Abstract
Purpose
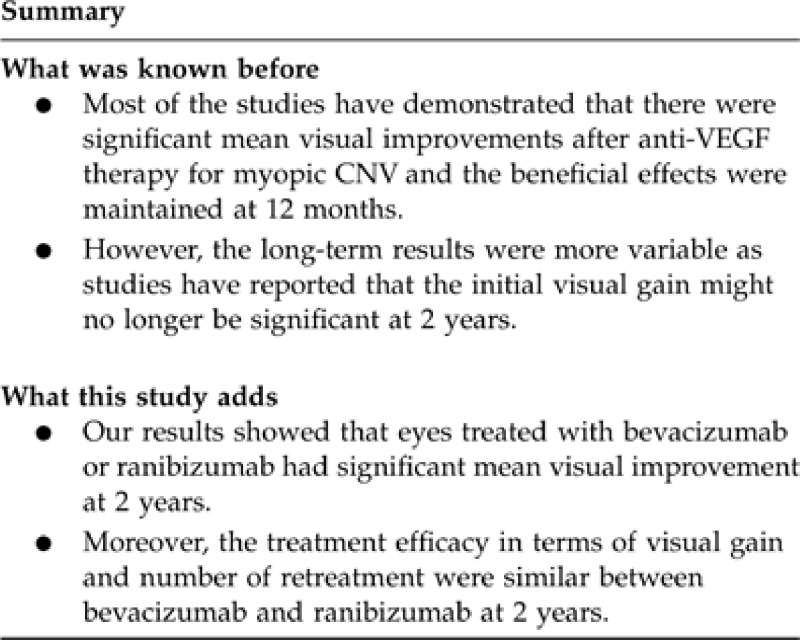
To evaluate the long-term efficacy of intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy as primary treatment for subfoveal myopic choroidal neovascularization (CNV).
Methods
In all, 37 treatment-naïve eyes of 37 patients with subfoveal myopic CNV who received intravitreal bevacizumab (n=22) or ranibizumab (n=15) injections with at least 2 years of follow-up were reviewed. All eyes received initial three loading doses of anti-VEGF at monthly intervals and retreatment was performed in persistent or recurrent CNV. Multivariate regression analyses were performed to determine the prognostic factors for visual outcome.
Results
The mean age was 57.3 years and the mean refractive error was −11.7 D. For all eyes, the mean logMAR best-corrected visual acuity improved from 0.86 (20/145) at baseline to 0.48 (20/60) at 2 years (P<0.001). The mean visual improvement for the bevacizumab and ranibizumab groups at 2 years was 2.8 and 5.1 lines, respectively (P=0.073). There was no significant difference in the proportion of eyes having visual gain of three or more lines or visual loss of three or more lines between the two groups. The mean number of injections was 3.8 for both bevacizumab and ranibizumab groups. Multivariate analyses showed that eyes with higher myopic refractive error were less likely to have visual gain after treatment (P=0.043), while size of CNV was negatively correlated with mean change in vision (P=0.046).
Conclusions
Intravitreal anti-VEGF therapy resulted in long-term visual improvement in myopic CNV. The treatment efficacy in terms of visual gain and number of retreatment appeared to be similar between bevacizumab and ranibizumab.
Keywords: ranibizumab, bevacizumab, anti-VEGF therapy, choroidal neovascularization, pathologic myopia, high myopia
Introduction
Choroidal neovascularization (CNV) is one of the most sight-threatening complications in patients with pathologic myopia.1, 2 The visual prognosis is generally poor without treatment, as a substantial proportion of patients will have progression of myopic maculopathy resulting in significant visual loss.3, 4 Photodynamic therapy (PDT) with verteporfin has been used for treating myopic CNV in the past decade and studies have shown that PDT might reduce the risk of visual loss compared with placebo.5, 6 However, the long-term outcome of PDT is not favourable as patients generally had no improvement in mean visual acuity following treatment and the beneficial effect of PDT in preventing visual loss was no longer significant at 2 years.6, 7
In the past few years, various studies have demonstrated the short-term efficacy of intravitreal anti-vascular endothelial growth factor (VEGF) agents in treating myopic CNV, including both bevacizumab8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 and ranibizumab.18, 19, 20, 21, 22, 23, 24, 25 Most of the studies have demonstrated significant mean visual improvement after anti-VEGF therapy and the beneficial effects were maintained at 12 months. In addition, several more recent studies have also reported the longer term visual outcomes of up to 2 years following intravitreal bevacizumab treatment for myopic CNV.14, 26, 27 In contrast with the short-term results, these longer term results were more variable, as studies have reported that the initial visual gain might no longer be significant at 2 years.14, 27 Previous studies that have evaluated the use of anti-VEGF therapy in myopic CNV were also rather heterogeneous, as the studies have included prior treated eyes as well as non-subfoveal CNV.28, 29, 30 In order to further assess the long-term efficacy of anti-VEGF therapy for myopic CNV, we evaluated the 2-year outcomes in the use of intravitreal bevacizumab and ranibizumab as the primary treatment for subfoveal myopic CNV. We also evaluated the prognostic factors that might influence the visual outcomes following anti-VEGF therapy for myopic CNV.
Patients and methods
This was a retrospective study of consecutive patients with subfoveal CNV secondary to pathologic myopia who received intravitreal bevacizumab or ranibizumab injections in the Department of Ophthalmology and Visual Sciences, the Chinese University of Hong Kong. The inclusion criteria included patients with follow-up of at least 2 years; myopia with spherical equivalent refractive error of −6 D or more; subfoveal CNV location; best-corrected visual acuity (BCVA) of 20/800 or better; and evidence of CNV leakage on fluorescein angiography (FA). Exclusion criteria included juxtafoveal or extrafoveal CNV, prior treatment of CNV including PDT or thermal laser photocoagulation, features suggesting CNV secondary to AMD or other causes such as trauma, choroiditis, angioid streaks and hereditary diseases in the study or fellow eye. Informed consent was obtained from all patients before treatment and the study was approved by an institutional review board and carried out in adherence to the tenets of the Declaration of Helsinki.
At baseline and all visits, BCVA was measured with ETDRS logMAR chart at 4 m or Snellen chart at 6 m being converted to logMAR unit for analysis. Fundus photography and FA were performed at the baseline with the CNV lesion size, location and composition noted. All patients were given three initial loading doses of intravitreal bevacizumab or ranibizumab injections at baseline, 1 and 2 months. Intravitreal injections of 1.25 mg bevacizumab (Avastin, Roche, Basel, Switzerland) or 0.5 mg ranibizumab (Lucentis, Novartis, Basel, Switzerland) in 0.05 ml were carried out in an out-patient setting using a 30-gauge needle at 4 mm post-limbus under strict aseptic techniques. The choice of using either bevacizumab or ranibizumab was based on the patient's financial preference. Patients were given topical 0.5% levofloxacin qid (Cravit, Santen, Osaka, Japan) for 1 week after each injection. Retreatments with three anti-VEGF injections at monthly intervals were performed in eyes with new symptoms and persistent or recurrent angiographic leakage after 3 months.
Patients were seen 1 week after injection and then every month for 3 months with examinations performed as with baseline. Fundus photography and FA were performed at 3 months after the first anti-VEGF injection and additional FA was performed after 3 months in patients with new symptoms or evidence of recurrence. Time-domain or spectral-domain optical coherence tomography (OCT) was also performed in most patients to evaluate the treatment response and to guide retreatment in cases of recurrence. The main outcome measures included mean changes in logMAR BCVA, mean number of anti-VEGF injections and proportion of patients requiring retreatment during the 2 years. Statistical analysis was performed using StatsPlus:mac 2009 (AnalystSoft Inc., Vancouver, BC, Canada). Continuous variables were compared using Wilcoxon signed-rank test and Mann–Whitney U-test, and categorical variables were analysed using the χ2 test. Multivariate logistic regression analysis and forward stepwise regression analysis were performed to identify prognostic factors for final visual outcomes. Variables evaluated in the multivariate analyses included age, gender, anti-VEGF agent, baseline visual acuity, lens status, refractive error, duration of symptoms and size of CNV. A P-value of <0.05 was considered as statistically significant.
Results
Patients demographics
In all, 37 eyes of 37 patients were included in the study, of which 22 were given bevacizumab and 15 were given ranibizumab. The baseline characteristics of the two groups are displayed in Table 1. All the baseline characteristics were comparable between the two groups except there was a longer duration of symptoms and worse baseline visual acuity in the ranibizumab group compared with the bevacizumab (χ2 test, P=0.035 and Mann–Whitney U-test, P=0.041, respectively). The mean±standard deviation (SD) age of all patients was 57.3±13.0 years (range, 35–84 years) and 23 (62.2%) were female. The mean±SD spherical equivalent refractive error was −11.7±4.0 D (range, −6.0 to −20.0 D). All patients were of Chinese ethnicity. In all, 29 (78.4%) eyes were phakic and the remaining 8 (21.6%) eyes were pseudophakic. All CNV were predominately classic in angiographic appearance and the mean greatest linear dimension of the CNV was 960±450 μm (range, 250–2000 μm). The mean±SD baseline logMAR BCVA of all 59 eyes was 0.86±0.43 (Snellen equivalent of 20/145).
Table 1. Baseline demographics of 37 eyes of 37 patients with myopic choroidal neovascularization treated with intravitreal bevacizumab or ranibizumab.
| All eyes (n=37) | Bevacizumab (n=22) | Ranibizumab (n= 15) | P-value | |
|---|---|---|---|---|
| Mean±SD age (years) | 57.3±13.0 | 56.3±14.6 | 58.9±10.5 | 0.50a |
| Mean±SD spherical equivalent refractive error (D) | –11.7±4.0 | –12.5±4.0 | –10.5±3.7 | 0.11a |
| Gender | ||||
| Male | 14 (37.8%) | 11 (50.0%) | 3 (20.0%) | 0.065b |
| Female | 23 (62.2%) | 11 (50.0%) | 12 (80.0%) | |
| Lens status | ||||
| Phakic | 29 (78.4%) | 16 (72.7%) | 13 (86.7%) | 0.31b |
| Pseudophakic | 8 (21.6%) | 6 (27.3%) | 2 (13.3%) | |
| Mean±SD duration of symptoms (months) | 1.7±1.1 | 1.3±0.8 | 2.2±1.3 | 0.046a |
| Mean±SD baseline greatest linear dimension of lesion (μm) | 960±450 | 1030±480 | 850±400 | 0.27a |
| Mean±SD baseline logMAR BCVA | 0.86±0.43 | 0.73±0.39 | 1.04±0.43 | 0.041a |
| (Snellen equivalent) | (20/145) | (20/107) | (20/219) | |
Abbreviations: logMAR BCVA, logarithm of minimal angle of resolution best-corrected visual acuity; SD, standard deviation.
Mann–Whitney U-test.
χ2 Test.
Visual and angiographic outcomes
At 3 months, the mean±SD logMAR BCVA improved significantly from 0.73±0.39 to 0.48±0.37 and from 1.04±0.43 to 0.66±0.43 in the bevacizumab and ranibizumab groups, respectively (Wilcoxon sign-rank test, P<0.001 for both groups). The mean BCVA improvements for the bevacizumab and ranibizumab groups were 2.5 and 3.8 lines, respectively, and the difference between the two groups was not significantly different (Mann–Whitney U-test, P=0.25). FA at 3 months showed absence of leakage in 33 (89.2%) eyes, with fibrosis of the CNV in 21 (56.8%) eyes and complete regression of CNV in 12 (32.4%) eyes. Two eyes in each of the bevacizumab and ranibizumab groups had reduced but persistent leakage at 3 months and required additional anti-VEGF injections. Examples of the fundus and FA changes after bevacizumab and ranibizumab injections are displayed in Figures 1 and 2.
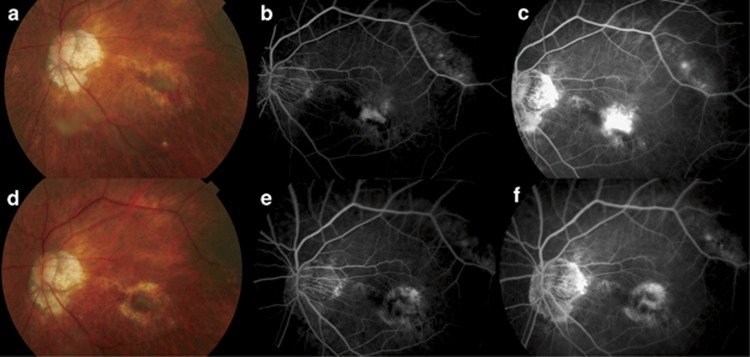
Figure 1.
(a) Fundus photo of the left eye of a 78-year-old man with subfoveal myopic choroidal neovascularization (CNV) before intravitreal bevacizumab injection. Retinal haemorrhage can be seen nasal and inferior to the CNV with surrounding retinal pigment epithelial (RPE) atrophy. The patient's best-corrected visual acuity was 20/250. (b) Early phase fluorescein angiogram showed a subfoveal CNV with (c) leakage in the late phase. (d) Fundus photo at 3 months after commencement of intravitreal bevacizumab injections showed resolution of the retinal haemorrhage with mild fibrosis of the CNV. The patient's vision improved to 20/70. (e) Early and (f) late phases fluorescein angiogram showed staining of the CNV due to fibrosis with surrounding RPE atrophy.
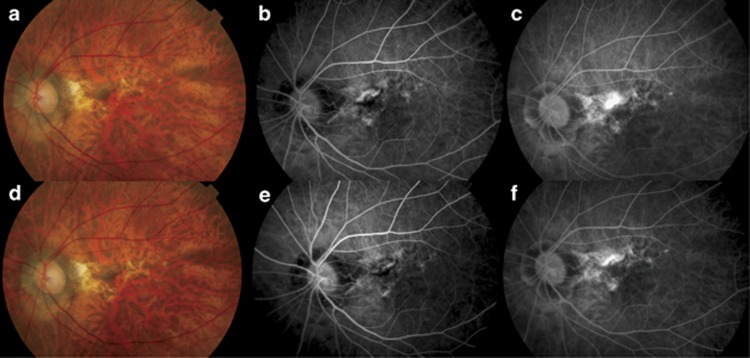
Figure 2.
(a) Fundus photo of the left eye of a 64-year-old woman with subfoveal myopic choroidal neovascularization (CNV) before intravitreal ranibizumab injection. Retinal haemorrhage inferior to the CNV with surrounding retinal pigment epithelial (RPE) atrophy. The patient's best-corrected visual acuity was 20/400. (b) Early phase fluorescein angiogram showed a subfoveal CNV with (c) extensive leakage in the late phase. (d) Fundus photo at 3 months after commencement of intravitreal ranibizumab injections showed resolution of the retinal haemorrhage. The patient's vision improved to 20/200. (e) Early and (f) late phases fluorescein angiogram showed mild leakage from the CNV and the patient was retreated with additional ranibizumab injections.
At 1 year, the mean±SD logMAR BCVA further increased to 0.41±0.32 and 0.49±0.37 in the bevacizumab and ranibizumab groups, respectively. There was no significant difference between the mean logMAR BCVA in the bevacizumab and ranibizumab group at 12 months (Mann–Whitney U-test, P=0.51). At 2 years, there was a slight decline in the mean±SD logMAR BCVA in both groups, with 0.45±0.36 in the bevacizumab group and 0.53±0.40 in the ranibizumab group (Mann–Whitney U-test, P=0.55). The mean improvements in logMAR BCVA for the bevacizumab and ranibizumab groups at 2 years were 2.8 and 5.1 lines, respectively, and the improvement from baseline remained statistically significant (Wilcoxon signed-rank test, P=0.009 and P<0.001, respectively). No significant difference in mean visual improvement was observed between the two groups (Mann–Whitney U-test, P=0.073).
At 2 years, 34 (91.9%) eyes avoided moderate visual loss of three or more lines of BCVA, with 22 (86.4%) eyes in the bevacizumab group and 15 (100.0%) eyes in the ranibizumab group (χ2 test, P=0.14). A total of 24 (64.9%) eyes had moderate visual gain of three or more lines, with 13 (59.1%) eyes in the bevacizumab group and 11 (73.3%) eyes in the ranibizumab group (χ2 test, P=0.37).
Number of treatment
The mean number of bevacizumab injections during the 2-year period was 3.8 (range, 3–9 injections). Seventeen (77.3%) eyes required only three initial intravitreal bevacizumab injections during the 2-year period. Five (22.7%) eyes required additional intravitreal bevacizumab injections after 3 months. One patient developed two episodes of CNV recurrence during the 24 month period and required a total of nine intravitreal anti-VEGF injections (Figure 3). For the ranibizumab group, the mean number of injections was also 3.8 (range, 3–6). Eleven (73.3%) eyes required only three initial intravitreal ranibizumab injections. No significant difference in the mean number of injections during the 2-year period was found between the bevacizumab and ranibizumab groups (Mann–Whitney U-test, P=0.89).
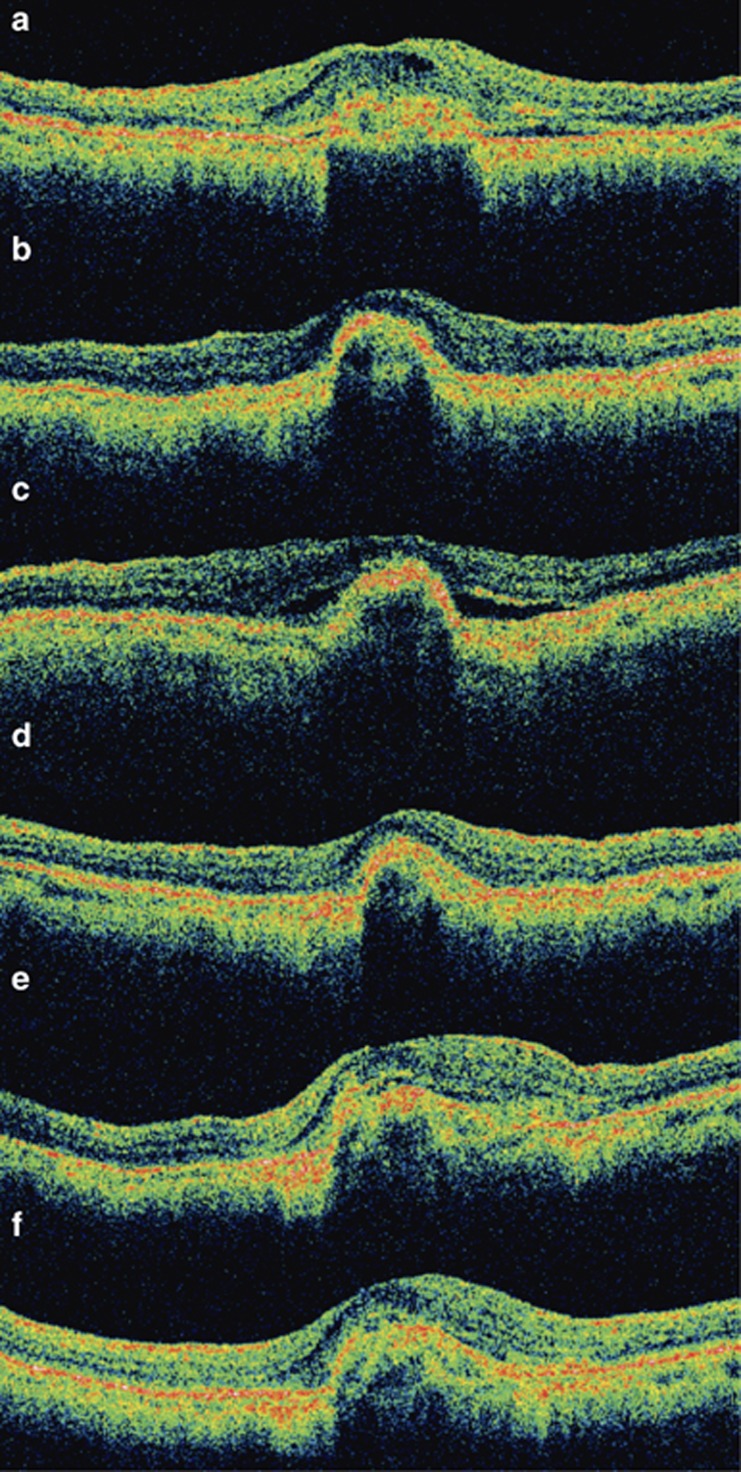
Figure 3.
Serial optical coherence tomography (OCT) examinations of a 71-year-old man with subfoveal myopic choroidal neovascularization (CNV) treated with intravitreal bevacizumab. (a) Baseline OCT showing subfoveal CNV with intraretinal and subretinal fluid adjacent to the CNV. (b) Following three intravitreal bevacizumab injections, there was complete resolution of intraretinal and subretinal fluid with regression of the CNV. The visual acuity improved from 20/400 at baseline to 20/50 at 3 months. (c) At 9 months after the first bevacizumab injection, OCT showed recurrence of CNV with subretinal fluid. (d) After a second course of three additional intravitreal bevacizumab injections, the subretinal fluid absorbed and the visual acuity increased to 20/40. (e) At 18 months after the first treatment, there was again slight recurrence CNV associated with retinal thickening and the visual acuity dropped to 20/50. (f) After the third course of intravitreal bevacizumab injections, the subretinal fluid resolved. Despite the subfoveal scarring on OCT, the patient's vision was 20/30 at 24 months.
Prognostic factors for visual outcome
Multivariate logistic regression analysis was performed to determine the prognostic factors for visual gain at 2 years (Table 2). Eyes with higher severity of myopia were less likely to develop visual gain of three or more lines at 2 years (P=0.043), whereas eyes with baseline logMAR BCVA of 0.8 or worse were more likely to gain three or more lines (P=0.017). Multivariate forward stepwise linear regression analysis also showed that the baseline CNV lesion size was negatively correlated with the change in visual acuity following anti-VEGF therapy at 2 years (P=0.046).
Table 2. Multivariate logistic regression analysis for prognostic factors of having three or more lines visual gain at 2 years.
| Adjusted odds ratio | 95% Confidence interval | P-value | |
|---|---|---|---|
| Age (per year) | 0.97 | 0.88–1.06 | 0.45 |
| Gender | |||
| Male | 0.82 | 0.08–8.15 | 0.86 |
| Female | Reference | ||
| Lens status | |||
| Phakic | Reference | ||
| Pseudophakic | 0.38 | 0.03–5.81 | 0.49 |
| Spherical equivalent of myopia (per dioptre) | 0.69 | 0.50–0.96 | 0.028 |
| Duration of symptoms (per month) | 0.61 | 0.20–1.83 | 0.38 |
| Anti-VEGF agent | |||
| Bevacizumab | 2.68 | 0.21–34.8 | 0.45 |
| Ranibizumab | Reference | ||
| Baseline greatest linear dimension of lesion (per mm) | 0.49 | 0.05–4.53 | 0.49 |
| Baseline logMAR BCVA | |||
| 0.8 or worse | 41.8 | 1.97–883.7 | 0.017 |
| Better than 0.8 | Reference | ||
Abbreviation: logMAR BCVA, logarithm of minimal angle of resolution best-corrected visual acuity.
Complications
None of the patients developed any systemic complications related to intravitreal bevacizumab or ranibizumab injection including cerebral vascular accident or cardiovascular event. Ocular complications developed in nine (24.3%) patients, with five in the bevacizumab group and four in the ranibizumab group. The most frequent ocular complication was increase in cataract (two in bevacizumab group and one in ranibizumab group) requiring cataract surgery during the 2-year period. Other complications included increase in myopic foveoschisis (one eye each in bevacizumab and ranibizumab group), cellophane maculopathy (one in ranibizumab group), macular hole (one in bevacizumab group), retinal detachment (one in bevacizumab group) and peripheral retinal thinning requiring barrier laser photocoagulation (one in ranibizumab group). Two patients developed visual loss of three or more lines as a result of the retinal complications (macular hole and retinal detachment).
Discussion
In the past few years, anti-VEGF therapy has gained increased popularity in the treatment of myopic CNV, as multiple studies have shown that anti-VEGF agents are effective in improving vision of patients with myopic CNV.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Although the short-term results have demonstrated significant visual improvement following anti-VEGF therapy, the longer term visual outcomes appeared more variable.14, 26, 27, 28 In addition, many previous studies have included both treatment naïve cases and previously treated eyes, as well as subfoveal and non-subfoveal CNV in the series, making comparison of results more difficult. The main strengths of our current study included the relatively long follow-up duration of 2 years and the homogeneity of cases with only treatment naïve subfoveal myopic CNV included in the series.
Previous studies by Gharbiya et al26 and Nakanishi et al28 demonstrated that patients with myopic CNV had significant visual improvement following bevacizumab injections at 24 months, whereas studies by Ruiz-Moreno et al27 and Ikuno et al14 showed that the initial visual improvements were no longer significant at 2 years after intravitreal bevacizumab therapy. The reasons for the lack of significant improvement observed in these two latter studies might be due to the relatively small sample size of 19 patients in the study by Ruiz-Moreno et al,27 and the inclusion of only patients aged 50 or more in the study by Ikuno et al.14 In our present study, we demonstrated that there were significant mean visual improvements in both the bevacizumab- and ranibizumab-treated groups at 2 years. Consistent with previous study by Ruiz-Moreno et al,27 there was also a slight decline in the mean BCVA in both groups between the first and the second year. Despite this slight visual loss, the visual improvements compared with baseline were still highly statistically significant in both groups at 2 years. The treatment efficacy of both bevacizumab and ranibizumab groups appeared to be similar as there was no significant difference in the mean visual improvement between the two groups and the proportions of eyes having moderate visual gain or moderate visual loss were also similar. There was also no significant difference in the mean number of anti-VEGF injections required and in the proportion of patients requiring retreatment during 2 years. Therefore, both anti-VEGF agents appeared to have similar efficacy for treating myopic CNV at 2 years.
Our findings showed that patients with myopic CNV had a mean improvement of 3.7 lines after anti-VEGF treatment at 2 years. This compared favourably with verteporfin PDT for treating myopic CNV as previous studies have demonstrated no significant visual improvement at 2 years after PDT.6, 7 Recent studies have also reported that anti-VEGF therapy with intravitreal bevacizumab appeared to result in better efficacy in terms of visual improvement compared with PDT.31, 32 Baba et al31 compared the 2-year outcome of intravitreal bevacizumab with PDT. It was shown that there was no significant change in vision following PDT, while intravitreal bevacizumab resulted in significant visual improvement at 2 years. The likely reason for the inferior visual outcome associated with PDT was likely to be due to the enlargement of chorioretinal atrophy around the CNV following PDT.31 Yoon et al32 also evaluated the use of anti-VEGF therapy with intravitreal bevacizumab or ranibizumab, PDT, and combined anti-VEGF with PDT in the treatment of myopic CNV. Results at 12 months showed that significantly higher proportion of patients in the anti-VEGF group lost fewer than 15 letters compared with both the combination and the PDT groups. As patients with pathologic myopia frequently have chorioretinal atrophy associated with the myopic CNV, it might not be advisable to perform PDT in these patients, as the PDT can further exacerbate the chorioretinal damage in these patients by damaging the already comprised choriocapillaris.
In this study, we also evaluated the prognostic factors for favourable visual outcome at 2 years after anti-VEGF therapy. Multivariate logistic regression analysis showed that patients with higher severity of myopia were less likely to gain three or more lines following anti-VEGF therapy, with a 31% less chance in gaining three or more lines per dioptre increase in myopic refractive error. This finding is consistent with a study by Kuo et al,33 in which higher severity of myopia was positively correlated with worse visual outcome following intravitreal bevacizumab. In addition, we also found that the size of myopic CNV was negatively correlated with the extent of visual improvement at 2 years. The result supports the findings by Nakanishi et al,28 in which the size of myopic CNV is a significant factor in influencing the final visual acuity after intravitreal bevacizumab treatment.
Patients with high myopia are well known to develop retinal complications such as retinal detachment, myopic foveoschisis and myopic macular hole. In our study, six (16.2%) patients developed various retinal complications during the 2-year period. Shimada et al34 reported that 5.4% of 74 eyes that had intravitreal bevacizumab for myopic CNV developed macular retinal detachment following treatment, and four eyes had foveoschisis around the CNV before the intravitreal injection. These complications might or might not be related to the intravitreal injections as they could develop even without intravitreal injection. Despite having these retinal complications, the majority of patients did not develop significant visual loss following anti-VEGF therapy. As patients with high myopia have increased risk of developing these retinal complications, they should be warned of the potential complications associated with intravitreal injections.
The main limitations of our study include its retrospective nature and the lack of untreated control for comparison. Despite the retrospective nature of our study, only four patients who received anti-VEGF therapy during the study review period were excluded from the analysis due to follow-up shorter than 2 years and therefore ascertainment bias was unlikely to occur. There was a slightly longer duration of symptoms and a trend of worse baseline visual acuity in the ranibizumab group compared with the bevacizumab group. Therefore some potential bias might exist when comparing the outcomes between the two anti-VEGF treatment groups. Nonetheless, multivariate analyses after adjusting for baseline visual acuity did not show a significant difference in visual outcome between the two anti-VEGF agents. Another limitation was the small number of cases for multivariate logistic regression analysis. As a general rule, 10 cases should be available for each predictor variable used in logistic regression analysis. Nonetheless, this rule can be relaxed in order to demonstrate adequate control of confounding variables,35 such as age, gender and refractive error in our series. Finally, we did not report the quantitative OCT findings in this study as not all patients had OCT assessment at each visit, and the OCT data were recorded using different time-domain and spectral-domain OCT machines, making serial comparisons invalid due to different measurement methods. Nonetheless, OCT examinations were performed to assess the treatment response and were used to guide retreatment in all cases with suspected CNV recurrence.
In summary, our results demonstrated that anti-VEGF therapy using either intravitreal bevacizumab or ranibizumab appeared to be effective as primary treatment for subfoveal myopic CNV, with significant visual improvement associated with both agents at 2 years. In view of these encouraging results, it is reasonable to recommend that anti-VEGF agents should be used as the first-line therapy for myopic CNV.36
Dr Lai has received honoria for lecture fees and for serving as a consultant in the advisory board of the Novartis Pharmaceutical Corporations. All the other authors declare no conflict of interest.
Footnotes
Presented in part at the 2010 Annual Meeting of the American Academy of Ophthalmology, Chicago, IL, October 2010.
References
- Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL. Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology. 1984;91:1573–1581. doi: 10.1016/s0161-6420(84)34116-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Matsui K, Yoshida T, Futagami S, Yasuzumi K, Shimada N, Kojima A, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularization in pathologic myopia. Br J Ophthalmol. 2003;87:570–573. doi: 10.1136/bjo.87.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ohno-Matsui K, Ohtake Y, Takashima T, Futagami S, Baba T, et al. Long-term visual prognosis of choroidal neovascularization in high myopia. A comparison between age groups. Ophthalmology. 2002;109:712–719. doi: 10.1016/s0161-6420(01)01007-7. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117:1595–1611. doi: 10.1016/j.ophtha.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donato G, Lewis H, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial-VIP report no. 3. Ophthalmology. 2003;110:667–673. doi: 10.1016/s0161-6420(02)01998-x. [DOI] [PubMed] [Google Scholar]
- Chan WM, Ohji M, Lai TY, Liu DT, Tano Y, Lam DS. Choroidal neovascularisation in pathological myopia: an update in management. Br J Ophthalmol. 2005;89:1522–1528. doi: 10.1136/bjo.2005.074716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho AM, Silva RM, Nunes SG, Cachuio ML, Figueira JP, Murta JN. Photodynamic therapy in high myopic eyes with choroidal neovascularization: 5 years of follow-up. Retina. 2011;31:1089–1094. doi: 10.1097/IAE.0b013e3181ff9546. [DOI] [PubMed] [Google Scholar]
- Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathologic myopia. Br J Ophthalmol. 2007;91:157–160. doi: 10.1136/bjo.2006.096776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi H, Ikuno Y, Gomi F, Kamei M, Sawa M, Tsujikawa M, et al. Intravitreal injection of bevacizumab for choroidal neovascularization associated with pathological myopia. Br J Ophthalmol. 2007;91:161–165. doi: 10.1136/bjo.2006.099887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization: six-month results of a prospective pilot study. Ophthalmology. 2007;114:2190–2196. doi: 10.1016/j.ophtha.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rojas ML, Quiroz-Mercado H, Dalma-Weiszhausz J, Fromow-Guerra J, Amaya-Espinosa A, Solis-Vivanco A, et al. Short-term effects of intravitreal bevacizumab for subfoveal choroidal neovascularization in pathologic myopia. Retina. 2007;27:707–712. doi: 10.1097/GIM.0b013e3180a03276. [DOI] [PubMed] [Google Scholar]
- Chan WM, Lai TY, Chan KP, Li H, Liu DT, Lam DS, et al. Changes in aqueous vascular endothelial growth factor and pigment epithelial-derived factor levels following intravitreal bevacizumab injections for choroidal neovascularization secondary to age-related macular degeneration or pathologic myopia. Retina. 2008;28:1308–1313. doi: 10.1097/IAE.0b013e31818358b2. [DOI] [PubMed] [Google Scholar]
- Chan WM, Lai TY, Liu DT, Lam DS. Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularization: 1-year results of a prospective pilot study. Br J Ophthalmol. 2009;93:150–154. doi: 10.1136/bjo.2008.145797. [DOI] [PubMed] [Google Scholar]
- Ikuno Y, Nagai Y, Matsuda S, Arisawa A, Sho K, Oshita T, et al. Two-year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol. 2010;149:140–146. doi: 10.1016/j.ajo.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Ruiz-Moreno JM, Montero JA, Arias L, Araiz J, Gomez- Ulla F, Silva R, et al. Twelve-month outcome after one intravitreal injection of bevacizumab to treat myopic choroidal neovascularization. Retina. 2010;30:1609–1615. doi: 10.1097/IAE.0b013e3181e22659. [DOI] [PubMed] [Google Scholar]
- Scupola A, Tiberti AC, Sasso P, Savastano MC, Mastrocola A, Marangoni D, et al. Macular functional changes evaluated with MP-1 microperimetry after intravitreal bevacizumab for subfoveal myopic choroidal neovascularization: one-year results. Retina. 2010;30:739–747. doi: 10.1097/IAE.0b013e3181c59725. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Ikuno Y, Gomi F. Different dosing of intravitreal bevacizumab for choroidal neovascularization because of pathologic myopia. Retina. 2011;31:880–886. doi: 10.1097/IAE.0b013e3181f2a293. [DOI] [PubMed] [Google Scholar]
- Gharbiya M, Giustolisi R, Allievi F, Fantozzi N, Mazzeo L, Scavella V, et al. Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab: a randomized controlled trial. Am J Ophthalmol. 2010;149:458–64.e1. doi: 10.1016/j.ajo.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Silva RM, Ruiz-Moreno JM, Nascimento J, Carneiro A, Rosa P, Barbosaa A, et al. Short-term efficacy and safety of intravitreal ranibizumab for myopic choroidal neovascularization. Retina. 2008;28:1117–1123. doi: 10.1097/iae.0b013e31817eda41. [DOI] [PubMed] [Google Scholar]
- Lai TY, Chan WM, Liu DT, Lam DS. Intravitreal ranibizumab for the primary treatment of choroidal neovascularization secondary to pathologic myopia. Retina. 2009;29:750–756. doi: 10.1097/IAE.0b013e31819ed6bd. [DOI] [PubMed] [Google Scholar]
- Mones JM, Amselem L, Serrano A, Garcia M, Hijano M. Intravitreal ranibizumab for choroidal neovascularization secondary to pathologic myopia: 12-month results. Eye (Lond) 2009;23:1275–1280. doi: 10.1038/eye.2009.88. [DOI] [PubMed] [Google Scholar]
- Lalloum F, Souied EH, Bastuji-Garin S, Puche N, Querques G, Glacet-Bernard A, et al. Intravitreal ranibizumab for choroidal neovascularization complicating pathologic myopia. Retina. 2010;30:399–406. doi: 10.1097/IAE.0b013e3181bcef24. [DOI] [PubMed] [Google Scholar]
- Silva RM, Ruiz-Moreno JM, Rosa P, Carneiro A, Nascimento J, Rito LF, et al. Intravitreal ranibizumab for myopic choroidal neovascularization: 12-month results. Retina. 2010;30:407–412. doi: 10.1097/IAE.0b013e3181c9691e. [DOI] [PubMed] [Google Scholar]
- Varano M, Tedeschi M, Oddone F, Perillo L, Coppe AM, Parravano M. Microperimetric retinal changes in myopic choroidal neovascularization treated with intravitreal ranibizumab. Retina. 2010;30:413–417. doi: 10.1097/IAE.0b013e3181bd2d23. [DOI] [PubMed] [Google Scholar]
- Heier JS, Brown D, Ciulla T, Abraham P, Bankert JM, Chong S, et al. Ranibizumab for choroidal neovascularization secondary to causes other than age-related macular degeneration: a phase I clinical trial. Ophthalmology. 2011;118:111–118. doi: 10.1016/j.ophtha.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Gharbiya M, Allievi F, Conflitti S, Esposito M, Scavella V, Moramarco A, et al. Intravitreal bevacizumab for treatment of myopic choroidal neovascularization: the second year of a prospective study. Clin Ter. 2010;161:e87–e93. [PubMed] [Google Scholar]
- Ruiz-Moreno JM, Montero JA. Intravitreal bevacizumab to treat myopic choroidal neovascularization: 2-year outcome. Graefes Arch Clin Exp Ophthalmol. 2010;248:937–941. doi: 10.1007/s00417-010-1340-y. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Tsujikawa A, Yodoi Y, Ojima Y, Otani A, Tamura H, et al. Prognostic factors for visual outcomes 2-years after intravitreal bevacizumab for myopic choroidal neovascularization. Eye (Lond) 2011;25:375–381. doi: 10.1038/eye.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Shimada N, Moriyama M, Hayashi W, Tokoro T, Ohno-Matsui K. Two-year outcomes of intravitreal bevacizumab for choroidal neovascularization in Japanese patients with pathologic myopia. Retina. 2012;32:687–695. doi: 10.1097/IAE.0b013e3182278bae. [DOI] [PubMed] [Google Scholar]
- Yoon JU, Kim YM, Lee SJ, Byun YJ, Koh HJ. Prognostic factors for visual outcome after intravitreal anti-VEGF injection for naïve myopic choroidal neovascularization. Retina. 2012;32:949–955. doi: 10.1097/IAE.0b013e318227a9ef. [DOI] [PubMed] [Google Scholar]
- Baba T, Kubota-Taniai M, Kitahashi M, Okada K, Mitamura Y, Yamamoto S. Two-year comparison of photodynamic therapy and intravitreal bevacizumab for treatment of myopic choroidal neovascularization. Br J Ophthalmol. 2010;94:864–870. doi: 10.1136/bjo.2009.166025. [DOI] [PubMed] [Google Scholar]
- Yoon JU, Byun YJ, Koh HJ. Intravitreal anti-VEGF versus photodynamic therapy with verteporfin for treatment of myopic choroidal neovascularization. Retina. 2010;30:418–424. doi: 10.1097/IAE.0b013e3181bd2fe4. [DOI] [PubMed] [Google Scholar]
- Kuo JZ, Ong FS, Yeung L, Wu WC, Chen YP, Wang NK, et al. Predictive factors for visual outcome to intravitreal bevacizumab in young Chinese patients with myopic choroidal neovascularization. Retina. 2011;31:1835–1840. doi: 10.1097/IAE.0b013e31821ba2dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada N, Ohno-Matsui K, Hayashi K, Yoshida T, Tokoro T, Mochizuki M. Macular detachment after successful intravitreal bevacizumab for myopic choroidal neovascularization. Jpn J Ophthalmol. 2011;55:378–382. doi: 10.1007/s10384-011-0034-2. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- Cohen SY. Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina. 2009;29:1062–1066. doi: 10.1097/IAE.0b013e3181b1bb1a. [DOI] [PubMed] [Google Scholar]