Abstract
Mothers of adolescents and adults with fragile X syndrome (FXS) are faced with high levels of parenting stress. The extent to which mothers are negatively impacted by this stress, however, may be influenced by their own genetic status. The present study uses a diathesis-stress model to examine the ways in which a genetic vulnerability in mothers with the premutation of the FMR1 gene interacts with child-related environmental stress to predict their morning cortisol levels. Seventy-six mothers of an adolescent or adult with FXS participated in an 8-day telephone diary study in which they reported on the behavior problems of their son or daughter with FXS each day. We analyzed salivary cortisol collected from mothers at awakening and 30 minutes after awakening on 4 of these days. The results indicated that mothers with greater genetic vulnerability had a lower level of cortisol on mornings following days when their son or daughter with FXS manifested more episodes of behavior problems, whereas mothers with less genetic risk evinced the opposite pattern of higher morning cortisol in response to their child’s behavior problems. This finding contributes to our understanding of gene-by-environment interactions and highlights the importance of interventions to alleviate parenting stress in mothers raising children with FXS.
Keywords: cortisol, fragile X syndrome, parenting stress
Fragile X syndrome (FXS) is a neurodevelopmental disorder involving an expansion to more than 200 repeats of the cytosine-guanine- guanine (CGG) sequence of nucleotides comprising the 5′ untranslated region of the fragile X mental retardation gene (FMR1) located on the X chromosome (Brown, 2002). The FMR1 gene and its protein products are important in neurological development and functioning. The full mutation of the FMR1 gene (defined as 200 or more repeats) is the leading inherited cause of intellectual disability (ID) and, when associated with ID, occurs in about 1 in 3,600 individuals (Hagerman et al., 2009). Individuals with FXS also present with a challenging profile of behavior problems and autism symptoms into adulthood, which can be highly stressful for parents (Bailey, Raspa, Olmsted, & Holiday, 2008; Cornish, Turk, & Hagerman, 2008). Moreover, mothers of children with FXS may themselves have the full mutation of FXS, or more frequently, the premutation of the FMR1 gene (defined as 55 to 200 CGG repeats), accompanied by varying degrees of biochemical alterations, leading to their own emotional and physical health problems (e.g., Bailey, Raspa, et al., 2008; Johnston et al., 2001). The ability of mothers to manage the stress of having a child with FXS may thus be impacted by the extent to which they are affected by either the premutation or the full mutation of the FMR1 gene. The present study focuses on mothers who have the premutation genotype, and uses a diathesis-stress model (Monroe & Simons, 1991) to examine the ways in which stress related to parenting a child with FXS interacts with a genetic vulnerability in these mothers to predict daily cortisol levels, a hormonal measure of stress.
Diathesis and stress
In the diathesis-stress model, a genetic vulnerability (diathesis) interacts with environmental adversity (stress) to affect functioning. This model has not been directly tested in women with the FMR1 premutation, but it has been of value for examining the development and expression of psychopathology in the general population (Caspi et al., 2002, 2003; Fowles, 1992; McKeever & Huff, 2003; Monroe & Simons, 1991).
In our application of the diathesis-stress model, maternal diathesis is measured by activation ratio (defined below) and the stressful challenge of parenting is indexed by child behavior problems. Although the activation ratio is just one of the several biomarkers of mutations in the FMR1 gene, we selected it as our measure of genetic vulnerability to stress because it is an individual-difference variable that reflects the degree of biochemical affectedness. Past research on the activation ratio has suggested that it is a biomarker of the FMR1 premutation that has a linear association with various biopsychosocial outcomes, whereas other biomarkers such as CGG repeat length, may have more complex curvilinear associations (Seltzer, Abbeduto, Greenberg, Almeida, Hong, & Witt, 2009). Similarly, although child behavior problems are just one source of stress experienced by mothers, they are a prominent stressor documented in past research to be of significance in FXS (Bailey, Sideris, Roberts, & Hatton, 2008; Cornish et al., 2008). This study uses the diathesis-stress model to examine gene-by-environment interactions.
Diathesis
Mothers with the premutation of the FMR1 gene vary widely in terms of their biochemical affectedness (e.g., Tassone, Hagerman, Chamberlain, & Hagerman, 2000). This variation is due in part to X inactivation. The process of X inactivation occurs early in embryological development in all females, and it results in the “turning off” of one X chromosome in each cell. In females with the FMR1 premutation, the relative proportion of active and inactive FMR1 expansion mutation-carrying X chromosomes varies from person to person (Berry-Kravis, Potanos, Weinberg, Zhou, & Goetz, 2000). The percentage of cells with a normal X as the active X is known as the activation ratio.
Specifically, some women have a higher percentage of cells that have the normal X as the active X (i.e., a higher activation ratio), whereas other women have a lower percentage of cells with the normal X as the active X (i.e., a lower activation ratio). The activation ratio has been identified as a potentially important biological indicator of the extent to which various biochemical pathways are altered, and a low activation ratio may put mothers at risk of poor psychological well-being and physical health (Seltzer et al., 2009). Thus, a lower activation ratio may serve as a diathesis, which increases the degree to which mothers with the premutation may be negatively impacted by child-related stress.
Stress
Individuals with FXS often have severe behavior problems, including inattention, hyperactivity, aggression, and anxiety, and autism symptoms.Bailey et al. (2008) found that 30% of the mothers of young children with FXS had clinically significant scores on the Parenting Stress Index. This pattern of elevated behavior problems and autism symptoms continues into adolescence and adulthood (Bailey, Raspa, et al., 2008; Hartley et al., 2011). Indeed, mothers of adolescents and adults with FXS, on average, report higher levels of parenting stress, lower psychological well-being, and more health problems than normative samples (Bailey, Sideris, et al., 2008; Franke et al., 1996; Johnston et al., 2003; Sarimski, 1997; van Lieshout, De Meyer, Leopold, Curfs, & Fryns, 1998; von Gontard et al., 2002). Mothers of adolescents and adults with FXS also report higher levels of parenting stress than do mothers of similarly aged children with Down syndrome, and levels of stress as high as mothers of adolescents and adults with autism spectrum disorders (Abbeduto et al., 2004).
Maternal cortisol
The experience of chronic and severe life stress can take a toll on an individual’s physiological functioning, and this impact is often indexed by dysregulation in the hypothalamic-pituitary-adrenal (HPA) axis (McEwen, 1998). Cortisol normally peaks shortly after waking in the morning and then gradually declines throughout the rest of the day. Diurnal cortisol (i.e., the pattern of cortisol secretion across the day) provides a window into an individual’s biorhythms or chronobiology (Keenan, Licinio, & Veldhuis, 2001). The early-morning and evening levels of cortisol reflect daily engagement and disengagement, respectively, of the brain with peripheral physiology, and hence with the external environment. The rise of cortisol in the morning prepares individuals for engagement with the external environment (Fries, Dettenborn, & Kirschbaum, 2009). Failure to activate the HPA axis in the morning may result in difficulty in responding to the ordinary challenges that are faced every day (Sapolsky, Krey, & McEwen, 1986).
When exposed to a stressful event, the HPA axis is acutely activated and cortisol is released from the adrenal cortex, which in turn, helps the body to adapt by regulating protein synthesis and glucose, immune functioning, and mental activity. A pattern of increased cortisol secretion in the face of a stressful situation is normal and generally seen when exposed to acute stressful events (Dickerson & Kemeny, 2004).
However, individuals exposed to chronic stress often evince lower cortisol and a flatter slope in the diurnal decline across the day, and may exhibit a blunted cortisol response or even transient decrease in cortisol secretion following a stressor (for reviews see Gunnar & Vazquez, 2001; Heim, Ehlert, & Hellhamner, 2000; Miller, Chen, & Zhou, 2007). For instance, lower diurnal patterns of cortisol have been reported for parents of children with cancer (Miller, Cohen, & Ritchey, 2002), individuals with posttraumatic stress disorder (PTSD; Boscarino, 1996; Yehuda, Boisoneau, Lowy,&Giller, 1995; Yehuda, Kahana, et al., 1995), and other stress-related disorders (e.g., Griep et al., 1998; Pruessner, Hellhammer, & Kirschbaum, 1999; Roberts, Wessely, Chalder, Papadopoulos, & Cleare, 2004). Blunted cortisol responses, or transient decreases in cortisol following a stressful situation, have been reported in maltreated children on high-conflict days at nursery school (Hart, Gunnar, & Cicchetti, 1995), women with fibromyalgia (Wingenfeld et al., 2008), and on weeks with high stress in adults experiencing work overload (Dahlgren, Akerstedt, & Kecklund, 2004). These patterns of hypocortisolism are not yet fully understood, but are theorized to result from having had a history of prolonged exposure to stress and elevated cortisol levels, which subsequently led to down-regulation of the HPA system and decreased cortisol secretion (Fries, Hesse, Hellhammer, & Hellhammer, 2005; Heim et al., 2000).
In a recent study, mothers of coresiding adolescents and adults with autism spectrum disorders (ASD) were found to have reduced diurnal cortisol levels as compared to mothers of unaffected coresiding adolescent and adult children (Seltzer et al., 2010). This pattern of low cortisol was interpreted to be indicative of the chronic and severe levels of parenting stress experienced by mothers of grown children with ASD. Furthermore, these mothers also had an attenuated cortisol response in relation to chronic and severe stress at a within-person level. If the grown child with ASD did not have a history of clinically significant behavior problems, mothers reacted to the uncharacteristic event of child behavior problems with a heightened morning rise in cortisol on the following morning. However, if the grown child with ASD had a history of clinically significant behavior problems, and thus behavior problems were a chronic stressor, mothers demonstrated a blunted morning rise in cortisol following the experience of multiple child behavior problems during the prior day.
Our diathesis-stress model predicts that the extent to which mothers of individuals with FXS are negatively affected by their child’s behavior problems will be influenced by their own genetic vulnerability. Mothers with a greater genetic vulnerability (i.e., those who have a lower activation ratio) will be more negatively impacted by this child-related stress, leading to a hypocortisolemic response on mornings following the experience of multiple behavior problems by their son or daughter with FXS. In contrast, mothers with less genetic vulnerability (i.e., those who have a higher activation ratio) may have a more typical response to their son or daughter’s behavior problems, resulting in a pattern of hypercortisolemic activity following a day with multiple behavior problems.
Present study
In the present study, we examined the extent to which the mothers’ activation ratio (i.e., diathesis) interacts with their experience of the adolescent or adult child’s behavior problems (i.e., stress) to predict maternal cortisol levels on the following morning. Researchers documenting these interactions usually examine between-person differences in measured aspects of the environment, where relative contributions of genetic and environmental effects on affective experience and behavior are compared across adults in different situations (see review by Shanahan & Hofer, 2005). Rarely do these genetically informed studies examine emotional experience within the same person across different contexts and situations. The current paper extends prior approaches by using a daily diary study of mothers and examining intraindividual variation in physiological responses (i.e., cortisol) across different naturally occurring stressful situations (i.e., child behavior problems) (Almeida, 2005). The unique design of this study permits an examination of how genetic and environmental effects on physiological response may shift according to day-to-day fluctuation of parenting stress.
Mothers participated in an 8-day telephone diary study, in which they reported on the behavior problems of their son or daughter at the end of each day. Salivary cortisol was collected at four time points (awakening, 30 minutes after awakening, before lunch, and before bed) each day on Days 2 through 5 of the diary study; the first two time points were analyzed for the present study, for reasons explained below. Maternal activation ratio was measured through DNA analysis of blood samples. Multilevel models were used to analyze daily variables nested within individuals across time.
We hypothesized that there would be an interaction between activation ratio and child behavior problems in predicting morning cortisol levels. Specifically, we hypothesized that mothers with lower activation ratios would have lower cortisol on mornings following a day when their child had multiple episodes of behavior problems, than mothers with higher activation ratios, who would evidence a more typical pattern of higher cortisol on mornings following a day when their child had multiple episodes of behavior problems.
Method
Participants
Participants were drawn from an ongoing longitudinal study of 147 mothers of adolescent and adult children with the full mutation of FXS. Data for the present analyses were collected in the first of the three waves of data collection during 2009–2010. Families lived in 32 states across the United States and one lived in Canada. In order to qualify for the study, mothers had to be the biological parent of an adolescent or adult (aged 12+ years) child with FXS. Mothers had to live with their adolescent or adult child with FXS or have at least weekly contact with him or her. Genetic verification confirming that the grown child had the full mutation of FXS was required. In families with multiple children with FXS within the specified age range, one child was designated as the target child. To select a target child, mothers were asked to identify the child with FXS who resided at home (if one child resided at the family home and the others did not), or to identify the most severely affected child (if all children with FXS resided at home). Families were recruited through advertisements posted to listservs and websites maintained by national organizations supporting families with FXS, brochures distributed at parent and professional conferences and to health providers, and from national research registries.
The larger study involved three components: (a) completion of a telephone interview and self-administered standardized measures, (b) participation in the daily diary study, and (c) supplying a blood sample for the analysis of CGG repeats and activation ratio or providing records documenting CGG repeat number and activation ratio. Because the present study is focused on mothers with the premutation, mothers who either had the full mutation or a normal FMR1 gene were not included in the analysis. Of the 138 mothers with the premutation, 129 participated in the diary and cortisol components of the study. However, cortisol values were flagged as problematic due to a failure to follow collection procedures for five mothers. Of the remaining 124 mothers, activation ratio was available on 99 mothers. Of these 99 mothers, 23 mothers supplied past records of activation ratio, whereas the others (n = 76) provided a blood sample that was analyzed for activation ratio by Kimball Genetics, Inc. Given the potential for lab-to-lab variability in assays, we restricted the present analysis to the 76 mothers for whom activation ratio was measured by Kimball Genetics. Independent sample t tests indicated that there were no differences in household income, child residence, maternal marital status, maternal ethnicity, maternal education, number of children with a disability, child behavior problems, or cortisol levels at awakening or 30 minutes after awakening between the mothers included in the present analyses and those who were not included. However, those who were included in the present analysis had fewer children (M = 1.59, SD = 0.79; M = 2.00, SD = 1.49, respectively, t (137) = 2.08, p = .040), older children (M = 21.71, SD = 7.77; M = 19.30, SD = 6.10, respectively, t (137) = 2.41 p = 0.032), and were themselves older (M = 51.47, SD = 7.73; M = 48.83, SD = 7.01, respectively, t (137) = 2.15 p = 0.040) than those not included.
Table 1 presents characteristics of the 76 mothers and their adolescent or adult children with FXS. Mothers ranged in age from 37 to 70 years with a mean age of 51, and their son or daughter with FXS ranged in age from 12 to 43 years (M = 21). (Note that although we use the term “child” in this paper, it is meant in the sense that the adolescent or adult son or daughter is the offspring of the mother.) The majority of the mothers were married (86%) and more than half (54%) had an additional child with disabilities among their other children. The majority of the sons and daughters were male (83%) and lived in the parental home (86%). The characteristics of this sample (higher proportion of males than females; high proportion of other children with disabilities in the family) are consistent with the well-documented profile of families with children who have FXS.
Table 1.
Participant characteristics (n = 76)
| Mother | |
|---|---|
| Activation ratio (M, SD) | .58 (.20) |
| Range | .11–1.00 |
| Age in years (M, SD) | 51.25 (7.14) |
| Range | 37–70 |
| Caucasian (n,[%]) | 74 (97.4%) |
| Maternal education (n,[%]) | |
| High school grad | 9 (11.84%) |
| Some college | 22 (28.95%) |
| College graduate or beyond | 45 (59.21%) |
| Married (n, [%]) | 65 (85.53%) |
| Family income | |
| <40,0000 | 6 (7.89%) |
| $79,999–40,001 | 23 (30.26%) |
| $80,000–99,999 | 14 (18.42%) |
| ≥$100,000 | 32 (42.11%) |
| Missing | 1 (1.32%) |
| Number of children (M, SD) | 2.49 (1.04) |
| Range | 1–6 |
| Presence of additional child(ren) with disability (n,[%]) | 41 (53.95%) |
| FXS | 27 |
| Other | 14 |
| Adolescent/adult with FXS | |
| Age in years (M, SD) | 21.36 (7.23) |
| Range | 12–43 |
| Male (n, [%]) | 63 (82.9%) |
| Coreside with parents | 65 (85.5%) |
Measures
Activation ratio
Blood samples were analyzed by Kimball Genetics, Inc. Band intensities were measured by phosphorimaging, and those values were used in the following calculation: unmethylated normal/(unmethylated normal + methylated normal). In the present sample, activation ratio ranged from .11 to 1.00 (M = .58, SD = .20). Thus, some mothers had as few as 11% normal cells, while others had 100% normal cells, providing ample variability in this biomarker to test our hypotheses.
Cortisol
Saliva was collected at four points across four days (upon awakening, 30 minutes after getting out of bed, before lunch, and at bed time), resulting in 16 samples per mother, following the paradigm reported on previously by Almeida, McGonagle, and King (2009). The present analyses were based on only two of these time points: (a) level of cortisol at awakening and (b) level 30 minutes later. Our reasons for focusing on early-morning cortisol were twofold. First, examining the lagged (i.e., previous day) effect of behavior problems on next-morning maternal cortisol, as opposed to simultaneous effect (i.e., same day), allowed us to more confidently interpret the direction of the effects. This methodological strategy for understanding time-order effects was used in our previous study that similarly examined the effect of child-related stress on maternal cortisol levels in families of children with autism (Seltzer et al., 2010). Cortisol values later in the day (i.e., lunch and bedtime) are more likely to be influenced by same-day events. Thus, it would be difficult to assess the effect of prior-day events on next-day cortisol values at lunch and bedtime as these effects would be confounded with ongoing stress during that same day. Second, awakening cortisol levels, more so than cortisol assessed at other time points, are responsive to subtle changes in the HPA axis associated with environmental stressors and psychiatric conditions such as chronic fatigue syndrome, depression, and sleep disturbance (e.g., Wust et al., 2000). An altered awakening cortisol response has become accepted as the biological signature of many of these psychiatric conditions (e.g., Cleare, 2003).
Mothers received a home saliva collection kit 1 week prior to their initial phone call in the diary study. Saliva was collected using Sarstedt salivette collection devices. Sixteen numbered and color-coded vials were included in the collection kit, each containing a small absorbent wad, about three-quarters of an inch long. A detailed instruction sheet was also included in the collection kit. Data on the exact time mothers provided each saliva sample were obtained from the nightly telephone interviews and on a paper–pencil log sent with the collection kit. Mothers used prepaid courier packages for mailing back to the laboratory, where the salivary specimens were stored in an ultracold freezer at −60 °C. For analysis, they were thawed and centrifuged at 3,000 rpm for 5 minutes yielding a clear fluid with low viscosity. Cortisol concentrations were quantified with a commercially available luminescence immunoassay (IBL, Hamburg, Germany) by the laboratory of Dr. C. Kirschbaum in Dresden, Germany.
In prior research (as described in Seltzer et al., 2009), approximately 25% of the respondents received a “smart box” to store their salivettes. These boxes contained a computer chip that recorded the time respondents open and close the box. The correlations between self-reported times and the times obtained from the “smart box” ranged from .75 for the evening occasion to .95 for the morning, substantiating the reliability of the self-reported times of saliva collection.
In our multilevel models, we used the natural log of cortisol to adjust for the positively skewed distribution found in raw cortisol values. Fourmothers in the present sample had cortisol values higher than 60 nmol, which were considered to be extreme outliers and were recoded as 61 prior to log transformations, following the Winsorization statistical approach (Dixson & Yuen, 1974; Wainer, 1976).
Behavior problems
Our measure of daily behavior problems was based on the Scales of Independent Behavior—Revised (SIB-R; Bruininks, Woodcock, Weatherman, & Hill, 1996). The SIB-R assesses eight types of behavior problems: (a) self-injurious behavior, (b) unusual or repetitive behaviors, (c) withdrawn or inattentive behavior, (d) behavior that is hurtful to others, (e) property destruction, (f) disruptive behavior, (g) socially offensive behavior, and (h) uncooperative behavior. On each day of the 8-day diary study, mothers reported on the presence or absence of episodes of each type of behavior problem (coded as 1 if present and 0 if absent) during the previous 24 hours. The total number of types of behavior problems experienced during each day of the diary study was computed and used in the present analyses. This is a modification of the standard approach to administering the SIB-R, but this approach has been used in our other daily studies of behavior problems in adolescents with intellectual and developmental disabilities (Seltzer et al., 2010).
Control variables
We controlled for several characteristics of the mother and the adolescent or adult child with FXS in our multilevel models. Characteristics of the mother were age, education, marital status, presence of other children with FXS, and medication use. Saliva collection time (coded in hours) also was controlled as it has been shown to influence cortisol levels (Almeida, Piazza, & Stawski, 2009; Keenan et al., 2001). Maternal age was coded in years. Maternal education was coded: 1= less than high school, 2 = high school graduate, 3 = some college, and 4 = college graduate. Marital status was coded: 1 = married and 0 = not married. Presence of other children with a disability was coded: 1= additional children with a disability and 0 = no additional children with a disability. We included two variables to control for the effects of medication. The first variable indicated whether the mother took prescription antidepression and antianxiety medications during the diary study, coded: 1 = took at least one antidepressant or antianxiety medication and 0 = took no antidepressant or antianxiety medications during the diary study period. The second variable indicated whether the mother took any prescription nonpsychoactive medication, that is, allergy medication, steroids, or birth control/hormonal medication during the diary study period, coded: 1= took at least one of these medications, 0 = took none of these medications. Characteristics controlled for the son or daughter with FXS in the multilevel models were gender and residential status. Gender of the adolescent or adult child with FXS was coded: 1 = male and 0 = female. Residential status of the son or daughter with FXS was coded: 0 = resides in family home, 1 = resides outside of family home.
Data analysis plan
Following descriptive analyses, we examined the extent to which mothers’ activation ratio interacted with the experience of child behavior problems to predict cortisol levels on the following morning, both at awakening and 30 minutes later, using multilevel models with the Hierarchical Linear Modeling software (Raudenbush & Bryk, 2002). In the multilevel models, covariates included maternal education, age, and marital status, whether there was an additional child with a disability in the family, whether the mother was taking psychotropic (antidepression and antianxiety medications) and/or nonpsychotropic medications during the diary study period, whether the child lived with the parents, the child’s sex, and the saliva collection time. We first estimated the main effects of maternal activation ratio (Model 1) and child behavior problems manifested on the previous day (Model 2). Next, we estimated a model testing the interaction effect between activation ratio and behavior problems (Model 3) to predict cortisol at awakening and at 30 minutes after awakening.
Results
Behavior problems and cortisol
Table 2 presents descriptive data on the daily behavior problems manifested by the adolescent or adult child with FXS during the 8-day diary study. Unusual or repetitive behavior was the most frequently occurring behavior problem, followed by uncooperative behavior, which occurred on approximately one-third and one-quarter of diary days, respectively. Socially offensive behavior, withdrawn or inattentive behavior, and disruptive behavior were less frequent, evident on 12 to 14% of days. Behaviors involving hurting oneself, hurting others, or destroying property occurred much less frequently, on approximately 3 to 5% of days. On average, mothers reported at least one episode of behavior problems on 54% of the days in the diary study, with only 14.3% of mothers reporting that their son or daughter had no episode of behavior problems during the 8-day diary study. There were no significant correlations (ranging from r=−.02 to .10; p > .05) between mothers’ activation ratio and the frequency of any of the behavior problems or the overall number of behavior problems during the daily diary study.
Table 2.
Behavior problems during the 8-day diary study
| Mean (SD) | Behavior problem on at least one day |
|
|---|---|---|
| Unusual or repetitive behavior | .33 (.47) | 59.7% |
| Uncooperative behavior | .25 (.43) | 61.0% |
| Socially offensive behavior | .14 (.35) | 59.7% |
| Withdrawn or inattentive behavior | .13 (.34) | 40.3% |
| Disruptive behavior | .12 (.33) | 39.0% |
| Hurtful to self | .05 (.22) | 16.9% |
| Hurtful to others | .05 (.21) | 15.6% |
| Destructive to property | .03 (.18) | 14.3% |
| Any behavior problem | .54 (.50) | 85.7% |
Table 3 presents the mean, standard deviations, and range of maternal cortisol values at awakening and 30 minutes after awakening. There was not a significant correlation between mothers’ activation ratio and cortisol values at awakening (r=−.06, p = .32) or 30 minutes after awakening (r =.01, p = .94).
Table 3.
Mean, standard deviation, and range of cortisol values at awakening and 30 minutes after awakening
| Awakening | 30 min. after awakening | |
|---|---|---|
| Overall sample (M, SD) | 15.21 (8.76) | 20.81 (10.43) |
| Range | 0.13 to 61.00 | 0.17–61.00 |
Activation ratio (diathesis) × Behavior problems (stress) and cortisol
Table 4 presents the multilevel models in which the interaction of mothers’ activation ratio by child behavior problems was evaluated. The dependent variables were cortisol at awakening and at 30 minutes after awakening. Specifically, the effects of mothers’ activation ratio (Model 1), previous-day child behavior problems (Model 2), and the interaction between activation ratio and previous-day child behavior problems (Model 3) were used to predict cortisol levels at these two time points, and covariate controls were included in the models.
Table 4.
Multilevel models of activation ratio and daily behavior problems predicting cortisol levels
| Awakening |
30 minutes after awakening |
|||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Fixed effect | ||||||
| Intercept | 3.06 (0.34)** | 3.06 (0.34)** | 3.06 (0.33)** | 3.23 (0.37)** | 3.23 (0.37)** | 3.23 (0.37)** |
| Person-level predictors | ||||||
| Maternal education | −0.05 (0.03) | −0.05 (0.03) | −0.05 (0.03) | 0.003 (0.03) | 0.003 (0.03) | 0.003 (0.03) |
| Maternal age | −0.003 (0.007) | −0.003 (0.007) | −0.003 (0.007) | −0.01 (0.007) | −0.01 (0.007) | −0.01 (0.007) |
| Marital status (married = 1) | −0.11 (0.17) | −0.11 (0.17) | −0.09 (0.17) | 0.07 (0.15) | 0.07 (0.15) | 0.07 (0.15) |
| Additional child w/disability (yes = 1) | −0.22 (0.11)* | −0.22 (0.11)* | −0.21 (0.11)* | −0.23 (0.10)* | −0.23 (0.10)* | −0.23 (0.10)* |
| Nonpsychoactive medication (yes = 1) | 0.22 (0.11)* | 0.22 (0.11)* | 0.23 (0.11)* | 0.10 (0.10) | 0.10 (0.10) | 0.10 (0.10) |
| Antidepressant/antianxiety medication | −0.21 (0.13) | −0.21 (0.13) | −0.21 (0.13) | −0.18 (0.10) | −0.18 (0.10) | −0.18 (0.10) |
| Child residence (parents = 1) | −0.01 (0.17) | −0.01 (0.17) | −0.02 (0.17) | −0.13 (0.19) | −0.13 (0.19) | −0.13 (0.19) |
| Child’s sex (male = 1) | −0.21 (0.20) | −0.21 (0.21) | −0.21 (0.21) | −0.08 (0.15) | −0.08 (0.19) | −0.08 (0.19) |
| Activation ratio (AR) | −0.42 (0.29) | −0.42 (0.29) | −0.42 (0.33) | −0.23 (0.24) | −0.23 (0.37) | −0.23 (0.24) |
| Day-level predictors | ||||||
| Collection time | −0.009 (0.04) | −0.006 (0.04) | −0.004 (0.04) | −0.13 (0.03)** | −0.13 (0.03)** | −0.13 (0.03)** |
| Previous-day behavior problems | — | 0.02 (0.03) | 0.04 (0.02) | — | −0.01 (0.02) | −0.007 (0.02) |
| Cross-level interaction | ||||||
| AR × Previous-day behavior problems | — | — | 0.40 (0.11)** | — | — | 0.07 (0.11) |
| Random effect (variance) | ||||||
| Between-person intercept (Level 2) | 22.43 (d.f. = 64) χ2 = 410.41** |
22.40 (d.f. = 64) χ2 = 409.07** |
22.55 (d.f. = 64) χ2 = 423.22** |
17.08 (d.f. = 64) χ2 = 337.19** |
17.08 (d.f. = 64) χ2 = 335.98** |
17.06 (d.f. = 64) χ2 = 334.40** |
| Within-person (Level 1) | 12.95 | 12.98 | 12.54 | 13.59 | 13.64 | 13.71 |
Note.
p < .05;
p < .01.
Among the covariates, having additional children with disabilities was a significant predictor of cortisol. At both awakening and at 30 minutes after awakening, having additional children with disabilities in the family predicted lower levels of maternal cortisol. Taking allergy, steroids, or hormonal medications predicted higher awakening cortisol level, while the collection time of the saliva sample predicted lower cortisol level at the 30-minute point.
As shown in Model 1, a mother’s activation ratio did not predict her cortisol level as a main effect, either at awakening or 30 minutes later, and as shown in Model 2, the within-person main effect of the child’s previous-day behavior problems was not a significant predictor of maternal cortisol at either time point. However, in Model 3, as hypothesized, the interaction between mothers’ activation ratio and the number of previous-day behavior problems by the child was a significant predictor of cortisol at awakening. However, this interaction effect was not significant in predicting cortisol 30 minutes after awakening.
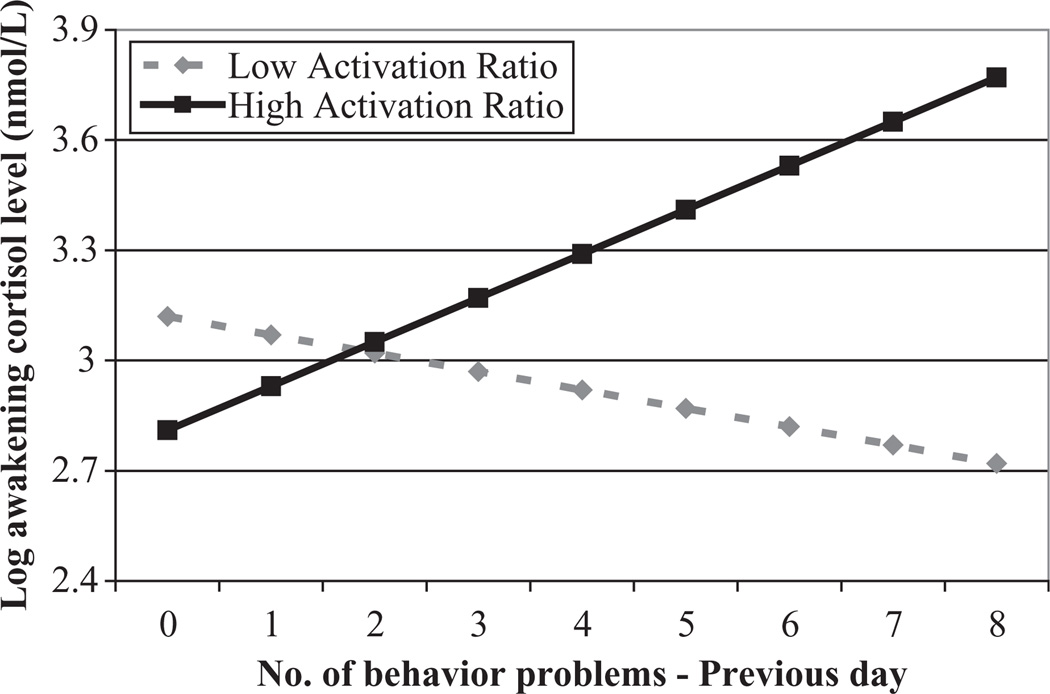
Figure 1 illustrates the significant interaction effect for awakening cortisol level. As shown in Figure 1, mothers with lower (graphed at 1 SD below the mean) and higher (graphed at 1 SD above the mean) activation ratios showed markedly different awakening cortisol values depending on the prior-day’s experience of child behavior problems. For mothers with low activation ratios, the greater the number of behavior problems on the previous day, the lower the morning cortisol level; whereas for mothers with a high activation ratio, the greater the number of behavior problems yesterday, the higher the morning level of cortisol, which is a more typical neuroendocrine response to environmental stress.
Figure 1.
Number of behavior problems by the son or daughter with FXS and log of awakening cortisol on the following morning for mothers with low (1 SD below the mean) and high (1 SD above the mean) activation ratios.
Discussion
Our study has confirmed that mothers of children with FXS are at elevated risk of experiencing high parenting stress, which concurs with prior reports of poor psychological and health outcomes as compared to normative samples (e.g., Bailey, Raspa, et al., 2008; Johnston et al., 2003) and to mothers of children with other types of disabilities (Abbeduto et al., 2004).We used a diathesis-stress model to examine the ways in which child-related challenges may interact with genetic vulnerability in mothers with the premutation of the FMR1 gene to predict maternal cortisol levels, a hormonal measure of stress. A daily diary methodology was used to track how the experience of behavior problems by the son or daughter with FXS impacted the mothers’ cortisol levels on the following morning.
We found that adolescents and adults with FXS exhibited high levels of behavior problems, with the large majority (85.7%) reported to have exhibited at least one episode of behavior problems during the 8-day diary study period. The prevalence of unusual or repetitive behaviors, uncooperative behaviors, and socially offensive behaviors is particularly concerning, with more than half the adolescents and adults with FXS reported to display these behaviors on at least one day. In addition, more than one-third of adolescents and adults with FXS were reported to manifest at least one episode of withdrawn or inattentive behavior or disruptive offensive behavior during the diary study. Given the high frequency and severe nature of these behavior problems, it is not surprising that past research has focused on behavior problems as a primary source of psychological stress in parents.
Our findings, however, indicate that the activation ratio of mothers with the premutation is an important biological vulnerability factor that influences the extent to which mothers are affected at the neuroendocrine level by the behavior problems of their adolescent or adult child with FXS. Previous research also has found a low activation ratio to confer greater vulnerability in women with the premutation (e.g., Hessl et al., 2005). Specifically, as hypothesized, mothers with lower activation ratios (i.e., who were more biochemically affected) evidenced a lower level of cortisol at awakening on days following more behavior problems than on days following behavior-problem-free days. In contrast, mothers with higher activation ratios had a higher level of cortisol at awakening on days following more behavior problems, which is the more typical hormonal reaction to environmental stress. The pattern of hypocortisolism following exposure to stress, which was characteristic of the mothers with lower activation ratios, has also been found in adults with PTSD and other stress-related disorders including fibromyalgia, adults experiencing work overload, and in children with histories of maltreatment (Dahlgren et al., 2004; Hart et al., 1995; Wingenfeld et al., 2008). In addition, a low morning cortisol level is the “biological signature” reported in the research literature to be associated with feelings of fatigue and exhaustion among people experiencing chronic stress (Cleare, 2003; Fries et al., 2005; Sonnenschein et al., 2007). These symptoms have also been reported in females with the FMR1 premutation, along with an increased prevalence of fibromyalgia and chronic pain (Coffey et al., 2008; Hagerman, 2002).
Furthermore, low morning cortisol (both at awakening and at the 30-minutes-after-awakening point) was characteristic of mothers in the present study who had more than one child with a disability. In our sample, 54% of the mothers had another child with a disability (including 36% with at least one additional child who had FXS and 18% with at least one child with another disability). This further evidence that chronic exposure to stressful parenting is associated with hypocortisolism is consistent with our previous research on mothers coping with adolescent and adult children with ASD (Seltzer et al., 2010). Notably, whether the adolescent or adult with FXS lived with the mother was not a predictor of the low cortisol pattern; 86% coresided, and the other mothers in the present sample had at least weekly contact with their son or daughter with FXS if he or she lived elsewhere, which may explain why residential status was not a significant predictor.
Why mothers’ cortisol levels at 30 minutes after awakening did not vary according to activation ratio or child behavior problems cannot be determined from the present analysis, and should be the subject of future research. Another limitation of the present study concerns the possible nonrepresentative nature of this sample of mothers. They were largely from the United States, Caucasian, well educated, had a high family income, and all volunteered to participate in a research study. Population-based studies indicate that FXS occurs across ethnic and racial groups, although the prevalence varies (Song, Barton, Sleightholme, Yao, & Fry-Smith, 2003). The extent to which findings from the present study generalize across these diverse groups is unknown. Furthermore, women with the FMR1 premutation in our study were ascertained via their full-mutation children. They might not be representative of women with the premutation who do not have children, or whose children do not have the full mutation of FXS.
There are also several strengths of the present study. We included a relatively large sample of mothers with the FMR1 premutation, and this study is the first to report on hormonal patterns in mothers with the premutation who have a child with FXS across multiple days. Our daily diary methodology allowed for a micro-level, repeated-measures examination of the time–order relation between events and experiences as they unfold on a daily basis in their natural and spontaneous context. The availability of data on medications taken during the diary study also minimized any potential confounds of medication use on the cortisol findings. The activation ratio of mothers with the premutation appears to be a useful biological marker of vulnerability. In future research, other markers (e.g., CGG repeat and methylation) should also be explored.
In summary, our findings indicate that a genetic vulnerability (measured by the activation ratio) interacts with the experience of life stress evoked by the son or daughter’s behavior problems to account for a differential influence on the physiology of mothers with the FMR1 premutation. Mothers with the premutation who have a lower activation ratio appear to be particularly vulnerable to the negative impact of behavior problems of their son or daughter, leading to a physiological response characteristic of chronic stress. Interventions focused on reducing and managing behavior problems are needed to lessen parental stress in these families. Moreover, services aimed at helping mothers learn more effective ways to cope with parenting stress are also important. Thus, our study revealed an example of a gene-by-environment interaction that has clinical significance and also determined a novel hormonal biomarker to assess the success of interventions and services.
Acknowledgments
The first two authors contributed equally to this research report. We are grateful to Kimball Genetics, Inc., which performed the activation ratio assays, and to the Kirschbaum Laboratory in Dresden, Germany, which performed the cortisol assays. We are also extremely appreciative of the families who participated in this study, without whom our research would not have been possible. We would like to thank the National Fragile X Foundation for providing informational materials to share with families. Requests for reprints should be sent to the corresponding author.
Funding
This study was supported by a grant from the National Institute of Child Health and Human Development to the Intellectual and Developmental Disability Research Center (IDDRC) at the University of North Carolina (P30 HD003100-S1) to support a Fragile X Research Center at three additional sites (Research Triangle Institute International, the University of Wisconsin-Madison, and University of Kansas). The present analysis was based on data collected by the UW-Madison Waisman Center site (M. M. Seltzer, principle investigator). We are also grateful for the support we received from the Waisman Center Core Grant (P30 HD03352, M. M. Seltzer, principal investigator).
References
- Abbeduto L, Seltzer MM, Shattuck PT, Krauss MK, Orsmond GI, Murphy MM. Psychological well-being and coping in mothers of youths with autism, Down syndrome, or fragile X syndrome. American Journal on Mental Retardation. 2004;9:237–254. doi: 10.1352/0895-8017(2004)109<237:PWACIM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Almeida D. Resilience and vulnerability to daily stressors assessed via diary methods. Current Directions in Psychological Science. 2005;14:64–68. [Google Scholar]
- Almeida DM, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009;55:220–238. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Piazza JR, Stawski RS. Inter-individual differences and intra-individual variability in the cortisol awakening response: An examination of age and gender. Psychology and Aging. 2009;24:819–827. doi: 10.1037/a0017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics Part A. 2008;146A:2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Sideris J, Roberts J, Hatton D. Child and genetic variables associated with maternal adaptation to fragile X syndrome: A multidimensional analysis. American Journal of Medical Genetics Part A. 2008;146A:720–729. doi: 10.1002/ajmg.a.32240. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Potanos K, Weinberg D, Zhou L, Goetz CG. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Annals of Neurology. 2005;57:144–147. doi: 10.1002/ana.20360. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder, exposure to combat and lower plasma cortisol among Vietnam veterans: Findings and clinical implications. Journal of Consulting and Clinical Psychology. 1996;64:191–201. doi: 10.1037//0022-006x.64.1.191. [DOI] [PubMed] [Google Scholar]
- Brown WT. The molecular biology of fragile X mutation. In: Hagerman R, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment, and research. 3rd ed. Baltimore, MD: John Hopkins University Press; 2002. pp. 110–135. [Google Scholar]
- Bruininks RH, Woodcock RW, Weatherman RF, Hill BK. Scales of Independent Behavior—Revised. Itasca, IL: Riverside; 1996. [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Poulton R. Role of genotype in the cycle of maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cleare AJ. The neuroendocrinology of chronic fatigue syndrome. Endocrine Reviews. 2003;24:236–252. doi: 10.1210/er.2002-0014. [DOI] [PubMed] [Google Scholar]
- Coffey SM, Cook K, Tartaglia N, Tassone F, Nguyen DV, Pan R, Hagerman RJ. Expanded clinical phenotype of women with the FMR1 premutation. American Journal of Medical Genetics Part A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish KJ, Turk J, Hagerman RJ. Annotation: The fragile X continuum: New advances and perspectives. Journal of Intellectual Disability Research. 2008;52:469–482. doi: 10.1111/j.1365-2788.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- Dahlgren A, Akerstedt T, Kecklund G. Individual differences in the diurnal cortisol response to stress. Chronobiology International. 2004;21:913–933. doi: 10.1081/cbi-200035937. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol response: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dixson WJ, Yuen KK. Trimming and winsorization: A review. Statistical Papers. 1974;15:157–170. [Google Scholar]
- Fowles DC. Schizophrenia: Diathesis-stress revisited. Annual Review of Psychology. 1992;43:303–336. doi: 10.1146/annurev.ps.43.020192.001511. [DOI] [PubMed] [Google Scholar]
- Franke P, Maier W, Hautzinger M, Weiffenbach O, Gänsicke M, Iwers B, Froster U. Fragile-X carrier females: Evidence for a distinct psychopathological phenotype? American Journal of Medical Genetics. 1996;64:334–339. doi: 10.1002/(SICI)1096-8628(19960809)64:2<334::AID-AJMG20>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Griep EN, Bersma JW, Lentjes EG, Prins AP, van derKorst JK, de Kloet ER. Function of the hypothalamic-pituitary-adrenal axis in patients with fibromyalgia and low back pain. Journal of Rheumatology. 1998;25:1374–1381. [PubMed] [Google Scholar]
- Gunnar MT, Vazquez DM. Low cortisol and a flattening of the expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Physical and behavioral phenotype. In: Hagerman RJ, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment, and research. Baltimore, MD: The Johns Hopkins University Press; 2002. pp. 3–109. [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Gunnar M, Cicchetti D. Salivary cortisol inmaltreated children: Evidence of relations between neuroendocrine activity and social competence. Developmental and Psychopathology. 1995;7:11–26. [Google Scholar]
- Hartley SL, Seltzer MM, Raspa M, Olmsted MG, Bishop EE, Bailey DB. Adult life for men and women with fragile X syndrome: Results from a national survey. American Journal of Intellectual and Developmental Disabilities. 2011;116:16–35. doi: 10.1352/1944-7558-116.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Hagerman PJ. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the Fragile X premutation. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2005;139B:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Johnston C, Eliez S, Dyer-Friedman J, Hessl D, Glaser B, Blasey C, Reiss A. Neurobehavioral phenotype in carriers of the fragile X premutation. American Journal of Medical Genetics. 2001;103:314–319. [PubMed] [Google Scholar]
- Johnston C, Hessl D, Blasey C, Eliez S, Erba H, Dyer-Friedman J, Reiss AL. Factors associated with parenting stress in mothers of children with fragile X syndrome. Journal of Developmental and Behavioral Pediatrics. 2003;24:267–275. doi: 10.1097/00004703-200308000-00008. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Licinio J, Veldhuis JD. A feedbackcontrolled ensemble model of the stress-responsive hypothalamopituitary- adrenal axis. Proceedings of the National Academy of Sciences. 2001;98:4028–4033. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McKeever VM, Huff ME. A diathesis-stress model of posttraumatic stress disorder: Ecological, biological, and residual stress pathways. Review of General Psychology. 2003;7:237–250. [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life-stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicine. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Thousand Oaks, CA: SAGE Publications; 2002. [Google Scholar]
- Roberts AD, Wessely S, Chalder T, Papadopoulos A, Cleare AJ. Salivary cortisol response to awakening in chronic fatigue syndrome. British Journal of Psychiatry. 2004;184:136–141. doi: 10.1192/bjp.184.2.136. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocrine Reviews. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Sarimski K. Behavioural phenotypes and family stress in three mental retardation syndromes. European Child and Adolescent Psychiatry. 1997;6:26–31. doi: 10.1007/BF00573637. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Abbeduto L, Greenberg JS, Almeida D, Hong J, Witt W. Biomarkers in the study of families of children with developmental disabilities. In: Glidden LM, Seltzer MM, editors. International review of research on mental retardation. Vol. 37. New York, NY: Academic Press; 2009. pp. 213–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Hong J, Smith LE, Almeida DM, Coe C, Stawski RS. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. Journal of Autism and Developmental Disorders. 2010;40:457–469. doi: 10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: Retrospect and prospect. Journals of Gerontology. 2005;60B:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A. Screening for fragile X syndrome: A literature review and modeling study. Health Technology Assessment. 2003;7:1–106. doi: 10.3310/hta7160. [DOI] [PubMed] [Google Scholar]
- Sonnenschein M, Mommersteeg PMC, Houtveen JH, Sorbi MJ, Schaufeli WB, van Doornen LJP. Exhaustion and endocrine functioning in clinical burnout: An in-depth study using the experience sampling method. Biological Psychology. 2007;75:176–184. doi: 10.1016/j.biopsycho.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Chamberlain WD, Hagerman PJ. Transcription of the FMRI gene in individuals with fragile X syndrome. American Journal of Medical Genetics. 2000;97:195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Van Lieshout CF, De Meyer RE, Leopold MG, Curfs LM, Fryns J-M. Family contexts, parental behavior, and personality profiles of children and adolescents with Prader-Willi, fragile-X, or Williams Syndrome. Journal of Child Psychology and Psychiatry. 1998;39:699–710. [PubMed] [Google Scholar]
- Von Gontard A, Backes M, Laufersweiler-Plass C, Wendland C, Lehmkuhl G, Zerres K, Rudnik-Schoneborn S. Psychopathology and familial stress: Comparison of boys with fragile X syndrome and spinal muscular atrophy. Journal of Child Psychology and Psychiatry. 2002;43:949–957. doi: 10.1111/1469-7610.00098. [DOI] [PubMed] [Google Scholar]
- Wainer H. Robust statistics: A survey and some prescriptions. Journal of Educational Statistics. 1976;1:285–312. [Google Scholar]
- Wingenfeld K, Heim C, Schmidt I, Wagner D, Meinlschmidt G, Hellhammer DH. HPA axis reactivity and lymphocyte glucocorticoid sensitivity in fibromyalgia. Psychosomatic Medicine. 2008;70:65–72. doi: 10.1097/PSY.0b013e31815ff3ce. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response: Normal values and confounds. Noise and Health. 2000;2:79–88. [PubMed] [Google Scholar]
- Yehuda R, Boisoneau D, Lowy MT, Giller EL. Doseresponse changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. A. rchives of General Psychiatry. 1995;52:583–593. doi: 10.1001/archpsyc.1995.03950190065010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. American Journal of Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]