Abstract
Oligodendrogenesis encompasses lineage specification of neural progenitor cells (NPCs) and differentiation into oligodendrocytes that ultimately culminates in the myelination of central nervous system axons. Each individual process must be tightly regulated by extracellular and cell-intrinsic mechanisms, whose identities are barely understood. We had previously demonstrated that soluble factors derived from rat mesenchymal stem cells (MSCs) induce oligodendrogenesis in differentiating adult NPCs under differentiation conditions. However, since lineage specification predominantly occurs in proliferating progenitors and not necessarily during early differentiation, we investigated if soluble factors derived from MSCs are able to prime NPCs to the oligodendroglial fate already under proliferation conditions. Therefore, we analyzed the effects of a 3 weeks stimulation of adult NPCs under proliferation conditions with conditioned media derived from MSCs (MSC-CM) in terms of cell morphology, proliferation, cell-specific marker expression profile, response to growth factor withdrawal (GFW), cell-lineage restriction, and expression of glial fate determinants. While MSC-CM did not affect the proliferation rate of NPCs, it boosted the formation of 2′, 3′-cyclic-nucleotide-3′-phosphodieesterase (CNPase)- and myelin basic protein-expressing oligodendrocytes after GFW, even when cells were exposed to an astrogenic milieu. Moreover, it reinforced the proper development of oligodendrocytes, since it ensured a sustained expression of the functional marker CNPase. Finally, the presence of MSC-CM reduced the anti-oligodendrogenic determinant Id2 in proliferating NPCs, thus increasing the relative proportion of the pro-oligodendrogenic factor Olig2 expression. In summary, MSCs prime proliferating progenitors and, thus, reinforce cell fate choice and accelerate differentiation toward the oligodendrocyte lineage. The present findings underscore the potential use of MSCs in cell therapies for remyelination such as in multiple sclerosis and spinal cord injury. Moreover, they urge the identification of the oligodendrogenic activity(ies) derived from MSCs to develop novel molecular therapies for demyelinating diseases.
Introduction
Oligodendrocytes are one of the 3 main neuroectodermal cell types within the central nervous system (CNS). Their main function is to form myelin that wraps axons to facilitate saltatory electric conductance [1]. Demyelinating diseases, such as multiple sclerosis (MS), are characterized by the loss of oligodendrocytes resulting in severe neurological symptoms such as hemiparesis or visual impairment. Enhancing oligodendrogenesis and promoting remyelination might be an attractive approach to counteract this situation. The feasibility of this approach is provided by the fact that oligodendrogenesis can occur during adulthood. Oligodendrocyte progenitors cells (OPCs) are widespread throughout the CNS in white and gray matter, representing 5% to 8% of total glial cells [2,3] and constitute the major cellular source of remyelinating cells [4]. Upon demyelination, endogenous OPCs start to proliferate and the expression of oligodendrogenic genes is induced. After OPCs activation, cells are recruited toward the lesion site where they differentiate and mature into myelinating oligodendrocytes [4,5]. However, OPCs are not unique in the ability to remyelinate, since subventricular zone (SVZ) derived neural stem cells (NSCs), beside their neurogenic potential, represent a source for new oligodendrocytes [6–9]. Under normal physiological conditions and in response to demyelinating insults, SVZ derived NSCs migrate into the corpus callosum, the striatum, and to the fimbria fornix where they give rise to myelinating oligodendrocytes [6–8,10]. In addition to this, cells residing in the subcallosum zone also enter the corpus collosum and generate oligodendrocytes [11]. Therefore, the adult CNS is equipped with different cellular sources for remyelination and regenerative mechanisms in response to demyelination.
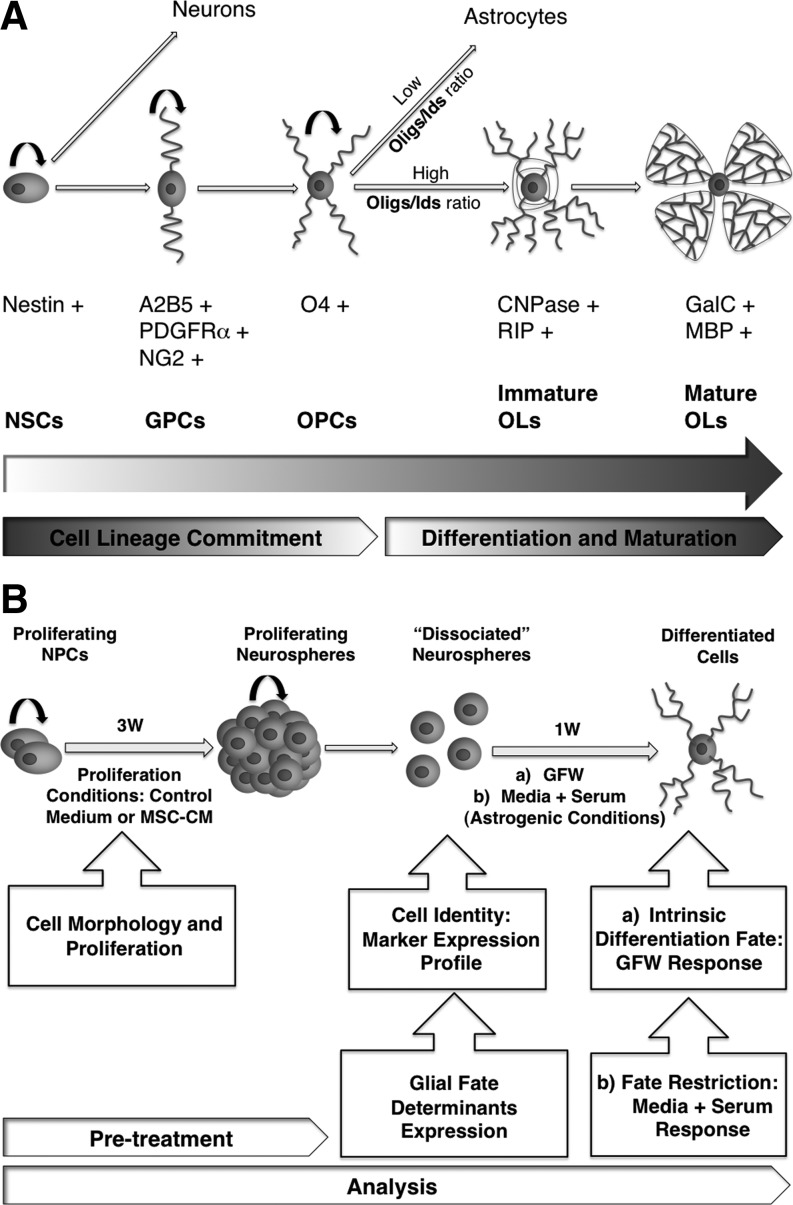
Oligodendrogenesis is a hierarchically structured process that involves (i) specification of proliferating neural stem/progenitor cells to the oligodendroglial lineage via glial and subsequently oligodendroglial progenitors, (ii) migration of progenitors and differentiation into oligodendrocytes, and (iii) myelination of axons [12–14] (Fig. 1A). This process involves the sequential generation of several cell types that display distinct proliferation and differentiation properties. Due to the cell-specific marker expression profile, each cell type can be monitored and identified in vivo and in vitro (Fig. 1A). Each individual step in oligodendrogenesis is tightly regulated by extracellular and cell-intrinsic mechanisms. For example, platelet derived growth factor (PDGF) and sonic hedgehog (Shh) are potent activators of oligodendrogenesis [15–18], while bone morphogenetic proteins (BMPs) inhibit the generation of oligodendrocytes. BMPs induce the expression of the inhibitors of differentiation 2 and 4 (Id2 and Id4), which sequester the pro-oligodendrogenic transcription factors Olig1 and Olig2 in the cytoplasm avoiding their entrance into the nucleus [19]. Therefore, Olig1/2 and Id2/4 proteins are glial fate determinants and the balance between their expression levels plays a crucial role in the astrocyte/oligodendrocyte decision.
FIG. 1.
The oligodendrogenic process of neural stem/progenitor cells and the experimental design. (A) In the oligodendroglial lineage, undifferentiated neural stem cells (NSCs) undergo sequential steps of cell lineage commitment, differentiation, and maturation involving different cell types, culminating in myelinating oligodendrocytes (OLs). Due to the specific marker expression profile each cell type can be identified during this process. Proliferating (circular small arrow) Nestin-expressing NSCs, follow a specification step to give rise to glial progenitor cells (GPCs), characterized by the expression of A2B5 epitope, platelet derived growth factor receptor-alpha (PDGFRα), and the chondroitin sulfate proteoglycan NG2. In turn, proliferating GPCs can give rise to O4-expressing cycling oligodendrocyte progenitor cells (OPCs) that undergo glial fate decision toward astrocytes or OLs. Several glial fate determinants regulate this step, in particular the relative expression levels of Oligs and Ids play an important role at the glial branch point. A high Oligs/Id2 ratio implies an oligodendrocyte fate decision and vice versa. Therefore, cell lineage commitment and restriction might occur in proliferating immature stem/progenitor cells. Next OPCs follow a differentiation process toward immature 2′,3′-cyclic nucleotide 3′ phosphodiesterase- (CNPase) and oligodendrocyte specific molecule (RIP)-expressing OLs. Finally, a maturation process takes place and will give rise to myelinating OLs, characterized by the expression of Galactocerebroside (GalC) and myelin basic protein (MBP). (B) The experimental setup of the present work uses a 3 week (3W) treatment of proliferating (circular small arrow) NPCs with MSC-CM. During this period, cell morphology and proliferation are followed. Proliferating neural progenitor cells (NPCs) give rise to neurospheres that are dissociated for subsequent experiments and analyses. Cell identity is directly analyzed after the 3 week stimulation by profiling cell type specific marker expression; the intrinsic differentiation fate of the cells is analyzed after a 1 week (1W) period of growth factor withdrawal (GFW); the oligodendroglial cell-lineage restriction is tested by exposing the primed cells for 1 week (1W) to serum, an astroglia inducing activity; finally, the expression of Olig2 and Id2 is directly determined in proliferating NPCs after the 3 weeks (3W) of exposure to MSC-CM. MSC-CM, conditioned media derived from mesenchymal stem cell.
Conditioned medium derived from mesenchymal stem cells (MSCs-CM) promotes expression of myelin basic protein (MBP) and oligodendrogenesis in differentiating neural progenitor cells (NPCs) [20–22]. Moreover, we demonstrated that MSCs promote oligodendroglial differentiation of NPCs that were co-transplanted into a hippocampal slice [23]. Thus, factors derived from MSCs strongly promote oligodendrogenesis of differentiating cells. However, it is unclear if MSC-CM targets progenitors and specifies them toward the oligodendroglial fate also under proliferation conditions. Obviously, this is of huge relevance, since proliferating progenitors commonly surround CNS lesions in high numbers, but often fail to remyelinate sparse axons [24,25]. Moreover, this situation becomes more evident and relevant in aged subjects [26–28]. While the deficiency in remyelination is partly derived from the failure to differentiate into oligodendroglia [24,26–28], a lack of sufficient oligodendroglial commitment in proliferating progenitors might contribute to this deficiency. Here, we test the hypothesis that MSC derived soluble factors are able to predispose or prime proliferating NPCs toward an oligodendroglial fate.
Materials and Methods
Animal subjects
Adult female Fischer 344 rats (Charles River Deutschland GmbH) were used as donors for the NPCs and MSCs cultures. All experiments were carried out in accordance with the European Communities Council Directive (86/609/EEC) and institutional guidelines. Animals had ad libitum access to food and water throughout the study.
Rat NPCs cultures and neurospheres formation
NPCs derived from the adult hippocampus were generated as described [20,29]. Briefly, 2–4 month-old rats (Charles River Deutschland GmbH) were decapitated. Hippocampi were aseptically removed, transferred to 4°C Dulbecco's phosphate buffered saline (DPBS) (PAN) with 4.5g/L glucose (Merck; DPBS/glu), washed once, transferred to petri dishes and mechanically dissociated. The cell suspension was washed in DPBS/glu and resuspended in Papain-protease-dispase (PPD) solution containing 0.01% Papain (Worthington Biochemicals), 0.1% dispase II (Boehringer), 0.01% DNase I (Worthington Biochemicals), and 12.4 mM MgSO4 in Hank's buffered salt solution (HBSS) (PAN) without Mg++/Ca++ (PAA) and digested for 30 to 40 min at 37°C. The cell suspension was triturated every 10 min. Dissociated cells were collected and resuspended in Neurobasal (NB) medium containing B27 (Gibco BRL), 2 mM l-glutamine, and 100 U/mL penicillin/100 μg/mL streptomycin (PAN) and washed 3 times. Finally, the single cell suspension was resuspended in NB medium (Gibco BRL) supplemented with B27 (Gibco BRL; NB/B27), 2 mM l-glutamine (PAN), 100 U/mL penicillin/100 μg/mL streptomycin (PAN), 2 μg/mL heparin (Sigma), human recombinant 20 ng/mL fibroblast growth factor (FGF)-2 (R&D Systems GmbH), and human recombinant 20 ng/mL epidermal growth factor (EGF; R&D Systems GmbH). Cultures were maintained at 37°C in an incubator with 5% CO2. Half of the medium was changed every 3 to 4 days. After 1 to 2 weeks NPCs formed neurospheres and were passaged. Thereafter, neurospheres were passaged once a week and were grown for 2 to 3 weeks to reach passage 2. Neurospheres cultures from passage number 2 to 6 were used throughout this study.
Rat MSCs cultures and conditioned medium preparation
MSCs were prepared as previously described [20]. Briefly, bone marrow plugs were harvested from femurs and tibias of 2–4 month-old female Fisher-344 rats (Charles River Deutschland GmbH). Plugs were mechanically dissociated in minimal essential medium alpha medium (αMEM) (Gibco Invitrogen) and recovered by centrifugation. Cell pellets were resuspended in αMEM-10% fetal bovine serum (FBS) and seeded at 1×106 cells/cm2. After 3 days, media was changed and nonadherent cells were removed. Adherent cells were incubated in fresh αMEM-10% FBS until a confluent layer of cells was achieved. Cells were trypsinized using 0.25% Trypsin (Gibco Invitrogen) and seeded in αMEM-10% FBS at 8,000 cells/cm2. After 3–5 days of culture, the resulting monolayer of cells, hereafter named rat bone marrow-derived MSCs, was trypsinized and further cultured for experiments or frozen for later use. As demonstrated in our previous work, this cell culture preparation is highly enriched in multipotent MSCs with virtually no hematopoietic contamination [20].
MSC-CM was similarly prepared as described [20] with the exception of the media used. MSCs were plated at 12,000 cells/cm2 and incubated in normal NPCs proliferation medium [NB medium supplemented with B27 (NB/B27), 2 mM l-glutamine, 100 U/mL penicillin/100 μg/mL streptomycin, 2 μg/mL heparin, human recombinant 20 ng/mL FGF-2, and human recombinant EGF 20 ng/mL]. After 3 days, the conditioned medium was collected and filtered using a 0.22 μm-pore filter. In some of the experiments, the conditioned medium was replenished with EGF and FGF-2 (20 ng/mL each) to exclude the possibility that MSCs might have consumed the growth factors.
NPCs stimulation with MSC-CM and cell number analysis
About 5×104 NPCs/mL were incubated up to 3 weeks either in normal NPCs proliferating medium (control) or in complete MSC-CM. Alternatively, cells were incubated in a half/half mixture of normal NPCs proliferation media and MSC-CM (50% MSC-CM) or in MSC-CM comprising 20 ng/mL FGF-2 and 20 ng/mL EGF [MSC-CM+growth factor (GF)]. Media was changed after 3 to 4 days.
Neurospheres proliferation was explored by determining the cell number for control condition, 50% MSC-CM, 100% MSC-CM, and MSC-CM+GF through Trypan blue (Sigma-Aldrich) exclusion and MTS (tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega) and by measuring the number of neurospheres and the mean neurosphere diameter.
For Trypan blue exclusion 5×104 cells/mL were seeded in the above mentioned media. After 7, 14, and 21 days, cell number was determined by Trypan blue exclusion. The neurospheres and the cells that were adherent after MSC-CM treatment were detached and dissociated with Accutase and treated with Trypan blue. Following, the number of living cells was determined using a light microscope (Zeiss).
For the MTS assay, untreated cells and cells that were treated for 1 week with 50% MSC-CM, 100% MSC-CM, or MSC-CM+GF were seeded in the respective media into 96-well plates at a density of 2×104 cells/cm2. The assay was performed according to the manufacturer's instructions. On day 0, 2, 4, and 7 the optical density was measured at 490 nm in a microplate reader (Berthold Technologies) after 3 h of incubation with the MTS solution.
Similar to the MTS assay cells in normal proliferation media and cells that were treated for 1 week with 50% MSC-CM, 100% MSC-CM, or MSC-CM+GF were used to determine the neurosphere number and diameter. Cells were seeded at a density of 1.5×104 cells/cm2 and incubated in the respective media for 2, 4, or 7 days. The number of neurospheres was analyzed by Trypan blue exclusion under a light microscope. The neurosphere diameter was determined using Olympus IX81 inverted microscope equipped with Hamamatsu digital camera and Volocity 5.4.1 software (PerkinElmer).
Expression profile analysis of cell-lineage-specific markers
NPCs were incubated in normal proliferation medium (control), 50% MSC-CM, 100% MSC-CM, and MSC-CM replenished with growth factors (MSC-CM+GF). After 3 weeks, marker expression profile was determined. For some experiments, RNA was directly isolated from the neurospheres after the 3 weeks treatment period and processed for quantitative real-time-polymerase chain reaction (qRT-PCR). Alternatively, the floating neurospheres and the cells that got adherent under the MSC-CM treatment were dissociated with Accutase (Innovative Cell Technologies Inc., distributed by PAA) and then plated on 100 μg/mL poly-l-ornithine (Sigma-Aldrich) and 5 μg/mL laminin-coated (Sigma-Aldrich) glass coverslips (2.5×104 cells/cm2) for subsequent immunofluorescence stainings or seeded in 100 μg/mL poly-l-ornithine and 5 μg/mL laminin-coated plates (2×104 cells/cm2) for subsequent RNA isolation. The dissociated cells were incubated in Dulbecco's modified essential medium (DMEM) Knockout-20% Serum Replacement supplement (SR; Gibco Invitrogen) for 12 h. This serum-free media has been used for embryonic stem cell maintenance and does not induce differentiation [30–32]. Under these conditions dissociated NPCs attach to the plate in the absence of serum. To analyze the expression of cell-lineage-specific markers, cells were either fixed for 30 min with 4% paraformaldehyde and processed for immunofluorescence or RNA was isolated from the cells and used for qRT-PCR.
Growth factor withdrawal response
NPCs were incubated in normal proliferation medium (control), 50% MSC-CM, 100% MSC-CM, and MSC-CM replenished with growth factors (MSC-CM+GF). After 3 weeks, the response to growth factor withdrawal (GFW) was determined. First, floating neurospheres and the cells that were adherent after MSC-CM treatment were detached and dissociated with Accutase (Innovative Cell Technologies Inc., distributed by PAA). Then, the dissociated cells were plated on 100 μg/mL poly-l-ornithine (Sigma-Aldrich) and 5 μg/mL laminin-coated (Sigma-Aldrich) glass coverslips (2.5×104 cells/cm2) for subsequent immunofluorescence stainings or directly in 100 μg/mL poly-l-ornithine and 5 μg/mL laminin-coated plates (2.5×104 cells/cm2) for subsequent RNA isolation. The cells were incubated in DMEM Knockout-20% Serum Replacement supplement (SR; Gibco Invitrogen) for 3 or 7 days. Cells were then fixed for 30 min with 4% paraformaldehyde and processed for immunofluorescence or, alternatively, RNA was isolated from the cells and used for qRT-PCR.
Astrogenic potential and cell-lineage restriction analysis
NPCs were incubated with normal proliferation medium (control), 50% MSC-CM and 100% MSC-CM. After 3 weeks, the astrogenic potential was determined. Floating neurospheres and the cells that were adherent after MSC-CM treatment were detached and dissociated with Accutase (Innovative Cell Technologies Inc., distributed by PAA) and plated on 100 μg/mL poly-l-ornithine (Sigma-Aldrich) and 5 μg/mL laminin-coated (Sigma-Aldrich) glass coverslips at a density of 2.5×104 cells/cm2 in αMEM (Gibco Invitrogen) and 10% FBS (Lonza) was added as an astrogenic stimulus. After 7 days, cells were fixed for 30 min with 4% paraformaldehyde and processed for immunofluorescence to detect the expression of the different cell-lineage-specific markers.
Immunofluorescence
Fixed cells were washed in tris-buffered saline (TBS) (0.15 M NaCl, 0.1 M Tris-HCl, pH 7.5), then blocked with a solution composed of TBS; 0.1% Triton-X100 (only for intracellular antigens); 1% bovine serum albumin; 0.2% Teleostean gelatin (Sigma; fish gelatin buffer [FGB]). The same solution was used during the incubations with antibodies. Primary antibodies were applied overnight at 4°C. Fluorochrome-conjugated species-specific secondary antibodies were used for immunodetection. The following antibodies and final dilutions were used. Primary antibodies: rabbit antiglial fibrillary acidic protein (anti-GFAP) 1:1,000 (Dako); mouse anti-ratNestin 1:500 (Pharmingen); goat anti-Sox2 1:1,000 (Santa Cruz); IgM mouse anti-O4 1:100 (Chemicon); rabbit anti-platelet-derived growth factor receptor alpha (PDGFRα) 1:100 (Santa Cruz); IgM mouse anti-A2B5 1:200 (Chemicon); mouse anti-Map 2a+2b 1:250 (Sigma); rabbit antidoublecortin (anti-DCX) 1:500 (NEB); mouse anti-2′, 3′-cyclic-nucleotide-3′-phosphodieesterase (CNPase) 1:200 (Millipore); rabbit anti-NG2 1:200 (Millipore); rabbit anti-Galactocerebroside (GalC) 1:200 (Millipore); mouse anti- oligodendrocyte specific molecule (RIP) 1:200 (Millipore); mouse anti-MBP 1:750 (SMI-94, Sternberger Monoclonals Incorporated). Secondary antibodies: donkey anti-mouse, rabbit conjugated with Alexa Fluor 488 (Molecular Probes), rhodamine ×1:1,000 (Dianova), and donkey anti-goat conjugated with Alexa Fluor 568 1:1,000 (Invitrogen). In cases of detergent-sensitive antigens (ie, O4, A2B5, GalC, and NG2), Triton X-100 was omitted from FGB. Nuclear counterstaining was performed with 4′, 6′-diamidino-2-phenylindole dihydrochloride (DAPI) hydrate at 0.25 μg/μl (Sigma). Specimens were mounted on microscope slides using Prolong Antifade kit (Molecular Probes). Epifluorescence observation and photo-documentation were realized using a Leica microscope (Leica Mikroskopie und Systeme GmbH) equipped with a Spot™ digital camera (Diagnostic Instrument Inc.) or an Olympus IX81 inverted microscope equipped with Hamamatsu digital camera and Volocity 5.4.1 software (PerkinElmer). For each culture condition, 10 randomly selected observation fields, containing in total 500–1,000 cells, were photographed for cell fate analysis. Expression frequency of selected cell type markers was determined for every condition in 3 independent experiments.
Quantitative PCR
RNA extraction from NPCs was performed using RNeasy Mini Kit (Qiagen) and cDNA was synthetized using Promega reverse transcription Kit (Promega). Expression analysis was performed by TaqMan gene expression assays kits (Applied Biosystems) for the following rat genes: Olig1, Olig2, Id2, NG2, Nestin, PDGFRα, CNPase, and MBP. Probes and primers were provided by the manufacturer (Applied Biosystems). Rat glyceraldehyde 3-phosphate dehydrogenase was used as a reference gene. The following temperature profile was used: activation of polymerase 95°C, 10 min; 40 cycles of denaturing 95°C, 15 s, and annealing/extension 60°C, 60 s. Data was obtained with a Rotor-Gene 6000 R Corbett Research (geneXpress) and analyzed by delta delta Ct method [33]. Gene expression under control conditions was used as a calibrator gene.
Statistical analysis
Data are presented as mean±standard deviation and statistical analysis was performed using PRISM4 (GraphPad). P values of <0.05 were considered to be significant acquired by parametric 1-way analysis of variance (ANOVA)-Tukey post hoc. For time course experiments 2-way ANOVA-Bonferroni post hoc was conducted. All experiments were performed at least in triplicate in 3 independent experiments.
Results
Here, we used a neurosphere culture model to analyze the effects of a 3 weeks MSC-CM treatment of NPCs under proliferation conditions with the hypothesis that the MSC-CM might prime the NPCs toward an oligodendroglial fate. To test whether the NPCs used in the present study are multipotent self-renewing progenitors, we grew primary and secondary neurospheres under stringent clonal condition (1 cell per well) and analyzed for neuronal, astroglial, and oligodendroglial differentiation potential of the cells (see Supplementary Data; available online at www.liebertonline.com/scd). Even though the majority of neurosphere derived cells expressed the oligodendroglial progenitor marker O4, as directly demonstrated after seeding of the cells, approximately half of the population was positive for the NSC markers Sox2 and Nestin. Moreover, the neurosphere derived cells generated primary and secondary spheres under clonal conditions and differentiated into DCX-expressing neurons, GFAP positive astrocytes, and MBP positive oligodendrocytes. Cell identity was directly analyzed after the 3 weeks stimulation by profiling the cell type specific marker expression. Cell intrinsic differentiation fate was analyzed after a 1 week period of GFW, and cell-lineage restriction was tested by exposing the primed cells to serum, an astroglia inducing activity. Finally, we directly determined the expression of Olig2 and Id2 glial fate determinants on proliferating NPCs after the 3 weeks of pretreatment (Fig. 1).
MSC-CM promotes adhesion of proliferating NPCs without interfering with cell proliferation
To study the effects of soluble factors derived from MSCs on proliferating NPCs, adult rat neurosphere derived single cells suspensions were treated with increasing concentrations of MSC-CM under proliferation conditions, that is, in the presence of EGF and FGF-2 for 7, 14, or 21 days. Importantly, the basic composition of the control medium and MSC-CM were identical. One additional control condition was used for some experiments to exclude the possibility that the MSC-CM derived effects might be due to the fact that MSCs might have consumed EGF and FGF resulting in CM without growth factors. Therefore, NPCs were incubated also with MSC-CM replenished with EGF and FGF-2.
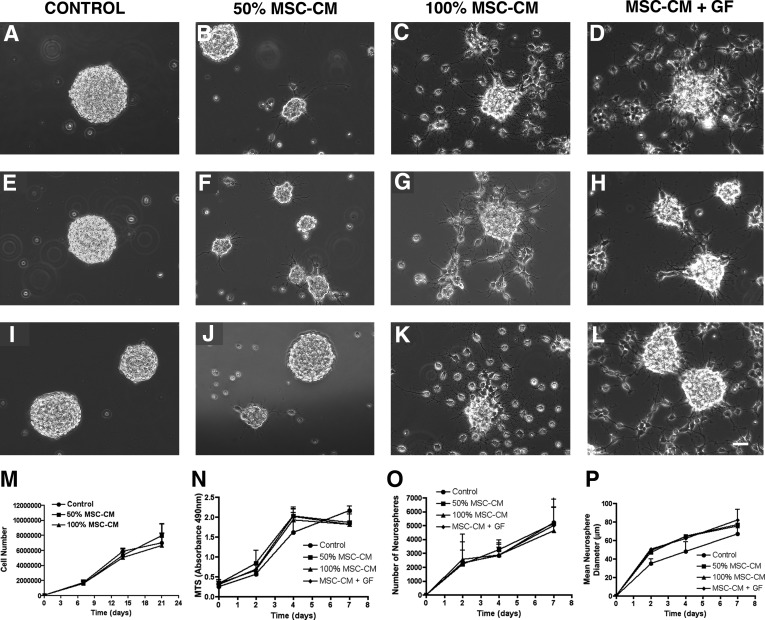
While proliferating NPCs readily regrew neurospheres in control proliferation medium (Fig. 2A, E, I), sphere formation was reduced in 50% MSC-CM, in 100% MSC-CM, and in MSC-CM+GF. Instead, cells attached to the plastic surface and formed cellular processes (Fig. 2B–D, F–H, J–L). The growth characteristics of the cultures were investigated by Trypan blue exclusion cell counting, MTS assay, and by determining the number of neurospheres and the mean neurosphere diameter. Regardless of the treatment, NPC cultures expanded along the 3 weeks of observation with comparable kinetics by generating similar cell numbers (Fig. 2M, N) and by forming similar neurosphere numbers (Fig. 2O) with neurospheres of comparable mean diameter (Fig. 2P). The mean diameter of untreated control neurospheres was slightly, but not significantly, less compared with the MSC-CM conditions. Moreover, there were no differences in the morphology and growth characteristics of cells treated with either 100% MSC-CM or MSC-CM+GF. Taken together, this indicates that MSC-CM had no obvious influence on the growth kinetics of NPCs.
FIG. 2.
Morphology and growth curve of proliferating NPCs. Phase contrast images of NPCs in normal proliferation medium (control) (A, E, I), treated with 50% MSC-CM (B, F, J), 100% MSC-CM (C, G, K), or MSC-CM replenished with growth factors (MSC-CM+GF) (D, H, L). Proliferating NPCs were incubated for 7 days (A–D), 14 days (E–H), and 21 days (I–L). Note that under control conditions proliferating NPCs grew as floating aggregates, but when the cells were treated with MSC-CM some NPCs attached to the surface of the flask and grew adherent. Scale bar=100 μm. The cell number was determined by Trypan blue exclusion after 7, 14, and 21 days. Growth curves for the different conditions were determined (M). The metabolic activity of the cells (N) and the number of neurospheres (O) and the mean neurosphere diameter (P) were analyzed on day 2, 4, and 7 during the second week of incubation with the respective media. Note that there is no significant effect of MSC-CM on the NPCs proliferation rate. All experiments were performed at least in triplicate in 3 independent experiments. Data are shown as mean±SD. For statistical analysis 2-way ANOVA-Bonferroni post hoc was performed. SD, standard deviation. ANOVA, analysis of variance; GF, growth factor.
MSC-CM pretreatment primes NPCs toward oligodendroglial differentiation under proliferation conditions
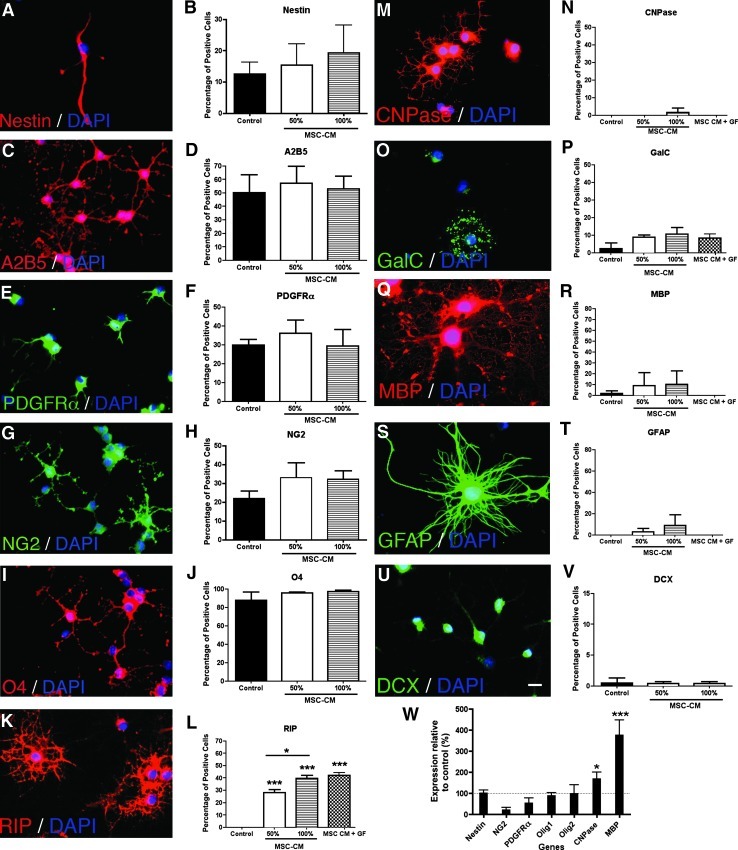
The striking effects of MSC-CM on the adherence of NPCs lead us to investigate the expression of progenitor markers in the cultures. Therefore, NPCs were cultured in EGF and FGF-2 for up to 3 weeks under control or MSC-CM conditions. Cells were then dissociated and seeded overnight under serum-free conditions to allow them to attach and fixed and stained for stem/progenitor markers. In consistence with our previous report [34], a subpopulation of NPCs in the cultures expressed the NSC marker Nestin (between 10% and 15% Fig. 3A, B). The majority of the NPCs expressed the oligodendrocyte progenitor marker O4 (∼90% Fig. 3I, J) and the glial progenitor markers A2B5 (∼50% Fig. 3C, D), PDFGRα (∼35% Fig. 3E, F), and NG2 (∼30% Fig. 3G, H). The MSC-CM treatment did not affect the relative proportion of cells expressing one of these stem/progenitor markers (Fig. 3B, D, F, H, J). However, the number of RIP positive oligodendrocytes was significantly increased after MSC-CM pretreatment in a dose-dependant manner from ∼0% in the control to 30% in 50% MSC-CM and to 40% in 100% MSC-CM (Fig. 3K, L). In general, fewer cells were positive for CNPase (∼0% in control, 2% after MSC-CM treatment; Fig. 3M, N), GalC (∼2% in control, 10% after MSC-CM treatment; Fig. 3O, P), or MBP (∼0% in control, 10% after MSC-CM treatment; Fig. 3Q, R). Finally, there were no GFAP positive cells (Fig. 3S, T) and only very few cells that expressed the neuronal precursor marker DCX in control conditions (Fig. 3U, V). The MSC-CM treatment did not affect the percentage of the DCX positive cells (Fig. 3V) and the slight increase of GFAP positive cells after MSC-CM treatment (Fig. 3T) was not significant. Also importantly, there was no difference in marker expression profile in cultures treated with 100% MSC-CM or MSC-CM+GF (Fig. 3L, N, P, R, T). Most of the NPCs expressed the stem/progenitor markers Nestin, A2B5, PDGFRα, NG2, and O4 indicating that the vast majority of cells in the cultures had indeed an immature character.
FIG. 3.
Cell identity: marker expression profile of pretreated proliferating NPCs. NPCs were dissociated after the 3 weeks exposure to MSC-CM or control conditions, and cells were seeded overnight under serum-free conditions. The marker expression profile was analyzed by immunocytochemistry and quantitative RT-PCR. Illustrative fluorescence images of the immunostainings are shown for: Nestin (red) and DAPI (blue) (A); A2B5 (red) and DAPI (blue) (C); PDGFRα (green) and DAPI (blue) (E); NG2 (green) and DAPI (G); O4 (red) and DAPI (blue) (I); RIP (red) and DAPI (blue) (K); CNPase (red) and DAPI (blue) (M); GalC (green) and DAPI (blue) (O); MBP (red) and DAPI (blue) (Q); GFAP (green) and DAPI (blue) (S); DCX (green) and DAPI (blue) (U). Scale bar=10 μm. Quantitative analysis show the percentage of positive cells for each marker (B, D, F, H, J, L, N, P, R, T, and V). Note that the number of RIP positive cells was significantly increased after MSC-CM treatments compared with control condition, while there was no significant change for the other tested markers after MSC-CM stimulation. (W) Quantitative RT-PCR for glial fate determinants and stem/progenitor cell markers. Delta delta Ct method was used for analysis considering glyceraldehyde 3-phosphate dehydrogenase as normalizer gene and control condition as calibrator. Relative expression levels of Nestin, NG2, PDGFRα, Olig1, Olig2, CNPase, and MBP after treatment with 100% MSC-CM compared with control condition (dashed line) are shown. After 3 weeks of pretreatment with MSC-CM there was a significant increase in the expression of CNPase and MBP, while there was a slight decrease in the expression levels of NG2 and PDGFRα. All experiments were performed at least in triplicate in 3 independent experiments. Data are shown as mean±SD. For statistical analysis 1-way ANOVA-Tukey post hoc was performed. Asterisks above individual columns indicate significant difference compared with control. Asterisks above a line spanning 2 columns indicate significant difference between MSC-CM treatments. *P<0.05; ***P<0.001. RT-PCR, real-time-polymerase chain reaction; DAPI, 4′, 6′-diamidino-2-phenylindole dihydrochloride; GFAP, glial fibrillary acidic protein; DCX, doublecortin.
Next, quantitative RT-PCR was performed to determine the expression levels of NSC and CNS lineage markers and to analyze the effects of the MSC-CM treatment. The 3 weeks MSC-CM treatment significantly enhanced the expression of CNPase- and of MBP-mRNA in the NPCs (Fig. 3W). The mRNA expression levels of the progenitor markers NG2 and PDGFRα were slightly, but not significantly, reduced, while the expression levels of Nestin and the Olig genes were not affected by the MSC-CM treatment (Fig. 3W).
These data suggest that MSC-CM primes NPCs under proliferation conditions to differentiate into oligodendrocytes. While the presence of MSC-CM induced major changes at the mRNA levels, in particular in MBP expression, the cells apparently did not yet express the oligodendrocyte phenotype at the protein level, suggesting that the oligodendrocytic phenotype at this stage is more advanced on mRNA level than on protein level.
Pretreatment of NPCs with MSC-CM enhances GFW induced oligodendroglial differentiation
We recently noticed that adult rat hippocampus and SVZ derived neurospheres, although being tripotent, predominantly differentiate into oligodendrocytes after GFW in serum free conditions [34]. To determine whether this default differentiation pathway is affected by MSC-CM, NPCs were pretreated with control proliferation medium or MSC-CM for 3 weeks, dissociated, and seeded, and incubated for 7 days in serum free conditions in the absence of FGF-2 and EGF. As expected, 1 week after GFW, control-treated NPCs, while still expressing O4 (Fig. 4A, B), primarily differentiated into mature oligodendrocytes expressing RIP and CNPase (∼30% Fig. 4C–F), GalC (∼40% Fig. 4G, H), and MBP (∼60% Fig. 4I, J). Interestingly, although MSC-CM treatment did not affect the proportion of O4-expressing oligodendrocyte progenitor cells (Fig. 4B), it increased the percentage of RIP-, CNPase-, GalC-, and MBP-expressing oligodendrocytes after GFW (Fig. 4D, F, H, J). We did not observe any MSC-CM effect on the percentage of cells that express the astrocyte marker GFAP (Fig. 4K, L). Finally, there were few cells that expressed the neuronal progenitor markers DCX (<4% Fig. 4M, N) and MAP2ab (<3% Fig. 4O, P) in response to GFW. MSC-CM pretreatment did not affect the percentage of DCX- and MAP2ab-expressing neurons generated after GFW (Fig. 4N, P). Moreover, regardless of the pretreatment of proliferating NPCs, <10% of the cells express neural stem/progenitor markers (data not shown), indicating that in the absence of growth factors NPCs are induced to differentiate. Again, in some of the experiments, the MSC-CM was replenished with EGF and FGF-2 to exclude the possibility that MSCs might have consumed these growth factors and thereby induced the pro-oligodendroglial effects. The results, as demonstrated in Fig. 4D, F, and H exclude this possibility. Interestingly, while the GFW condition demonstrated the increased oligodendroglial potential of the MSC-CM pretreated NPCs, the mRNA levels of the pro-oligodendroglial determinants Olig1 and Olig2 and of the oligodendroglial genes CNPase and MBP were not further enhanced (Fig. 4Q). Taken together, this suggests that MSC-CM enhances the oligodendrogenic ability of proliferating NPCs resulting in an increased level of oligodendroglial differentiation in response to GFW.
FIG. 4.
Cell intrinsic differentiation fate: GFW response of pretreated proliferating NPCs. NPCs that were exposed under proliferation conditions to MSC-CM or control conditions were dissociated, seeded, and incubated for 1 week under serum-free conditions in the absence of growth factors. Cells were fixed and the expression of cell-specific-lineage markers and fate determinants was analyzed by immunofluorescence and quantitative RT-PCR. Illustrative fluorescence images are shown for: O4 (red) and DAPI (blue) (A); RIP (red) and DAPI (blue) (C); CNPase (red) and DAPI (blue) (E); GalC (green) and DAPI (blue) (G); MBP (red) and DAPI (blue) (I); GFAP (green) and DAPI (K); DCX (green) and DAPI (blue) (M); MAP2ab (red) and DAPI (blue) (O). Scale bar=10 μm. Quantitative analysis show the percentage of positive cells for each marker (B, D, F, H, J, L, N, and P). Note the significant increase in the percentage of cells that express the oligodendroglial markers RIP, CNPase, GalC, and MBP when proliferating NPCs were pretreated with MSC-CM. (Q) Quantitative RT-PCR for glial fate determinants and oligodendrocyte markers. Delta delta Ct method was used for analysis considering glyceraldehyde 3-phosphate dehydrogenase as normalizer gene and control condition as calibrator. Relative expression levels Olig1, Olig2, Id2, CNPase, and MBP after treatment with 100% MSC-CM compared with control condition (dashed line) are shown. All experiments were performed at least in triplicate in 3 independent experiments. Data are shown as mean±SD. For statistical analysis 1-way ANOVA-Tukey post hoc was performed. Asterisks above individual columns indicate significant difference compared with control. Asterisks above a line spanning 2 columns indicate significant difference between MSC-CM treatments. *P<0.05; **P<0.01; ***P<0.001.
MSC-CM primes proliferating NPCs: acceleration of differentiation and reinforcement of oligodendroglial commitment
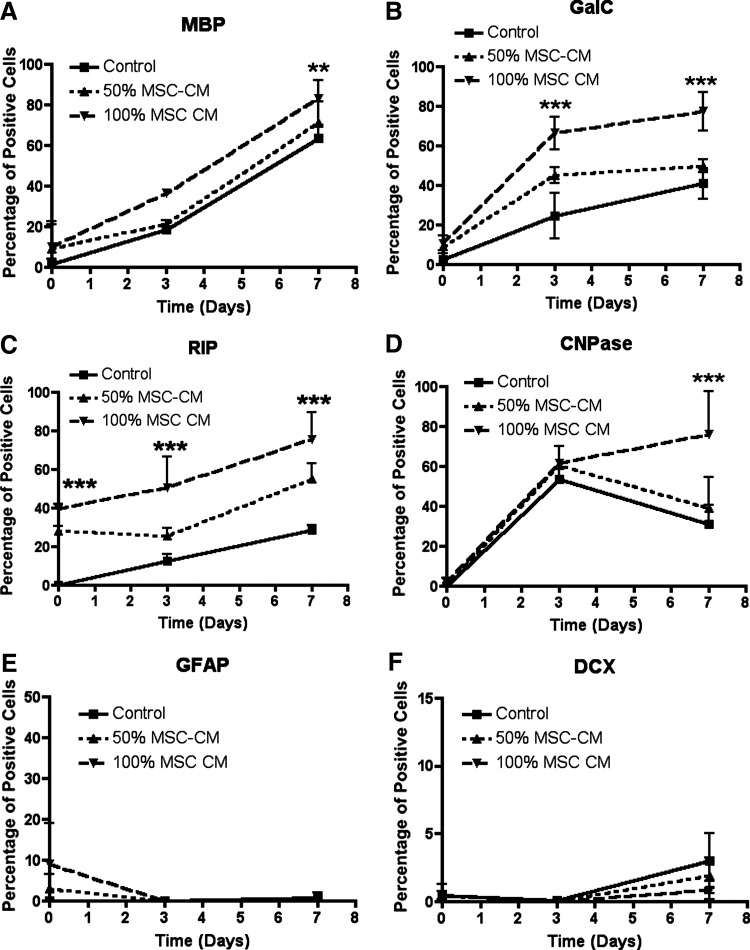
Based on the fact that MSC-CM encourages proliferating NPCs toward oligodendrogenesis we asked if this effect involves accelerated oligodendrocyte differentiation/maturation and/or enhanced oligodendrocyte lineage restriction. First, to determine whether MSC-CM modulates the kinetics of oligodendrogenesis in response to GFW, we analyzed the temporal expression pattern of markers that sequentially appear along the oligodendrogenic process and astrocytic and neuronal markers. GFW, regardless of the pretreatment, significantly increased the percentage of MBP-expressing mature oligodendrocytes with time (Fig. 5A). Importantly, the MSC-CM pretreatment triggered a significant increase in the generation of MBP-expressing oligodendrocytes 3 and 7 days after GFW (Fig. 5A). The expression of GalC and of RIP after GFW significantly increased over time under all conditions analyzed (Fig. 5B, C). Moreover, the MSC-CM pretreatment induced a dose-dependant increase in the percentage of GalC- and of RIP positive cells (Fig. 5B, C). Although the proportion of CNPase-expressing cells transiently increased in all conditions after GFW, only MSC-CM pretreated proliferating NPCs persistently produced such cells. This culminated in a significantly higher percentage of CNPase positive oligodendrocytes compared with the other conditions (Fig. 5D). In addition, in spite of the fact that MSC-CM pretreated proliferating NPCs displayed a slightly higher percentage of GFAP-expressing astrocytes compared with untreated NPCs, there was an abrupt decrease in GFAP expression after GFW independently of the pretreatment (Fig. 5E). Finally, although minor changes in the percentage of DCX-expressing neurons were observed along time after GFW, MSC-CM pretreatment had no significant effect on the generation of DCX positive cells (Fig. 5F). Together, these findings indicate that MSC-CM pretreatment of proliferating NPCs induces a faster and a more efficient differentiation into oligodendrocytes without affecting the generation of astrocytes and neurons.
FIG. 5.
Cell intrinsic differentiation fate: temporal expression of neural markers of pretreated proliferating NPCs in response to GFW. NPCs were grown for 3 weeks in the presence of MSC-CM, dissociated, and seeded overnight under serum-free conditions. Then, the cells were either fixed to analyze the marker expression profile at day 0 or the cells were incubated in the absence of growth factors for 3 and 7 days and fixed to analyze temporal changes in the marker expression profile. Immunofluorescence was performed for the presence of differentiating and mature oligodendrocyte-, and for astrocyte- and neuron-specific markers. Quantitative analysis show over time the percentage of cells that express: MBP (A), GalC (B), RIP (C), CNPase (D), GFAP (E), and DCX (F). NPCs pretreated with MSC-CM display more MBP-, GalC-, RIP-, and CNPase-expressing cells along time than untreated NPCs after growth factor withdrawal. All experiments were performed at least in triplicate in 3 independent experiments. Data are shown as mean±SD. For statistical analysis 2-way ANOVA-Bonferroni post hoc was performed. Asterisks indicate significant difference compared with control. **P<0.01; ***P<0.001.
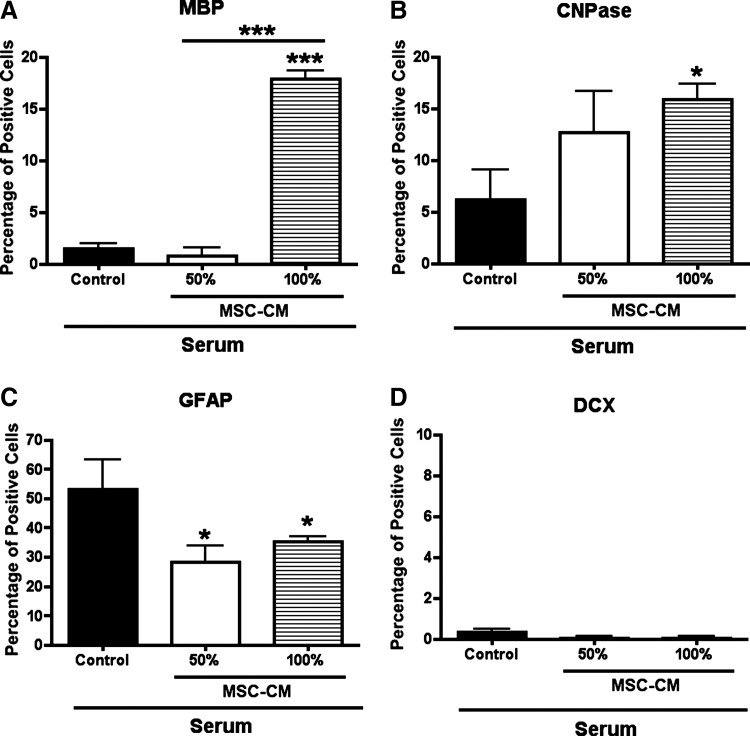
Second, we analyzed the possibility that MSC-CM pretreatment on proliferating NPCs might enhance oligodendrocyte lineage restriction. To test this hypothesis, MSC-CM pretreated and non pretreated proliferating NPCs were exposed to FBS, as serum has been described as a potent astrogenic stimulus on glial progenitors [35]. As expected, a 1 week stimulation with FBS predominantly induced astroglial differentiation in control-treated NPCs, since ∼60% of the cells expressed the astrocyte marker GFAP (Fig. 6C), while only ∼3% and 7% of the cells expressed the oligodendrocyte markers MBP and CNPase, respectively (Fig. 6A, B). In contrast, and despite the presence of an astrogenic milieu, the MSC-CM pretreatment generated significantly more MBP- and CNPase-expressing oligodendrocytes (Fig. 6A, B). Moreover, this effect was at the expense of astrogenesis, since significantly less GFAP-expressing cells could be observed under these conditions (Fig. 6C). The MBP- and the CNPase-expressing cells in the MSC-CM pretreated group appeared in clusters, suggesting a clonal effect. Finally, almost no DCX-expressing cells were found under this astrogenic condition, regardless of the pretreatment (Fig. 6D). In summary, MSC-CM pretreatment of proliferating NPCs boosts the oligodendrocyte lineage commitment and partially counteracts the astrogenic effect of FBS.
FIG. 6.
Cell fate restriction: neural differentiation of pretreated proliferating NPCs in response to astrogenic conditions. NPCs treated for 3 weeks under proliferation conditions with or without MSC-CM were dissociated, seeded, and incubated for 1 week in the presence of fetal bovine serum as an astrogenic stimulus. Cells were fixed and cell-specific-lineage marker expression was analyzed by immunofluorescence. Quantitative analysis shows the percentage of cells that express: MBP (A), CNPase (B), GFAP (C), and DCX (D). Note that despite astrogenic conditions, NPCs pretreated with MSC-CM display less GFAP-expressing astrocytes and more MBP- and CNPase-expressing oligodendrocytes than non pretreated NPCs. All experiments were performed at least in triplicate in 3 independent experiments. Data are shown as mean±SD. For statistical analysis 1-way ANOVA-Tukey post hoc was performed. Asterisks above individual columns indicate significant difference compared with control. Asterisks above a line spanning 2 columns indicate significant difference between MSC-CM treatments. *P<0.05; ***P<0.001.
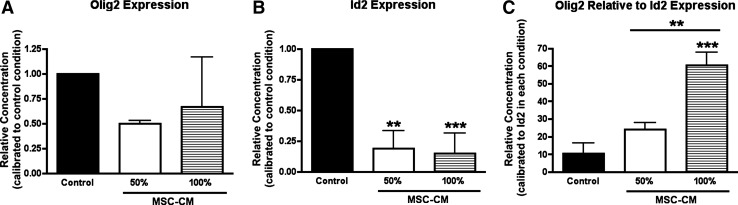
MSC-CM increases the Oligs/Id2 ratio in proliferating NPCs during priming
Next, we reasoned that oligodendroglial priming of NPCs by MSC-CM should involve altered expression of pro- and anti-oligodendrogenic fate determinants. Here, the relative expression of Olig2 and Id2 is a key determinant for oligodendrocyte/astrocyte fate decision [19] (Fig. 1). Therefore, proliferating NPCs were incubated up to 3 weeks under normal proliferation conditions (control), 50% and 100% MSC-CM, the expression level of Olig2 and Id2 were analyzed by quantitative RT-PCR, and the Olig2/Id2 ratio was determined for the different conditions. While the expression levels of Olig2 did not change within the different conditions (Fig. 7A), MSC-CM drastically reduced the expression of Id2 (Fig. 7B). To estimate the Olig2/Id2 ratio, the expression level of Olig2 was related to Id2 as a calibrator. MSC-CM dose-dependently increased the Olig2/Id2 ratio compared with control conditions (Fig. 7C) favoring oligodendrocyte fate decision. In conclusion, the increased Olig2/Id2 ratio in MSC-CM-treated NPCs might explain the enhanced oligodendrogenic response to GFW compared with nontreated NPCs.
FIG. 7.
Glial fate determinants expression of pretreated proliferating NPCs. Proliferating NPCs were grown for 3 weeks under normal conditions (control), 50%, or 100% MSC-CM. Quantitative RT-PCR was performed to analyze the expression of the pro-oligodendrogenic transcription factor Olig2 and the antioligodendrogenic determinant, Id2. Delta delta Ct method was used considering glyceraldehyde 3-phosphate dehydrogenase as normalizer gene and control conditions as a calibrator. Expression levels of Olig2 (A) and Id2 (B) under the different conditions are shown. To determine Olig2/Id2 ratio, Olig2 expression levels were quantified using Id2 as a calibrator gene in the respective condition (C). Note that while MSC-CM does not affect Olig2 expression it strongly decreases Id2 levels. MSC-CM-treated proliferating NPCs displays 6- to 7-fold increase on the Olig2/Id2 ratio compared with control conditions. All experiments were performed at least in triplicate in 3 independent experiments. Data are shown as mean±SD. For statistical analysis 1-way ANOVA-Tukey post hoc was performed. Asterisks above individual columns indicate significant difference compared with control. Asterisks above a line spanning 2 columns indicate significant difference between MSC-CM treatments. **P<0.01; ***P<0.001.
Discussion
Cell progenitor priming and cell fate and lineage decisions presumably occur while progenitors are still proliferating [12,36–38]. For instance, different combinations of growth factors such as PDGFα, FGF-2, and Shh can convert embryonic derived proliferating neurospheres into OPCs containing oligospheres [38,39]. Lithium chloride primes proliferating NPCs and increases the proportion of cells expressing neuronal markers at the expense of gliogenesis [37]. Moreover, FGF-2, heparin, and laminin set up adult human brain derived proliferating NPCs toward a neuronal fate [36]. Here, we have shown that MSCs prime proliferating NPCs, reinforce oligodendroglial cell fate decision and accelerate the differentiation toward oligodendrocytes.
The conditioned medium derived from MSCs did not impinge on cell proliferation and neurosphere formation. However, MSC-CM primed progenitors toward oligodendrocytes as the number of RIP positive cells and the mRNA expression levels of the oligodendrocyte markers CNPase and MBP were significantly enhanced after a 3 week incubation with MSC-CM under proliferation conditions. After GFW the MSC-CM induced elevated predisposition toward oligodengroglial differentiation at the mRNA levels consolidated into a higher percentage of RIP-, GalC-, CNPase-, and MBP-positive mature oligodendrocytes. In a number of experiments, we excluded the possibility that the oligodendroglial differentiation effect of the MSC-CM might derive from a consumption of EGF or FGF-2, suggesting that the MSC-CM indeed contains a pro-oligodendroglial activity, whose identity is still under investigation. Nevertheless, the present data clearly demonstrate that the pro-oligodendroglial effect of MSC-CM is dose dependent, although, depending on the gene or protein marker analyzed, this dose-dependency might vary. This might be a consequence of minor alterations in the quality of the CM resulting from slightly different features of the primary MSCs preparations. Nevertheless, in summary, regardless of the preparation, a pro-oligodendrogenic effect is always present in the MSC-CM.
The MSC-CM derived oligodendrogenic priming effect on proliferating NPCs reinforced the lineage restriction and enabled them to overcome an astrogenic milieu. A surprising observation within the present work is the notion that the majority of progenitors (∼60%) that were generated under control conditions differentiated into MBP-expressing oligodendrocytes in serum-free conditions. This suggests that the progenitors used in the present study where already partly committed to an oligodendroglial fate, which confirms our previous work [34]. This tendency to generate oligodendrocytes might have been overlooked in many previous studies, since most of the differentiation assays are typically performed in serum-containing, and thus astrocyte-inducing, media [20,29,40].
The temporal expression pattern analysis of cell type specific markers revealed that progenitors grown under control proliferation conditions generate a wave of CNPase-expressing immature oligodendrocytes in response to GFW. After the third day of GFW this CNPase population declines, while the numbers of RIP-, GalC-, and MBP-expressing mature oligodendrocytes increase with time (Fig. 5). In contrast to NPCs that were grown under control condition, progenitors pretreated with MSC-CM persistently generated an increasing number of CNPase positive cells in response to GFW. In addition, the MSC-CM pretreatment elevated the numbers of MBP, GalC, and RIP positive cells. The decline in the percentage of CNPase-expressing cells with a simultaneous increase in MBP, GalC, and RIP positive cells in the control condition is somewhat unexpected, since CNPase plays a functional role in oligodendrogenesis and its expression is normally maintained even in mature oligodendrocytes [41–44]. The reason for the decline in CNPase expression in the control condition and 50% MSC-CM treated cells is unclear at present. One attractive hypothesis might be that sustained expression of CNPase might require additional factors present in vivo but not in the control conditions. Nevertheless, MSC might express and secrete such a sustained pro-oligodendroglial activity, since the MSC-CM clearly sustainably elevated the percentage of CNPase-expressing cells.
The molecular mechanism of the MSC-CM induced oligodendroglial activity has still to be identified, but it seems to target the regulation of Id proteins. The ratio of the amount of Olig and Id2 proteins is a key determinant for oligodendrocyte versus astrocyte fate decision [19]. In our previous study, we had already demonstrated that MSC-CM induces Olig2 and reduces Id2 expression in NPCs that underwent serum-induced differentiation [20]. Under proliferation conditions and serum-free differentiation conditions, MSC-CM did not increase Olig1 and Olig2 expression, but constrained the expression of Id2 and thus raised the Olig2/Id2 ratio. As a consequence, Id2 appears to be a key regulator in the process, and a more detailed analysis of the molecular regulation of Id2 expression might be of interest.
CNS remyelination is the main endogenous regenerative mechanism that protects from demyelination, in particular in the case of MS. Although progenitors are present around MS lesions, migration and differentiation/maturation into myelinating cells are impaired [24,25]. The lack of myelin-promoting activities might contribute to the MS related remyelination impairment. In addition, aging represents a risk factor, since endogenous remyelination is drastically affected in aged subjects [26–28]. First, it has been shown that after demyelination in old rats remyelination occurs only in a slow and delayed fashion [26]. This age-related impairment in remyelination is due to a deficient progenitor recruitment and differentiation [27]. Thus, while remyelination impairment in MS and in particular during aging is partly derived from the failure to differentiate into oligodendrocytes, a lack of sufficient oligodendroglial commitment in proliferating progenitors might contribute to this deficiency. The fact that MSCs not only induce oligodendrocyte differentiation [20] but also prime proliferating NPCs toward an oligodendrocyte fate might have in vivo and clinical relevance when aiming to develop remyelinating therapies for MS in young and in old patients.
In conclusion, MSCs exert a potent oligodendrogenic effect on adult proliferating NPCs promoting and enhancing cell fate decision, differentiation, and maturation. The identification of the MSC derived oligodendrogenic activity and the clarification of the underlying molecular mechanism are crucial for the development of new therapies for the treatment of demyelinating diseases such as MS. Moreover, clinical situations that require remyelination of bare axons such as after spinal cord injury might also profit from such a development.
Supplementary Material
Acknowledgments
The authors would like to thank the following funding agencies for their support: the Bavarian State Ministry of Sciences, Humboldt Stiftung (FJR; Georg Forster Program), the Austrian Academy of Sciences (CS; Recipient of a DOC-fFORTE-fellowship of the Austrian Academy of Sciences at the Institute of Molecular Regenerative Medicine), the Christiane and Claudia Hempel Foundation for clinical stem cell research (PK), Research and the Arts (ForNeuroCell grant), the Germany Federal Ministry of Education and Research (BMBF grants #01GG0706 and #01GN0979), Deutsche Forschungsgesellschaft (DFG grant #AI31/3-1) and by the state of Salzburg.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bunge RP. Glial cells and the central myelin sheath. Physiol Rev. 1968;48:197–251. doi: 10.1152/physrev.1968.48.1.197. [DOI] [PubMed] [Google Scholar]
- 2.Chang A. Nishiyama A. Peterson J. Prineas J. Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine JM. Reynolds R. Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 4.Franklin RJ. Kotter MR. The biology of CNS remyelination: the key to therapeutic advances. J Neurol. 2008;255(Suppl. 1):19–25. doi: 10.1007/s00415-008-1004-6. [DOI] [PubMed] [Google Scholar]
- 5.Bruce CC. Zhao C. Franklin RJ. Remyelination—an effective means of neuroprotection. Horm Behav. 2010;57:56–62. doi: 10.1016/j.yhbeh.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Menn B. Garcia-Verdugo JM. Yaschine C. Gonzalez-Perez O. Rowitch D. Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Perez O. Romero-Rodriguez R. Soriano-Navarro M. Garcia-Verdugo JM. Alvarez-Buylla A. Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells. 2009;27:2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguirre A. Dupree JL. Mangin JM. Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- 9.Jablonska B. Aguirre A. Raymond M. Szabo G. Kitabatake Y. Sailor KA. Ming GL. Song H. Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nait-Oumesmar B. Decker L. Lachapelle F. Avellana-Adalid V. Bachelin C. Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 11.Seri B. Herrera DG. Gritti A. Ferron S. Collado L. Vescovi A. Garcia-Verdugo JM. Alvarez-Buylla A. Composition and organization of the SCZ: a large germinal layer containing neural stem cells in the adult mammalian brain. Cereb Cortex. 2006;16(Suppl. 1):i103–i111. doi: 10.1093/cercor/bhk027. [DOI] [PubMed] [Google Scholar]
- 12.Rivera FJ. Steffenhagen C. Kremer D. Kandasamy M. Sandner B. Couillard-Despres S. Weidner N. Kury P. Aigner L. Deciphering the oligodendrogenic program of neural progenitors: cell intrinsic and extrinsic regulators. Stem Cells Dev. 2010;19:595–606. doi: 10.1089/scd.2009.0293. [DOI] [PubMed] [Google Scholar]
- 13.Nicolay DJ. Doucette JR. Nazarali AJ. Transcriptional control of oligodendrogenesis. Glia. 2007;55:1287–1299. doi: 10.1002/glia.20540. [DOI] [PubMed] [Google Scholar]
- 14.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 15.Hu JG. Fu SL. Wang YX. Li Y. Jiang XY. Wang XF. Qiu MS. Lu PH. Xu XM. Platelet-derived growth factor-AA mediates oligodendrocyte lineage differentiation through activation of extracellular signal-regulated kinase signaling pathway. Neuroscience. 2008;151:138–147. doi: 10.1016/j.neuroscience.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 16.Nery S. Wichterle H. Fishell G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development. 2001;128:527–540. doi: 10.1242/dev.128.4.527. [DOI] [PubMed] [Google Scholar]
- 17.Orentas DM. Hayes JE. Dyer KL. Miller RH. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419–2429. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- 18.Tekki-Kessaris N. Woodruff R. Hall AC. Gaffield W. Kimura S. Stiles CD. Rowitch DH. Richardson WD. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- 19.Samanta J. Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- 20.Rivera FJ. Couillard-Despres S. Pedre X. Ploetz S. Caioni M. Lois C. Bogdahn U. Aigner L. Mesenchymal stem cells instruct oligodendrogenic fate decision on adult neural stem cells. Stem Cells. 2006;24:2209–2219. doi: 10.1634/stemcells.2005-0614. [DOI] [PubMed] [Google Scholar]
- 21.Rivera FJ. Kandasamy M. Couillard-Despres S. Caioni M. Sanchez R. Huber C. Weidner N. Bogdahn U. Aigner L. Oligodendrogenesis of adult neural progenitors: differential effects of ciliary neurotrophic factor and mesenchymal stem cell derived factors. J Neurochem. 2008;107:832–843. doi: 10.1111/j.1471-4159.2008.05674.x. [DOI] [PubMed] [Google Scholar]
- 22.Li QM. Fu YM. Shan ZY. Shen JL. Zhang XM. Lei L. Jin LH. MSCs guide neurite directional extension and promote oligodendrogenesis in NSCs. Biochem Biophys Res Commun. 2009;384:372–377. doi: 10.1016/j.bbrc.2009.04.147. [DOI] [PubMed] [Google Scholar]
- 23.Rivera FJ. Siebzehnrubl FA. Kandasamy M. Couillard-Despres S. Caioni M. Poehler A-M. Berninger B. Sandner B. Ulrich B, et al. Mesenchymal stem cells promote oligodendroglial differentiation in hippocampal slice cultures. Cell Physiol Biochem. 2009;24:317–324. doi: 10.1159/000233256. [DOI] [PubMed] [Google Scholar]
- 24.Williams A. Piaton G. Aigrot MS. Belhadi A. Theaudin M. Petermann F. Thomas JL. Zalc B. Lubetzki C. Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain. 2007;130:2554–2565. doi: 10.1093/brain/awm202. [DOI] [PubMed] [Google Scholar]
- 25.Solanky M. Maeda Y. Ming X. Husar W. Li W. Cook S. Dowling P. Proliferating oligodendrocytes are present in both active and chronic inactive multiple sclerosis plaques. J Neurosci Res. 2001;65:308–317. doi: 10.1002/jnr.1155. [DOI] [PubMed] [Google Scholar]
- 26.Shields SA. Gilson JM. Blakemore WF. Franklin RJ. Remyelination occurs as extensively but more slowly in old rats compared to young rats following gliotoxin-induced CNS demyelination. Glia. 1999;28:77–83. doi: 10.1002/(sici)1098-1136(199910)28:1<77::aid-glia9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Sim FJ. Zhao C. Penderis J. Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinks GL. Franklin RJ. Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats. Mol Cell Neurosci. 2000;16:542–556. doi: 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- 29.Wachs FP. Couillard-Despres S. Engelhardt M. Wilhelm D. Ploetz S. Vroemen M. Kaesbauer J. Uyanik G. Klucken J, et al. High efficacy of clonal growth and expansion of adult neural stem cells. Lab Invest. 2003;83:949–962. doi: 10.1097/01.lab.0000075556.74231.a5. [DOI] [PubMed] [Google Scholar]
- 30.Amit M. Carpenter MK. Inokuma MS. Chiu CP. Harris CP. Waknitz MA. Itskovitz-Eldor J. Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 31.Cheng J. Dutra A. Takesono A. Garrett-Beal L. Schwartzberg PL. Improved generation of C57BL/6J mouse embryonic stem cells in a defined serum-free media. Genesis. 2004;39:100–104. doi: 10.1002/gene.20031. [DOI] [PubMed] [Google Scholar]
- 32.Smith AG. Heath JK. Donaldson DD. Wong GG. Moreau J. Stahl M. Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Steffenhagen C. Kraus S. Dechant FX. Kandasamy M. Lehner B. Poehler AM. Furtner T. Siebzehnrubl FA. Couillard-Despres S. Strauss O. Aigner L. Rivera FJ. Identity, fate and potential of cells grown as neurospheres: species matters. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9251-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Trotter J. Schachner M. Cells positive for the O4 surface antigen isolated by cell sorting are able to differentiate into astrocytes or oligodendrocytes. Brain Res Dev Brain Res. 1989;46:115–122. doi: 10.1016/0165-3806(89)90148-x. [DOI] [PubMed] [Google Scholar]
- 36.Olstorn H. Varghese M. Murrell W. Moe MC. Langmoen IA. Predifferentiated brain-derived adult human progenitor cells migrate toward ischemia after transplantation to the adult rat brain. Neurosurgery. 2011;68:213–222. doi: 10.1227/NEU.0b013e3181fd2c11. discussion 222. [DOI] [PubMed] [Google Scholar]
- 37.Vazey EM. Connor B. In vitro priming to direct neuronal fate in adult neural progenitor cells. Exp Neurol. 2009;216:520–524. doi: 10.1016/j.expneurol.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Pedraza CE. Monk R. Lei J. Hao Q. Macklin WB. Production, characterization, and efficient transfection of highly pure oligodendrocyte precursor cultures from mouse embryonic neural progenitors. Glia. 2008;56:1339–1352. doi: 10.1002/glia.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibney SM. McDermott KW. Differentiation of oligodendrocytes in neurospheres derived from embryonic rat brain using growth and differentiation factors. J Neurosci Res. 2007;85:1912–1920. doi: 10.1002/jnr.21331. [DOI] [PubMed] [Google Scholar]
- 40.Palmer TD. Ray J. Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 41.de Castro F. Bribian A. The molecular orchestra of the migration of oligodendrocyte precursors during development. Brain Res Brain Res Rev. 2005;49:227–241. doi: 10.1016/j.brainresrev.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 42.McMorris FA. Kim SU. Sprinkle TJ. Intracellular localization of 2′,3′-cyclic nucleotide 3′-phosphohydrolase in rat oligodendrocytes and C6 glioma cells, and effect of cell maturation and enzyme induction on localization. Brain Res. 1984;292:123–131. doi: 10.1016/0006-8993(84)90896-5. [DOI] [PubMed] [Google Scholar]
- 43.Domanska-Janik K. Gajkowska B. de Nechaud B. Bourre JM. Myelin composition and activities of CNPase and Na+, K+-ATPase in hypomyelinated “pt” mutant rabbit. J Neurochem. 1988;50:122–130. doi: 10.1111/j.1471-4159.1988.tb13238.x. [DOI] [PubMed] [Google Scholar]
- 44.Scherer SS. Braun PE. Grinspan J. Collarini E. Wang DY. Kamholz J. Differential regulation of the 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene during oligodendrocyte development. Neuron. 1994;12:1363–1375. doi: 10.1016/0896-6273(94)90451-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.