Abstract
Core 2 β-1,6-N-acetylglucosaminyltransferase (C2GnT) forms an N-acetylglucosamine branch in O-glycans (core 2 O-glycans) of cell surface glycoproteins. C2GnT-expressing bladder tumors acquire highly metastatic phenotypes by surviving longer in host blood circulation. However, the detailed mechanisms underlying this increased survival remain unclear. In this study, we report that the expression of C2GnT in bladder tumors positively correlates with tumor progression and that bladder tumor cell-surface mucin 1 (MUC1) carrying core 2 O-glycans plays an important role in the evasion from natural killer (NK) cell attack. In C2GnT-expressing bladder tumor cells, heavily core 2 O-glycosylated MUC1 carries poly-N-acetyllactosamine in its O-glycans and galectin-3 binds to MUC1 through this poly-N-acetyllactosamine. The binding of galectin-3 to poly-N-acetyllactosamine in MUC1 core 2 O-glycans attenuates the interaction of the tumor cells with NK cells and interferes with the access of tumor necrosis factor-related apoptosis-inducing ligand to the tumor cell surface. These effects of MUC1 carrying core 2 O-glycans on NK cell attack facilitate C2GnT-expressing tumor cells to evade NK cell immunity and survive longer in host blood circulation. We reveal that MUC1 carrying core 2 O-glycans thus functions as a molecular shield against NK cell attack, thereby promoting bladder tumor metastasis.
Keywords: bladder tumor, metastasis, core 2 O-glycans, natural killer cell immunity, mucin 1
Introduction
In the case of patients with bladder tumors, the most common cause of the mortality is recurrence with metastasis. The process of metastasis involves various steps (1). Recently, it has been shown that cell-surface carbohydrates are involved in tumor metastasis (2,3). There are two classifications of cell-surface carbohydrates attached to proteins according to the nature of their linkage to the proteins, N-glycans (N-acetylglucosamine to asparagine) and O-glycans (N-acetylgalactosamine to serine or threonine). It has been reported that N-glycans are involved in several steps of metastasis (4); however, the roles of O-glycans remain unclear.
To understand the roles of O-glycans in tumor metastasis, in this study, we focused on mucins which carry the most abundant O-glycans on the cell surface. There is a growing body of evidence that cell-surface mucins are involved in tumor progression (5). The most extensively studied cell-surface mucin of this paradigm is mucin 1 (MUC1). The tandem repeat of MUC1 is highly O-glycosylated on serine and threonine residues. There is substantial evidence that MUC1 contributes to tumor metastasis (5). However, the roles of MUC1 O-glycans in metastasis depending on the structure of O-glycans have not yet been characterized.
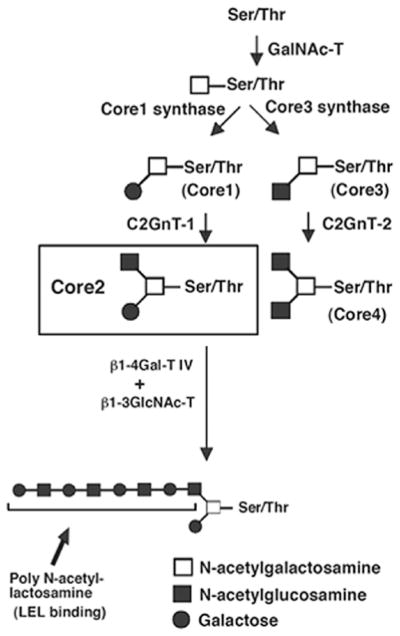
Fig. 1 presents the biosynthesis pathway of mucin-type O-glycans. N-acetylgalactosamine (GalNAc) is transferred to serine and threonine residues in the polypeptide, and then it can be extended with various carbohydrates, including galactose (Gal), N-acetylglucosamine (GlcNAc), fucose, or sialic acid. Depending on the carbohydrates added, four common O-glycan core structures, core1 through core 4 are expressed in mammalian tissues (Fig. 1). It has been shown that the expression of core 2 O-glycans positively correlates with highly metastatic phenotypes of several tumor types (6–10). MUC1 plays a key role in maintaining homeostasis and its structure and biochemical composition provide protection for the cell surface (5). Based on the above observations, we hypothesized that MUC1 carrying core 2 O-glycans plays an important role in the protection of core 2 β-1,6-N-acetylglucosaminyltransferase (C2GnT)-expressing bladder tumor cells against host tumor rejection responses. In the present study, we investigated the effects of MUC1 carrying core 2 O-glycans on natural killer (NK) cell tumor rejection responses, depending on its core structure.
Figure 1.
Biosynthesis pathway of mucin-type O-glycan core structures, core 1-4. GalNAc, N-acetylgalactosamine; GlcNAc, N-acetylglucosamine; Gal, galactose. GalNAc is transferred to serine (Ser) or threonine (Thr) residues in a polypeptide by peptide GalNAc transferase (GalNAc-T). The resultant GalNAcα1-Ser/Thr is converted by core 1 synthase to Galβ1-3GalNAcα1-Ser/Thr (core 1). Core 1 is then converted by Core 2 β-1,6-N-acetylglucosaminyltransferase-1 (C2GnT-1) to core 2. Core1 is also converted by core3 synthase to core 3. Core 3 is converted by C2GnT-2 to core 4. All of the three C2GnTs, C2GnT-1, -2 and -3 are capable of synthesizing core 2, although C2GnT-2 is the only one that can synthesize core 4. β-1,4-galactosyltransferase IV (β1-4Gal-T IV) together with β-1,3-N-acetylglucosaminyltransferase (β1-3GlcNAc-T) synthesize poly-N-acetyllactosamine in core 2 branched oligosaccharides. Lycoperiscon esculentum (tomato) lectin (LEL) binds specifically to poly-N-acetyllactosamine with at least three lactosamine unit repeats.
Materials and methods
Cells, reagents and antibodies
KK-47, a low-grade and non-metastatic bladder tumor cell line (11), was generously provided by Dr T. Masuko, Tohoku University (Sendai, Japan) and YTS-1, a high-grade and metastatic bladder tumor cell line (12) was a generous gift from Dr H. Kakizaki, Yamagata University (Yamagata, Japan). Cells were maintained in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS) (PAA Laboratories, Morningside, Australia). All the biochemical reagents were purchased from Sigma-Aldrich, unless otherwise noted. Recombinant human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The following mono-clonal antibodies to human antigens were used: anti-MUC1 (VU4H5) (Cell Signaling Technology, Danvers, MA, USA), anti-CD29 (9EG7), anti-galectin-3 (B2C10) and phycoerythrin (PE)-labeled anti-CD56 (BD Biosciences, San Jose, CA, USA). The following polyclonal antibodies were used: anti-lysosome-associated membrane glycoprotein 1 (LAMP1) (Lifespan Biosciences, Seattle, WA, USA), anti-death receptor 4 (DR4) (Millipore, Temecula, CA) and anti-actin (Sigma).
Stable transfectants
C2GnT expression in KK-47 cells was undetectable and C2GnT-expressing KK-47 cell lines were established as previously described (10). Briefly, KK-47 cells were transfected with pcDNA3-C2GnT-1 cDNA and three stable transfectants expressing C2GnT were selected in the presence of 400 μg/ml geneticin (Invitrogen, Carlsbad, CA, USA). Two clones (KK-47-C2-1 and -2) were used for all assays in the present study. The results obtained by KK-47-C2-1 (designated KK-47-C2) are shown here, since KK-47-C2-1 and -2 yielded almost identical results in all assays. As the control, KK-47 cells were transfected with pcDNA3 empty vector (mock transfectants), and the mock-transfected cells were designated as KK-47.
YTS-1 cells highly express C2GnT and YTS-1 cells with a reduced expression of C2GnT were generated by shRNA technology as previously described (10). A shRNA expression plasmid was constructed using pBAsi-hU6 Neo DNA (Takara Bio Inc., Shiga, Japan). The shRNA sequence for C2GnT-1 was: GATCCGAATCCTAGTAGTGATATTTAGTGCTCC TGGTTGAATATCACTACTAGGATTCTTTTTTA. The siRNA sequence is underlined. The sequence of the control shRNA containing the scrambled siRNA sequence was: GATCCGTC TTAATCGCGTATAAGGCTAGTGCTCCTGGTTGGCCTT ATACGCGATTAAGACTTTTTTA. The shRNA expression plasmids were subsequently introduced into YTS-1 cells using Lipofectamine 2000. Thirty-one drug-resistant colonies were selected in the presence of 200 μg/ml geneticin. Five C2GnT knockdown clones that had reduced C2GnT expression and three control clones were chosen. Two knockdown clones (YTS-1-C2KD-1 and -2) out of five and two control clones (YTS-1-1 and -2) out of three were used for the assays described in the present study. The results obtained by YTS-1-C2KD-1 and YTS-1-1 are shown here and designated as YTSC2KD and YTS, respectively, since two knockdown and control clones yielded almost identical results in all assays. Elevated and reduced C2GnT expression in KK-47-C2 and YTSC2KD cells was confirmed by the enzyme activity and the mRNA amount, respectively (10).
Human bladder tumor specimens
We collected 164 human bladder tumor specimens which were obtained by transurethral resection or radical cystectomy at the Departments of Urology, Tohoku University Graduate School of Medicine, Sendai; Akita University Graduate School of Medicine, Akita; and Hirosaki University Graduate School of Medicine, Hirosaki, Japan. Tumor stages were determined according to the American Joint Committee for Cancer Staging, 2002. Histopathological grading was performed according to the World Health Organization System (13). The bladder tumor specimens were fixed with 10% buffered formalin for 12 h. The paraffin-embedded samples were cut at 3 μm and subjected to hematoxylin and eosin staining and immunohistochemistry using an affinity-purified rabbit anti-C2GnT antibody prepared previously (14). Anti-rabbit immunoglobulin antibody conjugated with horseradish peroxidase (Nichirei, Tokyo, Japan) was used as the secondary antibody, and peroxidase activity was visualized with amino-ethylcarbazol (AEC) solution (Nichirei). Based on the staining status of the Golgi apparatus, specimens with 10% or more positive cancer cells were judged as C2GnT-positive. Written consents were obtained from all the patients. The Institutional Ethics Committees of Tohoku University, Akita University and Hirosaki University approved this study.
Cytotoxicity assay
Human primary NK cells were purified from human peripheral blood mononuclear cells using the the NK cell isolation kit (Myltenyi Biotech, Auburn, CA, USA). Cytotoxicity was measured by using the Cytotox 96 Non-radioactive Cytotoxicity Assay kit (Promega, Madison, MI, USA). NK cells (1×106 cells/ml) were cultured for three days in RPMI-1640 medium supplemented with 10% FBS in the presence of 1,000 units/ml of human recombinant IL-2 (Wako, Osaka, Japan). Target cells (tumor cells) were incubated with IL-2-activated NK cells for 4 h at 37°C. The release of lactate dehydrogenase from lysed target cells was measured. All assays were performed in quadruplicate. Percentage cytotoxicity = (experimental lactate dehydrogenase release − effector spontaneous release − target spontaneous release)/(target maximum release − target spontaneous release) × 100.
Western blot analysis
Total lysates of tumor cells were prepared by solubilization in 50 mM Tris-HCl buffer, pH 7.5, containing 1% Igepal CA-630, 150 mM NaCl and proteinase inhibitors. The lysates were resolved by SDS-PAGE on an 8–16% gradient gel (Invitrogen), and transferred to a polyvinylidene fluoride (PVDF) membrane. Western blot analyses were performed using specific primary antibodies and a horseradish peroxidase-conjugated secondary antibody. Signals were visualized using the ECL Plus detection system (GE Healthcare, Little Chalfont, UK).
Immunoprecipitation
For lectin immunoprecipitation, lysates from bladder tumor cells were incubated with Lycoperiscon esculentum (tomato) lectin (LEL)-agarose (Vector Laboratories, Burlingame, CA, USA). For immunoprecipitation of MUC1, cell-surface proteins were biotinylated and then cross-linked using the homobifunctional cross-linker dithio-bis-sulfosuccinimydylpropionate (DTSSP) (Pierce Biotechnology Inc., Rockford, IL, USA). Lysates from biotinylated cells were incubated with 2 μg/ml anti-MUC1 monoclonal antibody and then incubated with anti-mouse IgG agarose. The resin binding the immune complex was eluted with 1X Laemmle’s SDS-PAGE sample buffer.
Conjugate formation assay
Heterotypic cell conjugates were quantitatively determined by a double fluorescence assay (15) with some modifications. Bladder tumor target cells (2×106 cells/ml) were transfected with a green fluorescence protein (GFP)-expression plasmid, pmaxGFP (Lonza Walkersville Inc., Walkersville, MD, USA). After 1 h of incubation of GFP-expressing tumor cells with NK cells at 37°C, cells were stained with PE-labeled anti-CD56 antibody and then the number of conjugate was counted by cytometer, FACSCantoII (BD Biosciences). Double-colored (green and red) conjugates were calculated as a percentage of the total GFP-positive cells.
Cell viability assay
For cell viability assay, cells (1×104 cells/well in 96-well plates) were incubated with the indicated concentration of recombinant TRAIL at 37°C for indicated time. Cell viability was assayed using the Cell Counting kit-8 (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s specifications.
Statistical analysis
We used the statistical program SPSS 12.0 (SPSS, Chicago, IL, USA). Statistically significant differences were determined using the Student’s t-test. Differences were considered significant, at P<0.05.
Results
Correlation of C2GnT expression with tumor progression
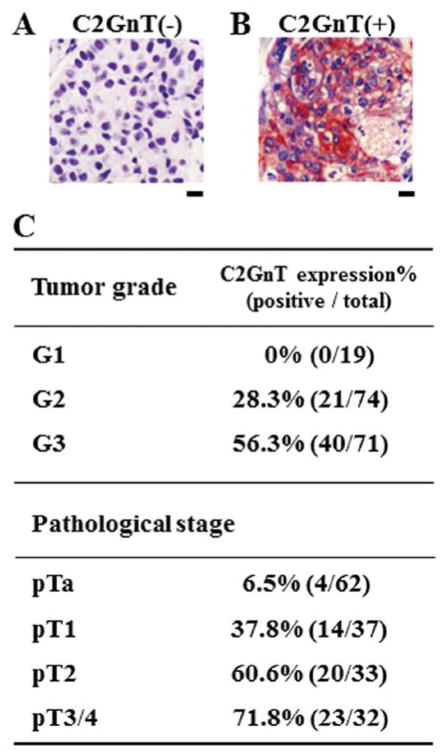
C2GnT is responsible for the formation of core 2 O-glycans (Fig. 1). To determine the correlation between the C2GnT expression status and bladder tumor progression, we immunohistochemically examined the C2GnT expression in bladder tumor specimens using anti-C2GnT antibody. In our previous study, we analyzed 57 patients with bladder tumors for the expression status of C2GnT to determine the correlation between C2GnT expression and bladder tumor patient survival. Patients with C2GnT-expressing bladder tumors had significantly shorter survival than those with C2GnT-non-expressing tumors (10). To obtain the information on the correlation between C2GnT expression and tumor progression in the present study, we performed clinicopathological analyses by examining 164 patients. The C2GnT staining examples were obtained from 164 patients (according to the results of the clinicopathological analyses) (Fig. 2A and B). The typical C2GnT-negative and -positive staining patterns are shown in Fig. 2A and B. Based on the staining status of the Glogi apparatus as previously observed (10,14), specimens with 10% or more positive cancer cells were judged as C2GnT-positive specimens. The specimens were then divided into two groups, C2GnT-negative and -positive specimens (Fig. 2). The table in Fig. 2C represents the correlation between C2GnT expression and pathological status of patients, indicating that C2GnT expression positively correlates with both tumor grade and pathological stage. These results show that C2GnT expression is an excellent indicator of bladder tumor progression, suggesting that C2GnT-expressing bladder tumor cells are highly metastatic.
Figure 2.
Correlation of C2GnT expression with progression of bladder tumors. (A and B) Typical examples of immunohistochemical analyses of bladder tumors using anti-C2GnT antibody. (A) C2GnT-negative tumor. (B) C2GnT-positive tumor. Bars, 10 μm. (C) The correlation between C2GnT expression and pathological status of patients. C2GnT expression in 164 specimens which were obtained by transurethral resection or radical cystectomy, was examined using anti-C2GnT antibody.
Core 2 O-glycosylation of MUC1
Growing evidence indicates that mucin O-glycosylation is important in many biological processes including the protection of epithelial cell surfaces, the immune responses, cell adhesion, inflammation, tumori-genesis and tumor metastasis (5). These observations, taken together with the results shown in Fig. 2, led us to postulate that core 2 O-glycans carried by MUC1 play an important role in bladder tumor metastasis. We first analyzed MUC1 from the bladder tumor cells for O-glycosylation by western blot analysis. MUC1 from the C2GnT-expressing KK-47-C2 cells exhibited a larger molecular weight than MUC1 from C2GnT-non-expressing KK-47 cells (Fig. 3, lanes 1 and 2). By contrast, there was no significant difference in molecular weight of the non-O-glycosylated cell-surface protein, LAMP1 between KK-47 and KK-47-C2 cells (Fig. 3, lanes 5 and 6). These results indicate that MUC1 from KK-47-C2 cells carries core 2 O-glycans. Similarly, MUC1 from C2GnT-expressing YTS cells carries more core 2 O-glycans than YTSC2KD cells with reduced C2GnT expression (Fig. 3, lanes 9 and 10).
Figure 3.
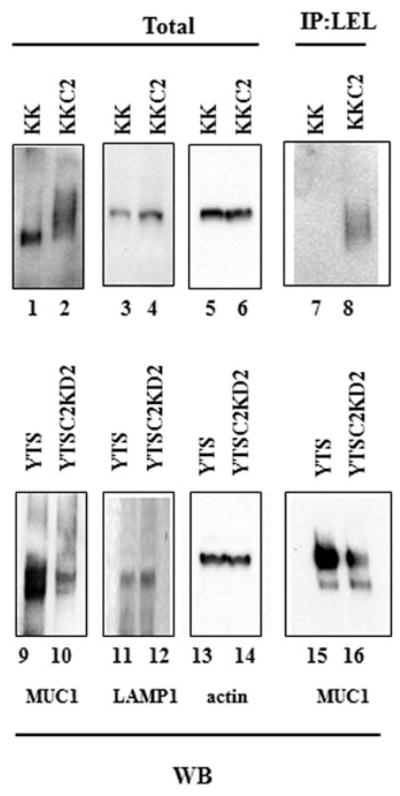
C2GnT-expressing bladder tumor cells express MUC1 carrying poly-N-acetyllactosamine in O-glycans. Total cell lysates from bladder tumor cells, KK-47, KK-47-C2, YTS and YTSC2KD were analyzed by western blot analysis with anti-MUC1 (lanes 1, 2, 9 and 10), anti-LAMP1 (lanes 3, 4, 11 and 12) and anti-actin (lanes 5, 6, 13 and 14). Total lysates were immunoprecipitated with LEL-agarose followed by western blot analysis with anti-MUC1 (lanes 7, 8, 15 and 16).
The core 2 branch is a scaffold of subsequent production of lactosamine disaccharide repeats, poly-N-acetyllactosamine (Galβ1-4GlcNAc)n (Fig. 1) (16). To determine whether MUC1 from KK-47-C2 cells carries poly-N-acetyllactosamine in O-glycans, we excluded N-glycans from bladder tumor cells by treatment with tunicamycin, an N-glycosylation inhibitor, and then analyzed the cell lysates by immunoprecipitation using LEL. LEL binds specifically to poly-N-acetyllactosamine with at least three lactosamine unit repeats. The LEL immunoprecipitates were subjected to western blot analysis with anti-MUC1 antibody. MUC1 was barely detectable in the LEL immunoprecipitates from KK-47 cells, but MUC1 was detected in the LEL immunoprecipitates from KK-47-C2 cells (Fig. 3, lanes 7 and 8). MUC1 was also detected at higher levels in the LEL immunoprecipitates from YTS than YTSC2KD cells (Fig. 3, lanes 15 and 16). These results indicate that MUC1 from C2GnT-expressing bladder tumor cells carries poly-N-acetyllactosamine in core 2 O-glycans.
Glectin-3 binds to MUC1 through poly-N-acetyllactosamine in O-glycans
Poly-N-acetyllactosamine is a ligand for galectins (17,18). Among the 15 members of the galectin family, we focused on glectin-3 on the surface of bladder tumor cells, since galectin-3 is widely distributed in normal tissues and has been shown to be involved in the survival of disseminating cancer cells in host blood circulation during metastasis (10,19,20). Cell-surface proteins were labeled with biotin and the cells were lysed. Biotinylated total cell-surface proteins were recovered by streptavidin-agarose beads and analyzed by western blot analysis for galectin-3. Galectin-3 was detected in the total cell-surface proteins from the bladder tumor cells at similar levels, regardless of C2GnT expression (Fig. 4, lanes 1 and 2). Subsequently, tunicamycin-treated cells were subjected to DTSSP cross-linking of cell-surface proteins to stabilize complexes. MUC1 was immunoprecipitated from the cell lysates of KK-47 and KK-47-C2 cells (Fig. 4, lanes 3 and 4). The MUC1 immunoprecipitates were analyzed by western blot analysis for galectin-3. Galectin-3 co-immunoprecipitated with MUC1 from KK-47-C2 cells, whereas the co-immunoprecipitation of galectin-3 with MUC1 from KK-47 cells was undetectable (Fig. 4, lanes 5 and 6). We also confirmed that higher levels of galectin-3 co-immunoprecipitated with MUC1 from YTS cells than YTSC2KD cells (Fig. 4, lanes 7–12). These results taken together with those shown in Fig. 3 suggest that galectin-3 binds to MUC1 through poly-N-acetyllactosamine in core 2 O-glycans in C2GnT-expressing tumor cells.
Figure 4.
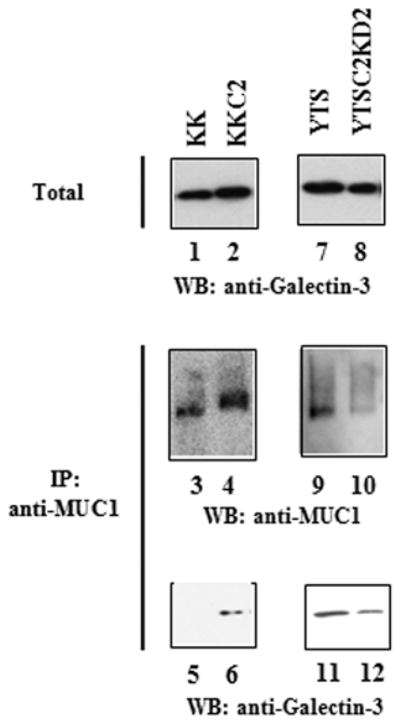
Galectin-3 binds to MUC1 through poly-N-acetyllactosamine in core 2 O-glycans at the cell surface. Bladder tumor cells, KK-47, KK-47-C2, YTS and YTSC2KD were subjected to biotinylation with sulfo-NHS-biotin. Biotinylated cell-surface proteins were recovered using streptavidin-agarose and analyzed by western blot analysis with anti-galectin-3 (lanes 1, 2, 7 and 8). Bladder tumor cells were also subjected to dithio-bis sulphosuccinimydyl propionate (DTSSP) cross-linking of cell-surface proteins and the total lysates were immunoprecipitated with anti-MUC1 followed by western blot analysis with anti-MUC1 (lanes 3, 4, 9 and 10) and anti-galectin-3 (lanes 5, 6, 11 and 12).
Fibronectin binding to bladder tumor cells is affected by C2GnT expression
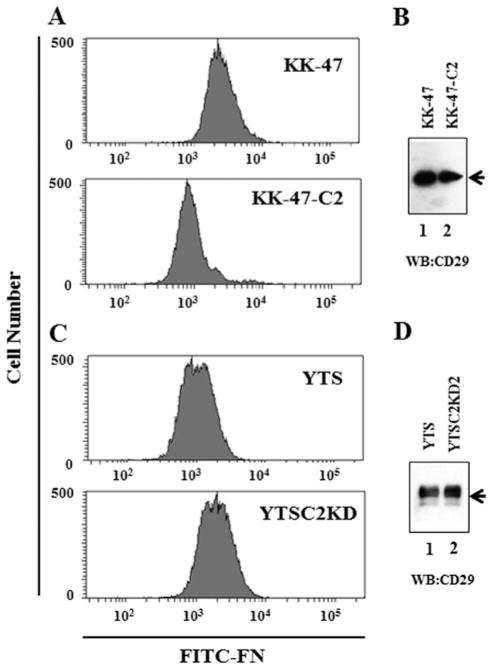
It has been reported that mucins contain the domain that mediates anti-adhesion (5,28). To examine the anti-adhesion property of MUC1 carrying core 2 O-glycans, the binding of fibronectin to bladder tumor cells was analyzed. Fibronectin was labeled with fluorescein isothiocyanate (FITC) by incubation with 4 mg/ml FITC in 50 μM Na2CO3 (pH 9.5) at room temperature for 16 h (21). Bladder tumor cells were incubated with FITC-labeled fibronectin and then analyzed on a flow cytometer, FACSCanto II (BD Biosciences). Fibronectin binding to KK-47-C2 cells was reduced, compared with KK-47 cells (Fig. 5A), although there was no difference in the expression levels of CD29, which is a fibronectin receptor between KK-47 and KK-47-C2 cells (Fig. 5B). Fibronectin binding to YTS cells was also reduced, compared with YTSC2KD cells (Fig. 5C), although there was no difference in the expression levels of CD29 between YTS and YTSC2KD cells (Fig. 5D). These results indicate that the fibronectin binding to C2GnT-expressing cells is lower than that to cells with reduced C2GnT expression. On the whole, the results shown in Figs. 3–5 suggest that the binding of galectin-3 to MUC1 through poly-N-acetyl-lactosamine in C2GnT-expressing tumor cell surface increases the anti-adhesiveness of MUC1, interfering with fibronectin binding to cells.
Figure 5.
Effect of C2GnT expression on fibronectin binding to bladder tumor cells. Binding of FITC-labeled fibronectin to bladder tumor cells was analyzed by FACS. (A) Fibronectin binding to KK-47 (upper panel) and KK-47-C2 cells (lower panel). (B) Expression levels of CD29, a fibronectin receptor subunit in the total lysates from bladder tumor cells were analyzed by western blotting using anti-CD29 monoclonal antibody. Lysates from KK-47 (lane 1) and KK-47-C2 cells (lane 2). (C) Fibronectin binding to YTS (upper panel) and YTSC2KD cells (lower panel). (D) Expression levels of CD29 in the total lysates from bladder tumor cells were analyzed. Lysates from YTS (lane 1) and YTSC2KD cells (lane 2).
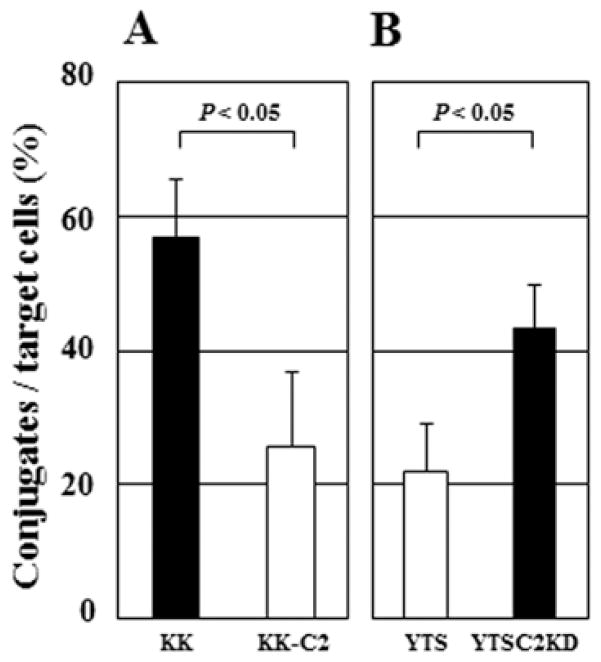
Conjugate formation of bladder tumor cells with NK cells is affected by C2GnT expression
NK cells play a critical role in tumor rejection responses in host circulation. NK cell attack to tumor cells is initiated by the NK cell-tumor cell interaction mediated through the NK receptor-tumor ligand interaction (22). Based on the results showing that C2GnT expression impaired the binding of ECM proteins to C2GnT-expressing tumor cells (Fig. 5), we investigated whether C2GnT expression affects the NK cell-tumor cell interaction. To address this question, we made a comparison of the conjugate formation with NK cells between C2GnT-expressing tumor cells and tumor cells with reduced C2GnT expression. After 1-h incubation of target tumor cells with NK cells, KK-47 cells formed more conjugates with NK cells than KK-47-C2 cells and YTSC2KD cells also formed more conjugates than YTS cells (Fig. 6A and B), indicating that C2GnT-non-expressing tumor cells or cells with reduced C2GnT expression (KK-47 and YTSC2KD cells) were able to form more conjugates with NK cells than C2GnT-expressing tumor cells (KK-47 and YTSC2KD cells). These results taken together with those shown in Figs. 4 and 5 suggest that in C2GnT-expressing tumor cells, the binding of galectin-3 to MUC1 through poly-N-acetyllactosamine in its O-glycans increases the anti-adhesiveness of MUC1, thereby impairing the NK cell-tumor cell interaction.
Figure 6.
Conjugate formation of NK cells with target tumor cells. (A) Hetero-typic conjugate formation of NK cells with KK-47 (closed bar) and KK-47-C2 (open bar) cells. (B) Heterotypic conjugate formation of NK cells with YTS (open bar) and YTSC2KD (closed bar) cells. Mean values ± SE of three independent experiments.
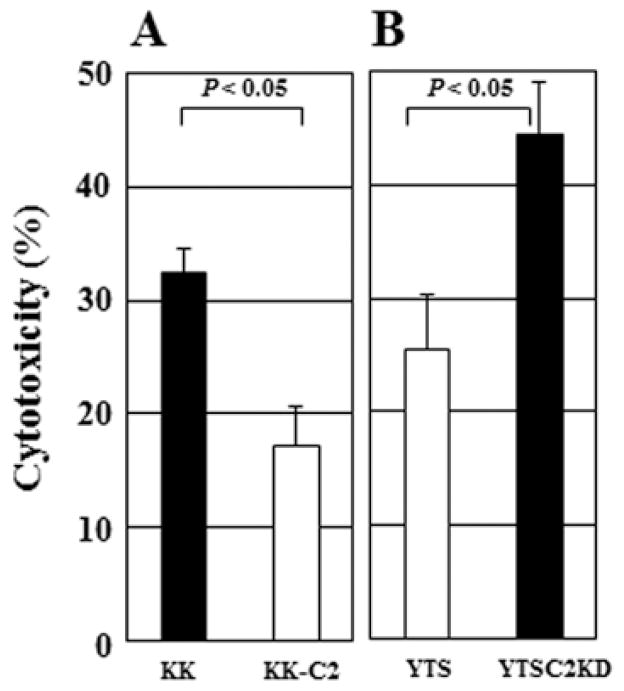
Effect of C2GnT expression on NK cell cytotoxicity
We then investigated whether C2GnT expression in tumor cells affects the cytotoxic activity of NK cells, since the interaction of NK cells with C2GnT-expressing tumor cells was impaired (Fig. 6). To address this question, we assayed the cytotoxicity of NK cells against tumor cells. NK cells killed KK-47 cells more efficiently than KK-47-C2 cells and NK cells also killed YTSC2KD cells more efficiently than YTS cells (Fig. 7A and B), indicating that C2GnT-expressing tumor cells (KK47-C2 and YTS cells) are more resistant to NK cell cytotoxicity than tumor cells with reduced C2GnT expression (KK-47 and YTSC2KD cells). These results suggest that C2GnT expression reduces the interaction of the tumor cells with NK cells (Fig. 6) and that C2GnT-expressing tumor cells become more resistant to NK cell cytotoxicity than tumor cells with reduced C2GnT expression, due to their reduced NK cell-tumor cell interaction (Fig. 7).
Figure 7.
Effect of C2GnT expression on NK cell cytotoxicity. (A) Cytotoxicity of human NK cells against KK-47 (closed bar) and KK-47-C2 (open bar) was assayed. (B) Cytotoxicity of NK cells against YTS (open bar) and YTSC2KD (closed bar) was also assayed. The assays were performed at an effector:target ratio of 5:1. Mean values ± SE of three independent experiments.
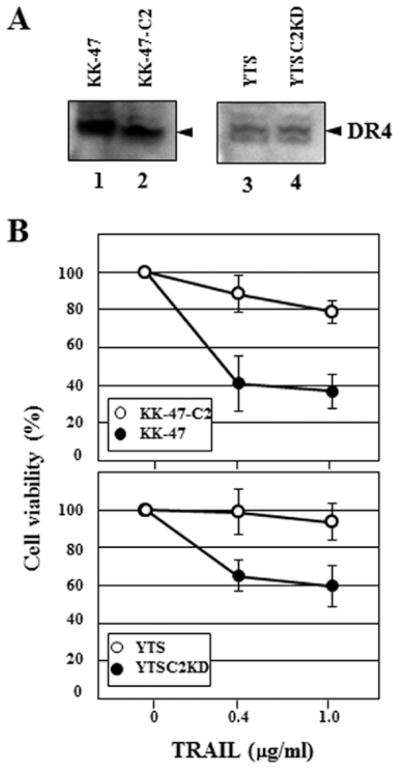
Core 2 O-glycan is a molecular shield against TRAIL
NK cells use two main mechanisms to kill tumor cells. One is that NK cells are activated through the NK cell receptor-tumor ligand interaction to release cytotoxic granules containing perforin, granzymes and other constituents. The other one is the induction of tumor cell apoptosis by death ligands including TRAIL (23). We previously demonstrated that C2GnT expression in bladder tumor cells impairs the NK receptor-tumor ligand interaction, thereby reducing the release of cytotoxic granules (10). However, the effect of C2GnT expression in target tumor cells on the attack of the death ligands has not been examined. TRAIL induces target cell apoptosis through its interaction with death receptors, such as DR4. We examined the expression of DR4 in bladder tumor cells by western blot analysis. There were no significant differences in the DR4 expression levels between KK-47 and KK-47-C2 (Fig. 8A, lanes 1 and 2) and YTS and YTSC2KD cells (Fig. 8A, lanes 3 and 4). Unlike MUC1, no significant difference in O-glycosylation of DR4 between C2GnT-expressing tumor cells (KK-47-C2 and YTS) and cells with reduced C2GnT expression (KK-47 and YTSC2KD) were observed on the western blots (Figs. 3 and 8A). To evaluate the effect of C2GnT expression in the bladder tumor cells on TRAIL sensitivity, we measured TRAIL-induced cell death by using soluble recombinant TRAIL (R&D Systems). The cell viability of KK-47-C2 cels was higher than KK-47 cells in the presence of TRAIL and cell viability of YTS cells was also higher than YTSC2KD cells (Fig. 8B). These results indicate that C2GnT-expressing tumor cells are more resistant to TRAIL than tumor cells with reduced C2GnT expression. MUC1 mediates anti-adhesion, as MUC1 is one of the most extended molecules above the cell surface (5,28). Our results suggest that MUC1 modified by poly-N-acetyllactosamine and galectin-3 in C2GnT-expressing tumor cells increases its anti-adhesiveness. Increased anti-adhesiveness reduces the accessibility of TRAIL to the death receptors, resulting in evasion from NK cell attack in the circulation. Thus, our results suggest that MUC1 carrying core 2 O-glycans functions as a molecular shield against TRAIL to promote C2GnT-expressing bladder tumor metastasis.
Figure 8.
Molecular shield function of the core 2 O-glycan against TRAIL. (A) Expression of death receptor 4 (DR4), a receptor for TRAIL in the bladder tumor cells, KK-47 (lane 1), KK-47-C2 (lane 2), YTS (lane 3) and YTSC2KD (lane 4) was analyzed by western blot analysis. (B) Cell viability of KK-47 (closed symbols) and KK-47-C2 (open symbols) cells (upper panel). Cell viability of YTS (open symbols) and YTSC2KD (closed symbols) cells (lower panel). Cells were incubated with TRAIL for 24 h at the indicated concentrations. Cell viability was determined using Cell Counting kit-8. Mean values ± SE of three independent experiments.
Discussion
By analyzing bladder tumor cell lines and human bladder tumor specimens, we show that galectin-3 binds to MUC1 through the poly-N-acetyllactosamine in O-glycans in C2GnT-expressing bladder tumor cells. Our results reveal that this MUC1 modification causes the immune evasion of tumor cells from NK cell immunity. In our previous study, when bladder tumor cells were intravenously injected into nude mice, C2GnT-expressing tumor cells produced more metastatic foci in the lungs than C2GnT-non-expressing tumor cells (10). Our present data taken together with data from our previous study, strongly suggest that this immune evasion results in increased survival of C2GnT-expressing tumor cells, promoting bladder tumor metastasis.
The immunomodulatory function of galectin-3 that we present in this study is supported by previous studies. Peng et al showed that tumor cell-surface-associated galectin-3 promotes tumor growth in vivo by inhibiting T cell immune responses (24,25). The contribution of galectin-3 to the barrier functions of the cell-surface MUC1 is also supported by a previous study. Arguesto et al reported that galectin-3 is associated with cell-surface mucins through O-glycans, stabilizing mucosal barriers of the ocular surface epithelia (26). The present study also suggests that inhibition of the immunomodulatory functions of galectin-3 may improve the therapeutic potential of cancer immunotherapy.
Wagner et al reported that tumor-cell sensitivity to TRAIL was controlled by O-glycosylation of death receptors (DR4 and DR5). In 22 out of 28 TRAIL-sensitive cancer cell lines, the expression of a peptidyl O-glycosyltrasferase which catalyzes the initial step of O-glycosylation was elevated (27). It has been shown that the O-glycosylation of death receptors promotes TRAIL-stimulated clustering of the receptors, mediating recruitment and activation of the apoptosis-initiating protease, caspase-8. Although their O-glycan structures have not been analyzed, O-glycosylation increases TRAIL-sensitivity of cancer cells. Wagner et al observed O-glycosylation of death receptors on western blots in the 22 out of 28 TRAIL-sensitive cell lines (27). However, no significant O-glycosylation of DR4 was observed in the present study. This suggests that C2GnT-expressing bladder tumor cells use a different mechanism to control tumor-cell sensitivity to TRAIL from the mechanism reported by Wagner et al.
Our investigation will provide a new insight into the roles of the carbohydrates which tumor cell-surface mucins carry in tumor metastasis. Tumor cells take advantage of the functions of mucins to maintain homeostasis and promote their survival in the variable conditions. Further analyses of the roles of tumor cell-surface carbohydrates will contribute to the better understanding of the process of tumor metastasis.
Acknowledgments
This study was supported by the Grants-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (22570131) (to S.T.) and (B22390301) (to C.O.), the Ministry of Education, Culture, Sports, Science and Technology of Japan (21791483) (to C.O.) and (21791484) (to T.K.), Japan Science and Technology Agency (CREST) (to C.O.) and NIH grants P01CA71932 and R01CA3000 (to M.F.).
References
- 1.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Fuertes MB, Girart MV, Molinero LL, et al. Intracellular retention of the NKG2D ligand MHC class I chain-related gene A in human melanomas confers immune privilege and prevents NK cell-mediated cytotoxicity. J Immunol. 2008;180:4606–4614. doi: 10.4049/jimmunol.180.7.4606. [DOI] [PubMed] [Google Scholar]
- 3.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 4.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 5.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 6.Yousefi S, Higgins E, Daoling Z, Pollex-Krüger A, Hindsgaul O, Dennis JW. Increased UDP-GlcNAc: Gal beta 1-3GaLNAc-R (GlcNAc to GaLNAc) beta-1, 6-N-acetylglucosaminyltransferase activity in metastatic murine tumor cell lines. Control of poly-lactosamine synthesis. J Biol Chem. 1991;266:1772–1782. [PubMed] [Google Scholar]
- 7.Shimodaira K, Nakayama J, Nakamura N, Hasebe O, Katsuyama T, Fukuda M. Carcinoma-associated expression of core 2 beta-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res. 1997;57:5201–5206. [PubMed] [Google Scholar]
- 8.Machida E, Nakayama J, Amano J, Fukuda M. Clinicopathological significance of core 2 beta1,6-N-acetyl-glucosaminyltransferase messenger RNA expressed in the pulmonary adenocarcinoma determined by in situ hybridization. Cancer Res. 2001;61:2226–2231. [PubMed] [Google Scholar]
- 9.Hagisawa S, Ohyama C, Takahashi T, et al. Expression of core 2 beta1,6-N-acetylglucosaminyltransferase facilitates prostate cancer progression. Glycobiology. 2005;15:1016–1024. doi: 10.1093/glycob/cwi086. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi S, Sutoh M, Hatakeyama S, et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011;30:3173–3185. doi: 10.1038/emboj.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hisazumi H, Uchibayashi T, Katoh M, et al. Anticancer drug sensitivity in vitro in the bladder cancer cell line, KK-47 and prophylactic use of carbazilquinone and urokinase in bladder cancer. Urol Res. 1981;9:231–235. doi: 10.1007/BF00256892. [DOI] [PubMed] [Google Scholar]
- 12.Kubota Y, Nakada T, Yanai H, Kakizaki H, Sasagawa I, Watanabe M. Electropermeabilization in bladder cancer chemotherapy. Cancer Chemother Pharmacol. 1996;39:67–70. doi: 10.1007/s002800050539. [DOI] [PubMed] [Google Scholar]
- 13.Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press; Lyon: 2004. [Google Scholar]
- 14.Skrincosky D, Kain R, El-Battari A, Exner M, Kerjaschki D, Fukuda M. Altered Golgi localization of core 2 beta-1,6-N-acetyl-glucosaminyltransferase leads to decreased synthesis of branched O-glycans. J Biol Chem. 1997;272:22695–22702. doi: 10.1074/jbc.272.36.22695. [DOI] [PubMed] [Google Scholar]
- 15.van de Wiel-van Kemenade E, Ligtenberg MJ, de Boer AJ, et al. Episialin (MUC1) inhibits cytotoxic lymphocyte-target cell interaction. J Immunol. 1993;151:767–776. [PubMed] [Google Scholar]
- 16.Maemura K, Fukuda M. Poly-N-acetyllactosaminyl O-glycans attached to leukosialin. The presence of sialyl Le(x) structures in O-glycans. J Biol Chem. 1992;267:24379–24386. [PubMed] [Google Scholar]
- 17.Sato S, Hughes RC. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem. 1992;267:6983–6990. [PubMed] [Google Scholar]
- 18.Knibbs RN, Agrwal N, Wang JL, Goldstein IJ. Carbohydrate-binding protein. [PubMed] [Google Scholar]
- 35.II. Analysis of the interaction of the recombinant polypeptide with saccharides. J Biol Chem. 1993;268:14940–14947. [PubMed] [Google Scholar]
- 19.Fukumori T, Kanayama HO, Raz A. The role of galectin-3 in cancer drug resistance. Drug Resist Updat. 2007;10:101–108. doi: 10.1016/j.drup.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 21.Tsuboi S. Calcium integrin-binding protein activates platelet integrin alpha IIbbeta 3. J Biol Chem. 2002;277:1919–1923. doi: 10.1074/jbc.M110643200. [DOI] [PubMed] [Google Scholar]
- 22.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nature Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cretney E, Shanker A, Yagita H, Smyth MJ, Sayers TJ. TNF-related apoptosis-inducing ligand as a therapeutic agent in autoimmunity and cancer. Immunol Cell Biol. 2006;84:87–98. doi: 10.1111/j.1440-1711.2005.01413.x. [DOI] [PubMed] [Google Scholar]
- 24.Peng W, Wang HY, Miyahara Y, Peng G, Wang RF. Tumor-associated galectin-3 modulates the function of tumor-reactive T cells. Cancer Res. 2008;68:7228–7236. doi: 10.1158/0008-5472.CAN-08-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 26.Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009;284:23037–23045. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner KW, Punnoose EA, Januario T, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 28.Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, Hilkens J. Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol. 1995;129:255–265. doi: 10.1083/jcb.129.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]