Abstract
Elafin is a 6 kDa innate immune protein present at several epithelial surfaces including the pulmonary epithelium. It is a canonical protease inhibitor of two neutrophil serine proteases (neutrophil elastase (NE) and proteinase 3) with the capacity to covalently bind extracellular matrix proteins by transglutamination. In addition to these properties, elafin also possesses antimicrobial and immunomodulatory activities. The aim of the present study was to investigate the effect of Pseudomonas aeruginosa proteases on elafin function. We found that P. aeruginosa PAO1-conditioned medium and two purified Pseudomonas metalloproteases, pseudolysin (elastase) and aeruginolysin (alkaline protease), were able to cleave recombinant elafin. Pseudolysin was shown to inactivate the anti-NE activity of elafin by cleaving its protease-binding loop. Interestingly, antibacterial properties of elafin against PAO1 were found to be unaffected after pseudolysin treatment. In contrast to pseudolysin, aeruginolysin failed to inactivate the inhibitory properties of elafin against NE. Aeruginolysin cleaved elafin at the amino-terminal Lys6-Gly7 peptide bond resulting in a decreased ability to covalently bind purified fibronectin following transglutaminase activity. In conclusion, this study provides evidences that elafin is susceptible to proteolytic cleavage at alternative sites by P. aeruginosa metalloproteinases, which can affect different biological functions of elafin.
Keywords: Antimicrobials, antiproteases, cleavage, microbial, proteases, transglutaminase
Introduction
Elafin, also known as SKALP (Skin-derived AntiLeukoProtease) or ESI (Elastase-Specific Inhibitor), is a small-size (6 kDa, 57 amino acids), cationic protease inhibitor firstly discovered in the skin (Schalkwijk et al., 1990;Wiedow et al., 1990) and the lung (Sallenave and Ryle, 1991). Like SLPI (secretory leucoprotease inhibitor), elafin belongs to the chelonianin family, one of the 18 families of canonical inhibitors. Structurally, this inhibitor is a member of the WAP (Whey Acidic Protein) family which is characterised by the presence of one or more domain that contains eight highly conserved cysteine residues organised into four disulphide bridges. Elafin is generated from a longer molecule known as trappin-2 or pre-elafin, (10 kDa) possessing, in addition to the WAP domain, an amino-terminal cementoin domain, rich in transglutaminase substrate motifs with the consensus sequence GQDPVK. These sequences allow the inhibitor to covalently bind to a number of extracellular matrix (ECM) proteins following the catalytic action of transglutaminases (Guyot et al., 2005b;Nara et al., 1994). The presence of elafin in vivo indicates that the inhibitory WAP domain (elafin) is released from trappin-2 by proteolytic cleavage. A previous study showed that the mast cell protease tryptase may be involved in this process as the enzyme is able to generate elafin from trappin-2 (Guyot et al., 2005a). Both elafin and trappin-2 are potent inhibitors of two neutrophil serine proteases: neutrophil elastase (NE) and proteinase 3 (Pr3) (Ying and Simon, 1993;Zani et al., 2004). Trappin-2/elafin is mainly produced by epithelial cells and its expression is up-regulated during inflammation. IL-1β, TNF-α, lipopolysaccharide (LPS) and NE are inflammatory mediators known to increase expression of the inhibitor in lung and skin epithelial cells (Bingle et al., 2001;Meyer-Hoffert et al., 2003;Pfundt et al., 2000;Reid et al., 1999;Sallenave et al., 1994;Simpson et al., 2001). The inhibitory properties of trappin-2/elafin confer a protective role for this protein in shielding mucosal surfaces against excessive neutrophil-mediated proteolysis during inflammation. While elafin and trappin-2 were originally identified as antiproteases, recent studies indicate that elafin/trappin-2 possesses immunomodulatory (Butler et al., 2006;Henriksen et al., 2004), anti-bacterial (Baranger et al., 2008;Simpson et al., 1999), anti-fungal (Baranger et al., 2008) and anti-viral activities. Readers are directed to a recent review of the functional activities of elafin/trappin-2 (Moreau et al., 2008).
The regulation of excessive proteolytic activity of neutrophil serine proteases during lung inflammation is crucial to prevent serious tissue damage. Multiple factors in the pulmonary environment can inactivate endogenous protease inhibitors (alpha-1-antitrypsin (AAT), SLPI, elafin) and affect the local protease-antiprotease balance. Two distinct mechanisms are known to inactivate neutrophil serine protease inhibitors: oxidation and proteolytic inactivation. The lung environment during inflammation is oxidative due to reactive oxygen species produced by neutrophils. AAT, SLPI and elafin contain a methionine residue in their inhibitory loop that can be oxidised into a methionine sulphoxide. This modification was shown to considerably decrease the affinity of the antiproteases for their target enzymes (Beatty et al., 1980;Boudier and Bieth, 1994;Nobar et al., 2005). Host and exogenous proteases can also mediate the inactivation of inhibitors. A number of matrix metalloproteases are known to cleave and inactivate AAT (Banda et al., 1980;Mast et al., 1991;Michaelis et al., 1990). SLPI was shown to be cleaved and inactivated by elastolytic cysteine cathepsins B, L and S in the epithelial lining fluid from emphysematous patients (Taggart et al., 2001). Elafin and AAT (but not human SLPI) are susceptible to proteolytic activity of the house dust mite protease Der p 1 (Brown et al., 2003). We have recently demonstrated that excessive levels of NE activity in the Pseudomonas-infected Cystic Fibrosis lung can degrade and inactivate activities associated with elafin function (Guyot et al., 2008). Bacterial proteases may also be involved in maintaining inflammation by breaking down the lung anti-elastase screen. Pseudomonas aeruginosa, an opportunistic pathogen involved in a number of severe human infections, produces metalloproteases able to cleave a large variety of substrates including ECM proteins, cytokines, chemokines, immunoglobulins, complement components, antimicrobial peptides/proteins and protease inhibitors. Among the metalloproteases secreted by P. aeruginosa, pseudolysin (also called Pseudomonas elastase or LasB) has previously been reported to cleave and inactivate AAT and SLPI (Johnson et al., 1982;Morihara et al., 1979). To date, the proteolytic inactivation of elafin by bacterial proteases remains poorly studied. Only a recent study reports the cleavage of elafin within its protease-binding loop by the cysteinyl protease RgpB from Porphyromonas gingivalis (Kantyka et al., 2009). Given the virulence of P. aeruginosa and the vulnerability of the pulmonary anti-elastase screen to proteolytic inactivation, the aim of the present work was to evaluate the effect of Pseudomonas proteases on elafin.
Results
Effect of PAO1-conditioned medium on the integrity of elafin, SLPI and AAT
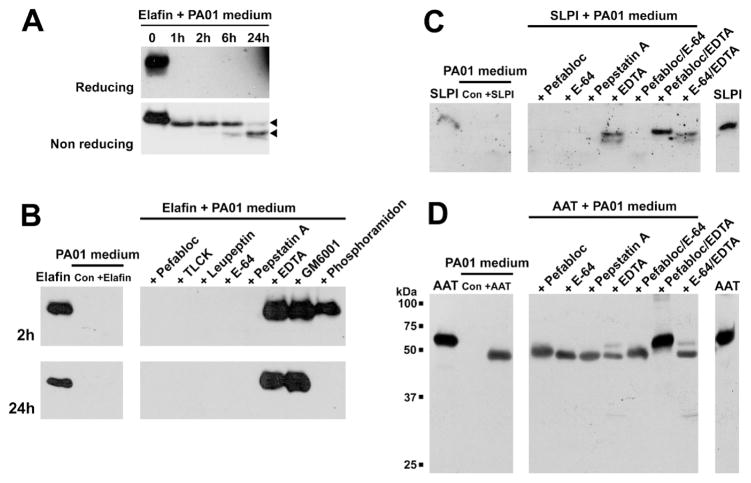
PAO1-conditioned medium was used as a complex medium to evaluate the effect of proteases secreted by P. aeruginosa on elafin. The elastase inhibitor was incubated with the PAO1-conditioned medium and analysed by Western blot using a biotinylated anti-elafin antibody. As shown in Figure 1A, elafin was quickly cleaved following 1h exposure to the Pseudomonas medium. The class of protease(s) involved in the degradation of elafin was investigated by pre-treating the medium with protease inhibitors. Figure 1B shows that only metalloprotease inhibitors (EDTA, GM6001 and phosphoramidon) could prevent the cleavage of elafin by PAO1-conditioned medium. Interestingly, phosphoramidon, an inhibitor of the Pseudomonas metalloprotease pseudolysin, was able to prevent elafin degradation after 2h incubation but not 24h. These results indicate that one or several Pseudomonas metalloprotease(s) including pseudolysin were involved in this process. The effect of PAO1-conditioned medium on SLPI and AAT was determined as positive controls as both inhibitors were previously shown to be cleaved by pseudolysin. As demonstrated in Figures 1C and 1D, SLPI and AAT were cleaved after 24h incubation with Pseudomonas-conditioned medium. Interestingly, none of the individual non-specific inhibitors used in the study could prevent the cleavage of these antiproteases. However, the use of the serine protease inhibitor Pefabloc combined to the metalloprotease inhibitor EDTA was able to protect both SLPI and AAT against proteolysis (Figures 1C and 1D).
Figure 1. Effect of Pseudomonas aeruginosa (PAO1)-conditioned medium on the integrity of elastase inhibitors elafin, SLPI and AAT.
8.3×10−7 M of elastase inhibitors (elafin, SLPI and AAT) were incubated with 10 μL PAO1- conditioned medium at 37°C and analysed by Western blot. (A) Time-course incubation of recombinant elafin with PAO1-conditioned medium for 0, 1, 2, 6 and 24h. Incubation products were analyzed by Western blot under reducing (upper panel) and non-reducing (lower panel) conditions. Arrow heads indicates cleavage products of elafin. (B) Identification of the Pseudomonas proteases involved in elafin cleavage. PAO1-conditioned medium was pre-incubated with the indicated proteases inhibitors (right panels) and mixed with recombinant elafin for 2h (upper panels) and 24h (lower panels). Elafin alone, PAO1-conditioned medium incubated in the absence (Con) or presence of elafin (+Elafin) are indicated in the left panels. Effect of PAO1-conditioned medium on (C) SLPI and (D) AAT integrity, and identification of the Pseudomonas proteases involved in their cleavage. PAO1-conditioned medium was pre-incubated with the indicated proteases inhibitors (right panels) before incubation with recombinant elafin for 24h. SLPI or AAT alone, PAO1-conditioned medium incubated in the absence (Con) or presence of SLPI (+SLPI) or AAT (+AAT) are indicated in the left panels.
Effect of PAO1-conditioned medium on the antiprotease activities of elafin
As PAO1-conditioned medium was capable of cleaving elafin, we investigated whether this cleavage had any effects on elafin’s ability to inhibit NE using the chromogenic substrate N-(Methoxysuccinyl)-Ala-Ala-Pro-Val-paranitroanilide (MeOSuc-AAPV-pNA). Full length elafin was ~100% inhibitory towards NE but its efficacy decreased to about 15% inhibition of NE activity after 1h exposure to PAO1-conditioned medium (Figure 2). This decrease in inhibition was completely prevented by pre-treating the medium with EDTA and phosphoramidon. Our result indicates that Pseudomonas metalloprotease(s) play a crucial role in cleaving and inactivating elafin and its ability to inhibit its cognate protease NE.
Figure 2. Effect of Pseudomonas aeruginosa (PAO1)-conditioned medium on the anti- elastase activity of elafin.
Recombinant elafin was incubated with Tryptic Soy Broth (control) or PAO1-conditioned medium (pre-incubated in absence or in presence of the metalloproteinase inhibitors EDTA and phosphoramidon (Pa)) for 0, 1, 2 and 6h and analysed for its inhibitory activity against neutrophil elastase (NE) by spectrophotometry using the NE substrate MeOSuc-AAPV-pNA.
Effect of purified Pseudomonas metalloproteases on elafin integrity and antiprotease activity
Given the previous findings, purified Pseudomonas metalloproteases were investigated for their ability to cleave and inactivate elafin. Dose-response incubations of pseudolysin, staphylolysin (LasA) or aeruginolysin (Pseudomonas alkaline protease) were carried out for 2h at 37°C and analysed by SDS-PAGE. As shown in Figure 3, recombinant elafin is cleaved by 10 nM pseudolysin (Figure 3A) and by 400 nM aeruginolysin (Figure 3C). In contrast, staphylolysin does not cleave elafin even at the highest concentration tested (10 μM) (Figure 3B).
Figure 3. Effect of purified Pseudomonas aeruginosa metalloproteases on the integrity of recombinant elafin.
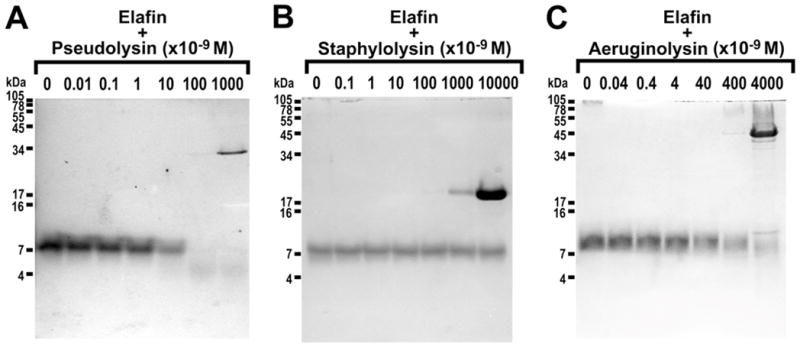
Recombinant elafin (3 μg, 25 μM) was incubated with various concentrations of purified (A) pseudolysin (0–1 μM), (B) staphylolysin (0–10 μM) and (C) aeruginolysin (0–4 μM) for 2h at 37°C. Incubation products were separated by SDS-PAGE and visualised by Coomassie Blue staining.
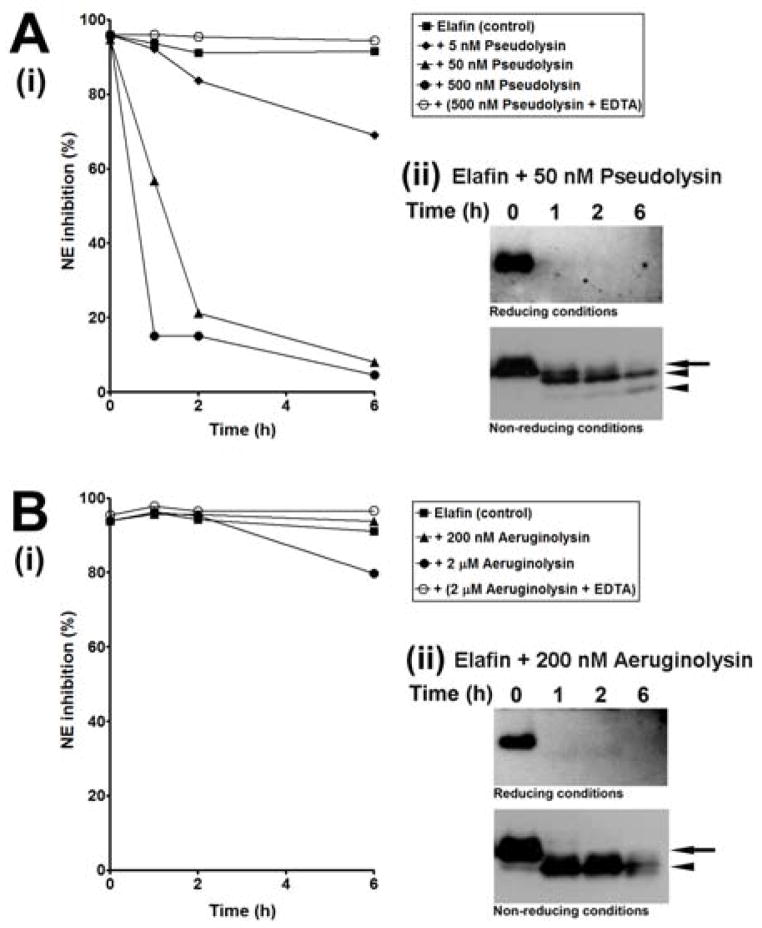
The effect of proteolytic cleavage on the inhibitory activity of elafin were investigated by measuring the anti-NE activity of elafin pre-incubated with various concentrations of pseudolysin (5–500 nM) and aeruginolysin (20–2000 nM) for various times (0, 1, 2 and 6h) at 37°C. As shown in Figure 4A(i), pseudolysin gradually inactivated the antiprotease activity of elafin as a function of the enzyme concentration and the incubation time. While the inhibition of NE by elafin was about 94–96% at the initial time of incubation and about 92% after 6h at 37°C, we found that both 50 and 500 nM pseudolysin led to the rapid inactivation of elafin as NE inhibition was reduced to 21% and 15% after 2h of incubation, respectively, and still continued to decrease to 5–8% inhibition after 6h. Pre-incubation of 500 nM pseudolysin with EDTA completely inhibited pseudolysin-mediated inactivation of elafin since 95% of NE inhibition by elafin is remained after 6h at 37°C. Western blot analysis of samples with 50 nM of pseudolysin was performed to correlate the loss of activity with proteolytic cleavage. As shown in Figure 4A(ii), the majority of elafin was cleaved within 1h of incubation. A fragment with an apparent mass slightly lower to that of native elafin was detectable under non-reducing conditions. Further cleavage of this fragment was detected with prolonged exposure as the amount of this fragment decreased from 1h to 6h incubation while another fragment with a lower apparent mass was generated during the same period of time. Taken together, these results suggest that 50 nM pseudolysin rapidly cleaved elafin in such a way as to inactivate its anti-NE activity.
Figure 4. Effect of purified pseudolysin and aeruginolysin on the inhibitory activity of elafin.
Recombinant elafin was incubated alone (control) or with various concentrations of (A) pseudolysin or (B) aeruginolysin for 0, 1, 2 and 6h at 37°C. Samples were analysed (i) by spectrophotometry for their inhibitory activity toward neutrophil elastase (NE) using the NE substrate MeOSuc-AAPV-pNA and (ii) by Western blot under reducing (upper panels) and non-reducing (lower panels) conditions to confirm elafin cleavage in the presence of pseudolysin and aeruginolysin. Arrows and arrowheads indicate respectively native elafin and cleavage products of elafin.
In contrast to pseudolysin, aeruginolysin did not inactivate the antiprotease activity of elafin, despite its ability to cleave the protein. Indeed, Figure 4B(i) shows that over 90% of NE was still remaining inhibited by elafin when the inhibitor was incubated alone or with 20–200 nM aeruginolysin. A slight decrease of NE inhibition to 80% was however observed with the highest concentration of enzyme (2000 nM) used in our experiment after 6h incubation (Figure 4B(i)). Western blot analysis of samples with 200 nM aeruginolysin demonstrated that elafin was completely cleaved after 1h into a unique cleavage product (Figure 4B(ii)) that retained inhibitory properties as shown in Figure 4B(i).
Identification of pseudolysin and aeruginolysin generated cleavage sites
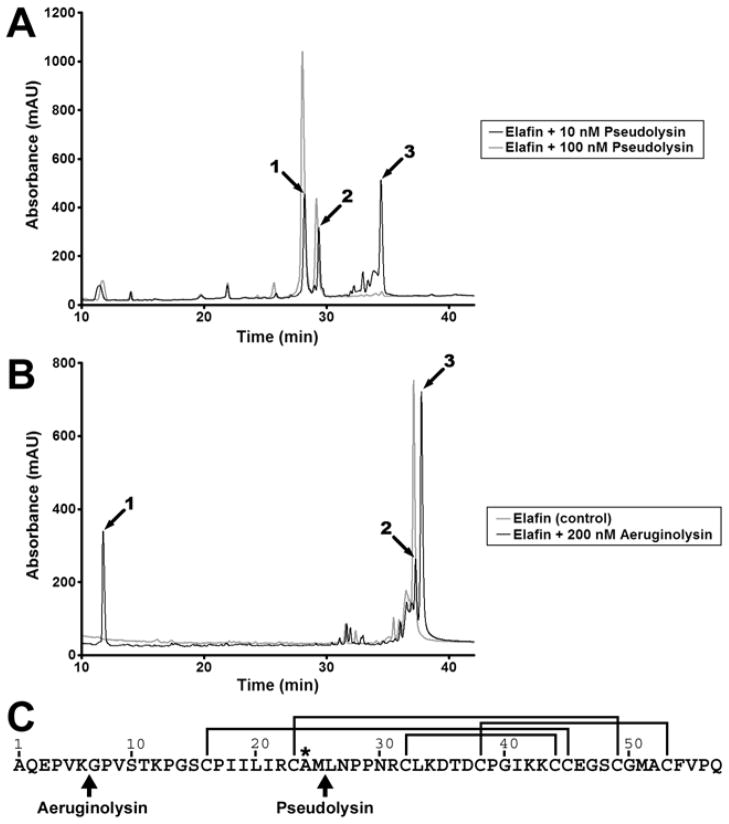
Elafin fragments generated by pseudolysin and aeruginolysin were separated by reverse phase HPLC (Figure 5) and identified by electrospray mass spectrometry. HPLC analysis of elafin incubated with 10 nM pseudolysin resulted in the generation of three main peaks (Figure 5A, peaks 1, 2 and 3). As shown in Table 1, product peptides of elafin were identified in peaks 1 and 2 while peak 3 was exclusively composed of the full length elafin. A single peptide was identified in peak 1; it contained residues 26–57. The major (94%) elafin product detected in peak 2 was identified as containing residues 1–23. A peptide containing residues 1–24 constituted 5% and one with residues 1–25 was detected at 0.4%. These results indicate that 10 nM pseudolysin cleaved the inhibitor between Met25 and Leu26. The use of a higher concentration of the pseudolysin (100 nM) led to the elimination of native elafin (peak 3) and an increase in peaks 1 and 2 (Figure 5A).A HPLC chromatogram of elafin incubated with 400 nM aeruginolysin also displayed three major peaks (Figure 5B). Measured masses for each peak are indicated in Table 2 with the corresponding assigned sequence. The measured masses of peaks 1 and 3 allowed their identification as elafin residues 1–6 and 7–57. Peak 2 was identified as the native elafin. These data indicate that 400 nM aeruginolysin cleaved elafin at the Lys6-Gly7 peptide bond within 2h incubation. Cleavage sites of elafin by peudolysin and aeruginolysin are summarised in Figure 5C.
Figure 5. HPLC-mass spectrometry analysis of elafin incubated with pseudolysin and aeruginolysin.
Recombinant elafin (3 μg, 25 μM) was incubated with various concentrations of pseudolysin or aeruginolysin for 2h at 37°C. Samples were then analysed by reverse phase HPLC coupled to electrospray mass spectrometry. (A) HPLC analysis of elafin incubated with 10 nM (black line) and 100 nM (grey line) pseudolysin. (B) HPLC analysis of elafin incubated alone (grey line) or with 200 nM (black line) aeruginolysin. (C) Identification of the cleavage sites in elafin. The amino acid sequence of elafin is represented from the amino-terminus (Ala1) to the carboxy-terminus (Gln57). The lines indicate the position of the disulphide bridges and the asterisk identifies the residue in P1 position within the protease-binding loop. Arrows highlight the cleavage sites generated by pseudolysin (at Met25-Leu26) and aeruginolysin (at Lys6-Gly7).
Table 1. Monoisotopic mass of elafin fragments generated by pseudolysin.
Elafin fragments obtained with pseudolysin and identified by HPLC (Peaks 1, 2 and 3, Figure 5A) were analyzed by mass spectrometry. In the table are represented the observed and calculated monoisotopic masses for the components constituting over 90% of each peak.
| Peak | Observed Mass, Da | Calculated Mass, Da | Assignment |
|---|---|---|---|
| 1 | 3,426.53 | 3,426.51 | P26–57 |
| 2 | 2,392.31 | 2,392.30 | P1–23 |
| 3 | 6,002.86 | 6,002.88 | Full length P1–57 |
Table 2. Monoisotopic mass of elafin fragments generated by aeruginolysin.
Elafin fragments obtained with aeruginolysin and identified by HPLC (Peaks 1, 2 and 3, Figure 5B) were analyzed by mass spectrometry. In the table are represented the observed and the calculated masses and the assigned sequence for each peak.
| Peak | Observed Mass, Da | Calculated Mass, Da | Assignment |
|---|---|---|---|
| 1 | 670.36 | 670.36 | P1–6 |
| 2 | 6,002.86 | 6,002.88 | Full length P1–57 |
| 3 | 5,350.52 | 5,350.52 | P7–57 |
Effect of PAO1-conditioned medium on the anti-neutrophil elastase activity of normal BAL fluid
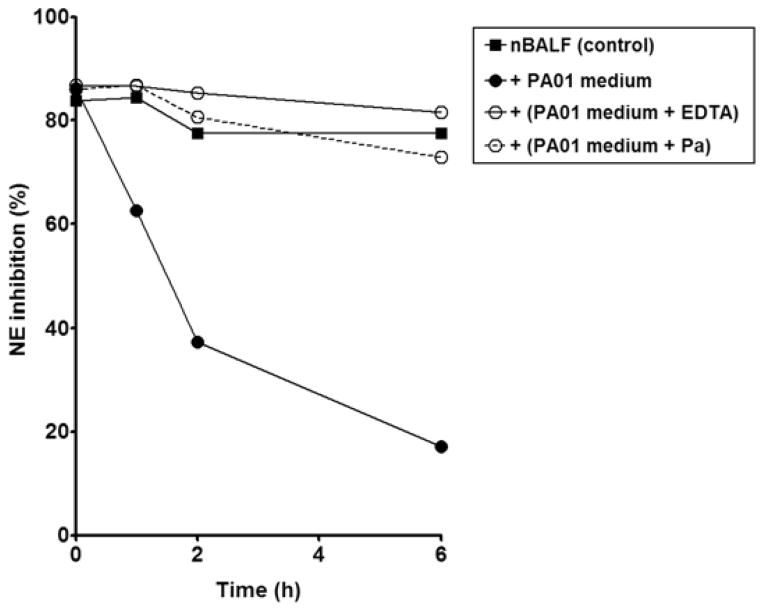
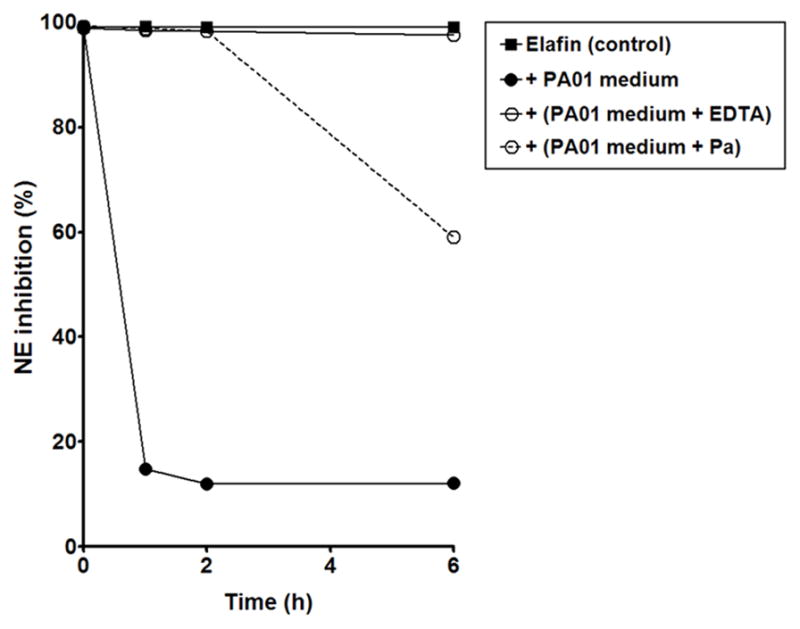
PAO1-conditioned medium was incubated with bronchoalveolar lavage (BAL) fluid from healthy patients to mimic the first steps of colonisation of the lung by P. aeruginosa. As shown in Figure 6, normal BAL fluid exhibited anti-NE activity. The incubation of normal BAL fluid with PAO1-conditioned medium led to a rapid decrease in the anti-NE activity of the BAL fluid as 63%, 37% and 17% of inhibition of NE were detected after 1, 2 and 6h of incubation, respectively, while normal BAL fluid displayed 78% of inhibition of NE after 6h. Pre-treatment of PAO1-conditioned medium with the metalloprotease inhibitors EDTA and phosphoramidon (a specific inhibitor of pseudolysin) resulted in the retention of anti-NE activity of the normal BAL fluid to a similar level of that observed for control: 73% and 82% of NE still remained inhibited by BAL fluid after 6h incubation with PAO1-conditioned medium pre-treated with phosphoramidon and EDTA respectively.
Figure 6. Inactivation of the anti-NE activity of normal BAL fluid by Pseudomonas aeruginosa PAO1-conditioned medium.
Normal BAL fluid was incubated with Tryptic Soy Broth (control) or PAO1-conditioned medium, pre-incubated in absence or presence of EDTA and phosphoramidon (Pa) for 0, 1, 2 and 6h and analysed for its inhibitory activity against neutrophil elastase (NE) by spectrophotometry using the NE substrate MeOSuc-AAPV-pNA.
Effect of aeruginolysin on the transglutaminase-mediated crosslinking of elafin to fibronectin
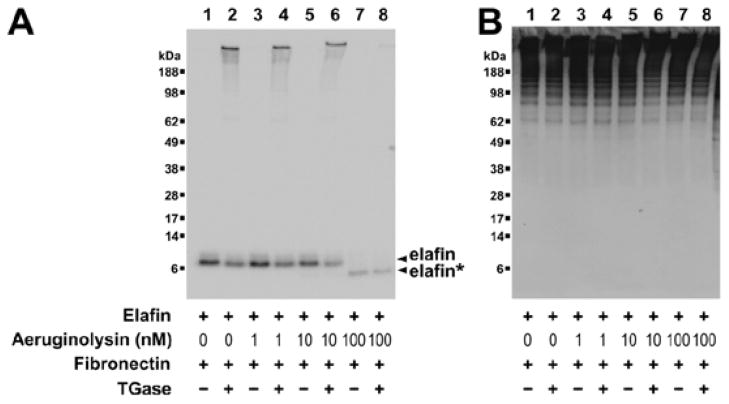
In contrast to our findings with pseudolysin, aeruginolysin cleaved recombinant elafin within its amino-terminal extremity at Lys6-Gly7 and this cleavage did not alter its inhibitory properties. However, the amino-terminal extremity of elafin contains a transglutaminase substrate motif (AQEPVK) that may be involved in crosslinking the inhibitor to ECM proteins by transglutamination. We thus hypothesised that the removal of this motif by aeruginolysin could suppress the transglutaminase-mediated binding of elafin to ECM proteins. Elafin has previously been shown to crosslink a number of ECM proteins including fibronectin. Therefore, we investigated the ability of the amino-terminal truncated form of elafin to crosslink fibronectin by transglutamination. Recombinant elafin was pre-treated with increasing concentrations of aeruginolysin and then incubated with GM6001. Samples were then incubated with plasma fibronectin in the presence or absence of guinea pig liver transglutaminase. Results were analysed by Western blot using anti-elafin antibodies (Figure 7A) and anti-fibronectin antibodies (Figure 7B). As shown in Figure 7A (lanes 1 to 6), native recombinant elafin had an apparent mass of 7 kDa under non-reducing conditions. However in the presence of fibronectin and transglutaminase (lanes 2, 4 and 6), part of the inhibitor was also detected at high molecular mass (>188 kDa) suggesting that the inhibitor was covalently crosslinked to fibronectin. Treatment of elafin with 100 nM aeruginolysin cleaved elafin resulting in the inhibition of its ability to crosslink to fibronectin by transglutamination (Figure 7A, lane 8). This effect is due to the truncation of elafin rather than fibronectin degradation since samples were treated with GM6001 to suppress metalloproteinase activity (Figure 7B).
Figure 7. Effect of purified aeruginolysin on transglutaminase-mediated crosslinking of elafin to fibronectin.
Recombinant elafin (100 ng, 3 μM) was incubated for 2h at 37°C with increasing amounts of aeruginolysin (lanes 1 and 2: no enzyme, 3 and 4: 1 nM, 5 and 6: 10 nM, 7 and 8: 100 nM, 9 and 10: 1000 nM). Samples were treated with GM6001 to inactivate aeruginolysin activity and incubated for 1h at 37°C with fibronectin in absence (lanes 1, 3, 5, 7, 9) or in presence (lanes 2, 4, 6, 8, 10) of 0.38 mU guinea pig liver transglutaminase (TGase). Samples were analyzed by SDS-PAGE followed by Western blot using (A) a biotinylated anti-elafin antibody and (B) an anti-fibronectin antibody. In (A), intact and cleaved elafin (respectively denoted elafin and elafin*) are indicated by arrow heads.
Effect of pseudolysin on the antibacterial activity of elafin
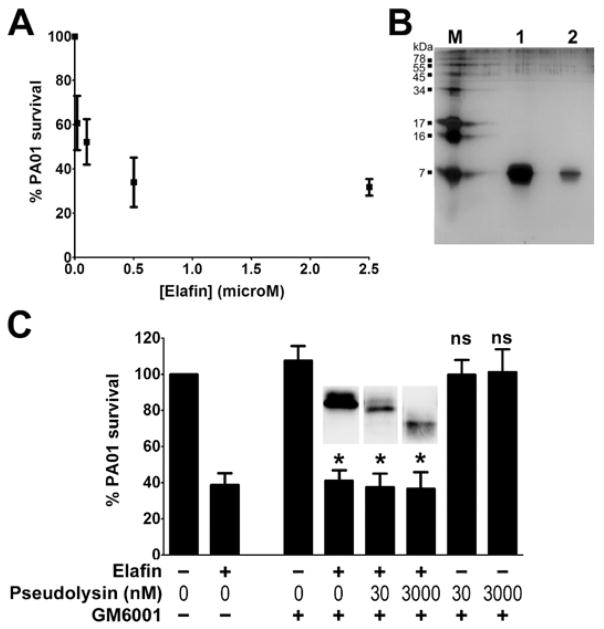
The ability of pseudolysin to inactivate the inhibitory activity of elafin by cleaving the protease-binding loop prompted us to investigate the effect of such cleavage on the antibacterial activity of elafin (Figure 8). Previous studies demonstrated antibacterial activities of elafin against various bacteria including P. aeruginosa and were confirmed in our study by dose-response experiments using recombinant elafin and P. aeruginosa PAO1. PAO1 survival was diminished as a function of elafin concentration used (Figure 8A). To ensure that the observed effect was only due to the recombinant elafin, we assessed the purity of the elafin preparation by SDS-PAGE. As shown in Figure 8B, no contamination was observed since only one single band corresponding to elafin was detected at around 7 kDa after silver staining. The antibacterial assay was then performed using elafin treated with 30 and 3000 nM pseudolysin to generate cleaved forms of elafin. Our results presented in Figure 8C demonstrated that elafin, even cleaved by 30 and 3000 nM pseudolysin, retained its antibacterial properties against P. aeruginosa PAO1.
Figure 8. Effect of purified pseudolysin on the antibacterial activity of recombinant elafin against Pseudomonas aeruginosa PAO1.
(A) Dose-response effect of elafin on P. aeruginosa PAO1 survival. Various concentrations of elafin (0 to 2.5 μM) were incubated with P. aeruginosa PAO1 for 2h. Colonies were counted and the results were expressed as percentage of bacteria survival. Values are means ±SEM (n=5). (B) Purity of recombinant elafin assessed by SDS-PAGE and silver staining. M: Molecular marker; lane 1: 1 μg elafin; lane 2: 200 ng elafin. (C) Effect of pseudolysin-cleaved elafin on P. aeruginosa PAO1 survival. Recombinant elafin (0 or 41.6 μM) was treated with increasing concentrations of pseudolysin (0, 30 and 3000 nM) for 2h and treated with or without GM6001 as indicated in the figure. Samples (diluted 41.6 times) were incubated with P. aeruginosa PAO1 for 2h. Bacterial survival was determined by counting the colony-forming units. Results are expressed as percentage of PAO1 survival. Values are means ±SEM (n=5). One-way ANOVA was performed to determine the statistical significance of the effects of elafin and concentrations of pseudolysin on PAO1 survival (* P<0.05; ns, non significant). Insets represent elafin integrity determined by Western blot under non-reducing conditions.
Discussion
P. aeruginosa is an opportunistic Gram-negative bacterium causing severe infections in vulnerable hosts. Such infections can affect various tissues like lung, cornea, skin or urinary tract. In the lung, P. aeruginosa is responsible for acute respiratory infections common in ventilated and immunocompromised patients and causes chronic respiratory infections in patients with Cystic Fibrosis. The severity of Pseudomonas infections is due to the ability of the pathogen to secrete extracellular factors associated with virulence, including toxins (exotoxin A, exoenzyme S), hemolysins (phospholipase C, lecithinase) and proteolytic enzymes. Pseudomonas proteases can cleave and inactivate a variety of lung proteins with important biological functions and are thus thought to play a role in promoting bacterial invasion. To date, one serine protease (arginyl peptidase) and three metalloproteases (pseudolysin, staphylolysin, aeruginolysin) have been associated with virulence of P. aeruginosa. Among these proteases, pseudolysin (Pseudomonas elastase, LasB) has been demonstrated to cleave and inactivate AAT and SLPI, two inhibitors of neutrophil serine proteases found in the lung. We confirmed in our study that both inhibitors are cleaved by proteases secreted by P. aeruginosa PAO1 following 24h incubation. Interestingly, we found that only a combination of inhibitors of metalloproteases (EDTA) and serine proteases (Pefabloc) prevented this cleavage of SLPI and AAT. These results demonstrate that, in addition to pseudolysin, a serine protease secreted by P. aeruginosa PAO1 is able to cleave these inhibitors. We hypothesise that arginyl peptidase (protease IV) and/or LasD may be involved in this process. However, there is no evidence to date that Pseudomonas serine protease(s) can inactivate SLPI or AAT.
Our study indicates that elafin is also susceptible to proteolytic cleavage by Pseudomonas proteases, as the inhibitor is rapidly degraded in the presence of the supernatant of P. aeruginosa PAO1 culture. Additionally, we found that Pseudomonas supernatant is able to decrease the anti-NE activity of elafin. In contrast to SLPI and AAT, we found that only metalloprotease inhibitors (EDTA, GM6001 and phosphoramidon) prevented elafin cleavage. This result demonstrates that elafin is susceptible to one or more Pseudomonas metalloproteases. The observed resistance of elafin to proteolysis mediated by Pseudomonas serine protease(s) suggests that it is not as sensitive as SLPI and AAT, or may be explained by the capacity of elafin to inhibit serine protease(s) secreted by the pathogenic microorganism. Indeed, a recent study suggests that elafin inhibits growth of P. aeruginosa in complex media via the inhibition of a serine protease produced by the pathogen, presumably the arginyl peptidase protease IV (Bellemare et al., 2008). Identification of the Pseudomonas metalloprotease(s) involved in the cleavage and inactivation of elafin was carried out using purified Pseudomonas metalloproteases: pseudolysin, staphylolysin (LasA) and aeruginolysin (Pseudomonas alkaline protease, serralysin). Our study provides evidence that pseudolysin and aeruginolysin, but not staphylolysin, are able to cleave recombinant elafin. Although two Pseudomonas metalloproteases were shown to degrade elafin, functional investigations demonstrate that these proteases have distinct effects on elafin’s functional activities.
Elafin is a protein inhibitor belonging to the chelonianin family, one of the 18 families of canonical inhibitors (Krowarsch et al., 2003;Laskowski, Jr. et al., 2000). The canonical inhibitors are a group of protein inhibitors characterised by a conserved protease-binding loop. The inhibitory loop is convex and matches perfectly with the concave active site of the target enzyme. The inhibition of proteases by the canonical inhibitors is reversible and obeys the standard mechanism of canonical inhibitors well described elsewhere (Krowarsch et al., 2003; Laskowski, Jr. et al., 2000). The crystallised structure of the complex between elafin and porcine pancreatic elastase (Tsunemi et al., 1996) indicates that seven residues of the inhibitory loop (Leu20-Leu26) are in contact with the enzyme and that the scissile bond is located at Ala24-Met25 also described as P1-P1′ peptide bond according to the nomenclature of Schechter and Berger (Schechter and Berger, 1967). In the present study, we report that pseudolysin cleaves elafin within its protease-binding loop giving two peptides as major products, P1–23 and P26–57. The key consequence of pseudolysin-mediated cleavage of elafin is the removal of both P1 and P1′ amino-acid residues (Ala24 and Met25) from the protease-binding loop. This is consistent with the slight shift of pseudolysin-treated elafin observed by western blot under non-reducing conditions. The gap generated by the lack of the P1 and P1′ residues within the canonical loop may prevent association with target enzymes and thus prevent elafin inhibiting NE, as demonstrated in our study. The role of pseudolysin in the inactivation of elafin and the pulmonary anti-NE defence is underlined in our study by the capacity of phosphoramidon, a specific inhibitor of pseudolysin with no effect on staphylolysin and aeruginolysin activities, to prevent anti-elastase inactivation in normal BAL by PAO1-conditioned medium. The reason for using normal BAL in the study was to mimic the initial phases of the lung infection by P. aeruginosa. However, a severe infection will lead to a rapid and a massive influx of neutrophils into the lungs followed by a secretion of large amounts of neutrophil serine proteases in the airways including the target enzymes of elafin: NE and Pr3. In this context, a competition may then occur between pseudolysin and neutrophil serine proteases for the interaction with elafin. In addition, pseudolysin can inactivate neutrophil elastase making it difficult to evaluate the inactivation of elafin by pseudolysin in the presence of neutrophil elastase (Doring et al., 1985). In contrast to pseudolysin, aeruginolysin does not inactivate elafin. This enzyme was shown to cleave the inhibitor at Lys5-Gly6. This peptide bond is located in the amino-terminal extremity of the inhibitor and structural data indicate that the amino-terminal region (1–8) of elafin is flexible and is not involved in the antiprotease function of the inhibitor (Francart et al., 1997;Tsunemi et al., 1996). Curiously, the elafin fragment Gly6-Gln57 generated by aeruginolysin was detected by western blot under non-reducing conditions but not in the presence of reducing agents. This feature may be explained at least in part by the weak ability of the antibody to detect reduced elafin. Moreover, this observation may also suggest that the fragment Ala1-Gly6 contains a major epitope for the antibody. Similar observations were previously reported with the elafin fragments Lys6-Gln57 and Ser10-Gln57 generated by neutrophil elastase (Guyot et al., 2008).
A number of studies have demonstrated that elafin and its precursor trappin-2 have intrinsic antimicrobial activities against various pathogens including bacteria (such as P.aeruginosa, Staphylococcus aureus and Klebsiella pneumoniae) and fungi (Aspergillus fumigatus, Candida albicans). Nevertheless the mechanism by which elafin and trappin-2 act as antimicrobials remains unclear. It is thought that the cationic nature of both inhibitors as well as their structure (WAP domain) can play a key role in this process. Moreover it has been found recently that elafin/trappin-2 can use an antimicrobial mechanism dependent (Bellemare et al., 2008) or independent (Baranger et al., 2008) of their antiprotease properties. It seems that the antimicrobial effect against P. aeruginosa and the mechanism of action of elafin/trappin-2 differ depending on the bacterial strain used in the studies. In the present work, we tested the antibacterial properties of elafin against P. aeruginosa PAO1. Around 30–40% of control PAO1 was recovered after treatment with 1–2.5 μM elafin. These results are similar to those obtained previously by Simpson et al. (Simpson et al., 1999). Interestingly, our data demonstrate that the cleavage of elafin by pseudolysin within the protease-binding region did not alter antibacterial properties of elafin against PAO1, while its anti-NE activity was inhibited. These findings suggest an anti-PAO1 activity independent of its antiprotease properties for elafin, as previously demonstrated for trappin-2 (Baranger et al., 2008).
Elafin and its precursor trappin-2 are substrates of transglutaminases (Guyot et al., 2005b;Molhuizen et al., 1993;Nara et al., 1994). Transglutaminases are enzymes that catalyse the formation of an isopeptide bond between the lateral chains of a lysine residue and a glutamine residue. By this mechanism, trappin-2 and elafin can covalently associate with structural proteins. In vivo, elafin/trappin-2 was shown to be covalently crosslinked to components in the tracheal epithelium that have not yet been identified (Nara et al., 1994). In the epidermis, elafin and trappin-2 were shown to associate with a number of structural proteins of the cornified cell envelope (including keratin-1, loricrin, involucrin and desmoplakin I/II among others). In vitro, elafin can crosslink to elastin, fibronectin, collagen, laminin, fibrinogen (Guyot et al., 2005b;Muto et al., 2007;Nara et al., 1994). The amino-terminal sequence of elafin seems to be important in the capacity of the inhibitor to crosslink by transglutamination since as we have shown that the removal of Ala1-Lys6 sequence by aeruginolysin inhibited crosslinking of elafin to fibronectin following transglutaminase activity. This is in agreement with recent results demonstrating that elafin fragment depleted from its amino-terminal (Ser10-Gln57) is unable to bind fibronectin by transglutamination (Guyot et al., 2005b). Interestingly, elafin crosslinked to fibronectin retains its inhibitory properties and can protect fibronectin against NE (Guyot et al., 2005b) suggesting a role for elafin in the preservation of structural proteins against proteolysis mediated by neutrophil serine proteases. Given that aeruginolysin can inhibit the covalent binding of elafin to fibronectin in vitro, then it is likely that during infection, P. aeruginosa can regulate elafin crosslinking and subsequent protection against increased proteolysis mediated by neutrophils.
In conclusion, this study provides evidence that metalloproteases secreted by P. aeruginosa can cleave elafin at different sites and specifically alter functions associated with the inhibitor. Among these proteases, pseudolysin is able to inactivate the antiprotease properties of elafin. In contrast, aeruginolysin can potentially perturb the transglutaminase-mediated crosslinking of elafin to ECM proteins. Therefore, both proteases may affect the protective role of elafin against neutrophil-mediated proteolysis in the lung via different mechanisms, shedding further light on the pathogenesis of Pseudomonas infections. The inactivation of elafin and pulmonary anti-elastase defense by P. aeruginosa may considerably disturb lung epithelial homeostasis. In this respect, these findings may have pathological importance.
Experimental procedures
Materials
Recombinant human elafin was obtained from Proteo Biotech AG (Kiel, Germany). Biotinylated anti-human elafin antibody and recombinant SLPI were purchased from R&D Systems (Abingdon, Oxon, UK). Aprotinin, Nα-Tosyl-L-lysine chloromethyl ketone hydrochloride (TLCK), leupeptin, Pefabloc, pepstatin A, ethylenediamine-tetraacetic acid (EDTA) and N-Methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide (MeOSuc-AAPV-pNA) were purchased from Sigma-Aldrich. Pseudomonas elastase (EC 3.4.24.26), E-64, GM6001 and phosphoramidon were purchased from Merck Biosciences. Human neutrophil elastase (NE) (EC 3.4.21.37) was from Elastin Products Company, Inc. (Owensville, MO, USA). Purified alpha-1-antitrypsin (AAT) was from Athens Research (Athens, GA, USA). Complete protease inhibitor tablets were obtained from Roche. Horseradish peroxidase (HRP)-conjugated streptavidin was purchased from Cambridge BioSciences Ltd (Cambridge, UK). SuperSignal West Femto Maximum Sensitivity Substrate was purchased from Pierce. Pseudomonas alkaline protease (EC 3.4.24.40) was from Nagase Biochemicals. LasA (EC 3.4.24.-) was obtained from Prof. Efrat Kessler (Tel-Aviv University Sackler University of Medicine, Tel-Hashomar, Israel). All other reagents were of analytical grade.
Pseudomonas PAO1-conditioned medium
Pseudomonas aeruginosa strain 01 (PAO1) was a gift from R. Hancock (University of British Columbia). PAO1-conditioned medium was prepared by filter-sterilising culture supernatants from 72 h PAO1 Trypticase Soy Broth cultures.
Incubations and SDS-PAGE analysis
10 μl PAO1 conditioned-medium were pre-incubated for 0.5–1h at 37°C alone or with the following protease inhibitors: 10 mM Pefabloc, 0.13 mM TLCK, 13 μM aprotinin, 0.6 mM leupeptin, 0.4 mM E-64, 0.4 mM pepstatin A, 13 mM EDTA, 0.5 mM GM6001 or 0.2 mM phosphoramidon. Recombinant elafin, SLPI or purified AAT (8.5 × 10−7 M each) were mixed with 10 μl of pre-treated PAO1-conditioned medium in 30 mM TBS to a final volume of 20 μl for 24 h at 37°C. Additionally, recombinant elafin (2.5× 10−5 M) was incubated with various concentrations of purified Pseudomonas proteases for 2h in 30 mM TBS in a 20 μl final volume at 37°C and analysed by SDS-PAGE. All incubations were stopped by adding sample treatment buffer containing reducing agent and boiling samples for 5 min at 100°C. Samples were separated by Tricine SDS-PAGE using a 17.5% polyacrylamide gel and proteins analysed by staining the gel with Coomassie Blue G or by Western blotting.
Western blotting
Samples separated by Tricine SDS-PAGE were blotted onto a 0.2 μM nitrocellulose membrane (Sigma-Aldrich). The membrane was blocked for 1 h at room temperature with 3% BSA (elafin), 0.2% Iblock (SLPI) or 5% milk (AAT) in PBS containing 0.1% Tween 20. Elafin was detected by using a biotinylated anti-elafin antibody (R&D Systems, 1:500, overnight at 4°C) following by peroxidase-conjugated streptavidin (Zymed, 1:2500, 20 min at room temperature). SLPI and AAT were detected by incubating the membrane overnight at 4°C with rabbit anti-SLPI (1/1000) and anti-AAT (1/1000) antibodies respectively followed by a peroxidase-conjugated anti-rabbit antibody (1/2000) for 1h at room temperature. Peroxidase activity was detected with chemiluminescent substrates (Pierce).
HPLC-mass spectrometry analysis
Cleavage of elafin by Pseudomonas proteases was assessed by incubating 3 μg elafin with Pseudomonas elastase or alkaline protease in 30 mM TBS in 20 μl final volume for 2 hours at 37°C. Protease activity was neutralised with 1 μl of 0.1 g/ml EDTA for 30 min at room temperature. 14 μl of each sample was lyophilised for analysis when they were reconstituted in 10 μl 6M guanidine HCl, 100 mM Tris pH 8.5, 1 mM EDTA, and 10 mM dithiothreitol (DTT). Samples were incubated for 30 min at 37°C to ensure the reduction of disulfide bridges. One μl 10% trifluoroacetic acid was added to each sample to bring the pH to <3. Samples were then analysed by reverse phase HPLC coupled to electrospray mass spectrometry as described elsewhere (Liu et al., 2006). Spectra were deconvoluted with Mass Hunter version 2 (Agilent Technologies), using peaks whose heights were 1% or more of the largest peak.
Neutrophil elastase (NE) activity assays
The effect of Pseudomonas proteases on the anti-NE activity of elafin was assessed as following: PAO1-conditioned medium or purified proteases (pseudolysin, aeruginolysin) were pre-treated with or without protease inhibitors (10 mM EDTA or 0.17 mM phosphoramidon) for 20 min at 37°C in 30 mM TBS (20 μl final volume) and incubated with recombinant elafin for various times (0, 1, 2 or 6h) at 37°C. The reaction mix was diluted with 0.1 M Hepes, 0.5 M NaCl, pH 7.5 containing 0.1% Brij 35 and EDTA and incubated with NE in a 100 μl volume in such a way that elafin final concentration was two-times higher than NE concentration. The residual activity of NE was determined spectrophotometrically by adding 50 μl of 3 mM MeOSuc-AAPV-pNA and by measuring the absorbance at 405 nm over the time at 37°C. Inhibition of NE was expressed as a percentage of the NE present in control samples. The same method was applied to study the effect of PAO1-conditioned medium on the anti-NE activity of normal BAL fluid. 2 μl of PAO1-conditioned medium and 10 μl of a pooled fraction of normal BAL fluids (n=5) in the initial reaction.
Crosslinking of elafin to fibronectin by transglutamination
Recombinant elafin (100 ng, 3μM) was incubated with increasing concentrations of aeruginolysin (0–100 nM) in PBS for 2h at 37°C. Aeruginolysin was inactivated by 198 μM GM6001 for 30 min. The resulting mix was incubated in 200 mM Tris-acetate pH 6 containing 5 mM CaCl2 and 0.1 mM DTT for 1h at 37°C with 5 μg plasma fibronectin and 0.38 mU guinea pig liver transglutaminase (1 unit catalyses the formation of 1.0μmole of hydroxamate per minute from Nα-CBZ-Glutaminylglycine and hydroxylamine at pH 6.0 at 37°C). The reaction was stopped by adding sample treatment buffer without reducing agent and by boiling samples for 5 min at 100°C. Samples were separated by 4–12% Bis-Tris SDS-PAGE and analysed by Western blotting using a biotinylated anti-elafin antibody as described above.
Antibacterial assay
A single P. aeruginosa PAO1 colony was grown in 10 ml Tryptic Soy Broth at 37°C with agitation (250 rpm). After 4–6h of growth, the PAO1 culture was centrifuged (4000 rpm, 10 min) and the bacterial pellet was washed and resuspended in PBS. 50 μl of PAO1 diluted 10,000 times was mixed with 50 μl of recombinant elafin (0 to 2.5 μM) in PBS. The reaction mixture was incubated for 2h at 37°C with agitation (250 rpm). 10 μl of various serial dilutions of the reaction mixtures were spread on Tryptic Soy Agar plates and incubated overnight at 37°C. The colonies were counted and expressed as PAO1 survival compared to PAO1 alone as control (100%). To study the effect of pseudolysin on the antibacterial activity of elafin, the inhibitor was treated as following: 41.6 μM recombinant elafin was incubated with increasing concentrations of pseudolysin (0, 30 and 3000 nM) in PBS for 2h at 37°C and treated with 60 μM GM6001 to stop the reaction. Controls were also carried out using pseudolysin alone and PBS alone without GM6001 in the same conditions. The reaction mixtures were diluted 41.6-fold in 100 μL PBS (final elafin concentration: 0 or 1 μM) containing PAO1 as described above.
Acknowledgments
This work was supported by the Health Research Board, the Alpha One Foundation, the Program for Research in Third levels Institutes administered by Higher Education Authority, Science Foundation Ireland, Cystic Fibrosis Hope Source, Cystic Fibrosis Research Trust, Cystic Fibrosis Association of Ireland (awarded to C.T.) and the Royal College of Surgeons in Ireland.
References
- Banda MJ, Clark EJ, Werb Z. Limited proteolysis by macrophage elastase inactivates human alpha 1-proteinase inhibitor. J Exp Med. 1980;152:1563–1570. doi: 10.1084/jem.152.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranger K, Zani ML, Chandenier J, let-Choisy S, Moreau T. The antibacterial and antifungal properties of trappin-2 (pre-elafin) do not depend on its protease inhibitory function. FEBS J. 2008;275:2008–2020. doi: 10.1111/j.1742-4658.2008.06355.x. [DOI] [PubMed] [Google Scholar]
- Beatty K, Bieth J, Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980;255:3931–3934. [PubMed] [Google Scholar]
- Bellemare A, Vernoux N, Morisset D, Bourbonnais Y. Human pre-elafin inhibits a Pseudomonas aeruginosa-secreted peptidase and prevents its proliferation in complex media. Antimicrob Agents Chemother. 2008;52:483–490. doi: 10.1128/AAC.00585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle L, Tetley TD, Bingle CD. Cytokine-mediated induction of the human elafin gene in pulmonary epithelial cells is regulated by nuclear factor-kappaB. Am J Respir Cell Mol Biol. 2001;25:84–91. doi: 10.1165/ajrcmb.25.1.4341. [DOI] [PubMed] [Google Scholar]
- Boudier C, Bieth JG. Oxidized mucus proteinase inhibitor: a fairly potent neutrophil elastase inhibitor. Biochem J. 1994;303 (Pt 1):61–68. doi: 10.1042/bj3030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Farmer K, MacDonald L, Kalsheker N, Pritchard D, Haslett C, et al. House dust mite Der p 1 downregulates defenses of the lung by inactivating elastase inhibitors. Am J Respir Cell Mol Biol. 2003;29:381–389. doi: 10.1165/rcmb.2003-0060OC. [DOI] [PubMed] [Google Scholar]
- Butler MW, Robertson I, Greene CM, O’Neill SJ, Taggart CC, McElvaney NG. Elafin prevents lipopolysaccharide-induced AP-1 and NF-kappaB activation via an effect on the ubiquitin-proteasome pathway. J Biol Chem. 2006;281:34730–34735. doi: 10.1074/jbc.M604844200. [DOI] [PubMed] [Google Scholar]
- DÖring G, Goldstein W, Roll A, Schiotz PO, Hoiby N, Botzenhart K. Role of Pseudomonas aeruginosa exoenzymes in lung infections of patients with cystic fibrosis. Infect Immun. 1985;49:557–62. doi: 10.1128/iai.49.3.557-562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francart C, Dauchez M, Alix AJ, Lippens G. Solution structure of R-elafin, a specific inhibitor of elastase. J Mol Biol. 1997;268:666–677. doi: 10.1006/jmbi.1997.0983. [DOI] [PubMed] [Google Scholar]
- Guyot N, Butler MW, McNally P, Weldon S, Greene CM, Levine RL, et al. Elafin, an elastase specific inhibitor, is cleaved by its cognate enzyme neutrophil elastase in sputum from individuals with cystic fibrosis. J Biol Chem. 2008 doi: 10.1074/jbc.M803707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot N, Zani ML, Berger P, let-Choisy S, Moreau T. Proteolytic susceptibility of the serine protease inhibitor trappin-2 (pre-elafin): evidence for tryptase-mediated generation of elafin. Biol Chem. 2005a;386:391–399. doi: 10.1515/BC.2005.047. [DOI] [PubMed] [Google Scholar]
- Guyot N, Zani ML, Maurel MC, let-Choisy S, Moreau T. Elafin and its precursor trappin-2 still inhibit neutrophil serine proteinases when they are covalently bound to extracellular matrix proteins by tissue transglutaminase. Biochemistry. 2005b;44:15610–15618. doi: 10.1021/bi051418i. [DOI] [PubMed] [Google Scholar]
- Henriksen PA, Hitt M, Xing Z, Wang J, Haslett C, Riemersma RA, et al. Adenoviral gene delivery of elafin and secretory leukocyte protease inhibitor attenuates NF-kappa B-dependent inflammatory responses of human endothelial cells and macrophages to atherogenic stimuli. J Immunol. 2004;172:4535–4544. doi: 10.4049/jimmunol.172.7.4535. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Carter-Hamm B, Dralle WM. Inactivation of human bronchial mucosal proteinase inhibitor by Pseudomonas aeruginosa elastase. Am Rev Respir Dis. 1982;126:1070–1073. doi: 10.1164/arrd.1982.126.6.1070. [DOI] [PubMed] [Google Scholar]
- Kantyka T, Latendorf T, Wiedow O, Bartels J, Glaser R, Dubin G, et al. Elafin is specifically inactivated by RgpB from Porphyromonas gingivalis by distinct proteolytic cleavage. Biol Chem. 2009;390:1313–1320. doi: 10.1515/BC.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowarsch D, Cierpicki T, Jelen F, Otlewski J. Canonical protein inhibitors of serine proteases. Cell Mol Life Sci. 2003;60:2427–2444. doi: 10.1007/s00018-003-3120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M, Jr, Qasim MA, Lu SM. Interaction of standard mechanism, canonical protein inhibitors with serine proteinases. In: Kleanthouse C, editor. Protein-protein recognition: The frontiers in molecular biology series. Oxford University Press; 2000. pp. 228–279. [Google Scholar]
- Liu X, Shu S, Hong MS, Levine RL, Korn ED. Phosphorylation of actin Tyr-53 inhibits filament nucleation and elongation and destabilizes filaments. Proc Natl Acad Sci U S A. 2006;103:13694–13699. doi: 10.1073/pnas.0606321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast AE, Enghild JJ, Nagase H, Suzuki K, Pizzo SV, Salvesen G. Kinetics and physiologic relevance of the inactivation of alpha 1-proteinase inhibitor, alpha 1-antichymotrypsin, and antithrombin III by matrix metalloproteinases-1 (tissue collagenase), -2 (72-kDa gelatinase/type IV collagenase), and -3 (stromelysin) J Biol Chem. 1991;266:15810–15816. [PubMed] [Google Scholar]
- Meyer-Hoffert U, Wichmann N, Schwichtenberg L, White PC, Wiedow O. Supernatants of Pseudomonas aeruginosa induce the Pseudomonas-specific antibiotic elafin in human keratinocytes. Exp Dermatol. 2003;12:418–425. doi: 10.1034/j.1600-0625.2002.120409.x. [DOI] [PubMed] [Google Scholar]
- Michaelis J, Vissers MC, Winterbourn CC. Human neutrophil collagenase cleaves alpha 1-antitrypsin. Biochem J. 1990;270:809–814. doi: 10.1042/bj2700809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molhuizen HO, Alkemade HA, Zeeuwen PL, De Jongh GJ, Wieringa B, Schalkwijk J. SKALP/elafin: an elastase inhibitor from cultured human keratinocytes. Purification, cDNA sequence, and evidence for transglutaminase cross-linking. J Biol Chem. 1993;268:12028–12032. [PubMed] [Google Scholar]
- Moreau T, Baranger K, Dade S, let-Choisy S, Guyot N, Zani ML. Multifaceted roles of human elafin and secretory leukocyte proteinase inhibitor (SLPI), two serine protease inhibitors of the chelonianin family. Biochimie. 2008;90:284–295. doi: 10.1016/j.biochi.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Morihara K, Tsuzuki H, Oda K. Protease and elastase of Pseudomonas aeruginosa: inactivation of human plasma alpha 1-proteinase inhibitor. Infect Immun. 1979;24:188–193. doi: 10.1128/iai.24.1.188-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto J, Kuroda K, Wachi H, Hirose S, Tajima S. Accumulation of elafin in actinic elastosis of sun-damaged skin: elafin binds to elastin and prevents elastolytic degradation. J Invest Dermatol. 2007;127:1358–1366. doi: 10.1038/sj.jid.5700647. [DOI] [PubMed] [Google Scholar]
- Nara K, Ito S, Ito T, Suzuki Y, Ghoneim MA, Tachibana S, et al. Elastase inhibitor elafin is a new type of proteinase inhibitor which has a transglutaminase-mediated anchoring sequence termed “cementoin”. J Biochem. 1994;115:441–448. doi: 10.1093/oxfordjournals.jbchem.a124357. [DOI] [PubMed] [Google Scholar]
- Nobar SM, Zani ML, Boudier C, Moreau T, Bieth JG. Oxidized elafin and trappin poorly inhibit the elastolytic activity of neutrophil elastase and proteinase 3. FEBS J. 2005;272:5883–5893. doi: 10.1111/j.1742-4658.2005.04988.x. [DOI] [PubMed] [Google Scholar]
- Pfundt R, Wingens M, Bergers M, Zweers M, Frenken M, Schalkwijk J. TNF-alpha and serum induce SKALP/elafin gene expression in human keratinocytes by a p38 MAP kinase-dependent pathway. Arch Dermatol Res. 2000;292:180–187. doi: 10.1007/s004030050475. [DOI] [PubMed] [Google Scholar]
- Reid PT, Marsden ME, Cunningham GA, Haslett C, Sallenave JM. Human neutrophil elastase regulates the expression and secretion of elafin (elastase-specific inhibitor) in type II alveolar epithelial cells. FEBS Lett. 1999;457:33–37. doi: 10.1016/s0014-5793(99)01004-2. [DOI] [PubMed] [Google Scholar]
- Sallenave JM, Ryle AP. Purification and characterization of elastase-specific inhibitor. Sequence homology with mucus proteinase inhibitor. Biol Chem Hoppe Seyler. 1991;372:13–21. doi: 10.1515/bchm3.1991.372.1.13. [DOI] [PubMed] [Google Scholar]
- Sallenave JM, Shulmann J, Crossley J, Jordana M, Gauldie J. Regulation of secretory leukocyte proteinase inhibitor (SLPI) and elastase-specific inhibitor (ESI/elafin) in human airway epithelial cells by cytokines and neutrophilic enzymes. Am J Respir Cell Mol Biol. 1994;11:733–741. doi: 10.1165/ajrcmb.11.6.7946401. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J, Chang A, Janssen P, De Jongh GJ, Mier PD. Skin-derived antileucoproteases (SKALPs): characterization of two new elastase inhibitors from psoriatic epidermis. Br J Dermatol. 1990;122:631–641. doi: 10.1111/j.1365-2133.1990.tb07285.x. [DOI] [PubMed] [Google Scholar]
- Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Cunningham GA, Porteous DJ, Haslett C, Sallenave JM. Regulation of adenovirus-mediated elafin transgene expression by bacterial lipopolysaccharide. Hum Gene Ther. 2001;12:1395–1406. doi: 10.1089/104303401750298553. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Maxwell AI, Govan JR, Haslett C, Sallenave JM. Elafin (elastase-specific inhibitor) has anti-microbial activity against gram-positive and gram-negative respiratory pathogens. FEBS Lett. 1999;452:309–313. doi: 10.1016/s0014-5793(99)00670-5. [DOI] [PubMed] [Google Scholar]
- Taggart CC, Lowe GJ, Greene CM, Mulgrew AT, O’Neill SJ, Levine RL, et al. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J Biol Chem. 2001;276:33345–33352. doi: 10.1074/jbc.M103220200. [DOI] [PubMed] [Google Scholar]
- Tsunemi M, Matsuura Y, Sakakibara S, Katsube Y. Crystal structure of an elastase-specific inhibitor elafin complexed with porcine pancreatic elastase determined at 1.9 A resolution. Biochemistry. 1996;35:11570–11576. doi: 10.1021/bi960900l. [DOI] [PubMed] [Google Scholar]
- Wiedow O, Schroder JM, Gregory H, Young JA, Christophers E. Elafin: an elastase-specific inhibitor of human skin. Purification, characterization, and complete amino acid sequence. J Biol Chem. 1990;265:14791–14795. [PubMed] [Google Scholar]
- Ying QL, Simon SR. Kinetics of the inhibition of human leukocyte elastase by elafin, a 6-kilodalton elastase-specific inhibitor from human skin. Biochemistry. 1993;32:1866–1874. doi: 10.1021/bi00058a021. [DOI] [PubMed] [Google Scholar]
- Zani ML, Nobar SM, Lacour SA, Lemoine S, Boudier C, Bieth JG, et al. Kinetics of the inhibition of neutrophil proteinases by recombinant elafin and pre-elafin (trappin-2) expressed in Pichia pastoris. Eur J Biochem. 2004;271:2370–2378. doi: 10.1111/j.1432-1033.2004.04156.x. [DOI] [PubMed] [Google Scholar]