Abstract
Galectins are characterized by their binding affinity for β-galactosides, a unique binding site sequence motif, and wide taxonomic distribution and structural conservation in vertebrates, invertebrates, protista, and fungi. Since their initial description, galectins were considered to bind endogenous (“self”) glycans and mediate developmental processes and cancer. In the past few years, however, numerous studies have described the diverse effects of galectins on cells involved in both innate and adaptive immune responses, and the mechanistic aspects of their regulatory roles in immune homeostasis. More recently, however, evidence has accumulated to suggest that galectins also bind exogenous (“non-self”) glycans on the surface of potentially pathogenic microbes, parasites, and fungi, suggesting that galectins can function as pattern recognition receptors (PRRs) in innate immunity. Thus, a perplexing paradox arises by the fact that galectins also recognize lactosamine-containing glycans on the host cell surface during developmental processes and regulation of immune responses. According to the currently accepted model for non-self recognition, PRRs recognize pathogens via highly conserved microbial surface molecules of wide distribution such as LPS or peptidoglycan (pathogen-associated molecular patterns; PAMPs), which are absent in the host. Hence, this would not apply to galectins, which apparently bind similar self/non-self molecular patterns on host and microbial cells. This paradox underscores first, an oversimplification in the use of the PRR/PAMP terminology. Second, and most importantly, it reveals significant gaps in our knowledge about the diversity of the host galectin repertoire, and the subcellular targeting, localization, and secretion. Furthermore, our knowledge about the structural and biophysical aspects of their interactions with the host and microbial carbohydrate moieties is fragmentary, and warrants further investigation.
Keywords: galectin, C-type lectin, microbial recognition, glycan ligands
INTRODUCTION
The functional interplay between lectins and their “self” or “non-self” carbohydrate receptors implicated in various aspects of immune responses of both vertebrates and invertebrates have been characterized in considerable detail in recent years (Akira et al., 2001; Liu and Rabinovich, 2005; Ludwig et al., 2006). It is now firmly established C-type lectins, ficolins, siglecs, and galectins are not only key players in innate immune processes that lead to pathogen recognition, endocytosis, complement activation, and antigen processing, but are also involved in adaptive immune functions, including B and T cell clonal selection, maturation, activation, and apoptosis. In addition, lectins also participate in other intracellular and extracellular biological processes such as glycoprotein trafficking, protein folding, cell–cell or cell–ECM interactions, signal transduction, fertilization, and development (reviewed in Vasta and Ahmed, 2009). The identification of strikingly diverse lectin repertoires in virtually every animal species, including the presence of multiple lectin families with numerous members and lectin isoforms, has enabled rationalization of their roles in immunity. Unlike immunoglobulins, however, lectins do not generate diversity in recognition by genetic recombination and therefore, considerable interest has arisen on the germline-encoded diversity of the lectin repertoires, the somatic mechanisms leading to expansion of their ligand recognition spectrum, and the structural/functional aspects their carbohydrate recognition domains (CRDs; Vilches and Parham, 2002; Garred et al., 2006).
It is now firmly established that in vertebrates innate immunity carries a substantial burden of the defense functions against infections disease, and in the past few years the instructive roles of innate immunity on adaptive immunity have been widely recognized. Furthermore, invertebrates and protochordates rely solely on innate immunity for defense against microbial infection. Thus, great interest has been generated in the structural-functional aspects of its various components, particularly on complement, lectins, and Toll-like receptors (Fujita et al., 2004; Khalturin et al., 2004; Iliev et al., 2005). Both the Drosophila Toll and the mammalian Toll-like receptors recognize pathogens via highly conserved and widely distributed microbial surface molecules such as lipopolysaccharide, flagellin, lipoteichoic acid, or peptidoglycan (“pathogen-associated molecular patterns”; PAMPs), which are essential for the microbe but absent in the host. By recognizing such non-self molecular patterns, these receptors were designated as “pattern recognition receptors” (PRRs; Medzhitov and Janeway, 2002). Given that non-pathogenic microbes also share these surface molecules it has been suggested that these may be more accurately described as “microbe-associated molecular patterns” (MAMPs; Bittel and Robatzek, 2007). More recently, the term “virulence-associated molecular pattern” (VAMP) has been introduced to describe those factors (e.g., microbial toxins, flagellin) that enable the host to discriminate pathogenic microbes from the non-pathogenic ones (Miao and Warren, 2010). Finally, endogenous factors such as nuclear or cytosolic components that are released during tissue stress or necrosis can trigger inflammatory responses have been designated as “danger-associated molecular patterns” (DAMPs; Seong and Matzinger, 2004).
THE MANNOSE-BINDING LECTIN AS A PROTOTYPICAL PATTERN RECOGNITION RECEPTOR
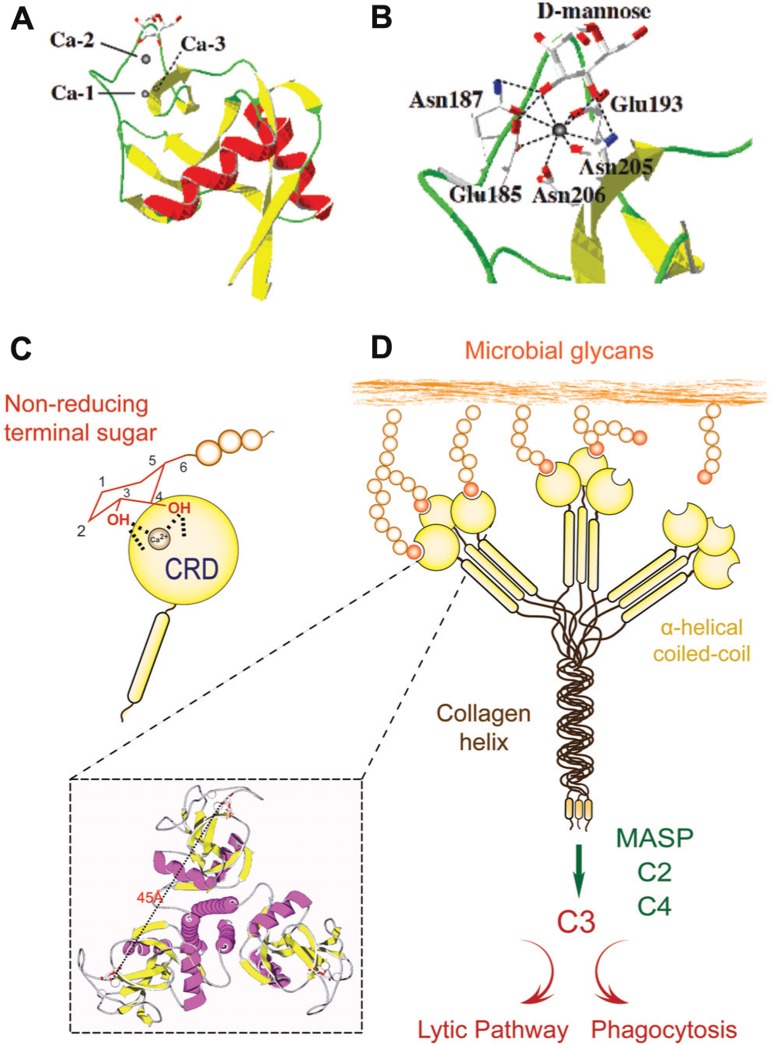
Since the PRR/PAMP paradigm was initially established for Toll and TLRs, it has been progressively extended to other innate immune recognition proteins. Among the best-characterized animal lectins, the mannose-binding lectin (MBL) a member of the C-type lectin family has been described as a prototypical PRR (Garred et al., 2006). C-type lectins are characterized by their Ca2+ requirement for ligand binding and their structural fold (C-type lectin domain fold, CTLD), and in most family members, the presence of multiple, unrelated structural domains in the polypeptide (Zelensky and Gready, 2005). They comprise the collectins (MBLs, ficolin, conglutinin, pulmonary surfactant), proteoglycan core proteins, selectins, endocytic receptors, the mannose-macrophage receptor, and DC-SIGN (Zelensky and Gready, 2005; Ip et al., 2009). Although some C-type lectins such as selectins and DC-SIGN bind “self” glycans, others such as collectins recognize exposed sugar ligands on the microbial surface. Collectins are lectins with a collagenous region linked to the CRD that recognizes sugars on microbial surfaces, and upon binding to a serine protease (MBL-associated serine proteases; MASPs) may activate the complement cascade (Weis et al., 1998; Wallis, 2002; Nonaka, 2011; Kingeter and Lin, 2012; Figure 1). Several lectins homologous of MBLs and ficolins, MASPs, and complement components have been identified in invertebrates and ectothermic vertebrates, suggesting that C-type lectins and the complement system played a pivotal role in innate immunity long before the emergence of adaptive immunity in vertebrates (Weis et al., 1998; Wallis, 2002; Nonaka, 2011). The CTLD fold has a double-loop structure with its N- and C-terminal β strands (β1, β5) coming close together to form an antiparallel β-sheet (Figure 1A). The second loop that lies within the domain is long and it enters and exits the core domain at the same location. Four cysteine residues (C1–C4), the most conserved residues in the CTLD, form disulfide bridges at the bases of the loops. The residues C1 and C4 link β5 and α1 (the whole domain loop), while C2 and C3 residues link β3 and β5 (the long loop region). The rest of the chain contains two flanking α helices (α1 and α2) and the second β-sheet, formed by strands β2, β3, and β4 (Weis et al., 1998; Feinberg et al., 2000; Liu and Eisenberg, 2002). The long loop region is involved in Ca2+-dependent carbohydrate binding, and in domain-swapping dimerization of some CTLDs. Four Ca2+-binding sites are present in the CTLD structures, of which only one (site 2) is known to participate in binding to the carbohydrate ligand (Loeb and Drickamer, 1988; Weis et al., 1991; Feinberg et al., 2000). Resolution of the structure of the rat MBP-A/Man6-GalNAc2-Asn complex revealed that a ternary complex is formed between the protein, the Ca2+ ion bound in site 2, and the terminal mannose moiety of the oligosaccharide (Weis et al., 1992). The complex is stabilized by a network of coordination and hydrogen bonds: oxygen atoms from 4- and 3-hydroxyls of the mannose form two coordination bonds with the Ca2+ ion and four hydrogen bonds with the carbonyl side chains that form the Ca2+-binding site 2 (Figure 1B). The amino acid residues flanking the conserved cis-proline in the long loop region, which are involved in Ca2+-binding site 2 formation, determine the specificity for either galactose or mannose (Figure 1C). In most mannose-binding CTLDs, the sequence of the motif is EPN (E185 and N185 in MBP-A), while in the galactose-specific CTLDs it is QPD. The oligomerization of the MBL subunits results in binding multivalency that enables the protein to recognize ligands that are displayed 45 Å apart on the microbial surface, thereby increasing the MBL’s avidity (Figure 1D). The density of the surface ligands and their scaffolding (as glycoproteins or glycolipids) modulates affinity of the interaction via negative cooperativity (Dam and Brewer, 2008). Thus, the binding of MBL to multiple non-reducing terminal carbohydrate ligands on the microbial surface, which are not readily exposed in the mammalian host, leads to agglutination and immobilization of the potential pathogen. Further, the interactions of other MBL domains with additional factors such as the MASP trigger downstream effector functions including complement activation and opsonization or lysis of the agglutinated microbes (Weis et al., 1998; Nonaka, 2011). In addition to C-type lectins, other lectins families have been identified as PRRs, including ficolins, F-lectins, pentraxins, and more recently, the galectins (reviewed in Vasta and Ahmed, 2009).
FIGURE 1.
Recognition and effector activities of the mannose-binding lectin (MBL). (A) A schematic representation of CTLD organization; (B) Crystallographic model of CTLD; (C) Ca2+-dependent carbohydrate binding of CTLD. The CRD recognizes equatorial hydroxyls on C3 and C4 of non-reducing terminal mannose with participation of the Ca2+ atom. (D) The MBL trimer binds to ligands that are displayed about 45 Å apart on the microbial surface, and via association with the MASP) may activate the complement cascade, leading to opsonization or lysis of the microbe.
GALECTINS: A STRUCTURALLY CONSERVED LECTIN FAMILY
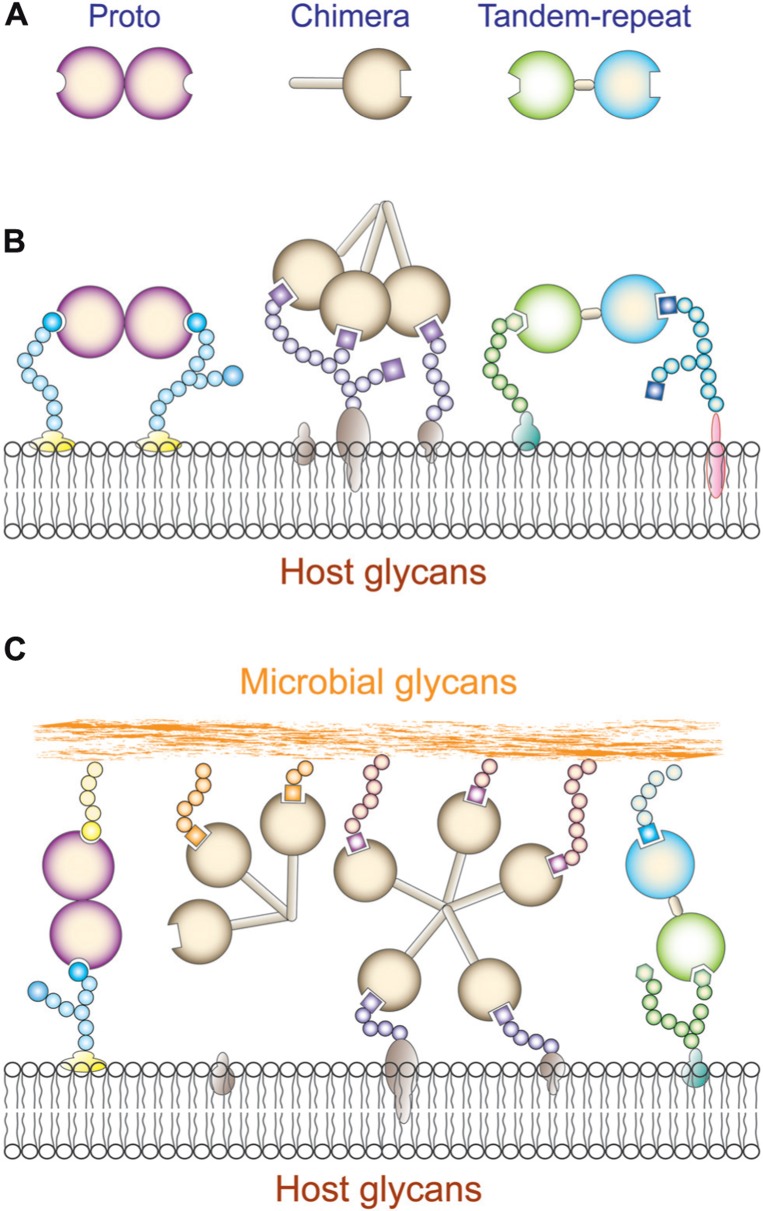
Galectins constitute a family of animal lectins defined by their affinity for β-galactosides, and a characteristic CRD sequence motif (Cooper, 2002). The galectin family members are widely distributed in eukaryotic taxa from fungi and sponges to both protostome and deuterostome lineages of metazoans and from the structural standpoint are strikingly conserved (Vasta et al., 1999; Cooper, 2002). Based on their domain organization, galectins have been classified in three types: “proto,” “chimera,” and “tandem-repeat” (Hirabayashi and Kasai, 1993; Figure 2A). Proto-type galectins contain one CRD per subunit and are non-covalently linked homodimers. The chimera galectins have a C-terminal CRD and an N-terminal domain rich in proline and glycine. In tandem-repeat (TR) galectins, two CRDs are joined by a functional linker peptide. Recently, a novel TR-type galectin with four CRDs has been described (Tasumi and Vasta, 2007). Proto and TR types comprise several distinct galectin subtypes, which have been numbered following the order of their discovery. At present time, 15 galectin subtypes have been identified in mammals. Galectin-1, -2, -5, -7, -10, -11, -13, -14, and -15 are proto type. Galectin-3 is the only chimera type. Galectin-4, -6, -8, -9, and -12 are TR type. In solution, galectins can form multivalent species in a concentration-dependent equilibrium (Morris et al., 2004). The association of proto-type galectin monomers as non-covalently bound dimers via a hydrophobic interphase is critical for their function in mediating cell–cell or cell–ECM interactions, lattice formation at the cell surface, and downstream effector functions (Rabinovich et al., 2002b). For the chimera-type galectins, oligomerization takes place via the N-terminus domain to form trimers or pentamers that in the presence of multivalent oligosaccharides in solution or at the cell surface display binding cooperativity (Brewer et al., 2002; Rabinovich et al., 2002b; Dam and Brewer, 2008). Proto-type galectins associate as non-covalently bound dimers via a hydrophobic interphase, while the bivalent TR-type galectins can recognize different saccharide ligands with a single polypeptide, although they can also form higher order aggregates that enhances their avidity (Liu et al., 2012; Troncoso et al., 2012). Most galectins are non-glycosylated soluble proteins, although a few exceptions have transmembrane domains (Gorski et al., 2002; Lipkowitz et al., 2004). Although galectins lack a typical secretion signal peptide, they are present not only in the cytosol and the nucleus, but also in the extracellular space (Cho and Cummings, 1995). From the cytosol, galectins may be targeted for secretion by non-classical mechanisms, possibly by direct translocation across the plasma membrane (Sato and Hughes, 1994; Cleves et al., 1996; Vyakarnam et al., 1997). In the extracellular space, galectins can bind to glycans at the cell surface and/or the extracellular matrix (Elola et al., 2007; Rabinovich and Toscano, 2009) and to potential pathogens (Mercier et al., 2008; Vasta, 2009). Galectins preferentially bind to N-acetyllactosamine (LacNAc; Galβ1,4GlcNAc) and related disaccharides, including lactose (Lac), T-disaccharide (Galβ1,3GalNAc), and human ABH blood group oligosaccharides. Thus, glycans that contain N-acetyllactosamine and polylactosamine chains [(Galβ1,4GlcNAc)n], such as laminin, fibronectin, lysosome-associated membrane proteins, and mucins, are the preferred endogenous glycans recognized by galectins (Fang et al., 1993; Seetharaman et al., 1998). The biological function of a particular galectin, however, may vary from site to site, depending on the availability of suitable ligands. The binding properties and biological functions of galectins in the oxidative extracellular environment, however, may depend on their immediate binding to ligand, which prevents the oxidation of free cysteine residues, as well as galectin susceptibility to proteolysis (Lobsanov et al., 1993; Liao et al., 1994). The binding of galectins to cell surface β-galactoside-containing glycolipids and glycoproteins can lead to the formation of lattices that cluster these ligands into lipid raft microdomains required for optimal transmission of signals relevant to cell function (Brewer et al., 2002; Partridge et al., 2004; Rabinovich et al., 2007b; (Figures 2B and C). Galectin-mediated lipid raft assembly may modulate turnover of endocytic receptors, signal transduction pathways leading to T cell activation and cytokine secretion, or apoptosis, B cell maturation, activation and tolerance, and neutrophil activation leading to phagocytosis, oxidative burst, and protease and cytokine release. Thus, galectin-glycoprotein lattices at the cell surface have been proposed to function as an “on-an-off switch” that regulates cell proliferation, differentiation and survival, including immune cell responsiveness and tolerance (Brewer et al., 2002; Dam and Brewer, 2008).
FIGURE 2.
Galectin recognition. (A) Schematic representation of galectin domain organization; (B) Schematic illustration of cis-interactions of proto, chimera, and tandem repeat galectins with host cell surface glycans. (C) Schematic illustration of trans-interactions of proto, chimera, and tandem repeat galectins with host cell surface and microbial glycans. Proto- and trandem repeat-type galectins can cross-link host and microbial glycans. Chimera galectins (galectin-3) can recognize microbial glycans but it is not clear that they can cross-link them to the host cell surface.
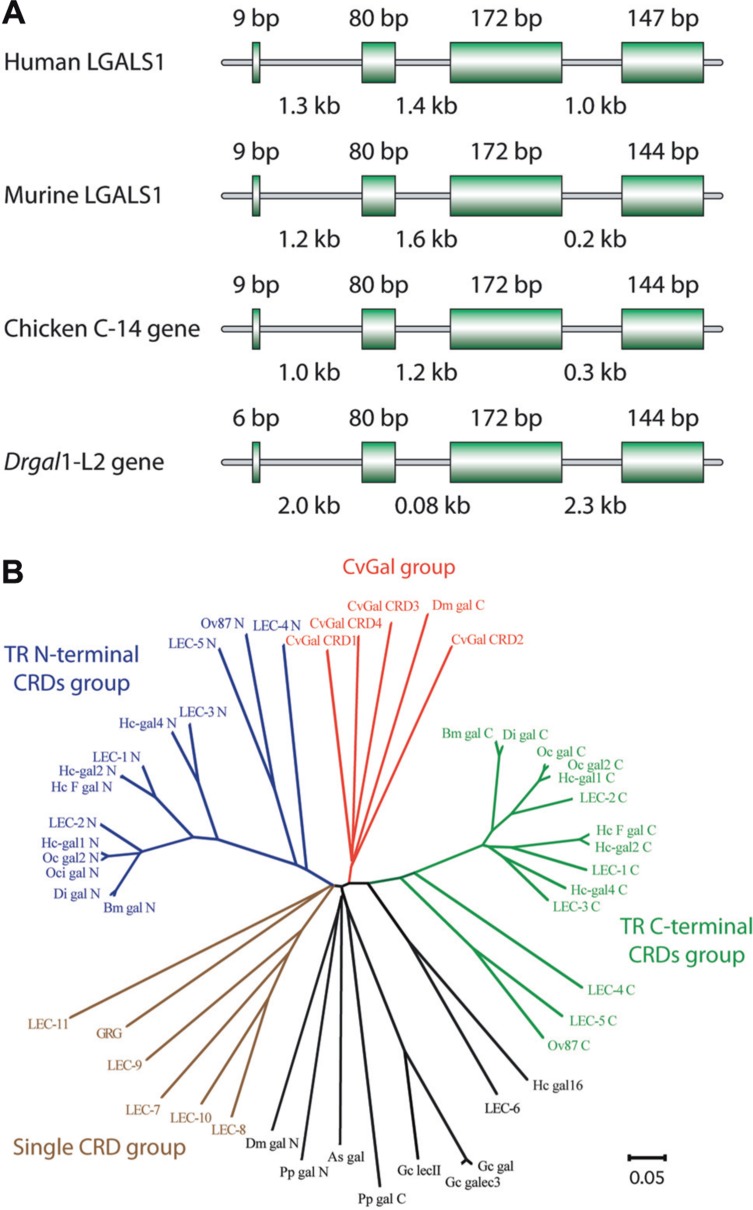
Among mammalian galectins, gene organization and primary structures of the encoded proteins are substantially conserved (Figure 3A). Prior to or during early in chordate evolution, duplication of a mono-CRD galectin gene would have led to a bi-CRD galectin gene, in which the N- and C-terminal CRDs subsequently diverged into two different subtypes, defined by exon–intron structure (F4-CRD and F3-CRD). All vertebrate single-CRD galectins belong to either the F3 (e.g., gal-1, -2, -3, -5) or F4 (e.g., gal-7, -10, -13, -14) subtype, whereas TR galectins such as gal-4, -6, -8, -9, and -12 contain both F4 and F3 subtypes (Houzelstein et al., 2004). However, phylogenetic analysis of the galectin (CvGal) from the eastern oyster Crassostrea virginica, that displays four tandemly arrayed CRDs, revealed that these are closely related to the single CRD galectins, suggesting that the CvGal gene is the product of two consecutive gene duplications of a single-CRD galectin gene (Tasumi and Vasta, 2007; Figure 3B). Like for C- and F-type lectins, most mammals are endowed with a complex galectin repertoire, including members that exhibit multiple isoforms and more or less subtle variations in carbohydrate specificity, which together with a certain degree of plasticity in sugar binding of each CRD, suggests a substantial diversity in recognition properties (Sparrow et al., 1987; Zhou and Cummings, 1990; Sato and Hughes, 1992; Fang et al., 1993; Ahmed et al., 2002; Shoji et al., 2003). In general, ectothermic vertebrates and invertebrates, and earlier taxa such as parazoa (sponges), fungi, and protista appear to have a smaller galectin repertoire. The presence of a galectin fold in the protistan parasite Toxoplasma gondii, and galectin-like proteins in the fungus Coprinopsis cinerea and in the sponge Geodia cydonium reveals the early emergence and structural conservation of galectins in eukaryotic evolution (Saouros et al., 2005; Walser et al., 2005; Stalz et al., 2006). Among the ectothermic vertebrates, proto-type galectins have been characterized in toads (Bufo arenarum), frogs (Rana catesbeiana, Xenopus laevis), salamanders (Ambystoma mexicanum), and zebrafish, among numerous other species (Ahmed et al., 1996, 2004; Vasta et al., 1997; Cooper, 2002; Shoji et al., 2003). The proto-type galectin from B. arenarum resembles the mammalian galectin-1 with respect to its carbohydrate-binding profiles and its carbohydrate-binding sequence motif (all nine residues responsible for carbohydrate binding are present: His45, Asn47, Arg49, His53, Asp55, Asn62, Trp69, Glu72, and Arg74; Ahmed et al., 1996). The structures of the B. arenarum galectin complexed with LacNAc or TDG further support this close similarity (Bianchet et al., 2000). Multiple proto-type galectins from X. laevis and zebrafish contain all the above nine amino acid residues for carbohydrate binding, and are likely to exhibit a binding profile similar to the mammalian galectin-1. Some divergent family members exhibit unique features, ranging from sequence replacements in the CRD to major structural differences. In a proto galectin from X. laevis, His52 and Arg73 (numbered as the bovine galectin-1) are replaced by Ser and Lys, respectively (Marschal et al., 1992; Shoji et al., 2003), leading to a distinct sugar-binding profile (Marschal et al., 1992; Ahmed and Vasta, 1994). In congerin I, a galectin from the conger eel Conger myriaster, one of the β strands is exchanged between the two subunits (Shirai et al., 1999, 2002). This “strand-swap” contributes to stabilize the dimer by increasing inter-subunit interactions, and perhaps explains the high thermostability of the protein. CGL2, a galectin from the fungus Coprinus cinereus forms a tetramer characterized by two perpendicular twofold axes of rotation, with the C-terminal amino acids of the four monomers meeting at the center of the tetramer interface (Walser et al., 2004). Furthermore, some galectin-like proteins such as the mammalian lens crystalline protein GRIFIN (galectin-related inter-fiber protein) and the galectin-related protein GRP (previously HSPC159; hematopoietic stem cell precursor) lack carbohydrate-binding activity, and are considered products of evolutionary co-option (Ogden et al., 1998; Ahmed and Vasta, 2008).
FIGURE 3.
Gene organization and phylogeny of galectins. (A) Genomic structures of galectin-1 or galectin-1 like protein from human, murine, chicken, and zebrafish (Danio rerio). Coding sequences (exons) are represented by boxes, with their sizes noted above each box. Intron sizes are shown below. (B) Phylogenetic analysis of invertebrate galectins modified based on Tasumi and Vasta (2007). The unrooted tree constructed by the N-J distance method is shown.
Resolution of the structure of galectin-1–LacNAc complex revealed a jellyroll topology typical of legume lectins, and enabled the identification of the amino acid residues and the hydroxyl groups of the ligands that participate in protein–carbohydrate interactions (Lobsanov et al., 1993; Liao et al., 1994; Bianchet et al., 2000). The subunit of galectin-1 is composed of an 11-strand antiparallel β-sandwich and contains a single CRD (Figure 4A). The carbohydrate binding site is formed by three continuous concave strands (β4–β6) containing all important residues such as histidine 44, asparagine 46, arginine 48, histidine 52, asparagine 61, tryptophan 68, glutamic acid 71, and arginine 730 that are involved in direct interactions with LacNAc (Liao et al., 1994; Figure 4B). Additional interactions of water molecules are involved in bridging the nitrogen of the NAc group with His52, Asp54, and Arg73 resulting in the increased affinity of LacNAc over Lac. Unlike galectin-1, galectin-3 has an extended carbohydrate-binding site formed by a cleft open at both ends, in which the LacNAc is positioned in such a way that the reducing end of the LacNAc (GlcNAc) is open to solvent, but the non-reducing Gal moiety is in close proximity to residues in the β3 strand (Seetharaman et al., 1998). The extended binding site leads to increased affinity for glycans with multiple lactosamine units, and with their substitution of the non-reducing terminal galactose moiety such as ABH blood group oligosaccharides [Fucα1, 2; GalNAcα1,3(Fucα1,2); and Galα1,3(Fucα1,2)]. Thermodynamic approaches have been used not only to assess the galectins’ carbohydrate-binding properties, but also the oligomeric organization of the protein. On microcalorimetric studies, the dissociation constants for the interactions of bovine galectin-1 with the preferred ligands (lactose, N-acetyllactosamine, thiodigalactoside) were in the range of 10-5 M, with two binding sites per molecule (Schwarz et al., 1998).
FIGURE 4.
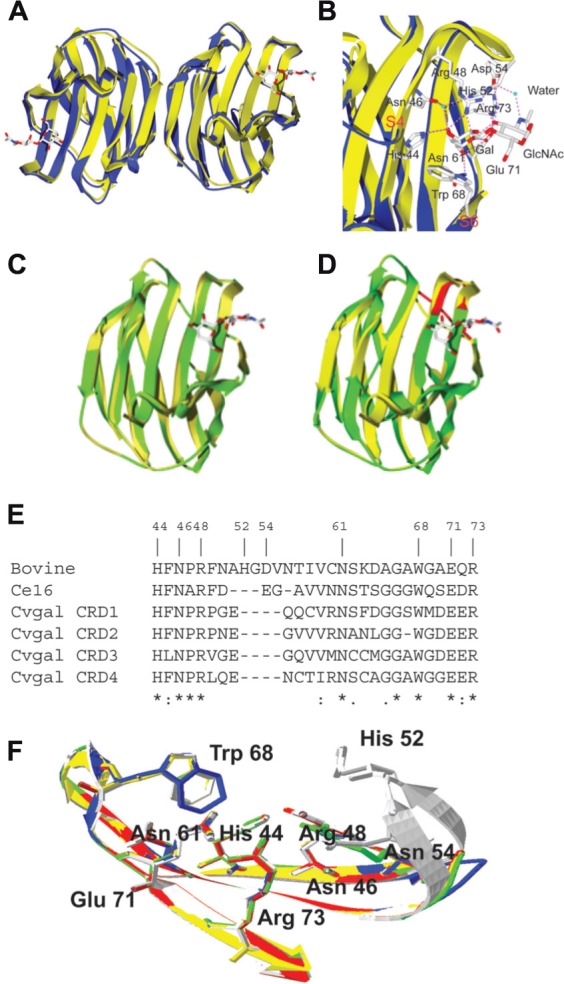
Structures of bovine galectin-1 and Bufo arenarum galectin-1 like protein. (A) The ribbon diagram shows the overlap of the toad (B. arenarum) galectin-1 like protein (blue, PDB 1GAN) and bovine (Bos taurus) galectin-1 (yellow, PDB 1slt) in complex with LacNAc (stick representation). (B) Carbohydrate-binding sites of B. arenarum galectin-1 like protein (blue) and bovine galectin-1 (yellow). The interactions of amino acid residues with LacNAc are shown for the bovine galectin-1. The OH at C4’ of Gal (in LacNAc) makes hydrogen bond interactions with the highly conserved residues His44, Asn46, and Arg48. The OH at C6’ makes similar interactions with the Asn61 and Glu71. Trp68 participates in a stacking interaction with the Gal ring carbons and restricts orientation of the OH at C4’ to the axial form. In GlcNAc moiety of the LacNAc, the hydrogen bond interactions are involved with the protein through the C3-OH with Agr48, Glu71, and Arg73. Additional interactions are involved via a water molecule that bridges the nitrogen of the NAc group with His52, Asp54, and Arg73. (C) Drgal1-L2 (green) was modeled at the SWISS-MODEL Protein Modeling Server (http://swissmodel.expasy.org) based on the bovine galectin-1 structure (yellow, PDB 1slt). All nine residues that form the carbohydrate-binding cassette in mammalian galectin-1 are present in the putative binding site of Drgal1-L2. All side chains of these residues were within 0.5 Å of the equivalent side chains of the bovine galectin-1. (D) C. elegans 16-kDa galectin (Lec-6; shown in green) was modeled at the SWISS-MODEL Protein Modeling Server (http://swissmodel.expasy.org) based on the bovine galectin-1 structure (shown in yellow, PDB 1slt). The model reveals that a shorter loop (indicated by arrow) between strands 4 and 5 is responsible for its unique binding profile. (E) Alignment of bovine galectin-1, Ce16 (C. elegans 16 kDa galectin), and CRD1 to -4 of CvGal. (F) Homology modeling of CvGal CRDs. Bovine galectin-1, CRD-1, -2, -3, and -4 are shown in white, blue, yellow, red, and green, respectively. Numbering of amino acid residues is based on bovine galectin-1.
The galectin-1 like protein (Drgal-L2) from zebrafish (Danio rerio) shows extensive sequence homology and structural similarity to vertebrate galectin-1 (Figure 4C). All nine residues that form the carbohydrate-binding cassette in the mammalian galectin-1 are present in the putative binding site of Drgal1-L2 (Ahmed et al., 2002). All side chains of these residues were within 0.5 Å of the equivalent side chains of the bovine galectin-1. Although, the model of the Caenorhabditis elegans 16-kDa galectin (Ce16) is similar to the bovine galectin-1 structure (Figure 4D), the carbohydrate specificity of the Ce16 is interesting compared to the bovine galectin-1 (Ahmed et al., 2002). In Ce16, amino acid substitutions at positions 46 and 48 (29 and 31 in bovine galectin) suggest that unlike in bovine galectin-1, these positions (strand 3) are involved in sugar binding. R46 (S29 in the bovine galectin sequence) interacts with 3′- and 4′-OH of the Gal residue. Interestingly, a shorter loop 2 (containing residues 66–69 between strands 4 and 5; Figure 4E) allows E67 to interact with equatorial -OH at C-3 of GlcNAc (in Galβ1,4GlcNAc) as well as axial -OH at C-4 of GalNAc (in Galβ1,3GalNAc). The model of the binding site of the C. elegans 16-kDa galectin also provides a rationale for the binding to α and β derivatives of Galβ1,3GalNAc (Ahmed et al., 2002). The sequence alignment of the oyster Crassostrea virginica galectin CvGal with the bovine galectin-1 revealed that among nine aa residues responsible for ligand binding, seven are conserved in all four CvGal CRDs (His44, Asn46, Arg48, Asn61, Trp68, Glu71, and Arg73; numbers corresponding to the bovine galectin-1; Tasumi and Vasta, 2007; Figure 4E). Homology modeling of all four CvGal CRDs revealed that the seven conserved residues from all four CvGal CRDs maintain their positions and orientations in the binding cleft, relative to the bovine galectin-1 (Figure 4F).
Although relatively conserved from a structural standpoint, galectins display a surprising functional diversification (Figure 5). Proto-type galectins such as galectin-1 have been reported as mediators of cell adhesion (Weis et al., 1998; Wallis, 2002), B cell differentiation (He and Baum, 2004; Martinez et al., 2004), development (Poirier, 2002), inflammation (Rubinstein et al., 2004), mRNA splicing (Patterson et al., 2004), leukocyte apoptosis (Perillo et al., 1998; Rabinovich et al., 2002a), neutrophil turnover (Dias-Baruffi et al., 2003), and cancer metastasis (Takenaka et al., 2004; Liu and Rabinovich, 2005). Furthermore, given the complexity of the mammalian galectin repertoire and the initial difficulties in identifying clear phenotypes in null mice for selected galectin family members, it was believed that a certain degree of functional redundancy exists among the members of the galectin repertoire. More recently, however, as the subtle aspects of their binding properties and natural ligands are identified and characterized, and their biological roles are elucidated in increasing detail, it has become clear that this is not the case (Rabinovich and Toscano, 2009). Soon after their discovery, galectins were characterized as developmentally regulated proteins, and proposed to participate in embryogenesis and early development. This was based on their binding to “self” carbohydrate moieties, such as polylactosamine-containing glycans, abundant at the cell surface and the ECM (Figure 5). Chicken galectins have been proposed to participate in myoblast fusion, whereas murine galectin-1 and galectin-3 have roles in notochord development, somitogenesis, and development of muscle tissue and central nervous system (Poirier et al., 1992; Colnot et al., 1996; Puche et al., 1996; Shoji et al., 2009).
FIGURE 5.
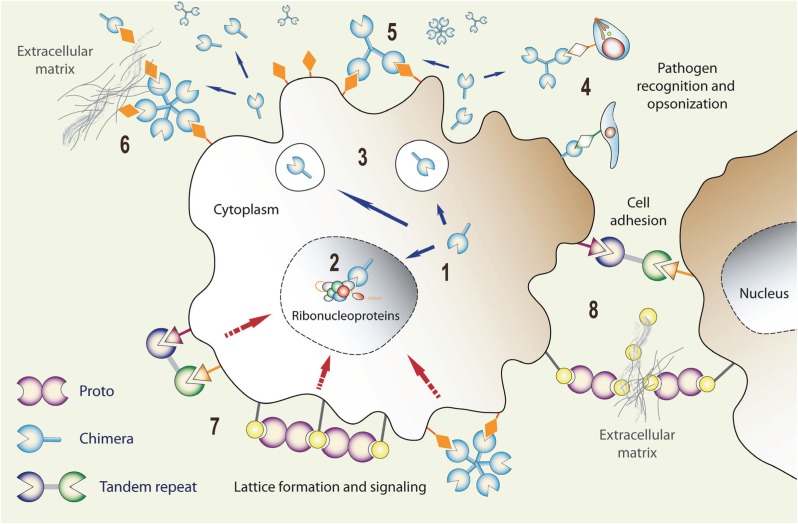
Expression, secretion, and functional diversification of galectins: (1) Galectin transcripts are translated in the cytoplasm, and the proteins can be translocated into the nucleus (2) where they can associate with ribonucleoproteins. Via unconventional mechanism(s), galectins can be secreted to the extracellular space (3) where they can function as pattern recognition receptors for microbial glycans (4), bind to the host cell surface glycans (5), and cross-link them with ECM glycans (6) thereby, for example, promoting cell migration. Galectins can also cross-link cell surface glycans and induce clustering of microdomains and lattice formation at the cell surface (7) that can trigger signaling cascades, or cross-link neighboring cells (8) and promote cell–cell interactions/adhesion.
Recent studies show that the gene encoding human galectin-2, a close relative of galectin-1, is mutated in patients with increased susceptibility to myocardial infarction, implicating galectin functions in vascular inflammation and atherosclerosis (Ozaki et al., 2004). Further, expression of galectin-3 at the onset of chondrification suggests its role in bone development, which was supported by finding of abnormalities of the hypertrophic zones of cells and a reduction of the total hypertrophic chondrocytes in galectin-3 null mice (Colnot et al., 2001).
GALECTINS AS REGULATORS OF IMMUNE HOMEOSTASIS
It is now firmly established that galectins participate in both innate and adaptive immune responses (Lund and Olafsen, 1999; Sato et al., 2003; Cartwright et al., 2004; Ohtsubo and Marth, 2006; Marth and Grewal, 2008; Mascanfroni et al., 2011). Galectins are ubiquitously expressed and distributed in mammalian tissues, including most cells of the innate (dendritic cells (DCs), macrophages, mast cells, natural killer cells, gamma/delta T cells, and B-1 cells) and adaptive (activated B and T cells) immune system, and as in other cell types (Li et al., 2011; Cedeno-Laurent and Dimitroff, 2012).
Since the early 1990s a growing body of experimental (in vivo and in vitro) evidence has accumulated to support the roles of galectins expressed by these cells and neighboring stromal cells in the development and regulation of innate and adaptive immune homeostasis as well as responses to infectious and allergic challenge, and cancer. Galectins released by stromal cells in central compartments contribute to the differentiation of immune cell precursors. Immune challenge and several pathological conditions may lead to further activation and differentiation of immune cells, and modulate the expression and release of galectins to the extracellular space where they may have autocrine or paracrine effects on immune regulation. Galectins released by immune cells can oligomerize and form lattices at the cell surface leading to activation of transmembrane signaling pathways that modulate immune cell functions, including for example, cell adhesion and migration, T cell apoptosis, and the Th1/Th2 cytokine balance (Rabinovich and Toscano, 2009). Further, galectins released into the extracellular environment under abnormal situations may themselves constitute “danger signals,” or by exerting their activities on other cells, such as mast cells, induce degranulation and release of factors (e.g., histamine) that represent the “danger signals” leading to activation of immune mechanisms in the absence of antigenic challenge (Sato and Nieminen, 2004).
Galectins have diverse effects on cells involved in innate immune responses (Rabinovich et al., 2007a; Di Lella et al., 2011) including macrophages and DCs, neutrophils, eosinophils, and mast cells. Galectin-1 participates in acute and allergic inflammation and displays anti-inflammatory activities by blocking or attenuating signaling events that lead to leukocyte infiltration, migration, and recruitment (Guevremont et al., 2004). It also displays various other effects on innate immunity, including cell surface exposure of phosphatidylserine in activated neutrophils, a process that leads to neutrophil removal by phagocytic cells without causing apoptosis, and activation/deactivation of macrophages on a concentration-dependent manner. In contrast to the anti-inflammatory effects of galectin-1, galectin-3 shows pro-inflammatory activity. Galectin-3 is normally expressed in various epithelia and inflammatory cells, such as activated macrophages, DCs, and Kupffer cells, and is upregulated during inflammation, cell proliferation, and cell differentiation. Galectin-3 also exhibits anti-apoptotic activity for macrophages and enhances their interactions with basal lamina glycans, such as laminin and fibronectin. Taken together, these observations strongly suggest that galectin-3 enhances macrophage survival, and positively modulates their recruitment and anti-microbial activity. Galectin-9 is a selective chemoattractant for eosinophils, highly expressed in various tissues of the immune system, such as bone marrow, spleen, thymus, and lymph nodes. Gal-9 released from activated T cells induces chemotaxis, activation, oxidative activity, and degranulation of eosinophils, and monocyte-derived DC maturation (Partridge et al., 2004; Ohtsubo et al., 2005; Rabinovich et al., 2007b; Stowell et al., 2008). Galectin-9 also promotes tissue inflammation through interaction with TIM-3 on macrophages (Anderson et al., 2007; Di Lella et al., 2011).
Concerning adaptive immune responses galectins function as regulators of immune cell homeostasis (Cartwright et al., 2004; Rabinovich et al., 2007a; Ilarregui et al., 2009; Di Lella et al., 2011). Interactions between stromal cells from the bone marrow and thymic compartments and lymphocyte precursors are critical to their development, selection, and further progression to the periphery. In this regard, interactions mediated by galectins can modulate B cell maturation and differentiation both at the central and peripheral immune compartments (Rabinovich et al., 2007a). Similarly, from their early developmental stages in the thymic compartment to the removal of the mature activated T cells in the periphery, the regulation of T cell survival is critical to a controlled immune response. Galectin-1 can regulate T cell proliferation and apoptosis through binding and clustering of lactosamine-rich cell surface glycoconjugates into segregated membrane microdomains (Rabinovich and Toscano, 2009). Galectin-1 may have pro- or anti-apoptotic effects on T cells depending on the developmental stage and activation status of the cell, and the microenvironment in which the exposure takes place. An immunoregulatory circuit involving galectin-1, DCs, and T cells has recently been described by Rabinovich and his associates (Ilarregui et al., 2009). Upon exposure to galectin-1, DCs acquired an IL-27-dependent regulatory function and promoted IL-10-mediated T cell tolerance, Th1, and Th17 responses, and suppressed autoimmune neuroinflammation (Ilarregui et al., 2009). Effects of galectin-3 in T cell survival are dependent on whether protein is produced endogenously (anti-apoptotic) or by exogenous exposure (pro-apoptotic; Shoji et al., 2003). Several studies suggest that intracellular galectin-3 confers resistance to apoptosis in response to chemotherapeutic drugs (Fukumori et al., 2007). In contrast, extracellular galectin-3 has been shown to induce T cell apoptosis through caspase-3 activation (Fukumori et al., 2003). Galectin-3 can also modulate T cell activation. Recent studies demonstrated that endogenous galectin-3 can directly control T cell activation at sites of immunological synapse (Chen et al., 2009).
Galectins also exert regulatory functions in T cell homeostasis, and signaling cascades triggered by their binding and lattice formation at the T cell surface has implications in a variety of downstream events that modulate their differentiation, functional activation, and production of pro- and anti-inflammatory cytokines. The effects of galectins on T cell cytokine synthesis and secretion ultimately determine the Th1/Th2 polarization of the immune response. By reducing IFN-γ and IL-2 and enhancing IL-5, IL-10, and TGF-β production, galectin-1 skews the balance from a Th1- toward a Th2-polarized response, whereas by reducing IL-5 levels, galectin-3 drives have the opposite effect (Liu and Hsu, 2007). Finally, given the regulatory roles of galectins on cells that mediate both innate and adaptive immune responses, their effects can be beneficial or detrimental to pathological conditions that have a basis on exacerbated or depressed immune function, such as inflammatory, allergic, and autoimmune disorders, and cancer (Liu and Hsu, 2007).
GALECTINS AS PATTERN RECOGNITION RECEPTORS
Insight into the multiple roles of galectins in both innate and adaptive immune functions has further expanded recently by discovery of their ability to directly recognize microbial pathogens (Lund and Olafsen, 1999), a property shared with other lectin types, such as C- and F-lectins, ficolins, and pentraxins. The roles of lectins in recognition of microbial glycans are particularly critical in invertebrates, since these organisms lack immunoglobulins and rely solely in innate immune mechanisms for recognition of potential microbial pathogens (Gerwick et al., 2007). This seems to be the case even in vertebrate taxa, since it has been reported that susceptibility/resistance to several infectious diseases in humans are determined by the presence of certain lectin alleles (Fuller et al., 2004). As discussed above, recognition of “non-self” carbohydrate moieties on the surface of microbial pathogens and parasites by lectins such as the MBL can be rationalized under the PRR/PAMP concept. In contrast, for those lectins that recognize endogenous glycans such as galectins, understanding recognition of microbial glycans requires additional considerations. In some cases, the ligands on foreign cells can be similar to those displayed on host cells such as ABH or Le blood group oligosaccharides (Cartwright et al., 2004; Stowell et al., 2010) and LacNAc present in viral and bacterial glycans, or structurally different and absent from the host glycome, such as α1-2-mannans in Candida (Cook et al., 2005) and LacdiNAc in Schistosoma (Cummings and Nyame, 1999). While the first scenario can be conceptualized as molecular mimicry by the microbial pathogens (Vasta, 2009), understanding the molecular basis of galectin binding to distinct self and non-self glycans via the same CRD requires further discussion. In this regard, galectins with tandemly arrayed CRDs such as the TR galectins from vertebrates, and the 4-CRD galectins from invertebrates are intriguing both in their binding properties and functional aspects. Vertebrate TR galectins such as galectin-4, -8, and -9 differ from the proto and chimera types in that they display two tandemly arrayed CRDs, and like the binary CRD F-type lectins, the N- and C-CRDs of TR galectins are similar but not identical, suggesting that they have distinct recognition properties (Carlsson et al., 2007). The structures of the TR galectin-4, -8, and -9 have been partially resolved either by crystallization of NMR analysis of their isolated N- or C-CRDs and revealed differences in their binding specificity and/or affinity for oligosaccharides or their scaffolding as glycolipids or glycoproteins (Tomizawa et al., 2005; Nagae et al., 2009; Krejcirikova et al., 2011). The structure of the N-CRD of the mouse galectin-4 revealed binding sites for lactose with different affinities, while the galectin-8 binds preferentially to larger glycans such as glycosphingolipids (Tomizawa et al., 2005; Nagae et al., 2009; Krejcirikova et al., 2011). The capacity of TR galectins to cross-link cells with different synthetic glycoconjugates (Tomizawa et al., 2005; Ideo et al., 2011) strongly suggests significant differences in the binding properties of their N- and C-CRDs. From the functional standpoint, the most striking example is the recognition and killing of Escherichia coli O86 that display B-blood group oligosaccharides (BGB+ E. coli) by the TR galectin-4 and -8. Mutation of key residues in either CRD revealed that the C-CRD mediates recognition the BGB+ E. coli but does not affect its viability, while the N-CRD was not affected, suggesting that N-CRD might be endowed with killing activity. This was confirmed by examining the binding and killing activity of the separate CRDs. This observation suggests that in galectin-4 and -8 the N- and C-CRDs not only have different recognition properties, but also they are functionally different in their interactions with the BGB+ E. coli (Stowell et al., 2009).
A different scenario is presented by the recognition of glycans on the phagocyte (self) and parasite (non-self) surface by the oyster galectin CvGal (Tasumi and Vasta, 2007). Although the four CRDs of CvGal are not identical, homology modeling suggests that the minor differences in sequence among the CRDs do not result in significant differences in binding properties. Therefore, the cross-linking of similar glycans on the host phagocytic cells and on the surface of the parasite trophozoite by CRDs of similar binding properties can only be rationalized by the cross-linking of similar self and non-self glycans by the folding of the 4-CRD polypeptide in a particular geometry, such as a tetrahedral architecture, where the CRDs are oriented in opposite directions. A similar model could be proposed by single-CRD proto type and chimera galectins for which the requisite multivalency and the spatial arrangement and orientation of the CRDs is achieved by oligomerization of the single-CRD subunits. However, questions about the molecular and structural basis for recognition and binding to either similar or distinct self or non-self glycans remain open, and several aspects concerning the protein, the carbohydrate ligands, the thermodynamics of lectin–ligand interaction, and the particular microenvironment in which this interaction takes place merit further discussion. With regards to the protein, recognition specificity and affinity are key aspects of the lectin–ligand interaction(s) that modulate downstream effector functions.
In addition to the structural aspects of the primary and extended carbohydrate binding sites by any CRD in monovalent recognition, multiple factors contribute to the energetics of the interaction, which are further complicated when addressing multivalent recognition (Ideo et al., 2011). Although as discussed above for C-, and F-lectins and TR galectins multivalency is a property of tandemly arrayed CRDs in the lectin polypeptide subunit, oligomerization of the monomers results in displays of clustered binding sites that either in their geometry or dimensions can be unique to a particular lectin. For example, the “bouquet”-like display of binding sites in the MBL trimer results in multivalency for sugars spaced about 45 Å apart, and increases avidity of the binding (Rapoport et al., 2008). Although a similar CRD display is presented in F-type lectins, the geometry of the ligand display requires sugars spaced 26 Å apart (Bianchet et al., 2002, 2010). The tissue localization and subcellular compartmentalization of lectins can influence their oligomerization and in turn, influence the selective binding of lectins to particular ligands. For galectins, oligomerization and clustering of CRDs enables binding to and cross-linking of multivalent glycoproteins and glycolipids on the cell surface leading to formation of microdomains and lattices that signal vial specific pathways depending on the glycans and galectins involved (Greenspan, 2001; Rabinovich et al., 2007b; Vokhmyanina et al., 2011). In addition, the redox properties of the intracellular and extracellular environments can modulate the activity of lectin–ligand interactions, and the biological outcome. Most galectins are active in the reducing environment, but are susceptible to inactivation by oxidation of their free Cys residues present in the CRD (Stowell et al., 2009). In the reducing intracellular environment, galectins remain stable, but upon secretion to the mostly oxidative extracellular space, the oxidation of the free cysteines compromises not only the binding activity but also the oligomerization of the galectin subunits (Stowell et al., 2009). It is noteworthy that the binding of galectin-9 to the protein disulfide isomerase at the cell surface, increases retention of the enzyme which in turn modulates the redox status at the plasma membrane (Rabinovich et al., 2007b). In addition to the lectin quaternary structure as established via structural or hydrodynamic approaches, higher orders of aggregation may occur in environments where the lectin concentration reaches above a certain threshold. The galactosyl-binding lectins DCL-I and DCL-II from the protochordate Didemnum candidum behave as homotetramers of 14.5 and 15.5 kDa subunits, respectively, as revealed by sedimentation velocity and sedimentation equilibrium studies. However, even at concentrations below 1 mg/ml, a positive dependence of the sedimentation coefficient with protein concentration was observed, suggesting that at increasing concentrations the protein associates at orders above the tetramer (Vasta et al., 1986). For the dimeric galectin-1, further association to form tetramers is temperature driven (Dam et al., 2009). Furthermore, the ligand density at the cell surface can drive oligomerization of the lectins subunits. In solution, galectin-3 monomers are in equilibrium with higher order oligomers, and when binding to multivalent glycoproteins or cell surface glycans they may recruit additional monomers to form a complex of multivalent interactions via galectin-3 trimers and pentamers (Ahmad et al., 2004). The density of the carbohydrate ligands and their protein or lipid scaffolding context on the cell surface are key factors that determine lectin recognition and affinity. As proposed by Dam et al. (2009), in contrast to the well-defined dissociation constants that describe the binding of lectin monomers to monovalent glycans, a wide range of relative dissociation constants is needed to describe lectin binding to multivalent surface glycans, that depends on the density and number of glycans on a surface and increased negative cooperativity. The authors propose that this relative affinity should replace the term avidity (Brewer et al., 2002). Galectin binding to ligand is enthalpically driven, exhibits enthalpy-entropy compensation, and follows a van’t Hoff dependence of the binding constant on temperature, properties that are shared by other lectins. Further, the solvation properties of the CRD, the reorganization or displacement of the water shell, and the establishment of defined water-mediated interactions between the protein and the carbohydrate are factors that affect the thermodynamic aspects of the recognition process and stability of the lectin–ligand interaction (Di Lella et al., 2007; Dam and Brewer, 2010; Echeverria and Amzel, 2011). As discussed above, many functional properties of lectins such as the triggering of signaling cascades result from clustered CRDs recognizing multivalent carbohydrate moieties in different scaffolds exposed at the cell surface, followed by the formation of microdomains and lattices (Rabinovich et al., 2007b). Therefore, a detailed knowledge of the thermodynamic aspects of lectin-mediated cross-linking of complex glycans is critical for the understanding of lectin-mediated selective recognition of self and non-self ligands. In this regard, recent studies have shown that high-affinity lectin–mucin interactions are driven by favorable binding entropy of binding associated with a “bind and jump” mechanism. This consists of a dynamic binding process in which the bound lectins jump from carbohydrate to carbohydrate moiety in the multivalent glycans, which by enhancing entropic effects facilitates binding and subsequent complex formation (Dam et al., 2009). The factors discussed above suggest that in the natural context, multiple factors that concern the protein binding site and lectin oligomerization, the display geometry and density of the recognized carbohydrate moieties in a particular protein or lipid scaffold, and the particular properties of the environment where the lectin–sugar interaction takes place, may determine not only the binding dynamics and affinity, but also the lectin’s preference for any one of the (self or non-self) ligands available.
GALECTINS AS RECEPTORS FOR MICROBIAL ADHESION AND INFECTION
Whether galectin-mediated recognition is an effective defence mechanism with a clear benefit for the host is not entirely clear, except for a few examples discussed above (Cartwright et al., 2004; Cook et al., 2005; Kohatsu et al., 2006; Stowell et al., 2010). In some cases, however, the microbe’s recognition by the vector or host galectins promotes its adhesion, host cell entry, or infection persistence, in addition to modulating the host’s immune responses. Thus, these pathogens and parasites would “subvert” the roles of host or vector galectins as PRRs, to attach to or gain entry into their cells. This is clearly illustrated by the participation of galectin interactions in the infection mechanisms of HIV. In contrast to the inhibitory role of galectin-1 in paramixovirus-mediated cell fusion, galectin-1, which is abundant in organs that represent major reservoirs for HIV-1, such as the thymus and lymph nodes, promotes infection by HIV-1 by facilitating viral attachment to CD4 receptor, and increasing infection efficiency (Ouellet et al., 2005; Mercier et al., 2008). Recent studies showed that galectin-1 enhances HIV adsorption kinetics on monocyte-derived host macrophages, which facilitates HIV-1 infectivity by shortening the time required to establish an infection. Further, galectin-1 would also function as a soluble scavenger receptor and enhance the uptake of the virus by macrophages, which together with evidence that galectin-1 is present in the ejaculate and the heads and tails of late spermatids, led to extend the proposal that galectin-1 may also facilitate sexual transmission of HIV-1 (Mercier et al., 2008). This would take place through enhancement of viral adsorption kinetics on the target cells’ surface by the galectin-1 released by sheared fibroblasts and epithelial cells following sex-related microabrasions. Gal-3 has no effect on HIV-1 adsorption, entry, or infection, although its expression is upregulated by the HIV Tat protein in several human cell lines, and in cells infected with other retroviruses, suggesting that it may participate in regulation of antiviral immunity (Schroder et al., 1995; Hsu et al., 1996; Fogel et al., 1999). This underscores the relevance of the subtle differences in galectin specificity and affinity that may determine very different recognition and effector outcomes. It is noteworthy that HIV also uses recognition by DC-SIGN, a C-type lectin, to enter DCs, thereby underscoring the multiple adaptations of the viral glycome for host infection (Hijazi et al., 2011; van der Vlist et al., 2011).
Several Leishmania species, which spend part of their life cycle in phlebotomine sandflies that constitute vectors for transmission to the vertebrate hosts, are also illustrative examples. Upon the sandfly feeding on blood from an infected host, the ingested amastigotes mature into promastigotes, which attach to the insect midgut epithelium to prevent their excretion along with the digested bloodmeal, and undergo numerous divisions before differentiating into free-swimming infective metacyclics (Volf et al., 2007). Although the involvement of the parasite LPG in this interaction had been suspected from prior studies, the specific Phlebotomus papatasi sandfly midgut receptor for the procyclic L. major LPG was identified as a 35.4-kDa TR galectin (PpGalec) only expressed by epithelial midgut cells, and upregulated in the blood-feeding females (Myskova et al., 2007). Because the binding specificity of PpGalec is restricted to Leishmania promastigotes bearing poly-Gal (β1–3) side chains on their LPG, it was proposed that it is the carbohydrate moiety responsible for specific binding of L. major to P. papatasi midgut linings. The assembly of polygalactose epitopes is downregulated during L. major metacyclogenesis, and thus, unable to bind to rPpGalec the free-swimming infective metacyclic promastigotes are released from the midgut for transmission from the sandfly to the mammalian host (Myskova et al., 2007).
The protozoan parasite Perkinsus marinus is a facultative intracellular parasite that causes “Dermo” disease in the eastern oyster Crassostrea virginica, and is responsible for catastrophic damage to shellfisheries and the estuarine environment in North America (Perkins, 1996). The infection mechanism remains unclear, but it is likely that while filter feeding, the healthy oysters ingest P. marinus trophozoites released to the water column by the infected neighboring individuals. Inside oyster phagocytic cells (hemocytes), trophozoites resist oxidative killing, proliferate, and spread throughout the host. It was recently discovered that oyster hemocytes recognize P. marinus via a novel galectin (CvGal) that displays four canonical galectin CRDs, a domain organization unlike any of the known galectin types (Tasumi and Vasta, 2007). Two amino acid residues (His53 and Asp55) that interact with the NAc group via a water molecule are missing in all four CvGal CRDs resulting in broader carbohydrate specificity. CvGal is present in the cytoplasm of circulating granulocytes, and upon their attachment and spreading it is translocated to the periphery, secreted, and binds to the cell surface. The remaining galectin is released to the extracellular environment, where it may bind to all other circulating (non-activated) granulocytes and hyalinocytes. The most surprising observation, however, was that the soluble CvGal also binds in a carbohydrate-specific manner to a wide variety of microorganisms, phytoplankton components, and preferentially, to Perkinsus spp. trophozoites, suggesting a direct role in recognition and opsonization of potential microbial pathogens, as well as algal food. The partial inhibition of phagocytosis of P. marinus trophozoites by pre-treatment of hemocytes with anti-CvGal revealed that the hemocyte surface-associated CvGal is a phagocytosis receptor for P. marinus. Thus, P. marinus may have evolved to adapt the trophozoite’s glycocalyx to be selectively recognized by the oyster hemocyte CvGal, thereby subverting the oyster’s innate immune/feeding recognition mechanism to gain entry into the host cells (Tasumi and Vasta, 2007). A galectin of similar domain organization was recently identified in the snail Biomphalaria glabrata, the intermediate host of the human blood fluke Schistosoma mansoni, and was proposed to mediate interactions with the parasite (Yoshino et al., 2008).
A recent study identified galectin-1 as the receptor for the protozoan parasite Trichomonas vaginalis (Okumura et al., 2008) the causative agent of the most prevalent non-viral sexually transmitted human infection in both women and men. As an obligate extracellular parasite, establishment and persistence of T. vaginalis infection requires adherence to the host epithelial cell surface. Like Leishmania spp., T. vaginalis displays a surface LPG rich in galactose and N-acetyl glucosamine, which is recognized in a carbohydrate-dependent manner by galectin-1 expressed by the epithelial cells in the cervical linings, as well as placenta, prostate, endometrial, and decidual tissue, also colonized by the parasite (Okumura et al., 2008).
CONCLUSION
By recognizing highly conserved and widely distributed microbial surface glycans (PAMPs or MAMPs) which are essential for the microbe but absent in the host, lectins like the MBL, a member of the C-type lectin family, fit accurately the definition of PRR. Recent studies clearly indicate that also galectins can function as PRRs that target oligosaccharides on the surface of virus, bacteria, protista and helminth pathogens, and parasites. A perplexing paradox arises, however, by the fact that galectins also recognize endogenous lactosamine-containing glycans on the host cell surface during development and regulation of immune homeostasis. For the TR-type galectins that display two CRDs of similar but distinct specificity in a single polypeptide monomer, the binding and cross-linking of endogenous and exogenous glycans can be rationalized by the distinct properties of their N- and C-terminus binding sites. For other lectins such as the proto- and chimera-type galectins that display a single CRD per monomer, their capacity to recognize both endogenous and exogenous glycans through the same binding site, can be explained by taking into consideration the multiple factors pertaining to the local lectin concentrations and oligomerization, the geometry of the presentation of the multivalent carbohydrate ligands on the host or microbial cell surface, and the properties of the microenvironment in which interactions take place. For example, in a local microenvironment where an infection is initiated, the particular concentration of galectins released to the extracellular space may lead to a distinctive oligomerization, CRD display geometry, and avidity that may shift galectin binding from the endogenous ligands to the glycans displayed on the microbial surface in a particular architectural presentation. It has been proposed that both the microbial and host glycomes and their receptors continuously evolve to escape mutual recognition, a process known as the “Red Queen effect” (reviewed in Vasta, 2009), by which the microbe avoids recognition by the host innate immune receptors (PRRs) and, the host by the microbial colonization factors (agglutinins, adhesins, and lectins). Given the key roles played by galectins in early development and regulation of immune homeostasis by the recognition of “self” glycans, strong functional constraints would prevent galectins from significant evolutionary changes in carbohydrate specificity. This is supported by the considerable structural conservation within this lectin family. Further, with the current evidence about how pathogens and parasites, which display a remarkable evolutionary plasticity, efficiently subvert the roles of galectins to attach or gain entrance into the host cells, it seems more plausible that instead of avoiding recognition by the host, they would have evolved their glycomes to mimic their hosts’ in a “Trojan horse” model (reviewed in Vasta, 2009), and rely on the host’s self-recognition molecules such as galectins for attachment to the vector or host invasion. It is noteworthy that most of (if not all) these pathogens and parasites are endowed with diverse and powerful mechanisms to evade intracellular killing by the host, and/or downregulate downstream immune responses. The complex strategies such as glycan mimicry developed by symbionts, commensals, and microbial pathogens to successfully colonize, enter, proliferate, and disseminate within and among their hosts or vectors, are the products of strong selective pressures that have led to adaptations that ensure their survival in the most hostile environment of all (Casadevall and Pirofski, 2000; Hughes and Sperandio, 2008). Thus, these fine tuned protein–carbohydrate interactions represent a significant challenge for the development of innovative strategies for intervention in infectious disease.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors’ research reviewed herein was supported by Grants IOS 1050518, IOB-0618409, and IOS0822257 from the National Science Foundation, and Grants 1R01GM070589-01 and 5R01GM070589-06 from the National Institutes of Health (NIH) to Gerardo R. Vasta, grant R41CA141970-01A2 from the NIH to Hafiz Ahmed, and grant 1R21AI076797-01A2 from the NIH to José A. Fernández-Robledo.
REFERENCES
- Ahmad N., Gabius H.-J., Andre S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C. F. (2004). Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279, 10841–10847 [DOI] [PubMed] [Google Scholar]
- Ahmed H., Bianchet M. A., Amzel L. M., Hirabayashi J., Kasai K., Giga-Hama Y., Tohda H., Vasta G. R. (2002). Novel carbohydrate specificity of the 16-kDa galectin from Caenorhabditis elegans: binding to blood group precursor oligosaccharides (type 1, type 2, Talpha, and Tbeta) and gangliosides. Glycobiology 12 451–461 [DOI] [PubMed] [Google Scholar]
- Ahmed H., Du S. J., O’Leary N., Vasta G. R. (2004). Biochemical and molecular characterization of galectins from zebrafish (Danio rerio): notochord-specific expression of a prototype galectin during early embryogenesis. Glycobiology 14 219–232 [DOI] [PubMed] [Google Scholar]
- Ahmed H., Pohl J., Fink N. E., Strobel F., Vasta G. R. (1996). The primary structure and carbohydrate specificity of a beta-galactosyl-binding lectin from toad (Bufo arenarum Hensel) ovary reveal closer similarities to the mammalian galectin-1 than to the galectin from the clawed frog Xenopus laevis. J. Biol. Chem. 271 33083–33094 [DOI] [PubMed] [Google Scholar]
- Ahmed H., Vasta G. R. (1994). Galectins: conservation of functionally and structurally relevant amino acid residues defines two types of carbohydrate recognition domains. Glycobiology 4 545–548 [DOI] [PubMed] [Google Scholar]
- Ahmed H., Vasta G. R. (2008). Unlike mammalian GRIFIN, the zebrafish homologue (DrGRIFIN) represents a functional carbohydrate-binding galectin. Biochem. Biophys. Res. Commun. 371 350–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Takeda K., Kaisho T. (2001). Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2 675–680 [DOI] [PubMed] [Google Scholar]
- Anderson A. C., Anderson D. E., Bregoli L., Hastings W. D., Kassam N., Lei C., Chandwaskar R., Karman J., Su E. W., Hirashima M., Bruce J. N., Kane L. P., Kuchroo V. K., Hafler D. A. (2007). Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318 1141–1143 [DOI] [PubMed] [Google Scholar]
- Bianchet M. A., Ahmed H., Vasta G. R., Amzel L. M. (2000). Soluble beta-galactosyl-binding lectin (galectin) from toad ovary: crystallographic studies of two protein-sugar complexes. Proteins 40 378–388 [DOI] [PubMed] [Google Scholar]
- Bianchet M. A., Odom E. W., Vasta G. R., Amzel L. M. (2002). A novel fucose recognition fold involved in innate immunity. Nat. Struct. Biol. 9 628–634 [DOI] [PubMed] [Google Scholar]
- Bianchet M. A., Odom E. W., Vasta G. R., Amzel L. M. (2010). Structure and specificity of a binary tandem domain F-lectin from striped bass (Morone saxatilis). J. Mol. Biol. 401 239–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel P., Robatzek S. (2007). Microbe-associated molecular patterns (MAMPs) probe plant immunity. Curr. Opin. Plant Biol. 10 335–341 [DOI] [PubMed] [Google Scholar]
- Brewer C. F., Miceli M. C., Baum L. G. (2002). Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 12 616–623 [DOI] [PubMed] [Google Scholar]
- Carlsson S., Oberg C. T., Carlsson M. C., Sundin A., Nilsson U. J., Smith D., Cummings R. D., Almkvist J., Karlsson A., Leffler H. (2007). Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology 17 663–676 [DOI] [PubMed] [Google Scholar]
- Cartwright J. R., Tharia H. A., Burns I., Shrive A. K., Hoole D., Greenhough T. J. (2004). Isolation and characterisation of pentraxin-like serum proteins from the common carp Cyprinus carpio. Dev. Comp. Immunol. 28 113–125 [DOI] [PubMed] [Google Scholar]
- Casadevall A., Pirofski L. A. (2000). Host-pathogen interactions: basic concepts of microbial commensalism, colonization, infection, and disease. Infect. Immun. 68 6511–6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedeno-Laurent F., Dimitroff C. J. (2012). Galectin-1 research in T cell immunity: past, present and future. Clin. Immunol. 142 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Y., Fermin A., Vardhana S., Weng I. C., Lo K. F., Chang E. Y., Maverakis E., Yang R. Y., Hsu D. K., Dustin M. L., Liu F. T. (2009). Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc. Natl. Acad. Sci. U.S.A. 106 14496–14501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M., Cummings R. D. (1995). Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J. Biol. Chem. 270 5207–5212 [DOI] [PubMed] [Google Scholar]
- Cleves A. E., Cooper D. N., Barondes S. H., Kelly R. B. (1996). A new pathway for protein export in Saccharomyces cerevisiae. J. Cell Biol. 133 1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C., Ripoche M. A., Scaerou F., Foulis D., Poirier F. (1996). Galectins in mouse embryogenesis. Biochem. Soc. Trans. 24 141–146 [DOI] [PubMed] [Google Scholar]
- Colnot C., Sidhu S. S., Balmain N., Poirier F. (2001). Uncoupling of chondrocyte death and vascular invasion in mouse galectin 3 null mutant bones. Dev. Biol. 229 203–214 [DOI] [PubMed] [Google Scholar]
- Cook M. T., Hayball P. J., Nowak B. F., Hayball J. D. (2005). The opsonising activity of a pentraxin-like protein isolated from snapper (Pagrus auratus, Sparidae) serum. Dev. Comp. Immunol. 29 703–712 [DOI] [PubMed] [Google Scholar]
- Cooper D. N. (2002). Galectinomics: finding themes in complexity. Biochim. Biophys. Acta 1572 209–231 [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Nyame A. K. (1999). Schistosome glysoconjugates. Biochim. Biophys. Acta 1455 363–374 [DOI] [PubMed] [Google Scholar]
- Dam T. K., Brewer C. F. (2008). Effects of clustered epitopes in multivalent ligand-receptor interactions. Biochemistry 47 8470–8476 [DOI] [PubMed] [Google Scholar]
- Dam T. K., Brewer C. F. (2010). Lectins as pattern recognition molecules: the effects of epitope density in innate immunity. Glycobiology 20 270–279 [DOI] [PubMed] [Google Scholar]
- Dam T. K., Gerken T. A., Brewer C. F. (2009). Thermodynamics of multivalent carbohydrate-lectin cross-linking interactions: importance of entropy in the bind and jump mechanism. Biochemistry 48 3822–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lella S., Marti M. A., Alvarez R. M., Estrin D. A., Ricci J. C. (2007). Characterization of the galectin-1 carbohydrate recognition domain in terms of solvent occupancy. J. Phys. Chem. B 111 7360–7366 [DOI] [PubMed] [Google Scholar]
- Di Lella S., Sundblad V., Cerliani J. P., Guardia C. M., Estrin D. A., Vasta G. R., Rabinovich G. A. (2011). When galectins recognize glycans: from biochemistry to physiology and back again. Biochemistry 50 7842–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Baruffi M., Zhu H., Cho M., Karmakar S., Mcever R. P., Cummings R. D. (2003). Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J. Biol. Chem. 278 41282–41293 [DOI] [PubMed] [Google Scholar]
- Echeverria I., Amzel L. M. (2011). Disaccharide binding to galectin-1: free energy calculations and molecular recognition mechanism. Biophys. J. 100 2283–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elola M. T., Wolfenstein-Todel C., Troncoso M. F., Vasta G. R., Rabinovich G. A. (2007). Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell. Mol. Life Sci. 64 1679–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R., Mantle M., Ceri H. (1993). Characterization of quail intestinal mucin as a ligand for endogenous quail lectin. Biochem. J. 293 (Pt 3) 867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg H., Park-Snyder S., Kolatkar A. R., Heise C. T., Taylor M. E., Weis W. I. (2000). Structure of a C-type carbohydrate recognition domain from the macrophage mannose receptor. J. Biol. Chem. 275 21539–21548 [DOI] [PubMed] [Google Scholar]
- Fogel S., Guittaut M., Legrand A., Monsigny M., Hebert E. (1999). The tat protein of HIV-1 induces galectin-3 expression. Glycobiology 9 383–387 [DOI] [PubMed] [Google Scholar]
- Fujita T., Matsushita M., Endo Y. (2004). The lectin-complement pathway – its role in innate immunity and evolution. Immunol. Rev. 198 185–202 [DOI] [PubMed] [Google Scholar]
- Fukumori T., Kanayama H. O., Raz A. (2007). The role of galectin-3 in cancer drug resistance. Drug Resist. Update 10 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumori T., Takenaka Y., Yoshii T., Kim H. R., Hogan V., Inohara H., Kagawa S., Raz A. (2003). CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 63 8302–8311 [PubMed] [Google Scholar]
- Fuller J. R., Pitzer J. E., Godwin U., Albertino M., Machon B. D., Kearse K. P., Mcconnell T. J. (2004). Characterization of the molecular chaperone calnexin in the channel catfish, Ictalurus punctatus, and its association with MHC class II molecules. Dev. Comp. Immunol. 28 603–617 [DOI] [PubMed] [Google Scholar]
- Garred P., Larsen F., Seyfarth J., Fujita R., Madsen H. O. (2006). Mannose-binding lectin and its genetic variants. Genes Immun. 7 85–94 [DOI] [PubMed] [Google Scholar]
- Gerwick L., Corley-Smith G., Bayne C. J. (2007). Gene transcript changes in individual rainbow trout livers following an inflammatory stimulus. Fish Shellfish Immunol. 22 157–171 [DOI] [PubMed] [Google Scholar]
- Gorski J. P., Liu F. T., Artigues A., Castagna L. F., Osdoby P. (2002). New alternatively spliced form of galectin-3, a member of the beta-galactoside-binding animal lectin family, contains a predicted transmembrane-spanning domain and a leucine zipper motif. J. Biol. Chem. 277 18840–18848 [DOI] [PubMed] [Google Scholar]
- Greenspan N. S. (2001). Dimensions of antigen recognition and levels of immunological specificity. Adv. Cancer Res. 80 147–187 [DOI] [PubMed] [Google Scholar]
- Guevremont M., Martel-Pelletier J., Boileau C., Liu F. T., Richard M., Fernandes J. C., Pelletier J. P., Reboul P. (2004). Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3. Ann. Rheum. Dis. 63 636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Baum L. G. (2004). Presentation of galectin-1 by extracellular matrix triggers T cell death. J. Biol. Chem. 279 4705–4712 [DOI] [PubMed] [Google Scholar]
- Hijazi K., Wang Y., Scala C., Jeffs S., Longstaff C., Stieh D., Haggarty B., Vanham G., Schols D., Balzarini J., Jones I. M., Hoxie J., Shattock R., Kelly C. G. (2011). DC-SIGN increases the affinity of HIV-1 envelope glycoprotein interaction with CD4. PLoS ONE 6 e28307 10.1371/journal.pone.0028307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi J., Kasai K. (1993). The family of metazoan metal-independent beta-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology 3 297–304 [DOI] [PubMed] [Google Scholar]
- Houzelstein D., Goncalves I. R., Fadden A. J., Sidhu S. S., Cooper D. N., Drickamer K., Leffler H., Poirier F. (2004). Phylogenetic analysis of the vertebrate galectin family. Mol. Biol. Evol. 21 1177–1187 [DOI] [PubMed] [Google Scholar]
- Hsu D. K., Hammes S. R., Kuwabara I., Greene W. C., Liu F. T. (1996). Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the beta-galactoside-binding lectin, galectin-3. Am. J. Pathol. 148 1661–1670 [PMC free article] [PubMed] [Google Scholar]
- Hughes D. T., Sperandio V. (2008). Inter-kingdom signalling: communication between bacteria and their hosts. Nat. Rev. Microbiol. 6 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideo H., Matsuzaka T., Nonaka T., Seko A., Yamashita K. (2011). Galectin-8-N-domain recognition mechanism for sialylated and sulfated glycans. J. Biol. Chem. 286 11346–11355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilarregui J. M., Croci D. O., Bianco G. A., Toscano M. A., Salatino M., Vermeulen M. E., Geffner J. R., Rabinovich G. A. (2009). Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat. Immunol. 10 981–991 [DOI] [PubMed] [Google Scholar]
- Iliev D. B., Roach J. C., Mackenzie S., Planas J. V., Goetz F. W. (2005). Endotoxin recognition: in fish or not in fish? FEBS Lett. 579, 6519–6528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip W. K., Takahashi K., Ezekowitz R. A., Stuart L. M. (2009). Mannose-binding lectin and innate immunity. Immunol. Rev. 230 9–21 [DOI] [PubMed] [Google Scholar]
- Khalturin K., Panzer Z., Cooper M. D., Bosch T. C. (2004). Recognition strategies in the innate immune system of ancestral chordates. Mol. Immunol. 41 1077–1087 [DOI] [PubMed] [Google Scholar]
- Kingeter L. M., Lin X. (2012). C-type lectin receptor-induced NF-kappaB activation in innate immune and inflammatory responses. Cell. Mol. Immunol. 9 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohatsu L., Hsu D. K., Jegalian A. G., Liu F.-T., Baum L. G. (2006). Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J. Immunol. 177 4718–4726 [DOI] [PubMed] [Google Scholar]
- Krejcirikova V., Pachl P., Fabry M., Maly P., Rezacova P., Brynda J. (2011). Structure of the mouse galectin-4 N-terminal carbohydrate-recognition domain reveals the mechanism of oligosaccharide recognition. Acta Crystallogr. D Biol. Crystallogr. 67 204–211 [DOI] [PubMed] [Google Scholar]
- Li Y., Feng J., Geng S., Wei H., Chen G., Li X., Wang L., Wang R., Peng H., Han G., Shen B. (2011). The N- and C-terminal carbohydrate recognition domains of galectin-9 contribute differently to its multiple functions in innate immunity and adaptive immunity. Mol. Immunol. 48 670–677 [DOI] [PubMed] [Google Scholar]
- Liao D. I., Kapadia G., Ahmed H., Vasta G. R., Herzberg O. (1994). Structure of S-lectin, a developmentally regulated vertebrate beta-galactoside-binding protein. Proc. Natl. Acad. Sci. U.S.A. 91 1428–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz M. S., Leal-Pinto E., Cohen B. E., Abramson R. G. (2004). Galectin 9 is the sugar-regulated urate transporter/channel UAT. Glycoconj. J. 19 491–498 [DOI] [PubMed] [Google Scholar]
- Liu F. T., Hsu D. K. (2007). The role of galectin-3 in promotion of the inflammatory response. Drug News Perspect. 20 455–460 [DOI] [PubMed] [Google Scholar]
- Liu F. T., Rabinovich G. A. (2005). Galectins as modulators of tumour progression. Nat. Rev. Cancer 5 29–41 [DOI] [PubMed] [Google Scholar]
- Liu F. T., Yang R. Y., Hsu D. K. (2012). Galectins in acute and chronic inflammation. Ann. N.Y. Acad. Sci. 1253 80–91 [DOI] [PubMed] [Google Scholar]
- Liu Y., Eisenberg D. (2002). 3D domain swapping: as domains continue to swap. Protein Sci. 11 1285–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobsanov Y. D., Gitt M. A., Leffler H., Barondes S. H., Rini J. M. (1993). X-ray crystal structure of the human dimeric S-Lac lectin, L-14-II, in complex with lactose at 2.9-A resolution. J. Biol. Chem. 268 27034–27038 [DOI] [PubMed] [Google Scholar]
- Loeb J. A., Drickamer K. (1988). Conformational changes in the chicken receptor for endocytosis of glycoproteins. Modulation of ligand-binding activity by Ca2+ and pH. J. Biol. Chem. 263 9752–9760 [PubMed] [Google Scholar]
- Ludwig I. S., Geijtenbeek T. B, Van Kooyk Y. (2006). Two way communication between neutrophils and dendritic cells. Curr. Opin. Pharmacol. 6 408–413 [DOI] [PubMed] [Google Scholar]
- Lund V., Olafsen J. A. (1999). Changes in serum concentration of a serum amyloid P-like pentraxin in Atlantic salmon, Salmo salar L., during infection and inflammation. Dev. Comp. Immunol. 23 61–70 [DOI] [PubMed] [Google Scholar]
- Marschal P., Herrmann J., Leffler H., Barondes S. H., Cooper D. N. (1992). Sequence and specificity of a soluble lactose-binding lectin from Xenopus laevis skin. J. Biol. Chem. 267 12942–12949 [PubMed] [Google Scholar]
- Marth J. D., Grewal P. K. (2008). Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8 874–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V. G., Pellizzari E. H., Diaz E. S., Cigorraga S. B., Lustig L., Denduchis B., Wolfenstein-Todel C., Iglesias M. M. (2004). Galectin-1, a cell adhesion modulator, induces apoptosis of rat Leydig cells in vitro. Glycobiology 14 127–137 [DOI] [PubMed] [Google Scholar]
- Mascanfroni I. D., Cerliani J. P., Dergan-Dylon S., Croci D. O., Ilarregui J. M., Rabinovich G. A. (2011). Endogenous lectins shape the function of dendritic cells and tailor adaptive immunity: mechanisms and biomedical applications. Int. Immunopharmacol. 11 833–841 [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C. A. , Jr (2002). Decoding the patterns of self and nonself by the innate immune system. Science 296 298–300 [DOI] [PubMed] [Google Scholar]
- Mercier S., St-Pierre C., Pelletier I., Ouellet M., Tremblay M. J., Sato S. (2008). Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology 371 121–129 [DOI] [PubMed] [Google Scholar]
- Miao E. A., Warren S. E. (2010). Innate immune detection of bacterial virulence factors via the NLRC4 inflammasome. J. Clin. Immunol. 30 502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S., Ahmad N., Andre S., Kaltner H., Gabius H. J., Brenowitz M., Brewer F. (2004). Quaternary solution structures of galectins-1, -3, and -7. Glycobiology 14 293–300 [DOI] [PubMed] [Google Scholar]
- Myskova J., Svobodova M., Beverley S. M., Volf P. (2007). A lipophosphoglycan-independent development of Leishmania in permissive sand flies. Microbes Infect. 9 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae M., Nishi N., Murata T., Usui T., Nakamura T., Wakatsuki S., Kato R. (2009). Structural analysis of the recognition mechanism of poly-N-acetyllactosamine by the human galectin-9 N-terminal carbohydrate recognition domain. Glycobiology 19 112–117 [DOI] [PubMed] [Google Scholar]
- Nonaka M. (2011). The complement C3 protein family in invertebrates. ISJ 8 21–32 [Google Scholar]
- Ogden A. T., Nunes I., Ko K., Wu S., Hines C. S., Wang A. F., Hegde R. S., Lang R. A. (1998). GRIFIN, a novel lens-specific protein related to the galectin family. J. Biol. Chem. 273 28889–28896 [DOI] [PubMed] [Google Scholar]
- Ohtsubo K., Marth J. D. (2006). Glycosylation in cellular mechanisms of health and disease. Cell 126 855–867 [DOI] [PubMed] [Google Scholar]
- Ohtsubo K., Takamatsu S., Minowa M. T., Yoshida A., Takeuchi M., Marth J. D. (2005). Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 123 1307–1321 [DOI] [PubMed] [Google Scholar]
- Okumura C. Y., Baum L. G., Johnson P. J. (2008). Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell. Microbiol. 10 2078–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet M., Mercier S., Pelletier I., Bounou S., Roy J., Hirabayashi J., Sato S., Tremblay M. J. (2005). Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J. Immunol. 174 4120–4126 [DOI] [PubMed] [Google Scholar]
- Ozaki K., Inoue K., Sato H., Iida A., Ohnishi Y., Sekine A., Odashiro K., Nobuyoshi M., Hori M., Nakamura Y., Tanaka T. (2004). Functional variation in LGALS2 confers risk of myocardial infarction and regulates lymphotoxin-alpha secretion in vitro. Nature 429 72–75 [DOI] [PubMed] [Google Scholar]
- Partridge E. A., Le Roy C., Di Guglielmo G. M., Pawling J., Cheung P., Granovsky M., Nabi I. R., Wrana J. L., Dennis J. W. (2004). Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306 120–124 [DOI] [PubMed] [Google Scholar]
- Patterson R. J., Wang W., Wang J. L. (2004). Understanding the biochemical activities of galectin-1 and galectin-3 in the nucleus. Glycoconj. J. 19 499–506 [DOI] [PubMed] [Google Scholar]
- Perillo N. L., Marcus M. E., Baum L. G. (1998). Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J. Mol. Med. (Berl) 76 402–412 [DOI] [PubMed] [Google Scholar]
- Perkins F. O. (1996). The structure of Perkinsus marinus (Mackin, Owen and Collier, 1950) Levine, 1978 with comments on taxonomy and phylogeny of Perkinsus spp. J. Shellfish Res. 15 67–87 [Google Scholar]
- Poirier F. (2002). Roles of galectins in vivo. Biochem. Soc. Symp. 69 95–103 [DOI] [PubMed] [Google Scholar]
- Poirier F., Timmons P. M., Chan C. T., Guenet J. L., Rigby P. W. (1992). Expression of the L14 lectin during mouse embryogenesis suggests multiple roles during pre- and post-implantation development. Development 115 143–155 [DOI] [PubMed] [Google Scholar]
- Puche A. C., Poirier F., Hair M., Bartlett P. F., Key B. (1996). Role of galectin-1 in the developing mouse olfactory system. Dev. Biol. 179 274–287 [DOI] [PubMed] [Google Scholar]
- Rabinovich G. A., Baum L. G., Tinari N., Paganelli R., Natoli C., Liu F. T., Iacobelli S. (2002a). Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 23, 313–320 [DOI] [PubMed] [Google Scholar]
- Rabinovich G. A., Liu F. T., Hirashima M., Anderson A. (2007a). An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand. J. Immunol. 66 143–158 [DOI] [PubMed] [Google Scholar]
- Rabinovich G. A., Rubinstein N., Toscano M. A. (2002b). Role of galectins in inflammatory and immunomodulatory processes. Biochim. Biophys. Acta 1572 274–284 [DOI] [PubMed] [Google Scholar]
- Rabinovich G. A., Toscano M. A. (2009). Turning “sweet” on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9 338–352 [DOI] [PubMed] [Google Scholar]
- Rabinovich G. A., Toscano M. A., Jackson S. S., Vasta G. R. (2007b). Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 17 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport E. M., Andre S., Kurmyshkina O. V., Pochechueva T. V., Severov V. V., Pazynina G. V., Gabius H.-J., Bovin N. V. (2008). Galectin-loaded cells as a platform for the profiling of lectin specificity by fluorescent neoglycoconjugates: a case study on galectins-1 and -3 and the impact of assay setting. Glycobiology 18 315–324 [DOI] [PubMed] [Google Scholar]
- Rubinstein N., Ilarregui J. M., Toscano M. A., Rabinovich G. A. (2004). The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens 64 1–12 [DOI] [PubMed] [Google Scholar]
- Saouros S., Edwards-Jones B., Reiss M., Sawmynaden K., Cota E., Simpson P., Dowse T. J., Jakle U., Ramboarina S., Shivarattan T., Matthews S., Soldati-Favre D. (2005). A novel galectin-like domain from Toxoplasma gondii micronemal protein 1 assists the folding, assembly, and transport of a cell adhesion complex. J. Biol. Chem. 280 38583–38591 [DOI] [PubMed] [Google Scholar]
- Sato A., Mayer W. E., Overath P., Klein J. (2003). Genes encoding putative natural killer cell C-type lectin receptors in teleostean fishes. Proc. Natl. Acad. Sci. U.S.A. 100 7779–7784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Hughes R. C. (1992). Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J. Biol. Chem. 267 6983–6990 [PubMed] [Google Scholar]
- Sato S., Hughes R. C. (1994). Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 269 4424–4430 [PubMed] [Google Scholar]
- Sato S., Nieminen J. (2004). Seeing strangers or announcing “danger”: galectin-3 in two models of innate immunity. Glycoconj. J. 19 583–591 [DOI] [PubMed] [Google Scholar]
- Schroder H. C., Ushijima H., Theis C., Seve A. P., Hubert J., Muller W. E. (1995). Expression of nuclear lectin carbohydrate-binding protein 35 in human immunodeficiency virus type 1-infected Molt-3 cells. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 9 340–348 [PubMed] [Google Scholar]
- Schwarz F. P., Ahmed H., Bianchet M. A., Amzel L. M., Vasta G. R. (1998). Thermodynamics of bovine spleen galectin-1 binding to disaccharides: correlation with structure and its effect on oligomerization at the denaturation temperature. Biochemistry 37 5867–5877 [DOI] [PubMed] [Google Scholar]
- Seetharaman J., Kanigsberg A., Slaaby R., Leffler H., Barondes S. H., Rini J. M. (1998). X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J. Biol. Chem. 273 13047–13052 [DOI] [PubMed] [Google Scholar]
- Seong S.-Y., Matzinger P. (2004). Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 4 469–478 [DOI] [PubMed] [Google Scholar]
- Shirai T., Matsui Y., Shionyu-Mitsuyama C., Yamane T., Kamiya H., Ishii C., Ogawa T., Muramoto K. (2002). Crystal structure of a conger eel galectin (congerin II) at 1.45A resolution: implication for the accelerated evolution of a new ligand-binding site following gene duplication. J. Mol. Biol. 321 879–889 [DOI] [PubMed] [Google Scholar]
- Shirai T., Mitsuyama C., Niwa Y., Matsui Y., Hotta H., Yamane T., Kamiya H., Ishii C., Ogawa T., Muramoto K. (1999). High-resolution structure of the conger eel galectin, congerin I, in lactose-liganded and ligand-free forms: emergence of a new structure class by accelerated evolution. Structure 7 1223–1233 [DOI] [PubMed] [Google Scholar]
- Shoji H., Deltour L., Nakamura T., Tajbakhsh S., Poirier F. (2009). Expression pattern and role of Galectin1 during early mouse myogenesis. Dev. Growth Differ. 51 607–615 [DOI] [PubMed] [Google Scholar]
- Shoji H., Nishi N., Hirashima M., Nakamura T. (2003). Characterization of the Xenopus galectin family. Three structurally different types as in mammals and regulated expression during embryogenesis. J. Biol. Chem. 278 12285–12293 [DOI] [PubMed] [Google Scholar]
- Sparrow C. P., Leffler H., Barondes S. H. (1987). Multiple soluble beta-galactoside-binding lectins from human lung. J. Biol. Chem. 262 7383–7390 [PubMed] [Google Scholar]
- Stalz H., Roth U., Schleuder D., Macht M., Haebel S., Strupat K., Peter-Katalinic J., Hanisch F. G. (2006). The Geodia cydonium galectin exhibits prototype and chimera-type characteristics and a unique sequence polymorphism within its carbohydrate recognition domain. Glycobiology 16 402–414 [DOI] [PubMed] [Google Scholar]
- Stowell S. R., Arthur C. M., Dias-Baruffi M., Rodrigues L. C., Gourdine J. P., Heimburg-Molinaro J., Ju T., Molinaro R. J., Rivera-Marrero C., Xia B., Smith D. F., Cummings R. D. (2010). Innate immune lectins kill bacteria expressing blood group antigen. Nat. Med. 16 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell S. R., Cho M., Feasley C. L., Arthur C. M., Song X., Colucci J. K., Karmakar S., Mehta P., Dias-Baruffi M., Mcever R. P., Cummings R. D. (2009). Ligand reduces galectin-1 sensitivity to oxidative inactivation by enhancing dimer formation. J. Biol. Chem. 284 4989–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell S. R., Qian Y., Karmakar S., Koyama N. S., Dias-Baruffi M., Leffler H., Mcever R. P., Cummings R. D. (2008). Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J. Immunol. 180 3091–3102 [DOI] [PubMed] [Google Scholar]
- Takenaka Y., Fukumori T., Raz A. (2004). Galectin-3 and metastasis. Glycoconj. J. 19 543–549 [DOI] [PubMed] [Google Scholar]
- Tasumi S., Vasta G. R. (2007). A galectin of unique domain organization from hemocytes of the Eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. J. Immunol. 179 3086–3098 [DOI] [PubMed] [Google Scholar]
- Tomizawa T., Kigawa T., Saito K., Koshiba S., Inoue M., Yokoyama S. (2005). Solution structure of the C-terminal gal-bind lectin domain from human galectin-4. Structural Genomics and Proteomics Initiative (RSGI), Riken [Google Scholar]
- Troncoso M. F., Elola M. T., Croci D. O., Rabinovich G. A. (2012). Integrating structure and function of “tandem-repeat” galectins. Front. Biosci. (Schol Ed) 4 864–887 [DOI] [PubMed] [Google Scholar]
- van der Vlist M., van der Aar A. M., Gringhuis S. I., Geijtenbeek T. B. (2011). Innate signaling in HIV-1 infection of dendritic cells. Curr. Opin. HIV AIDS 6 348–352 [DOI] [PubMed] [Google Scholar]
- Vasta G. R. (2009). Roles of galectins in infection. Nat. Rev. Microbiol. 7 424–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta G. R., Ahmed H. (2009). Animal Lectins: A Functional View. Boca Raton, FL: CRC Press (Taylor & Francis Group) [Google Scholar]
- Vasta G. R., Ahmed H., Amzel L. M., Biahchet M. A. (1997). Galectins from amphibian species: carbohydrate specificity, molecular structure and evolution. Trends Blycosci. Glycotechnol. 9 131–144 [Google Scholar]
- Vasta G. R., Hunt J. C., Marchalonis J. J., Fish W. W. (1986). Galactosyl-binding lectins from the tunicate Didemnum candidum. Purification and physicochemical characterization. J. Biol. Chem. 261 9174–9181 [PubMed] [Google Scholar]
- Vasta G. R., Quesenberry M., Ahmed H, O’Leary N. (1999). C-type lectins and galectins mediate innate and adaptive immune functions: their roles in the complement activation pathway. Dev. Comp. Immunol. 23 401–420 [DOI] [PubMed] [Google Scholar]
- Vilches C., Parham P. (2002). KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu. Rev. Immunol. 20 217–251 [DOI] [PubMed] [Google Scholar]
- Vokhmyanina O. A., Rapoport E. M., Ryzhov I. M., Korchagina E. Y., Pazynina G. V., Severov V. V., Kaltner H., Andre S., Gabius H. J., Bovin N. V. (2011). Carbohydrate specificity of chicken and human tandem-repeat-type galectins-8 in composition of cells. Biochemistry (Mosc) 76 1185–1192 [DOI] [PubMed] [Google Scholar]
- Volf P., Benkova I., Myskova J., Sadlova J., Campino L., Ravel C. (2007). Increased transmission potential of Leishmania major/Leishmania infantum hybrids. Int. J. Parasitol. 37 589–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyakarnam A., Dagher S. F., Wang J. L., Patterson R. J. (1997). Evidence for a role for galectin-1 in pre-mRNA splicing. Mol. Cell. Biol. 17 4730–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. (2002). Structural and functional aspects of complement activation by mannose-binding protein. Immunobiology 205 433–445 [DOI] [PubMed] [Google Scholar]
- Walser P. J., Haebel P. W., Kunzler M., Sargent D., Kues U., Aebi M., Ban N. (2004). Structure and functional analysis of the fungal galectin CGL2. Structure 12 689–702 [DOI] [PubMed] [Google Scholar]
- Walser P. J., Kues U., Aebi M., Kunzler M. (2005). Ligand interactions of the Coprinopsis cinerea galectins. Fungal Genet. Biol. 42 293–305 [DOI] [PubMed] [Google Scholar]
- Weis W. I., Drickamer K., Hendrickson W. A. (1992). Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature 360 127–134 [DOI] [PubMed] [Google Scholar]
- Weis W. I., Kahn R., Fourme R., Drickamer K., Hendrickson W. A. (1991). Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science 254 1608–1615 [DOI] [PubMed] [Google Scholar]
- Weis W. I., Taylor M. E., Drickamer K. (1998). The C-type lectin superfamily in the immune system. Immunol. Rev. 163 19–34 [DOI] [PubMed] [Google Scholar]
- Yoshino T. P., Dinguirard N., Kunert J., Hokke C. H. (2008). Molecular and functional characterization of a tandem-repeat galectin from the freshwater snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni. Gene 411 46–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelensky A. N., Gready J. E. (2005). The C-type lectin-like domain superfamily. FEBS J. 272 6179–6217 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Cummings R. D. (1990). The S-type lectin from calf heart tissue binds selectively to the carbohydrate chains of laminin. Arch. Biochem. Biophys. 281 27–35 [DOI] [PubMed] [Google Scholar]