Abstract
Since human mesenchymal stem cells (MSCs) are therapeutically attractive for tissue regeneration and repair, we examined the physiological responses of human umbilical cord blood–derived MSCs (hUCB-MSCs) to genotoxic stress. We found that that sublethal doses of reactive oxygen species (ROS) and ionizing radiation cause DNA damage and reduce DNA synthesis and cell proliferation in hUCB-MSCs, resulting in cellular senescence. In contrast, these physiological changes were limited in human fibroblast and cancer cells. Our data show that reduced activities of antioxidant enzymes, which may occur due to low gene expression levels, cause hUCB-MSCs to undergo cellular senescence in response to oxidative stress and ionizing radiation. Resistance of hUCB-MSCs to oxidative stresses was restored by increasing the intracellular antioxidant activity in hUCB-MSCs via exogenous addition of antioxidants. Therefore, the proliferation and fate of hUCB-MSCs can be controlled by exposure to oxidative stresses.
Introduction
Genotoxic stress, caused by factors such as ionizing radiation, ultraviolet light, reactive oxygen species (ROS), and chemical mutagens, leads to nucleotide modifications and DNA breaks [1,2]. Since DNA damage is deleterious to cell proliferation, genomic surveillance systems—including the DNA damage checkpoint signaling pathway—arrest cell cycle progression, thus allowing DNA repair and cell survival [3,4]. If DNA damage is not properly repaired, cells will progress to cellular senescence, apoptosis, or cancer. Cellular senescence is a phenomenon in which cells lose the ability to proliferate due to irreversible cell cycle arrest. Apoptosis is programmed cell death, a cellular form of suicide that removes damaged cells from a cell population. Cell fate may depend upon the cell type, as well as the intensity, duration, and nature of DNA damage [5,6].
Ionizing radiation generates a variety of DNA lesions, including oxidized base damage, abasic sites, single-strand breaks, and double-strand breaks. These lesions induce DNA damage response through activation or repression of distinct target proteins that prevent the proliferation of damaged cells and facilitate DNA repair [7]. ROS, such as oxygen ions, oxygen free radicals, and peroxide, induce oxidative stress that causes DNA and cell damage [8,9]. Although excess ROS are deleterious, a certain level of ROS is necessary for important cellular functions. Some cells generate ROS for destruction of invading microbes as well as for cell signaling [10]. Additionally, oxidative stress plays a central role in aging and cell death [11,12].
Oxidative stress has varying cellular effects. Hydrogen peroxide induces either apoptosis or cellular senescence in cultured cell lines [11,13]. Studies have shown that hydrogen peroxide induces apoptosis in cancer cells [14,15]. For example, in response to sublethal concentrations of hydrogen peroxide, early passage human fibroblasts undergo growth arrest and cellular senescence, but the cells show cellular apoptosis in response to lethal concentrations [16].
The response of stem cells to oxidative stress is not well understood. Mouse embryonic stem cells are sensitive to DNA damage agents and ROS, which cause them to undergo apoptosis [17,18]. However, other studies have shown that mouse embryonic stem cells possess a high level of antioxidant activity, which is attributable to upregulation of antioxidant and stress-inducible genes, and thereby exhibit more resistance to oxidative stress than differentiated cells [19,20]. In comparison with immortalized human keratinocytes, human skin–derived mesenchymal stem cells (MSCs), which have low antioxidant activity, exhibit more frequent cell death from hydrogen peroxide exposure [21]. However, the levels of antioxidant enzymes in human bone marrow–derived MSCs are similar to those in human skin fibroblasts, and the 2 types of cells exhibit the same degree of resistance to oxidative stress-induced cell death [22].
In this report, we demonstrate that human umbilical cord blood–derived MSCs (hUCB-MSCs) possess low levels of antioxidant enzyme activity because of low gene expression levels. Thus, hUCB-MSCs are particularly susceptible to oxidative stress and ionizing radiation. Sublethal doses of oxidative stress-inducing agents cause cellular senescence in hUCB-MSCs, while exogenous addition of antioxidants to hUCB-MSCs confers resistance against oxidative stress.
Materials and Methods
Cells and cell culture
hUCB-MSCs were obtained from MEDIPOST Co., Ltd. Experiments using hUCB-MSCs were approved by the Institutional Review Board of MEDIPOST Co., Ltd. The hUCB-MSC lines MSC1, MSC2, MSC3, and MSC4 were purified from 4 different donors as previously described [23,24]. Briefly, umbilical cord blood was collected from umbilical veins following neonatal delivery. Informed consent was obtained from the pregnant mothers. The gestational ages were 40+0 weeks for the hUCB-MSC1 donor, 40+4 weeks for the hUCB-MSC2 donor, 40+0 weeks for the hUCB-MSC3 donor, and 39+6 weeks for the hUCB-MSC4 donor. To isolate and grow MSCs from the cord blood, mononuclear cells were harvested using Ficoll–Hypaque solution (d=1.077 g/cm3; Sigma-Aldrich) and were seeded at 5×105 cells/cm2 into culture flasks. After the formation of spindle-shaped cell colonies, the cells were reseeded for expansion. Differentiation characteristics such as osteogenic, chondrogenic, and adipogenic features were confirmed before the onset of this study, as previously described [23]. hUCB-MSCs grown in alpha-minimum essential medium supplemented with 10% fetal bovine serum (FBS) and 50 μg/mL of gentamicin (Gibco) were used for the indicated experiments. Presenescent hUCB-MSCs (passages 3 to 5) were used in this study. U2OS (ATCC no. HTB-96), HeLa (ATCC no. CCL-2), HS68 (ATCC no. CRL-1635), and MRC5 (ATCC no. CCL-171) cell lines were grown in Dulbecco's modified Eagle's medium supplemented with 10% FBS and 1% penicillin/streptomycin (Welgene).
Immunostaining and SA-β-Gal staining
Cells grown on coverslips were fixed using 4% paraformaldehyde for 20 min at room temperature and permeabilized using 0.5% Triton X-100 in phosphate-buffered saline for an additional 20 min. Next, the cells were immunostained with the indicated antibodies for detection of Ki-67 (Abcam), BrdU (Amersham), and p53 (Santa Cruz). Antibodies to phospho-H2AX (Ser139), phospho-Chk1 (Ser137), and phospho-Chk2 (Thr68) were obtained from Cell Signaling. Antibodies to superoxide dismutase 1 (SOD1), SOD2, glutathione peroxidase 1 (GPx1), catalase (gifts from Dr. J.M. Kim), and β-actin (Sigma) were used in Western blot analysis. Senescence-associated β-galactosidase (SA-β-Gal) staining was performed using an SA-β-Gal staining kit (Cell Signaling) according to the manufacturer's instructions.
Comet assay
To detect DNA fragmentation, an alkaline comet assay was performed according to the instructions provided by the manufacturer (Comet Assay® Kit; Trevigen). Harvested cells were mixed with low-melting agarose and spread evenly on a slide, after which they were treated with a lysis buffer. Slides were then immersed in alkaline unwinding solution (pH>13, 200 mM NaOH, 1 mM EDTA) for 1 h at 4°C, submerged in cold electrophoresis buffer (pH>13, 200 mM NaOH, 1 mM EDTA), and subjected to electrophoresis at 300 mA for 30 min. The cells were stained with SYBR® Green I and viewed under a fluorescence microscope. The olive tail moment (OTM) values were quantified using Komet 7.0 software.
Quantification of cell death
Cell counts were performed using a cell suspension (1:1 dilution) in trypan blue (Invitrogen). Viable and nonviable cells were counted using a hemocytometer as trypan blue–unstained and trypan blue–stained cells, respectively, as previously described [25].
Measurement of intracellular ROS levels
Intracellular ROS levels were measured using the cell-permeable substrate, 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Sigma), which is converted to the fluorescent product, 2′,7′-dichlorodihydrofluorescein (DCF), by intracellular esterases and oxidation. Fluorescence intensity was measured using the FACS Calibur instrument (BD Bioscience), with excitation at 480 nm and emission at 530 nm.
Measurement of total antioxidant capacity and antioxidant enzyme activity
The total antioxidant capacity of cell lysates was measured using an Antioxidant Assay Kit (Cayman Chemical Co.) according to the manufacturer's instructions. Enzyme activities of SOD, GPx, and catalase were measured using assay kits (Cayman Chemical Co.).
RNA extraction and semiquantitative reverse transcriptase-polymerase chain reaction
RNA was extracted from the indicated cells by using the RNeasy® mini kit (Qiagen). A total of 1 μg of RNA was reverse transcribed using a ONE-STEP reverse transcriptase-polymerase chain reaction (RT-PCR) Premix Kit (Intron) and the following primers: SOD1, 5′-AAG GCC GTG TGC GTG CTG AA-3′ and 5′-CAG GTC TCC AAC ATG CCT CT-3′; SOD2, 5′-GCA CAT TAA CGC GCA GAT CA-3′ and 5′-AGC CTC CAG CAA CTC TCC TT-3′; GPx1, 5′-CCT CAA GTA CGT CCG ACC TG-3′ and 5′-CAA TGT CGT TGC GGC ACA CC-3′; catalase, 5′-GCA GAT ACC TGT GAA CTG TC-3′ and 5′-GTA GAA TGT CCG CAC CTG AG-3′; GADPH 1, 5′-GAG CTG AAC GGG AAG CTC ACT GG-3′ and 5′-CAA CTG TGA GGA GGG GAG ATT CAG-3′. The 24- or 26-cycle samples were subjected to electrophoresis to a 1.5% agarose gel and each band intensity was normalized against the GAPDH1 intensity.
Statistical analysis
For statistical analysis, average values and standard deviations were obtained from at least 3 independent experiments. Difference of effects between hUCB-MSCs and nonstem cells was evaluated by ANOVA. A P value of <0.05 was considered statistically significant. All analyses were performed with R for Windows, version 2.13.1.
Results
Proliferation of hUCB-MSCs is affected by genotoxic stress
Adult stem cells derived from the same tissue type often display varying properties. Therefore, we examined 4 different lineages of hUCB-MSCs (MSC1, MSC2, MSC3, and MSC4), each of which was derived from a different human donor, and compared these cells with untransformed human fibroblast and cancer cell lines. Because MSCs differentiate into fibroblasts in vitro [26], the human primary fibroblast cell lines, MRC5 and HS68, which originate from the lung and foreskin, respectively, were compared with hUCB-MSCs. Two human cancer cell lines, HeLa and U2OS, which are known to be relatively resistant to genotoxic stress, were also used in this study. To investigate cellular responses to genotoxic stress, cells were treated with hydrogen peroxide, which induces oxidative stress, or with γ-rays, which are a form of ionizing radiation [2,27].
To induce oxidative stress, cells were incubated for 2 h in the presence of hydrogen peroxide and then washed and incubated in fresh medium for 24 h. Next, cell proliferation was assessed by immunostaining for Ki-67, a marker of cell proliferation, by using an anti-Ki-67 antibody [28] (Fig. 1A and Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/scd). DNA synthesis was detected by assessing BrdU incorporation using an anti-BrdU antibody (Fig. 1B). Compared with the human fibroblast and cancer cell lines, all 4 types of hUCB-MSCs exhibited more dramatic reductions in both cell proliferation and DNA synthesis following hydrogen peroxide treatment. Ionizing radiation similarly decreased cell proliferation and DNA synthesis of hUCB-MSCs (Fig. 1C, D). These results suggest that the proliferation of hUCB-MSCs is affected by oxidative stress. Because all 4 different hUCB-MSCs responded similarly to hydrogen peroxide and ionizing radiation, we used MSC1 and MSC2 for subsequent experiments.
FIG. 1.
Proliferation of hUCB-MSCs is susceptible to genotoxic stresses. (A, B) hUCB-MSCs (MSC1, 2, 3, and 4), human fibroblast cells (MRC5 and HS68), and cancer cells (U2OS and HeLa) were treated with the indicated concentrations of hydrogen peroxide for 2 h as previously described [22]. The cells were washed twice with PBS and further incubated in fresh medium for 24 h, followed by immunostaining with the anti-Ki-67 (see also Supplementary Fig. S1) or anti-BrdU antibody for detection of cell proliferation or DNA synthesis, respectively. (C, D) Cells irradiated with γ-rays were grown for 24 h and immunostained as described previously. For the BrdU incorporation assay, cells were incubated for 30 min in the presence of 10 μM of BrdU prior to immunostaining as previously described [22]. DNA was visualized using DAPI staining. The proportion of Ki-67 or BrdU-positive cells was determined in each untreated or treated cell population; then the fold difference in proportion between treated and untreated cells is described on graphs as “Proliferation” or “DNA synthesis,” respectively. Graphs represent the averages of at least 3 independent experiments, detecting more than 100 cells each. The percentages (%) of untreated cells exhibiting Ki-67–positive staining were as follows: 90.5, U2OS; 87.5, HeLa; 73.9, HS68; 83.5, MRC5; 82.0, MSC1; 81.0, MSC2; 69.4, MSC3; and 58.8, MSC4. The percentages (%) of untreated cells exhibiting BrdU-positive staining were as follows: 33.3, U2OS; 37.3, HeLa; 36, HS68; 33.7, MRC5; 30.7, MSC1; 30.0, MSC2; 25.3, MSC3; and 28.5, MSC4. The P values were <0.0001 (A), <0.0001 (B), 0.0023 (C), and 0.0004 (D). MSCs, mesenchymal stem cells; hUCB-MSCs, human umbilical cord blood–derived MSCs; PBS, phosphate-buffered saline.
Oxidative stress produces more-severe DNA breaks in hUCB-MSCs
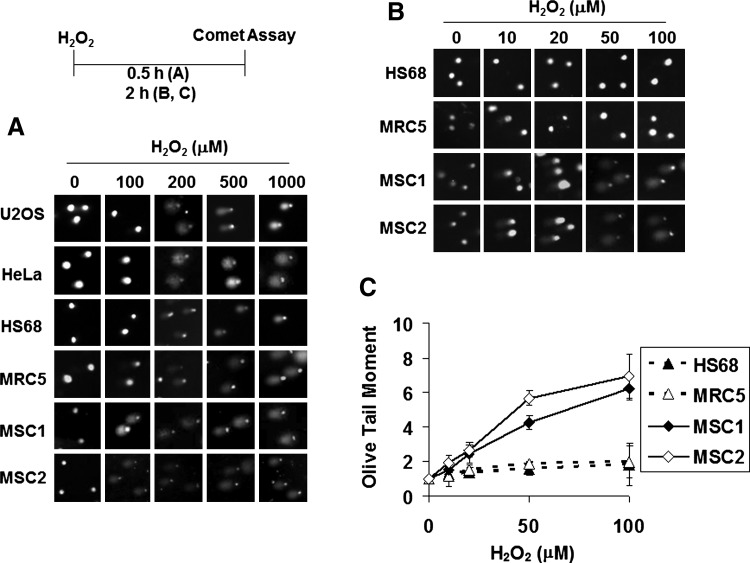
Intracellular ROS cause nucleotide base modification and DNA breaks [1]. This DNA damage inhibits or arrests cell proliferation. The sensitivity of hUCB-MSC proliferation to hydrogen peroxide was analyzed by using the comet assay to detect DNA breaks [29–31]. Each comet tail displayed in gel electrophoresis represents the extent of DNA breakage in a single cell. The comet assay was performed immediately after treating the cells with hydrogen peroxide (Fig. 2A, B). OTM, a parameter determined from the length and percentage of DNA in the tails [29,32], was used to quantify the DNA breaks (Fig. 2C). Whereas human fibroblast and cancer cells clearly exhibited comet tails only at or above 200 μM of hydrogen peroxide, the hUCB-MSCs, MSC1 and MSC2, showed tails at 10 μM of hydrogen peroxide, and the OTM increased in a dose-dependent manner. Although exposure to ionizing radiation followed by incubation of the irradiated cells for 24 h reduced DNA synthesis and proliferation of hUCB-MSCs (Fig. 1B, C), ionizing radiation alone, without further incubation, appeared to generate similar levels of comet tail production in hUCB-MSCs and other cells (Supplementary Fig. S2). These results suggest that DNA breaks are prone to occur in hUCB-MSCs under hydrogen peroxide–induced oxidative stress.
FIG. 2.
Oxidative stress causes more DNA breaks in hUCB-MSCs. (A, B) Cells incubated with the indicated concentration of hydrogen peroxide for 30 min (A) or 2 h (B) were analyzed in a comet assay to measure DNA damage. (C) The olive tail moment values of the 2-h samples were measured using the Komet 7.0 software and indicated as previously described [52,53]. The P value was 0.0124.
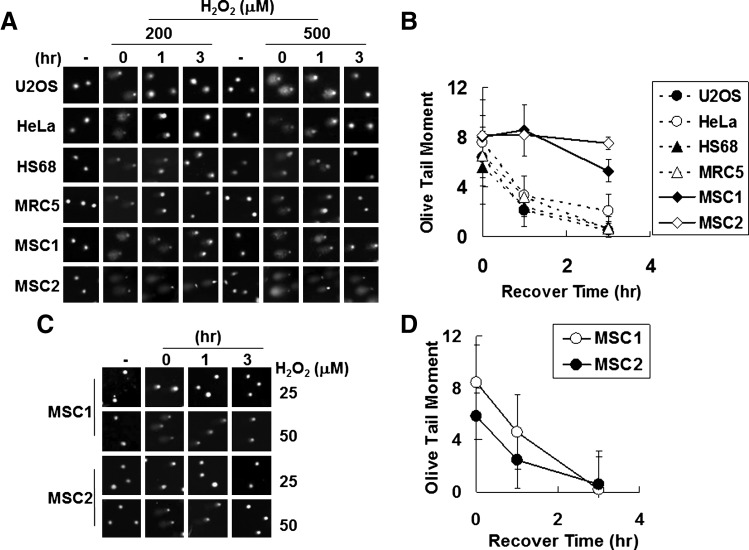
The comet assay can be used to estimate not only the extent of DNA breaks but also the degree of repair of these breaks [29,32]. The capability of recovering from DNA breaks, which reflects the ability to repair DNA damage, was evaluated by measuring the time needed for disappearance of comet tails (Fig. 3). When human fibroblast and cancer cells were treated with 500 μM of hydrogen peroxide, basal OTM levels were reached after approximately 3 h (Fig. 3A, B). However, comet tails persisted at 3 h in hUCB-MSCs, with high OTM values. Because the same hydrogen peroxide concentrations generated more-severe DNA breaks in hUCB-MSCs than in fibroblast and cancer cells (Fig. 2), hUCB-MSCs were then treated with 50 μM of hydrogen peroxide, at which they exhibited similar OTM levels to fibroblast and cancer cells treated with 500 μM of hydrogen peroxide (Fig. 3C, D). At 50 μM of hydrogen peroxide, the OTM values of hUCB-MSCs decreased to basal levels within 3 h.
FIG. 3.
Recovery following DNA damage in hUCB-MSCs. (A, C) Cells treated with the indicated concentration of hydrogen peroxide for 30 min were washed with PBS and then incubated in fresh media for the indicated number of hours. Comet assays were then performed. Values of olive tail moment after treatment with 500 μM (B) or 50 μM (D) of hydrogen peroxide are presented. The P values were <0.0001 (B) and >0.05 (D).
Additionally, we detected activation of proteins involved in DNA damage checkpoint signaling (Supplementary Fig. S3). Phosphorylation of H2AX, Chk1, and Chk2, and accumulation of p53, which is necessary for DNA damage checkpoint signaling [1,3–5,33], was observed in hUCB-MSCs following treatment with hydrogen peroxide, ionizing radiation, or ultraviolet light.
Oxidative stress induces cellular senescence in hUCB-MSCs
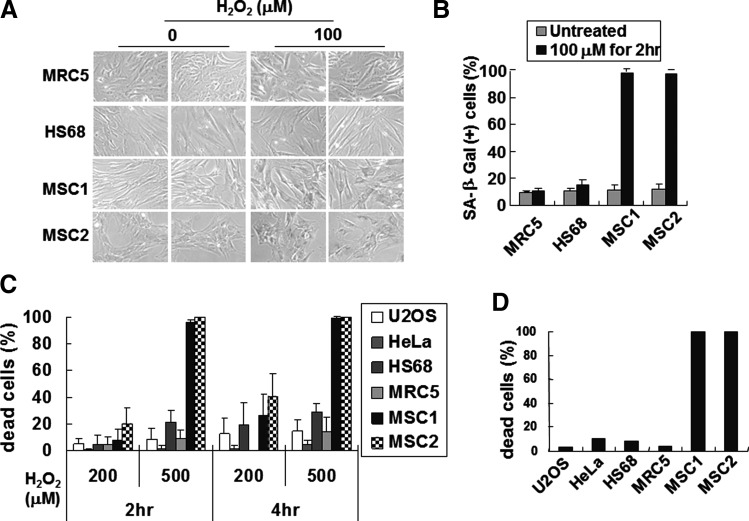
Detection of DNA damage leads to arrest of cell cycle progression until the damage is repaired [1,3,4]. Defects in the recovery of DNA damage often trigger DNA damage-induced cellular senescence or cell death, depending upon the extent of damage and the cell or tissue type [6,34]. Cells treated with hydrogen peroxide for 2 h were washed and further incubated in fresh medium for 4 days (Fig. 4A, B). While the morphologies of the human fibroblast cells did not change significantly, hUCB-MSCs became larger and flatter after treatment with hydrogen peroxide. Staining cells with SA-β-Gal, a marker for detecting cellular senescence [35], revealed that hUCB-MSCs underwent senescence. Moreover, ≥200 μM hydrogen peroxide caused cells to become round or smaller, or to float, indicating cell death (Supplementary Fig. S4). Trypan blue staining confirmed that hydrogen peroxide induces cell death in hUCB-MSCs (Fig. 4C). In contrast, human fibroblast cells were relatively resistant to cellular senescence and cell death. The human cancer cells were also resistant to cell death (Fig. 4C) and cellular senescence (data not shown). This suggests that sublethal doses of hydrogen peroxide induce cellular senescence in hUCB-MSCs.
FIG. 4.
Oxidative stress induces cellular senescence in hUCB-MSCs. (A) Cells incubated with 0 or 100 μM of hydrogen peroxide for 2 h were washed with PBS, and then incubated in fresh medium for 4 days. Cells were then stained with SA-β-Gal. (B) The percentage of cells stained blue (SA-β-Gal positive) is indicated. The data represent the average of 3 independent experiments, using over 150 cells each. (C) Cells incubated with 200 or 500 μM of hydrogen peroxide for 2 or 4 h were stained with trypan blue, and dead cells were scored using a hemocytometer. (D) Cells treated with 10 μM of phorbol-12-myristate-13-acetate for 4 h (see also Supplementary Fig. S5) were stained with trypan blue solution. The P values were <0.001 (B) and 0.0047 (D). SA-β-Gal, senescence-associated β-galactosidase.
Phorbol-12-myristate-13-acetate, which generates ROS through activation of protein kinase C [36], also reduced the viability of hUCB-MSCs (Fig. 4D and Supplementary Fig. S5), indicating that hUCB-MSC sensitivity to oxidative stress is not limited to hydrogen peroxide.
Low antioxidant activity in hUCB-MSCs
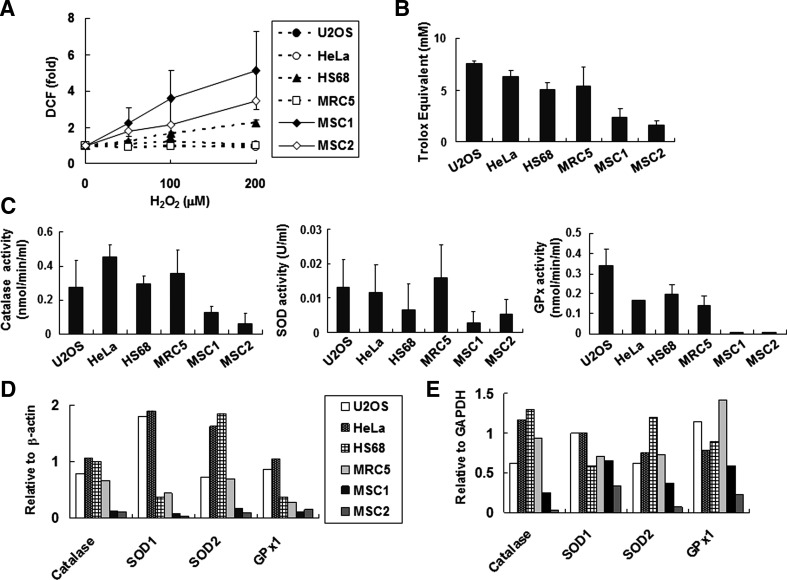
The sensitivity of hUCB-MSCs to oxidative stress suggests that these cells are defective in ROS scavenging. After treatment with hydrogen peroxide, the level of intracellular ROS was measured using H2DCFDA, which is converted to a fluorescent product, DCF, upon oxidation [37] (Fig. 5A). The ROS accumulation after hydrogen peroxide treatment in hUCB-MSCs was greater than that in human fibroblast and cancer cells. Even at 50 μM of hydrogen peroxide, ROS levels in hUCB-MSCs were much higher than those at 200 μM of hydrogen peroxide in fibroblast and cancer cells. These increased intracellular ROS levels suggest that hUCB-MSCs may be defective in ROS scavenging.
FIG. 5.
hUCB-MSCs show low antioxidant activity. (A) Cells incubated with 20 μM of 2′,7′-dichlorodihydrofluorescein diacetate for 30 min were washed, and then incubated with fresh media containing the indicated concentration of hydrogen peroxide for 30 min. Intracellular ROS levels were detected using the FACS Calibur instrument (BD Bioscience). Mean fluorescence intensity of DCF was used as measure of ROS, and values were normalized with the basal intensity of each cell line. (B) Total antioxidant activity in each cell lysate was measured using Trolox E as described in the Materials and Methods section. Values per 1 μg of cell lysate are shown. (C) Activities of catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPx) were measured as described in the Materials and Methods section. Enzyme activity per 1 μg of cell lysate is indicated. (D) 40 μg of total protein from each cell line was immunoblotted with the appropriate antibodies (see also Supplementary Fig. S6A). Relative band intensities normalized to the β-actin band were calculated using Multi Gauge 3.0 software (Fujifilm). SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2; GPx1, glutathione peroxidase 1. (E) 1 μg of mRNA from each cell line was reverse transcribed using specific primers (Materials and Methods). Relative band intensities from gel electrophoresis following RT-PCR (Supplementary Fig. S6B) were measured using Multi Gauge 3.0 software. Quantitative data for each RT-PCR product were normalized against the GAPDH level. The P values were 0.036 (A); 0.017 (B); 0.0081, catalase activity in (C); 0.0076, SOD activity in (C); and <0.0001, GPx activity in (C). DCF, 2′,7′-dichlorodihydrofluorescein; ROS, reactive oxygen species.
Total cellular antioxidant capacities were measured using Trolox, a water-soluble derivative of vitamin E [38] (Fig. 5B). Lysates of hUCB-MSCs grown in the absence of hydrogen peroxide possessed at least 3-fold lower antioxidant capacity than those of fibroblast and cancer cells. Cellular antioxidant activity is largely mediated by enzymes that scavenge ROS, including catalase, SOD, and GPx. Enzyme activities (Fig. 5C), protein amounts as determined using Western blotting (Fig. 5D and Supplementary Fig. S6A), and mRNA levels assessed by RT-PCR (Fig. 5E and Supplementary Fig. S6B) were lower in hUCB-MSCs than in fibroblast and cancer cells. These results suggest that the decreased antioxidant capacity of hUCB-MSCs is caused by low antioxidant enzyme activities due to reduced gene expression.
Exogenous antioxidant increases resistance of hUCB-MSCs to oxidative stress
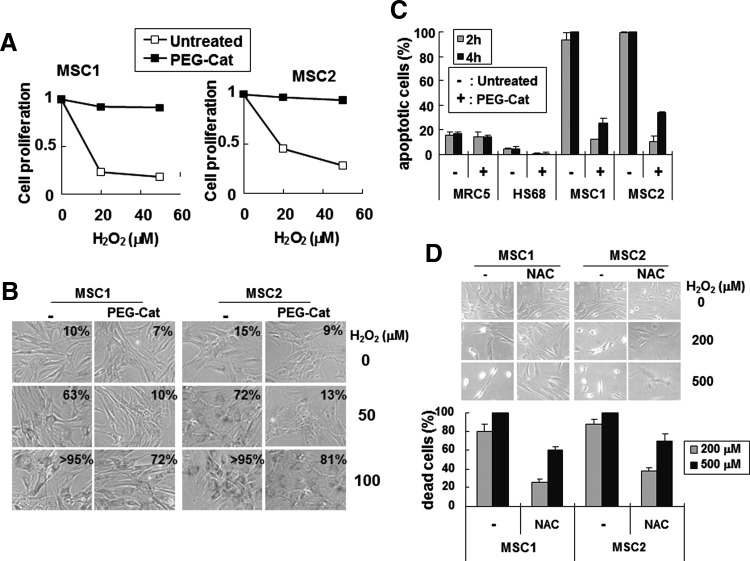
The data described previously indicate that hUCB-MSCs are sensitive to oxidative stress because they contain low levels of antioxidant proteins. Therefore, we examined whether exogenous addition of polyethylene glycol–conjugated catalase (PEG-catalase), a membrane-permeable form of catalase [39], increased resistance of these cells to oxidative stress. Pretreatment with PEG-catalase abrogated the effect of hydrogen peroxide on the proliferation of hUCB-MSCs (Fig. 6A and Supplementary Fig. S7A, B), and reduced the number of senescent cells (Fig. 6B). Additionally, this treatment decreased hydrogen peroxide–induced hUCB-MSC death (Fig. 6C). In contrast, PEG-catalase treatment of human fibroblast cells did not significantly alter cell viability (Supplementary Fig. S7C). Pretreatment with the antioxidant N-acetyl cysteine, an amino acid derivative that serves as a precursor for glutathione synthesis [40], also decreased hydrogen peroxide–induced death of hUCB-MSCs (Fig. 6D). Therefore, increased antioxidant activity confers resistance to oxidative stress in hUCB-MSCs, confirming that the sensitivity of hUCB-MSCs to oxidative stress results from low cellular antioxidant activity.
FIG. 6.
Exogenous addition of antioxidant prevents hUCB-MSCs from undergoing cellular senescence and cell death due to oxidative stress. (A) Cells incubated with 200 U/mL of PEG-catalase (PEG-Cat) for 24 h were further incubated for 2 h in fresh medium containing the indicated concentrations of hydrogen peroxide. The cells were then transferred to fresh medium, grown for 24 h, and immunostained using anti-Ki-67 antibodies (see also Supplementary Fig. S7A, B). (B) Cells pretreated with 200 U/mL of PEG-catalase were incubated in fresh medium for 2 h with hydrogen peroxide. Four days after hydrogen peroxide treatment, the cells were stained using SA-β-Gal. The percentage of SA-β-Gal–positive cells is indicated in each micrograph. (C) PEG-catalase (200 U/mL)–pretreated cells were incubated with 500 μM of hydrogen peroxide for 2 or 4 h. Cells were observed under a phase-contrast microscope (Supplementary Fig. S7C), and harvested cells were stained with trypan blue solution and counted. (D) hUCB-MSC lines, MSC1 and MSC2, were incubated with 1 mM of N-acetyl cysteine (NAC) for 6 h, and then incubated for 2 h in fresh medium containing the indicated concentrations of hydrogen peroxide. Cells were viewed under a phase-contrast microscope, and dead cells were counted using trypan blue staining. The graph shows the results from 3 independent experiments. The P values were <0.0001 (C) and 0.011 (D). PEG-catalase, polyethylene glycol–conjugated catalase.
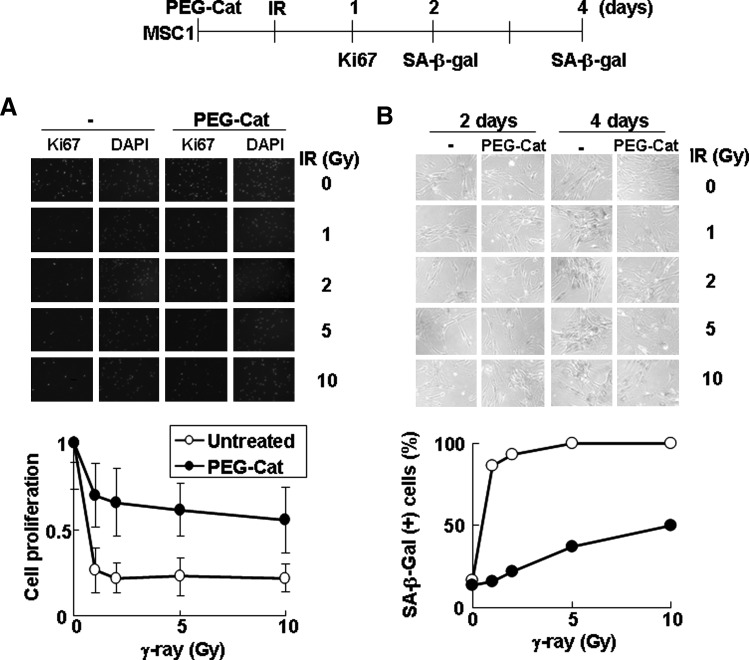
Ionizing radiation generates free radicals such as the hydroxyl radical (OH·), superoxide anion (O2−), and organic radicals (R·) in the presence of oxygen [41,42]. These free radicals, through reaction with oxygen, give rise to ROS, including hydrogen peroxide and organic hydroperoxides (ROOH) [41,43]. Ionizing radiation reduces proliferation and DNA synthesis in hUCB-MSCs to a greater extent than in fibroblast and cancer cells (Fig. 1C, D). Therefore, we tested whether increased antioxidant activity resulting from pretreatment with PEG-catalase confers resistance against ionizing radiation to hUCB-MSCs (Fig. 7). PEG-catalase pretreatment increased proliferation (Fig. 7A) and decreased cellular senescence (Fig. 7B) in hUCB-MSCs treated with radiation. However, PEG-catalase pretreatment did not affect MRC5 fibroblasts (Supplementary Fig. S8). These results suggest that the sensitivity of hUCB-MSC proliferation to ionizing radiation is attributable, at least in part, to their low antioxidant activity.
FIG. 7.
Increased antioxidant activity inhibits hUCB-MSCs from undergoing cellular senescence following ionizing radiation. (A) MSC1 hUCB-MSCs, pretreated with 200 U/mL of PEG-catalase for 24 h, were irradiated with γ-rays. One day after irradiation, cells were immunostained using the anti-Ki-67 antibody (upper panel). DAPI staining was used to visualize nuclei. The relative proportion of Ki-67–stained cells is shown in the plot (lower panel). The results of 3 independent experiments were averaged. P value was 0.037. (B) Two or 4 days after irradiation, MSC1 cells were stained using SA-β-Gal and viewed under a phase-contrast microscope (upper panel). The graph represents the percentage of SA-β-Gal–stained cells at 4 days after irradiation (lower panel). IR, γ-irradiation.
Discussion
In this study, we demonstrated that hUCB-MSCs possess low antioxidant capacity compared with human fibroblast and cancer cells, due to low levels of antioxidant protein expression. Because of this low antioxidant activity, hUCB-MSCs were defective in ROS scavenging, causing an increased number of DNA breaks in response to oxidative stress and ionizing radiation. DNA damage inhibits DNA replication and cell proliferation, resulting in cellular senescence. Increased intracellular antioxidant activity, resulting from exogenous antioxidant addition, conferred resistance to oxidative stress in hUCB-MSCs. Since all 4 hUCB-MSC lines (MSC1, 2, 3, and 4), derived from different donors, responded similarly to hydrogen peroxide and ionizing radiation, susceptibility to genotoxic stress is likely to be common in hUCB-MSCs.
Exposure to the same concentrations of hydrogen peroxide generated more severe DNA breaks in hUCB-MSCs than in human fibroblast cells, and the damage recovery period was longer in hUCB-MSCs (Figs. 2 and 3A, B). However, when both cell types contained similar levels of DNA breakage, the recovery time appeared to be similar (Fig. 3C, D). The similar duration of recovery from DNA damage and activation of DNA damage checkpoint proteins (Supplementary Fig. S3) implies that the DNA repair and damage checkpoint machinery are functional in hUCB-MSCs.
When cells are exposed to ionizing radiation, radiochemical damage can occur either directly or indirectly. Ions created by γ-rays can physically break one or both sugar phosphate DNA backbones or break DNA base pairs [44,45]. Additionally, radiation-induced ionizations produce free radicals such as the hydroxyl radical (OH·), superoxide anion (O2·−), and organic radicals (R·) in the presence of oxygen. These free radicals can oxidize molecules or modify chemical bonds [41,42]. Although ionizing radiation produces similar amounts of DNA breakage in hUCB-MSCs and human fibroblast and cancer cells (Supplementary Fig. S2), hUCB-MSCs grown for 24 h after ionizing radiation treatment exhibited reduced DNA synthesis and cell proliferation compared with human fibroblast and cancer cells (Fig. 1C, D). Because ROS produced by radiation were not efficiently scavenged in hUCB-MSCs, cellular senescence occurred (Fig. 7). Exogenous PEG-catalase added to hUCB-MSCs treated with radiation increased cell proliferation and decreased cellular senescence. These results support the hypothesis that hUCB-MSCs are defective in ROS elimination.
Cells in organisms living under aerobic conditions are continuously subjected to ROS, which are primarily generated in the mitochondria [46]. Oxidative stress is a key factor in the aging process [47]. ROS also appear to affect cell differentiation and maturation during embryogenesis [48,49]. Spontaneous differentiation of human embryonic stem cells is accompanied by ROS formation. Overexpression of antioxidant enzymes, including SOD, CAT, and peroxiredoxins, alters the differentiation pattern [50]. Continuous exposure of embryonic stem cells to ROS inhibits cardiomyogenesis and vasculogenesis. However, a low-level ROS pulse enhances differentiation in some lineages [51]. ROS inhibit cellular adhesion in mouse bone marrow–derived MSCs engrafted into ischemic myocardium [40].
Human skin–derived MSCs exhibit lower antioxidant activity than an immortalized human keratinocytic cell line and undergo apoptosis following treatment with 0.5 mM of hydrogen peroxide for 2 h [21]. In contrast, compared with human skin fibroblasts, human bone marrow–derived MSCs exhibit similar protein levels, activities, and gene expression, and are equally resistant to oxidative stress [22]. The discrepancy between the skin- and bone marrow–derived MSCs may be due to the different tissue or donor sources.
Susceptibility of hUCB-MSCs to ROS suggests that ROS in the surrounding microenvironment or niche may control the fate of hUCB-MSCs with regard to cellular senescence, cell death, or differentiation. Exogenously added antioxidants improved resistance to oxidative stress and ionizing radiation. This antioxidant treatment contributed to improved stability of hUCB-MSCs during isolation and maintenance and may have important therapeutic applications. Therefore, the proliferation and fate of hUCB-MSCs can be controlled using oxidative stresses.
Supplementary Material
Acknowledgments
The hUCB-MSCs were kindly donated by MEDIPOST Co., Ltd. We thank Dr. Dongmin Kang for providing catalase, SOD, and GPx antibodies. This work was supported by a grant from the Nuclear R&D Program of the National Research Foundation of Korea (2009-0078699). E.K. and K.Y.L. were supported by the second stage of the Brain Korea 21 Project.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hussain SP. Hofseth LJ. Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 2.Helleday T. Petermann E. Lundin C. Hodgson B. Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 3.Branzei D. Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 4.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 5.d'Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- 6.Campisi J. d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 7.Barcellos-Hoff MH. Park C. Wright EG. Radiation and the microenvironment—tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 8.Cooke MS. Evans MD. Dizdaroglu M. Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 9.Maynard S. Schurman SH. Harboe C. de Souza-Pinto NC. Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hensley K. Robinson KA. Gabbita SP. Salsman S. Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 11.Kujoth GC. Hiona A. Pugh TD. Someya S. Panzer K. Wohlgemuth SE. Hofer T. Seo AY. Sullivan R, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 12.D'Autreaux B. Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 13.Giorgio M. Trinei M. Migliaccio E. Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 14.Sonoda Y. Watanabe S. Matsumoto Y. Aizu-Yokota E. Kasahara T. FAK is the upstream signal protein of the phosphatidylinositol 3-kinase-Akt survival pathway in hydrogen peroxide-induced apoptosis of a human glioblastoma cell line. J Biol Chem. 1999;274:10566–10570. doi: 10.1074/jbc.274.15.10566. [DOI] [PubMed] [Google Scholar]
- 15.Ding B. Chi SG. Kim SH. Kang S. Cho JH. Kim DS. Cho NH. Role of p53 in antioxidant defense of HPV-positive cervical carcinoma cells following H2O2 exposure. J Cell Sci. 2007;120:2284–2294. doi: 10.1242/jcs.002345. [DOI] [PubMed] [Google Scholar]
- 16.Chen QM. Bartholomew JC. Campisi J. Acosta M. Reagan JD. Ames BN. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 1998;332(Pt 1):43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roos WP. Christmann M. Fraser ST. Kaina B. Mouse embryonic stem cells are hypersensitive to apoptosis triggered by the DNA damage O(6)-methylguanine due to high E2F1 regulated mismatch repair. Cell Death Differ. 2007;14:1422–1432. doi: 10.1038/sj.cdd.4402136. [DOI] [PubMed] [Google Scholar]
- 18.Tichy ED. Stambrook PJ. DNA repair in murine embryonic stem cells and differentiated cells. Exp Cell Res. 2008;314:1929–1936. doi: 10.1016/j.yexcr.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saretzki G. Armstrong L. Leake A. Lako M. von Zglinicki T. Stress defense in murine embryonic stem cells is superior to that of various differentiated murine cells. Stem Cells. 2004;22:962–971. doi: 10.1634/stemcells.22-6-962. [DOI] [PubMed] [Google Scholar]
- 20.Saretzki G. Walter T. Atkinson S. Passos JF. Bareth B. Keith WN. Stewart R. Hoare S. Stojkovic M, et al. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26:455–464. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- 21.Orciani M. Gorbi S. Benedetti M. Di Benedetto G. Mattioli-Belmonte M. Regoli F. Di Primio R. Oxidative stress defense in human-skin-derived mesenchymal stem cells versus human keratinocytes: different mechanisms of protection and cell selection. Free Radic Biol Med. 2010;49:830–838. doi: 10.1016/j.freeradbiomed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Valle-Prieto A. Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19:1885–1893. doi: 10.1089/scd.2010.0093. [DOI] [PubMed] [Google Scholar]
- 23.Yang SE. Ha CW. Jung M. Jin HJ. Lee M. Song H. Choi S. Oh W. Yang YS. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004;6:476–486. doi: 10.1080/14653240410005041. [DOI] [PubMed] [Google Scholar]
- 24.Jang YK. Jung DH. Jung MH. Kim DH. Yoo KH. Sung KW. Koo HH. Oh W. Yang YS. Yang SE. Mesenchymal stem cells feeder layer from human umbilical cord blood for ex vivo expanded growth and proliferation of hematopoietic progenitor cells. Ann Hematol. 2006;85:212–225. doi: 10.1007/s00277-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 25.Wei MC. Zong WX. Cheng EH. Lindsten T. Panoutsakopoulou V. Ross AJ. Roth KA. MacGregor GR. Thompson CB. Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vunjak-Novakovic G. Altman G. Horan R. Kaplan DL. Tissue engineering of ligaments. Annu Rev Biomed Eng. 2004;6:131–156. doi: 10.1146/annurev.bioeng.6.040803.140037. [DOI] [PubMed] [Google Scholar]
- 27.Pantopoulos K. Weiss G. Hentze MW. Nitric oxide and oxidative stress (H2O2) control mammalian iron metabolism by different pathways. Mol Cell Biol. 1996;16:3781–3788. doi: 10.1128/mcb.16.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scholzen T. Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Moneef MA. Sherwood BT. Bowman KJ. Kockelbergh RC. Symonds RP. Steward WP. Mellon JK. Jones GD. Measurements using the alkaline comet assay predict bladder cancer cell radiosensitivity. Br J Cancer. 2003;89:2271–2276. doi: 10.1038/sj.bjc.6601333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gueven N. Becherel OJ. Howe O. Chen P. Haince JF. Ouellet ME. Poirier GG. Waterhouse N. Fusser M, et al. A novel form of ataxia oculomotor apraxia characterized by oxidative stress and apoptosis resistance. Cell Death Differ. 2007;14:1149–1161. doi: 10.1038/sj.cdd.4402116. [DOI] [PubMed] [Google Scholar]
- 31.Djuzenova CS. Rothfuss A. Oppitz U. Spelt G. Schindler D. Hoehn H. Flentje M. Response to X-irradiation of Fanconi anemia homozygous and heterozygous cells assessed by the single-cell gel electrophoresis (comet) assay. Lab Invest. 2001;81:185–192. doi: 10.1038/labinvest.3780226. [DOI] [PubMed] [Google Scholar]
- 32.Kumaravel TS. Jha AN. Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat Res. 2006;605:7–16. doi: 10.1016/j.mrgentox.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Batchelor E. Loewer A. Lahav G. The ups and downs of p53: understanding protein dynamics in single cells. Nat Rev Cancer. 2009;9:371–377. doi: 10.1038/nrc2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Porath I. Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Dimri GP. Lee X. Basile G. Acosta M. Scott G. Roskelley C. Medrano EE. Linskens M. Rubelj I, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin JK. Shih CA. Inhibitory effect of curcumin on xanthine dehydrogenase/oxidase induced by phorbol-12-myristate-13-acetate in NIH3T3 cells. Carcinogenesis. 1994;15:1717–1721. doi: 10.1093/carcin/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong JS. Whiteman M. Rose P. Jones DP. The Coenzyme Q10 analog decylubiquinone inhibits the redox-activated mitochondrial permeability transition: role of mitcohondrial [correction mitochondrial] complex III. J Biol Chem. 2003;278:49079–49084. doi: 10.1074/jbc.M307841200. [DOI] [PubMed] [Google Scholar]
- 38.Mahfouz R. Sharma R. Sharma D. Sabanegh E. Agarwal A. Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertil Steril. 2009;91:805–811. doi: 10.1016/j.fertnstert.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 39.Beckman JS. Minor RL., Jr. White CW. Repine JE. Rosen GM. Freeman BA. Superoxide dismutase and catalase conjugated to polyethylene glycol increases endothelial enzyme activity and oxidant resistance. J Biol Chem. 1988;263:6884–6892. [PubMed] [Google Scholar]
- 40.Song H. Cha MJ. Song BW. Kim IK. Chang W. Lim S. Choi EJ. Ham O. Lee SY, et al. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28:555–563. doi: 10.1002/stem.302. [DOI] [PubMed] [Google Scholar]
- 41.Oberley LW. Lindgren LA. Baker SA. Stevens RH. Superoxide lon as the cause of the oxygen effect. Radiat Res. 1976;68:320–328. [PubMed] [Google Scholar]
- 42.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 43.Biaglow JE. Mitchell JB. Held K. The importance of peroxide and superoxide in the X-ray response. Int J Radiat Oncol Biol Phys. 1992;22:665–669. doi: 10.1016/0360-3016(92)90499-8. [DOI] [PubMed] [Google Scholar]
- 44.Cadet J. Bellon S. Douki T. Frelon S. Gasparutto D. Muller E. Pouget JP. Ravanat JL. Romieu A. Sauvaigo S. Radiation-induced DNA damage: formation, measurement, and biochemical features. J Environ Pathol Toxicol Oncol. 2004;23:33–43. doi: 10.1615/jenvpathtoxoncol.v23.i1.30. [DOI] [PubMed] [Google Scholar]
- 45.Siddiqi MA. Bothe E. Single- and double-strand break formation in DNA irradiated in aqueous solution: dependence on dose and OH radical scavenger concentration. Radiat Res. 1987;112:449–463. [PubMed] [Google Scholar]
- 46.Inoue M. Sato EF. Nishikawa M. Park AM. Kira Y. Imada I. Utsumi K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem. 2003;10:2495–2505. doi: 10.2174/0929867033456477. [DOI] [PubMed] [Google Scholar]
- 47.Skulachev VP. A biochemical approach to the problem of aging: “megaproject” on membrane-penetrating ions. The first results and prospects. Biochemistry (Mosc) 2007;72:1385–1396. doi: 10.1134/s0006297907120139. [DOI] [PubMed] [Google Scholar]
- 48.Allen RG. Venkatraj VS. Oxidants and antioxidants in development and differentiation. J Nutr. 1992;122:631–635. doi: 10.1093/jn/122.suppl_3.631. [DOI] [PubMed] [Google Scholar]
- 49.Sohal RS. Allen RG. Nations C. Oxidative stress and cellular differentiation. Ann N Y Acad Sci. 1988;551:59–73. doi: 10.1111/j.1749-6632.1988.tb22320.x. discussion 74. [DOI] [PubMed] [Google Scholar]
- 50.Cho YM. Kwon S. Pak YK. Seol HW. Choi YM. Park do J. Park KS. Lee HK. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006;348:1472–1478. doi: 10.1016/j.bbrc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Sauer H. Wartenberg M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal. 2005;7:1423–1434. doi: 10.1089/ars.2005.7.1423. [DOI] [PubMed] [Google Scholar]
- 52.Duez P. Dehon G. Dubois J. Validation of raw data measurements in the comet assay. Talanta. 2004;63:879–886. doi: 10.1016/j.talanta.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 53.Olive PL. Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1:23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.