow-like hematopoietic tissue; o, ossified tissue. μCT measurement of the bone volume in the pellets after subcutaneous implantation shows doubling of bone formation in the transient co-activation group compared with the TGFβ-treated group (O). *P<0.05 significant increase compared with TGFβ samples. Color images available online at www.liebertonline.com/scd
In the continuous co-activation group (n=7), more than half of the implanted pellets (57%, Table 1) were unretrievable, and the remaining 3 pellets retained cartilaginous features with positive staining for Alcian blue and type II collagen (Fig. 4C, F). These pellets have entered their early hypertrophic phase, staining positive for type X collagen (Fig. 4I) but were without detectable vessel formation (Fig. 4L). None of the retrievable pellets in this group was in the tissue remodeling or highly ossified stage.
Expression of PTHrP and cyclin D1
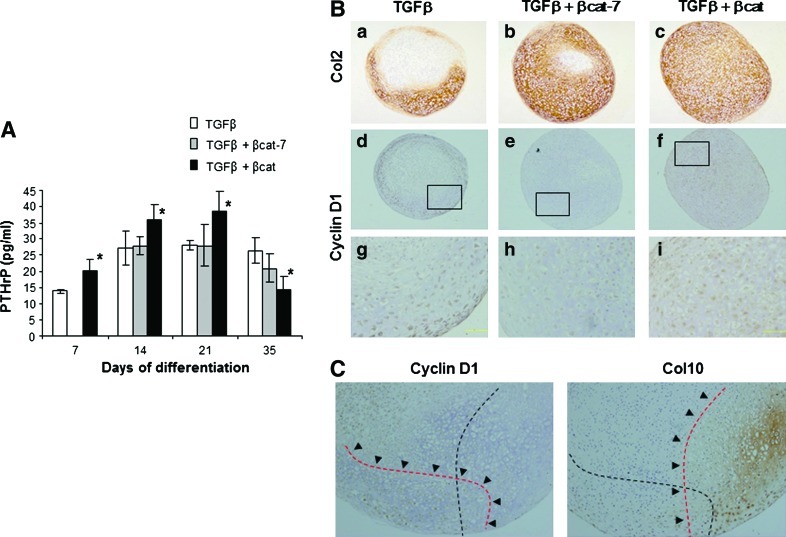
Expression of PTHrP and cyclin D1 has been associated with the hypertrophic development of chondrocytes [31]. We next examined the levels of PTHrP in the culture supernatant and the expression of cyclin D1 in the in-vitro differentiated samples. MSCs that had undergone chondrogenic differentiation with TGFβ treatment secreted an increased level of PTHrP but plateaued by day 14 (Fig. 5A). Cells in the transient co-activation sample secreted similar levels of PTHrP. Comparatively, the level of PTHrP in the continuous co-activation was significantly higher, up to day 21, than both the other groups. Cyclin D1 was differentially expressed in the 3 groups of treatment at differentiation stage of day 21 and day 28, with some donor variability. Even though both pellets of the transient and continuous co-activated groups expressed similar levels of type II collagen, cyclin D1 was not detected in chondrocytes of the transient co-activation pellet, but was prominently expressed in nuclei of most cells in the continuous co-activation pellet (Fig. 5B). In the TGFβ-treated pellet, chondrocytes in the hypertrophy zone, as marked by the expression of type X collagen, did not express cyclin D1. Instead, cyclin D1 cellular expression was detected in some regions with absence of type X collagen expression (Fig. 5C). Our data show that the continuous co-activation of TGFβ and β-catenin signaling pathway resulted in heightened secretion of PTHrP and expression of cyclin D1 in the chondrocytes which might have a role in the inhibition of chondrocyte hypertrophy.
FIG. 5.
Expression of PTHrP and cyclin D1. (A) PTHrP levels in the culture supernatants of MSC pellets at various time points during chondrogenesis were analyzed. Data shown are means±SD (n=3, *P<0.05 significant increase compared with TGFβ samples). (B) MSC pellet subjected to 4 weeks differentiation in TGFβ (a, b, g), TGFβ+transient (b, e, h), or continuous β-catenin (c, f, i) co-activation. Sections were immunostained for Col2 (a, b, c; taken at ×100 magnification) or Cyclin D1 (d, e, f; taken at ×100 magnification, g, h, i are the respective ×320 enlarged images). Despite a similar Col2 expression pattern, Cyclin D1 was not detected in chondrocytes of the transient co-activation pellet, but was prominently expressed in nuclei of most cells in the continuoused co-activation pellet. (C) Region of Cyclin D1 expression was exclusive from expression region of Col10. Images were taken at ×160 magnification. PTHrP, parathyroid hormone-related protein. Color images available online at www.liebertonline.com/scd
Discussion
The involvement of β-catenin signaling in the process of chondrogenic differentiation is complex, in which both inhibiting [16–18] and enhancing effects have been reported [19]. In TGFβ-induced chondrogenesis of human MSCs, β-catenin signaling has been implicated with the up-regulation of several components of Wnt-signaling pathways, the accumulation of β-catanin in the nuclear, and the promotion of β-catenin activated transcriptional activity [21,22]. Indeed, β-catenin together with TGFβ are 2 of the predicted pathways significant for the commitment of MSC to chondrogenic lineage [32].
In this study, we investigated the temporal requirement for the activation of the TGFβ and β-catenin pathway again by employing LiCl, and found that continuous activation of TGFβ-signaling is essential (data not shown), an observation previously reported [33]. We showed that a transient first 7 days co-induction of TGFβ and β-catenin activation is just as effective in enhancing MSC chondrogenic differentiation as continuous co-induction of both pathways. When following up the hypertrophic development of the induced cells, we found striking differences in chondrogenic maturation between the transient and the continuous β-catenin co-activation samples. Although transient co-activation did not show obvious effect on hypertrophy development in vitro, apart from increased type X collagen deposition, a heightened development towards hypertrophy was detected after ectopic implantation. We detected a higher number of pellets with mature ossification and robust formation of marrow-like hematopoietic tissue in the transient co-activation group compared with the control TGFβ group. Remarkably, continuous co-activation of TGFβ-induced MSC chondrogenesis with β-catenin activation consistently induced a significant reduction in hypertrophy in vitro, as evident from the down regulation of Col10 and BSP mRNA, the reduction in deposition of type X collagen, and alkaline phosphatase activity with prolonged in vitro culture up to 5 weeks. Comparatively, retrievable pellets from the continuous co-activation group, inspite of expressing type X collagen, none had signs of remodeling, vascular formation, or mature ossification. It was reported that the fate of the implanted MSC-derived cartilage was dependent on the differentiated stage and the maturity level of the in-vitro engineered cartilage [3,6,7]. Dickhut et al. show that MSC-derived chondrogenic pellets with high collagen type II but low ALP-activity before ectopic transplantation correlated with marginal calcification of explants and that nonmineralizing transplants have been found to be susceptible to ectopic site degradation [7]. This and our result indicate that unless chondrogenic differentiation of MSC has been locked in at the appropriate differentiation stage, dedifferentiation of cell and loss of cartilage tissue readily occurred at the ectopic site. The observation that pellets from the continuous co-activation group either disintegrated or did not enter into a mature ossification stage further indicates that chondrogenic cells in these pellets after 5 weeks of in vitro predifferentiation remained at the prehypertrophic developmental stage. The pellets from the transient co-activation group, although not showing a significant difference in hypertrophy development compared with the control in in-vitro circumstances, were, however, more prone to hypertrophy maturation in vivo.
To the best of our knowledge, this article is the first that provides evidence on the hypertrophic and maturation development affected by β-catenin during co-activation of TGFβ-induced chondrogenesis of MSCs. Progression of MSC-derived chondrogenic cells toward hypertrophy is inevitable in in vitro culture [3,27,34]. Inhibition of MSC hypertrophy has so far been achieved by the provision of a specific ECM environment such as chondroitin sulfate [28,35], type II collagen [36], collagen mimetic peptide niche [37], or novel nitrogen-rich plasma polymer coating [38]. Co-culturing with articular chondrocytes [39–41] has recently been shown to inhibit terminal differentiation of MSC-derived chondrocytes in which PTHrP, produced by articular chondrocytes, is the likely mediator [41]. PTHrP is a critical growth factor in regulating chondrocyte function in the growth plate; maintaining chondrocytes in a proliferative state; and preventing premature chondrocyte hypertrophy by suppressing Runx2, Col10, ALP activity, and matrix mineralization [42–44]. The mechanism of PTHrP in controlling chondrocyte maturation has been linked to the reduction of Runx proteins in a cyclin D1-dependent manner [31]. In our study, we detected significantly higher levels of PTHrP in the β-catenin continuous coactivation samples. In addition, we detected widespread nuclei expression of cyclin D1 in the chondrocytes of the continuous coactivation pellets. In contrast, cyclin D1 expression was absent in the chondrocytes of transient co-activation pellets. Our results suggest that increased secretion of PTHrP from chondrocytes might be one of the factors in the suppression of hypertrophic development in the continuous co-activation treatment, and its action might be, in part, through the autocrine regulation of cyclin D1 expression in the chondrocytes. A more confirmative implication of the role of PTHrP and cyclin D1 would require specific inhibitor blocking of PTHrP action and genetic regulation of cyclin D1 expression. Heightened expression of PTHrP and cyclin D1 might be responsible for the inhibition of chondrocyte hypertrophy during continuous TGFβ and β-catenin coactivation, but the underlying mechanism in the facilitation of hypertrophy after transient coactivation is unclear. It is worth noting that in vivo transient activation of β-catenin signaling was shown to induce abnormal growth plate closure in part due to the accelerated hypertrophic and endochondral ossification development [45].
It has been well documented that β-catenin signaling was involved in the induction of chondrocyte hypertrophy of growth plate chondrocytes [24–26]. It is important to note that these studies utilize mature chondrocytes isolated from the upper sternum which represents a source of chondrocytes at the verge of hypertrophic development, and that β-catenin signaling was activated in the absence of other pathways. β-catenin signaling has distinct and complex effects on stem cells and chondrocytes depending on the cellular phenotypic properties and developmental status. In our study, undifferentiated stem cells were used, and the effect of β-catenin was delivered in concert with TGFβ-activation. TGFβ plays an essential role in stimulating chondrocyte proliferation and the inhibition of chondrocyte hypertrophic differentiation, in which the former was shown to be through the β-catenin signaling-dependent cyclin D1 expression [46,47]. It is likely that with the continuous co-activation of β-catenin, MSC-derived chondrocytes were maintained in the proliferative, prehypertrophy stage via the action of cyclin D1. In vivo, the fate of these cells will be subjected to biochemical and mechanical factors in the microenvironmental niches. It is the subject of future study to find out how these predifferentiated cells fare in an in vivo orthotopic site and whether the maintenance of nonhypertrophy status will be upheld.
In summary, our article reports that temporal co-activation of TGFβ-induced MSC differentiation with β-catenin activation can yield cartilage with different phenotypes and represents the potential for devising MSC predifferentiation protocols before clinical implantation that might be applicable to the engineering of different types of cartilage tissue. Chondrocytes derived from the TGFβ and β-catenin co-activated MSCs having their hypertrophic development reduced might retain a degree of plasticity that can be further molded by the niche environment at the articular cartilage site. Continued studies that determine the optimal chondrogenic predifferentiation condition and the in vivo development of the predifferentiated MSC at a natural joint environment will be followed up.
Supplementary Material
Acknowledgments
The authors thank Jiabing Fan for assistance with the animal study. This study was supported by A*STAR/Singapore Stem Cell Consortium (grant number: SSCC-06-008).
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Goldring MB. Tsuchimochi K. Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33–44. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 2.Pelttari K. Steck E. Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58–S65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 3.Pelttari K. Winter A. Steck E. Goetzke K. Hennig T. Ochs BG. Aigner T. Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 4.De Bari C. Dell'Accio F. Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142–150. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- 5.Hennig T. Lorenz H. Thiel A. Goetzke K. Dickhut A. Geiger F. Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 6.Liu K. Zhou GD. Liu W. Zhang WJ. Cui L. Liu X. Liu TY. Cao Y. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials. 2008;29:2183–2192. doi: 10.1016/j.biomaterials.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Dickhut A. Pelttari K. Janicki P. Wagner W. Eckstein V. Egermann M. Richter W. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219–226. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 8.Guo X. Wang C. Zhang Y. Xia R. Hu M. Duan C. Zhao Q. Dong L. Lu J. Qing SY. Repair of large articular cartilage defects with implants of autologous mesenchymal stem cells seeded into beta-tricalcium phosphate in a sheep model. Tissue Eng. 2004;10:1818–1829. doi: 10.1089/ten.2004.10.1818. [DOI] [PubMed] [Google Scholar]
- 9.Wilke MM. Nydam DV. Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25:913–925. doi: 10.1002/jor.20382. [DOI] [PubMed] [Google Scholar]
- 10.Steck E. Fischer J. Lorenz H. Gotterbarm T. Jung M. Richter W. Mesenchymal stem cell differentiation in an experimental cartilage defect: restriction of hypertrophy to bone-close neocartilage. Stem Cells Dev. 2009;18:969–978. doi: 10.1089/scd.2008.0213. [DOI] [PubMed] [Google Scholar]
- 11.Zscharnack M. Hepp P. Richter R. Aigner T. Schulz R. Somerson J. Josten C. Bader A. Marquass B. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an Ovine Model. Am J Sports Med. 2010;38:1857–1869. doi: 10.1177/0363546510365296. [DOI] [PubMed] [Google Scholar]
- 12.Marquass B. Schulz R. Hepp P. Zscharnack M. Aigner T. Schmidt S. Stein F. Richter R. Osterhoff G, et al. Matrix-associated implantation of predifferentiated mesenchymal stem cells versus articular chondrocytes: in vivo results of cartilage repair after 1 year. Am J Sports Med. 2011;39:1401–1412. doi: 10.1177/0363546511398646. [DOI] [PubMed] [Google Scholar]
- 13.Wakitani S. Nawata M. Tensho K. Okabe T. Machida H. Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 14.Kuroda R. Ishida K. Matsumoto T. Akisue T. Fujioka H. Mizuno K. Ohgushi H. Wakitani S. Kurosaka M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15:226–231. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Chun JS. Oh H. Yang S. Park M. Wnt signaling in cartilage development and degeneration. BMB Rep. 2008;41:485–494. doi: 10.5483/bmbrep.2008.41.7.485. [DOI] [PubMed] [Google Scholar]
- 16.Church V. Nohno T. Linker C. Marcelle C. Francis-West P. Wnt regulation of chondrocyte differentiation. J Cell Sci. 2002;115:4809–4818. doi: 10.1242/jcs.00152. [DOI] [PubMed] [Google Scholar]
- 17.Stott NS. Jiang TX. Chuong CM. Successive formative stages of precartilaginous mesenchymal condensations in vitro: modulation of cell adhesion by Wnt-7A and BMP-2. J Cell Physiol. 1999;180:314–324. doi: 10.1002/(SICI)1097-4652(199909)180:3<314::AID-JCP2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Hwang SG. Yu SS. Lee SW. Chun JS. Wnt-3a regulates chondrocyte differentiation via c-Jun/AP-1 pathway. FEBS Lett. 2005;579:4837–4842. doi: 10.1016/j.febslet.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 19.Yano F. Kugimiya F. Ohba S. Ikeda T. Chikuda H. Ogasawara T. Ogata N. Takato T. Nakamura K. Kawaguchi H. Chung UI. Canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun. 2005;333:1300–1308. doi: 10.1016/j.bbrc.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 20.Fischer L. Boland G. Tuan RS. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002;277:30870–30878. doi: 10.1074/jbc.M109330200. [DOI] [PubMed] [Google Scholar]
- 21.Tuli R. Tuli S. Nandi S. Huang X. Manner PA. Hozack WJ. Danielson KG. Hall DJ. Tuan RS. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 22.Zhou S. Eid K. Glowacki J. Cooperation between TGF-beta and Wnt pathways during chondrocyte and adipocyte differentiation of human marrow stromal cells. J Bone Miner Res. 2004;19:463–470. doi: 10.1359/JBMR.0301239. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann C. Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- 24.Tamamura Y. Otani T. Kanatani N. Koyama E. Kitagaki J. Komori T. Yamada Y. Costantini F. Wakisaka S, et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 25.Dong YF. Soung DY. Schwarz EM. O'Keefe RJ. Drissi H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol. 2006;208:77–86. doi: 10.1002/jcp.20656. [DOI] [PubMed] [Google Scholar]
- 26.Wang L. Shao YY. Ballock RT. Thyroid hormone interacts with the Wnt/beta-catenin signaling pathway in the terminal differentiation of growth plate chondrocytes. J Bone Miner Res. 2007;22:1988–1995. doi: 10.1359/jbmr.070806. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone B. Hering TM. Caplan AI. Goldberg VM. Yoo JU. In vitro chondrogenesis of bone marrowderived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 28.Wu YN. Yang Z. Hui JH. Ouyang HW. Lee EH. Cartilaginous ECM component-modification of the micro-bead culture system for chondrogenic differentiation of mesenchymal stem cells. Biomaterials. 2007;28:4056–4067. doi: 10.1016/j.biomaterials.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 29.Lucas FR. Goold RG. Gordon-Weeks PR. Salinas PC. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci. 1998;111:1351–1361. doi: 10.1242/jcs.111.10.1351. [DOI] [PubMed] [Google Scholar]
- 30.van Noort M. Meeldijk J. van der Zee R. Destree O. Clevers H. Wnt signaling controls the phosphorylation status of beta catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M. Xie R. Hou W. Wang B. Shen R. Wang X. Wang Q. Zhu T. Jonason JH. Chen D. PTHrP prevents chondrocyte premature hypertrophy by inducing cyclin-D1-dependent Runx2 and Runx3 phosphorylation, ubiquitylation and proteasomal degradation. J Cell Sci. 2009;122:1382–1389. doi: 10.1242/jcs.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng F. Boucher S. Koh S. Sastry KS. Chase L. Lakshmipathy U. Choong C. Yang Z. Vemuri MC. Rao MS. Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 33.Weiss S. Hennig T. Bock R. Steck E. Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223:84–93. doi: 10.1002/jcp.22013. [DOI] [PubMed] [Google Scholar]
- 34.Mueller MB. Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377–1388. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varghese S. Hwang NS. Canver AC. Theprungsirikul P. Lin DW. Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Bosnakovski D. Mizuno M. Kim G. Takagi S. Okumura M. Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ. Yu C. Chansakul T. Hwang NS. Varghese S. Yu SM. Elisseeff JH. Enhanced chondrogenesis of mesenchymal stem cells in collagen mimetic peptide-mediated microenvironment. Tissue Eng Part A. 2008;14:1843–1851. doi: 10.1089/ten.tea.2007.0204. [DOI] [PubMed] [Google Scholar]
- 38.Mwale F. Girard-Lauriault PL. Wang HT. Lerouge S. Antoniou J. Wertheimer MR. Suppression of genes related to hypertrophy and osteogenesis in committed human mesenchymal stem cells cultured on novel nitrogen-rich plasma polymer coatings. Tissue Eng. 2006;12:2639–2647. doi: 10.1089/ten.2006.12.2639. [DOI] [PubMed] [Google Scholar]
- 39.D'Angelo M. Pacifici M. Articular chondrocytes produce factors that inhibit maturation of sternal chondrocytes in serum-free agarose cultures: a TGF-β independent process. J Bone Miner Res. 1997;12:1368–1377. doi: 10.1359/jbmr.1997.12.9.1368. [DOI] [PubMed] [Google Scholar]
- 40.Jikko A. Kato Y. Hiranuma H. Fuchihata H. Inhibition of chondrocyte terminal differentiation and matrix calcification by soluble factors released by articular chondrocytes. Calcif Tissue Int. 1999;65:276–279. doi: 10.1007/s002239900698. [DOI] [PubMed] [Google Scholar]
- 41.Fischer J. Dickhut A. Richter W. Rickert M. Articular chondrocytes secrete PTHrP and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62:2696–2706. doi: 10.1002/art.27565. [DOI] [PubMed] [Google Scholar]
- 42.Iwamoto M. Shimazu A. Pacifici M. Regulation of chondrocyte maturation by fibroblast growth factor-2 and parathyroid hormone. J Orthop Res. 1995;13:838–845. doi: 10.1002/jor.1100130606. [DOI] [PubMed] [Google Scholar]
- 43.O'Keefe RJ. Loveys LS. Hicks DG. Reynolds PR. Crabb ID. Puzas JE. Rosier RN. Differential regulation of type-II and type-X collagen synthesis by parathyroid hormone-related protein in chick growth-plate chondrocytes. J Orthop Res. 1997;15:162–174. doi: 10.1002/jor.1100150203. [DOI] [PubMed] [Google Scholar]
- 44.Riemer S. Gebhard S. Beier F. Poschl E. von der Mark K. Role of c-fos in the regulation of type X collagen gene expression by PTH and PTHrP: localization of a PTH/PTHrPresponsive region in the human COL10A1 enhancer. J Cell Biochem. 2002;86:688–699. doi: 10.1002/jcb.10260. [DOI] [PubMed] [Google Scholar]
- 45.Yuasa T. Kondo N. Yasuhara R. Shimono K. Mackem S. Pacifici M. Iwamoto M. Enomoto-Iwamoto M. Transient activation of Wnt/{beta}-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am J Pathol. 2009;175:1993–2003. doi: 10.2353/ajpath.2009.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong YF. Soung do Y. Chang Y. Enomoto-Iwamoto M. Paris M. O'Keefe RJ. Schwarz EM. Drissi H. Transforming growth factor-beta and Wnt signals regulate chondrocyte differentiation through Twist1 in a stage-specific manner. Mol Endocrinol. 2007;21:2805–2820. doi: 10.1210/me.2007-0199. [DOI] [PubMed] [Google Scholar]
- 47.Li TF. Chen D. Wu Q. Chen M. Sheu TJ. Schwarz EM. Drissi H. Zuscik M. O'Keefe RJ. Transforming growth factor-beta stimulates cyclin D1 expression through activation of beta-catenin signaling in chondrocytes. J Biol Chem. 2006;281:21296–21304. doi: 10.1074/jbc.M600514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.